Abstract
The next WHO classification should abandon “salivary duct carcinoma”; conventional salivary duct carcinoma should be classified as “high-grade salivary duct carcinoma”. Low-grade salivary duct carcinoma should replace the current nosology of “low-grade cribriform cystadenocarcinoma”. Cystadenocarcinoma should be classified with the descriptor “Not Otherwise Specified” and should be considered an exclusionary diagnostic category. On the other hand, “Not Otherwise Specified” does not fit for hyalinizing clear cell carcinoma (HCCC). The EWSR1-ATF1 fusion is specific for HCCC within the context of salivary neoplasia. We recommend adding “hyalinizing” even though this feature is not present in all cases; the benefit of which is the mental association with a salivary clear cell malignancy. Sinonasal Renal Cell-like Adenocarcinoma (SNRCLA) is a distinct clear cell neoplasm and should be added to the next WHO classification. Future studies will bear out whether SNRCLA is even a low-grade carcinoma, or may be reclassified as “adenoma”. Lastly, the next WHO monograph should include the Risk Model in the general introductory statements on oral squamous cell carcinoma, under a subheading of “Histological Prognosticators”. The positive predictive value for developing locoregional recurrence in patients with low-stage oral cavity squamous carcinoma (OSCC) and “worst pattern of invasion type-5” (WPOI-5) is 42 %. Low-stage high-risk OSCC with a combination of features other than WPOI-5 is associated with 32 % likelihood for locoregional progression. WPOI-5 also predicts occult metastatic disease (p = 0.0001, Chi squared, 2 DF). Thus the Risk Model can also be used to make decisions regarding staged elective neck dissections.
Keywords: WHO, Head and neck classification , Salivary duct, High-grade, Low-grade, Clear cell, Hyalinizing, Renal-cell-like
The Common Language of the World Health Organization (WHO)
Imagine the chaos that would reign if there was no universally accepted diagnostic nomenclature. If pathological diagnoses were subject to different regional nuances, imagine the fragmentation. We need not go further than the imagery of the Tower of Babel to convince the reader of the tremendous value of a uniformly adapted nomenclature as set out in the WHO series of “blue books”. The current “WHO Classification of Head and Neck Tumors” was published in 2005 after a meeting of the minds in Lyon, France in July, 2003. Ten years and many developments later, and in anticipation of the next WHO Head and Neck Working Group, the North American Society for Head and Neck Pathology and the European Working Group for Head and Neck Pathology have proactively developed “wish lists” of the changes and updates we would like to see in the next WHO Classification of Head and Neck Tumors.
The Classification of Salivary Ductal Carcinomas
The term “Low-grade Salivary Duct Carcinoma” (LGSDC) was introduced in 1996 by Delgado and colleagues to describe an entity resembling benign or atypical mammary duct hyperplasia [1]. These tumors were predominantly intraductal and characterized by three possible architectural patterns: (1) Cystically dilated ducts with tufted, micropapillary anastomosing proliferations, (2) Distended ducts with solid “pseudocribriform” (lacey) fenestrations or solid papillary proliferations, and (3) Intraductal proliferations with “conventional architectural atypia” (Roman bridges) akin to the cribriform architectural pattern typical of low-grade intraductal breast carcinoma (Fig. 1). Cytologically, the tumor cells were bland with heterogeneous morphology. Delgado noted that LGSDC and conventional “salivary duct carcinoma” were histologically and biologically quite distinct from one another. None of their six patients with follow-up developed locoregional recurrence or distant metastases. In 2004, we published a series of 16 patients with LGSDC [2]. Similarly, these were multifocal tumors composed of cytologically bland, heterogeneous cells forming intraductal proliferations with variable architectural patterns. Invasion beyond the intraductal component was documented in four cases. No LGSDC was associated with lymph node metastases. Follow-up on 13 of 16 patients demonstrated that all patients were disease-free. At the time the current WHO classification was being compiled in 2003, the Delgado manuscript was the only published description of LGSDC; thus it was not a well-accepted entity [1]. The decision, at that time, was to debut LGSDC in the 2005 WHO publication as “low-grade cribriform cystadenocarcinoma”, a variant of cystadenocarcinoma [3].
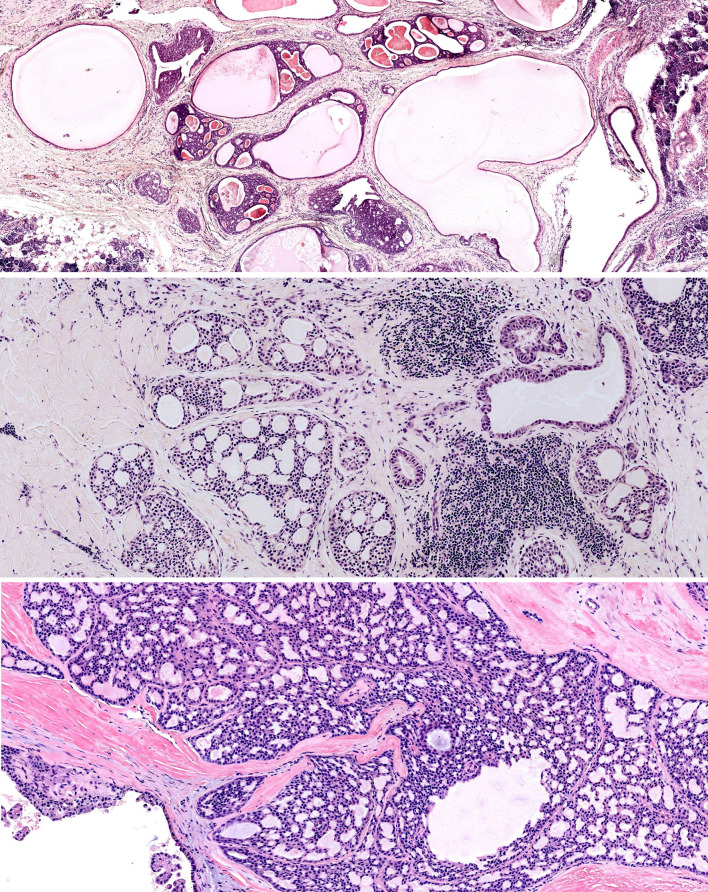
Fig. 1.
Low-grade salivary duct carcinoma—cystically dilated ducts with intraductal proliferation (top). Fenestrated, pseudocribriform architecture (middle and bottom)
Eight years and many publications later, LGSDC is increasingly recognized as a distinct entity. In 2013, two publications summarized all of the published findings of LGSDC in the English language literature [4, 5]. (The literature reviews did not overlap entirely; Wang [5] included two new patients and excluded a number of reports [6–10].) A wide age range is reported (27–93, mean 61.4 years) with a female predominance (F:M = 1.5:1) [4]. Most LGSDC arise in the parotid parenchyma (84.6 %), followed by intraparotid lymph nodes (5.1 %), minor salivary glands (5.1 %), accessory parotid (2.6 %), and submandibular gland (2.6 %) [4]. The typical low-power appearance reveals a well-circumscribed, nonencapsulated cystic tumor with prior hemorrhage. The bland tumor cells are epithelioid, with pale or eosinophilic cytoplasm and indistinct cell membranes (Fig. 2). They contain small, round to oval nuclei with finely dispersed or condensed chromatin and small nucleoli. The cellular heterogeneity includes tumor cells with apocrine differentiation (apical snouts, microvacuoles) and rare but pathognomonic luminal cells with fine yellow to brown cytoplasmic pigment. Invasive or micro-invasive carcinoma may be better appreciated after immunohistochemical (IHC) staining for calponin; this has been reported in 9 cases (23 %) of LGSDC. Perineural invasion or lymphovascular tumor emboli have not been observed. In total, five reported cases of LGSDC demonstrated limited areas of transition to higher cytologic grade including necrosis; [1, 2, 11, 12] this includes an unusual case demonstrating intraductal LGSDC, foci of typical intraductal and invasive high-grade salivary duct carcinoma (HGSDC), plus multiple regional lymph node metastases of high-grade carcinoma [12]. Importantly, all reported patients with LGSDC, to date, including this latter case, are disease-free [1–14].
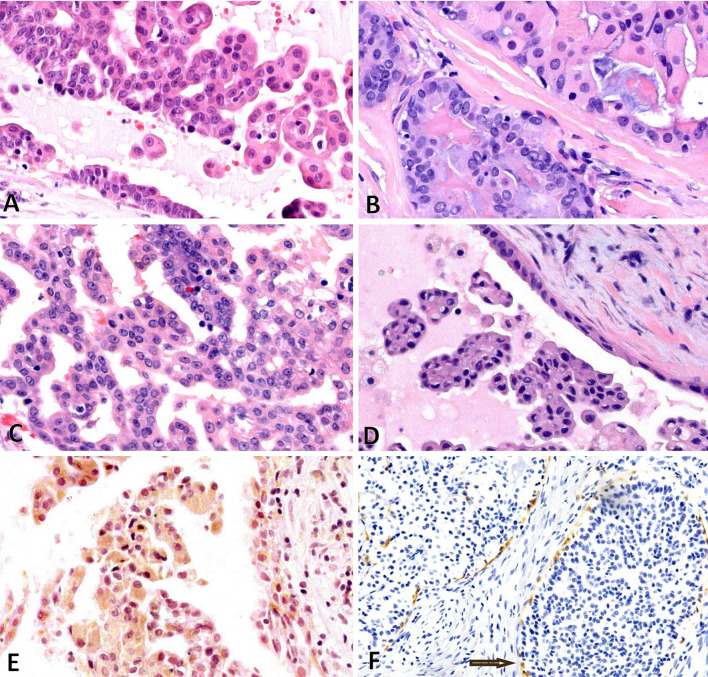
Fig. 2.
Low-grade salivary duct carcinoma—a through d demonstrate bland tumor cells with papillary or fenestrated architecture. Hobnail cells are seen (a, d). e Demonstrates tumor cells with yellow/brown pigment. f Demonstrates IHC for calponin, which delineates the ducts
What is the relationship between LGSDC and conventional HGSDC? The weight of the evidence argues against LGSDC being a precursor lesion to HGSDC. HGSDC appears uniformly high-grade (Fig. 3). Only one case, thus far, has documented the histological gamut of low-grade and high-grade duct carcinoma [12]. Therefore, LGSDC and HGSDC should be considered as separate entities. On the other hand, HGSDC which are entirely intraductal may recur and progress to invasive carcinoma [15].
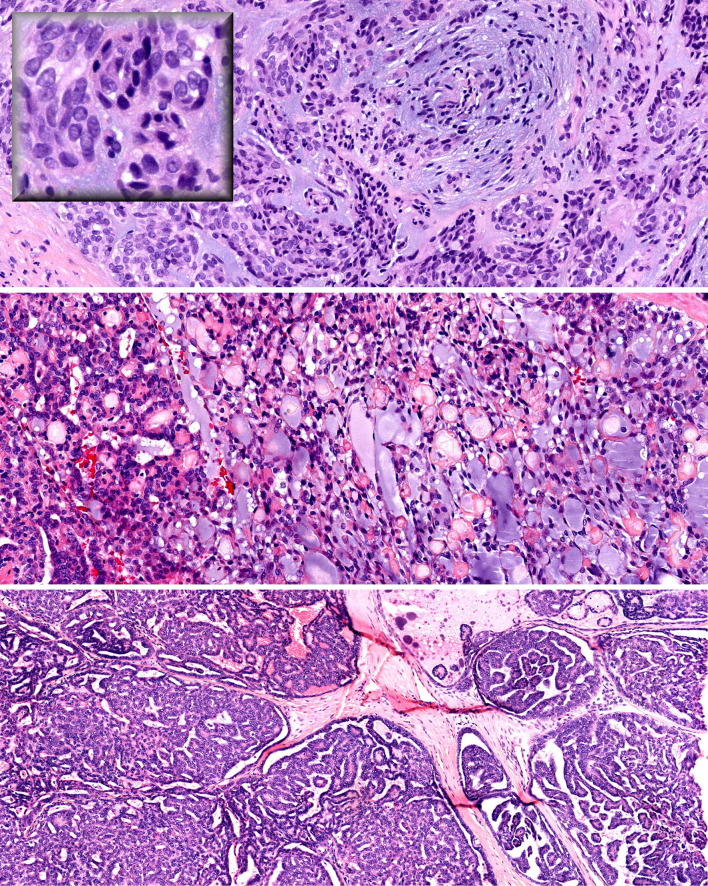
Fig. 3.
High-grade salivary duct carcinoma—necrosis (left) and high-grade grade tumor cells (right)
LGSDC Versus Cystadenocarcinoma
What then is the relationship between LGSDC and cystadenocarcinoma? Foss and colleagues published the largest series of (papillary) cystadenocarcinomas, 56 patients, in 1996 [16]. The difficulties in comparing these two entities lie in the fact that the latter clearly represents a heterogeneous group which did not benefit from the diagnostic refinements of IHC and molecular studies. Some of the illustrated cystadenocarcinomas do resemble LGSDC; yet other tumors were described as high-grade, contained comedonecrosis and likely represent HGSDC [16, 17]. All cystadenocarcinomas initially described by Foss were infiltrative and 20 % arose in the minor salivary glands [16]. In contrast, LGSDC is commonly entirely intraductal, and minor salivary origin is much less common (5.1 %) [4].
Therefore, the current nosology of cystadenocarcinoma begs for refinement. Importantly, defined entities require exclusion; these include HGSDC, LGSDC, low-grade papillary adenocarcinoma (LGPA), cribriform adenocarcinoma of tongue (CAT), which will be discussed later in this symposium, mammary analogue secretory carcinoma (MASC), and acinic cell carcinoma. HGSDC is a high-grade epithelioid infiltrative adenocarcinoma with eosinophilic glassy cytoplasm and high-grade nuclei. In situ high-grade cribriform ductal carcinoma with comedonecrosis is characteristic but not diagnostically requisite; this in situ morphology is frequently mimicked within the invasive or metastatic component. HGSDC is usually mitotically active, expresses androgen receptor, and overexpresses HER2. By contrast, LGSDC is characterized by a prominent intraductal cystic proliferation, with a pattern described as “pseudocribriform” or lacey fenestrations. If seen in the breast, these lesions would evoke a nonmalignant diagnosis (usual ductal hyperplasia or atypical ductal hyperplasia). Invasion may not be obvious and is better appreciated after immunohistochemistry (IHC) for calponin. Cytologically, LGSDC tumor cells are low-grade, bland, and may be morphologically heterogeneous. Necrosis and mitotic activity are not seen. Androgen receptors may be expressed in up to 62 % of LGSDC. However, HER2 is not overexpressed in LGSDC, and is probably the single best adjuvant test to distinguish between LGSDC and HGSDC, when adjuvant testing is necessary.
Low-grade papillary adenocarcinoma (LGPA) has also been included within the nosology of “papillary cystadenocarcinoma” [17, 18]. Although still controversial, LGPA is considered a variant of polymorphous low-grade adenocarcinoma (PLGA). LGPA may merit nosological distinction from PLGA based on higher rates of local recurrence and regional metastases [19–23]. There is tremendous morphologic overlap between PLGA and LGPA (Fig. 4). Both tumors are usually composed of bland epithelioid cells with vesicular nuclei, fine chromatin, and abundant cytoplasm. The characteristic features of blue/grey matrix, whorling fasicular arrangement, targetoid perineural invasion, and single cell infiltration can be seen in both PLGA and LGPA. A myoepithelial tumor component can be present in both tumors. PLGA is typically more basaloid than LGPA. The classification as LGPA is primarily based on the presence of papillary and solid tumor architectural components. The published outcome literature varies with respect to defined cut-offs for these papillary and solid components, hence this issue is unresolved. However, the morphologic features of PLGA/LGPA allow for exclusion of this entity from classification as papillary cystadenocarcinoma.
Fig. 4.
Low-grade papillary adenocarcinoma (LGPA) and polymorphous low-grade adenocarcinoma (PLGA) share characteristic features: low-grade epithelioid tumor cells with vesicular nuclei (top inset), whorling pattern and slate blue matrix (top), and lacey pseudocribriform pattern (middle). The bottom panel demonstrates a papillary pattern
Lastly, it is conceivable that some tumors formerly classified as papillary cystadenocarcinoma might represent mammary analogue secretory carcinoma (MASC) or acinic cell carcinomas. Mammaglobin (mamma) and S100 protein expression is usually sufficient to distinguish MASC (mamma and S100 positive) from acinic cell carcinoma (mamma negative and usually little or no S100 expression). However, mamma expression is not specific, as other salivary tumors may also express mamma [24, 25]. Fluorescent in situ hybridization (FISH) for the ETV-NTRK3 fusion remains the gold standard in establishing the diagnosis.
In summary, we suggest the next WHO classification abandon the current “salivary duct carcinoma”. Conventional salivary duct carcinoma should be classified as “high-grade salivary duct carcinoma”, further stratified as either “invasive” or “in situ”. In situ HGSDC is extremely rare and may have less aggressive potential [26, 27]. Low-grade salivary duct carcinoma should replace the current nosology of “low-grade cribriform cystadenocarcinoma”. No further stratification for invasion is necessary as all LGSDC reported to date are associated with excellent outcome. Cystadenocarcinoma should be classified with the additional descriptor of “Not Otherwise Specified”, and this should be considered an exclusionary diagnostic category.
Salivary Clear Cell Carcinoma: Not Otherwise Specified?
The origins of hyalinizing salivary clear cell carcinoma (HCCC) began with its separation from myoepithelial tumors and recognition as a low-grade malignancy. The AFIP fascicle first mentions salivary “clear cell adenoma” in the second series published in the pre-IHC era of 1974. However, clear-cell predominant myoepithelial tumors were being described and illustrated under this moniker [28]. Milchgrub and colleagues reported the first series of 11 patients with HCCC in 1994 [29]. They described a group of tumors lacking glandular/tubular formation, which infiltrated in strands or trabecular patterns. These tumors are cytologically characterized by clear, glycogen-rich cytoplasm and small dark bland nuclei. Importantly, there was no evidence of myoepithelial differentiation, thus separating HCCC from mimics such as epithelial–myoepithelial carcinoma and clear cell myoepithelial tumors [29]. Two HCCC patients presented with positive cervical lymph nodes and no patient with clinical follow-up developed local recurrence [29]. HCCC debuted in the 2005 WHO publication with the terminology “Clear Cell Carcinoma, Not Otherwise Specified” [30]. It was noted that, “unfortunately, a rationale for the qualifier “not otherwise specified” (was) not provided” [31]. In the AFIP fascicles published in 2008, HCCC is classified as “clear cell adenocarcinoma” [32]. However, HCCC rarely reveal any glandular differentiation and frequently demonstrate features of squamous differentiation (tonofilaments, prominent desmosomes, fine filopodia) [31]. Hence designating HCCC as an adenocarcinoma is somewhat misleading. Lastly, the exclusionary or “wastebasket” qualifier “not otherwise specified” for HCCC is also misleading. The specific association of HCCC with the t(12;22) translocation resulting in an EWSR1-ATF1 fusion clearly sets it apart from other salivary neoplasia [33].
The rationale for the initial investigation of the EWSR1 gene rearrangement in the context of HCCC was based on histologic similarities between HCCC and soft tissue myoepithelial tumor (SMET), which commonly contains the EWSR1-POU5F1 rearrangement [34]. SMET is one of many tumors, such as Ewing’s sarcoma, peripheral neuroectodermal tumor, and clear cell sarcoma of soft tissue, that harbor EWSR1 rearrangements [35]. Antonescu and colleagues demonstrated EWSR1 gene rearrangement in 82 % of HCCC by FISH; ATF1 was the fusion partner in 93 % of retested cases [33]. Importantly, EWSR1, ATF1, and FUS rearrangements were not found in any of the salivary HCCC mimics, such as epithelial–myoepithelial carcinoma, clear cell myoepithelial carcinoma, clear cell-predominant mucoepidermoid carcinoma, or in SCC with clear cell features. (The rationale for FUS translocation testing is that it is often a substitute for EWSR1 in fusion positive soft tissue sarcomas.) Conversely, it has been suggested that HCCC might represent a variant of clear cell predominant mucoepidermoid carcinoma (MEC). However, rearrangement of MAML2, which is common and characteristic to MEC, is not observed in HCCC [33].
What else have we learned in the last two decades? Accumulated experience bears out that HCCC is generally a low-grade malignancy with predilection for intraoral sites, most often palate and tongue base; origin from major salivary glands or extraoral minor salivary sites is very rare [36, 37]. Histologically, HCCC commonly elaborates hyalinized matrix and elicits a desmoplastic response, zonally decreasing at the tumor periphery [38]. There may be a sharp juxtaposition between the hyalinized and fibrocellular stromas [38]. HCCC invades in strands, trabeculae, and nests and ductoglandular formation is not observed. Perineural invasion has been seen in approximately one-third of tumors [39]. Necrosis and mitotic activity are usually not seen. Tumor cells are generally uniform with abundant, glycogen-positive cytoplasm. A second population of smaller tumor can be seen at periphery of tumor islands with (non-clear) eosinophilic cytoplasm, or little cytoplasm. The tumor nuclei are small and nucleoli are inconspicuous. Nuclei are usually situated peripherally and may appear shrunken and “raisinoid”. Although classification as an adenocarcinoma is unwarranted, intracytoplasmic mucin is observed in many translocation-confirmed HCCC [33]. Gland formation is a rare sighting (Fig. 5). HCCC may be observed to arise directly from mucosal epithelium and demonstrate intramucosal Pagetoid extension. Squamous differentiation and overt keratin pearls may also be observed [38, 40]. Review of the literature confirms that the rate of cervical lymph node metastases is approximately 25 % and one-third of patients have developed local recurrence. Although classified as a low-grade malignancy, pulmonary metastases, widespread metastases, and disease-related mortality are uncommon but documented [36, 37, 39, 41–44]. Progression in histological grade (high-grade transformation), e.g. increased mitotic rate, necrosis, and cytologic anaplasia, have been described in patients who develop disease-progression [41, 42, 44, 45].
Fig. 5.
Hyalinizing clear cell carcinoma—infiltrating cords and nests of clear tumor cells with hyaline production (top). Periodic Acid Schiff reveals granular cytoplasmic glycogen (top inset). A subtle biphasic tumor population is seen composed of cells with less cytoplasm (middle left and right). Nuclear pleomorphism and rare gland formation (bottom left and right)
Hyalinizing Clear Cell Carcinoma Versus Clear Cell Odontogenic Carcinoma?
Interestingly, clear cell odontogenic carcinoma (CCOC) bears many histologic similarities to HCCC; indeed the question as to whether CCOC actually represents “central HCCC” has been posed in the past. A recent interesting development is that these two morphologically similar tumors also share the EWSR1 rearrangement [46]. The EWSR1 rearrangement was demonstrated in 83 % of confirmed CCOC but not in other mandibular tumors with clear cell features (calcifying epithelial odontogenic tumor or Pindborg tumor, central SCC with clear cell features) [46].
CCOC is an expansile jaw malignancy with a predilection for the mandible and a female:male ratio of 2:1. Logoregional recurrence has been reported in approximately one-third of patients and the rate of distant metastases is 14 % [47–50]. Histologically, CCOC has been characterized as either monophasic or biphasic. Monophasic CCOC is composed of relatively small bland tumor cells with clear, glycogen-rich cytoplasm forming nests, and strands within a fibromyxoid stroma. The biphasic CCOC contains an additional component of smaller basaloid or squamoid cells which can surround tumor nests and islands (Fig. 6). This second component is identical to the smaller cells described in HCCC. The histological rationale for distinguishing “central clear cell carcinoma” from CCOC, proposed by Berho and Huvos, was the presence of strand-like rather than lobulated pattern of infiltration and tumor hyalinization in “central clear cell carcinoma” [51]. A detailed histological and immunohistochemical comparison between HCCC and CCOC revealed that the histochemical and immunohistochemical profiles were identical, myoepithelial differentiation was also excluded in CCOC. Only the histologic finding of peripheral palisading, present in more than half of CCOC could distinguish CCOC from HCCC [50]. The degree of “relatedness” overwhelms this minor distinction between CCOC and HCCC.
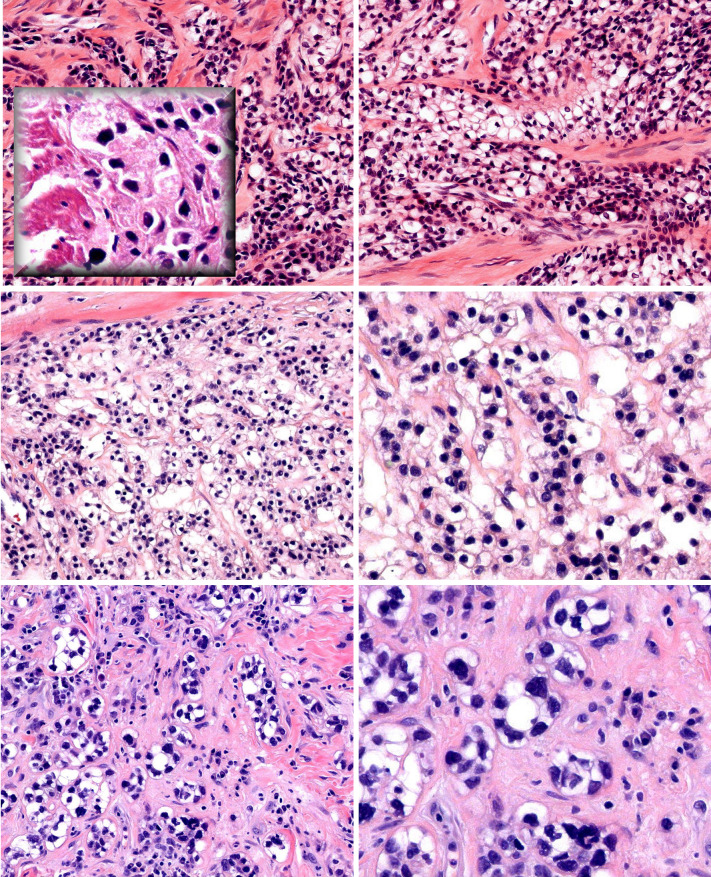
Fig. 6.
Clear cell odontogenic carcinoma—lobulated growth pattern (top). Strands and nests of clear tumor cells in a myxoid stroma (middle left and right). Cells at the periphery of this nest have less cytoplasm, reminiscent of the second population of smaller tumor cells in HCCC (bottom)
In summary, the qualifier, “Not Otherwise Specified” does not fit for HCCC, which is a distinct salivary malignancy. Thus far, the EWSR1-ATF1 fusion has not been detected in other salivary tumors, and is specific for HCCC within the context of salivary neoplasia. We recommend adding the descriptor “hyalinizing” even though this histologic feature is not present in all cases. The “hyalinizing” descriptor has only been associated with the nomenclature of salivary HCCC and thyroid trabecular hyalinizing adenoma. Thus the benefit of adding “hyalinizing” is the mental association with a salivary clear cell malignancy, as opposed to clear cell malignancies of other origins. The argument could also be made to extract “odontogenic” from CCOC, and classify it as intraosseous variant of HCCC, based on the genetic and histological overlap.
Sinonasal Renal Cell-Like Adenocarcinoma
It is fitting to follow the discussion above with another clear cell neoplasm currently bearing the moniker, “Sinonasal Renal Cell-Like Adenocarcinoma” (SNRCLA). In 2002, two independent case reports described a characteristic, unique low-grade clear cell neoplasm which did not fit any existing diagnostic category [52, 53]. In 2008, we reported two new patients and also updated the follow-up on the two original index patients [52–54]. Eight additional patients have been identified in the published literature which are summarized in Table 1, although the degree of detail varies. In addition, a new thirteenth patient is added to Table 1. Two case reports actually describe the same patient [58, 59]. Three other publications contain suboptimal histopathological documentation, and are considered possible SNRCLA [56, 57, 60].
Table 1.
Reported cases of sinonasal renal cell-like carcinoma
| Histological pattern | Cytokeratin | CK7 | CK20 | EMA | S100 | Vimentin | CEA | SMA | CD10 | RCC | Treatment | Outcome | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Heffner [63] | 62F | Nasal | Papillary, glandular | Resection, RT | Recurrence at 3 and 5 years. AWD at 7.5 years | ||||||||||
| 2 | Newman [62] | 77 M | Nasal, frontal sinuses | Nested | Resection | |||||||||||
| 3 | Moran [61] | 67 M | Sinonasal | Glandular, infiltrating | ||||||||||||
| 4 | Zur [52, 54] | 50F | Nasal | Glandular | + | + | − | + | + | − | + | − | − | − | Resection, RT | NED 8 years |
| 5 | Hadi [53, 54] | 22F | Nasal | Glandular | + | − | − | − | − | Resection | NED 5 years | |||||
| 6 | Stork [54] | 36F | Nasal | Glandular and solid | + | + | + | − | − | + | + | Resection, RT | NED 4 years | |||
| 7 | Stork [54] | 69 M | Nasopharynx | Glandular | + | − | − | − | RT | NED 2 years | ||||||
| 8 | Cheng [55] | 63F | Nasopharynx | + | Resection, RT | NED 1 years | ||||||||||
| 9 | Negahban [56] | 52F | Nasal, paranasal sinuses | + | + | − | + | − | − | Resection | LTF | |||||
| 10 | LI [59] Hong 2013 [58] | 34 M | Nasal | Glandular, nested | + | + | + | + | − | − | − | − | ChemoRT | NED 1 years | ||
| 11 | Huang [57] | 54F | Nasal | “Clear cell carcinoma” | + | + | − | − | +/− | Resection | NED 1 years | |||||
| 12 | Suzuki [60] | 59F | Sphenoid skull base | + | − | − | + | + | + | − | − | Primary RT | NED 2 years | |||
| 13 | Brandwein-Gensler | 56F | Nasal, skull base | Glandular, eosinophilic and basophilic cells | + | + | + | − | + | − | − | Resection, RT | NED 3 months | |||
RT radiotherapy, NED no evidence of disease, AWD alive with disease, LTF lost to followup
Epistaxis is a common presenting symptom, although not specific for SNRCLA. Seven tumors arose in the nasal cavity and two originated in the nasopharynx. A number of reports specify the absence of pre-existing, synchronous, or subsequent renal cell carcinomas, emphasizing a cursory resemblance to renal cell carcinoma (RCC). Histologically, SNRCLA is composed of monomorphous cuboidal to columnar glycogen-rich clear cells lacking mucin production (Fig. 7). The cellular cytoplasm may be “crystal clear”, or may be slightly eosinophilic. Patient #13 is unusual in that tumor cells with basophilic and eosinophilic cytoplasm were intermixed with the clear tumor cells. The nuclei are typically small, round or condensed, and may have a single prominent nucleolus; intranuclear holes may also be seen. Dilated vascular spaces are seen. The glandular structures are composed of simple back-to-back glands with a microfollicular pattern. Larger, longer, tubular glands can be present, containing eosinophilic secretory material and mimicking thyroid follicular carcinoma, clear cell variant. Nested, solid, or papillary patterns may also be seen. There is no hyalinized stroma or evidence of myoepithelial differentiation. No necrosis is seen. Nuclear pleomorphism and mitotic activity are usually limited. Some tumors are described as infiltrating. Histologically, these tumors are less vascular and pleomorphic as compared to RCC. The overall histologic impression is that of a low-grade neoplasm. Of note, no patients presented with positive lymph nodes.
Fig. 7.
Sinonasal renal cell-like adenocarcinoma—gland-forming tumor with pink secretions and increased vascularity reminiscent of CC-RCC (a). Bland tumor cells forming “thyroid-like” follicles (b). Intranuclear holes (c). This SNRCLA was unusual as the bland clear tumor cells were interspersed with cells with basophilic and eosinophilic cytoplasm (d, e, f)
Ten patients have clinical follow-up. One patient described by Heffner et al. [63] (their Case 6, low-grade carcinomas) developed multiple local recurrences. Nine other patients are disease-free after primary treatment (3 months to 8 years, mean 2.6 years). SNRCLA may not even be a low-grade malignancy, for as to date there have been no reports of metastases. Further experience with this rare lesion will bear out whether reclassification to “adenoma” is justified. Clearly, SNRCLA is associated with a better outcome than HCCC or CCOC, and represents a different disease entity.
The main differential diagnosis is with salivary hyalinizing clear cell carcinoma (HCCC); histologically, these two are very different. Strand-like growth and stromal hyalinization are characteristic of HCCC and absent in SNRCLA. Conversely, gland formation and expression of EMA, CEA, and S100 protein by IHC supports SNRCLA and rules out HCCC. One SNRCLA was found to be negative for EWSR1 rearrangements [46].
Clear cell predominant myoepithelial tumors and epithelial myoepithelial carcinomas are ruled out by demonstrating the absence of myoepithelial differentiation. Light microscopically, this tumor group is characterized by tumor heterogeneity which also include spindled, plasmacytoid, and epithelioid myoepithelial cells, ductule formation, and basement membrane deposition.
Metastatic clear cell RCC (CC-RCC) can be distinguished from SNRCLA by necrosis, nuclear pleomorphism, and frank hemorrhage. IHC is straight-forward in distinguishing RCC from SNRCLA. SNRCLA will typically express CK7 as well as other keratins, and 6/8 tumors are negative for vimentin. CC-RCC expresses vimentin, RCC antigen, PAX2, PAX-8 and CA9. CK7 is typically negative in CC-RCC but is diffusely and strongly expressed in a variant of RCC with exclusive clear cell cytology known as clear cell papillary RCC. However, this tumor is biologically likely to be indolent, with no lymph node or other metastases reported to date. One SNRCLA (Table 1, case 13) has been tested for CA9 and was strongly positive.
The differential diagnosis of “salivary clear cells” includes acinic cell carcinoma and oncocytoma with clear cell change. This became a relevant issue in diagnosing Case 13. Interspersed basophilic or oncocytic cells are not seen in Cases 4–7, nor have they been described. Additional studies ruled out the possibility of true acinar or oncocytic differentiation for this case: IHC for DOG-1 demonstrated limited apical and membranous staining whereas acinic cell typically demonstrates diffuse apical/luminal staining, and histochemistry for PTAH was negative.
In summary, SNRCLA is a clear cell neoplasm, which is distinct from HCCC, and other clear mimickers. We suggest this tumor be added to the next WHO classification as “Sinonasal Renal Cell-like Adenocarcinoma”. Two cases have been described in the nasopharynx; could these cases actually have arisen in the vicinity of the posterior choanae? As this neoplasm is gland-forming, we suggest “adenocarcinoma” rather than “carcinoma”, to further emphasize a distinction with HCCC. Future studies will bear out whether SNRCLA is even a low-grade carcinoma, or may be reclassified as “adenoma”.
The Risk Model for Oral Cavity Squamous Carcinoma (OSCC)
As pathologists, we all can innately recognize the phenotype of aggressive carcinomas. However, where do you define the “cut-points”? How can we meaningfully distinguish between oral carcinomas that are very-aggressive and not-so-very aggressive in a reproducible manner? In the Risk Model, we defined and validated those cut-point points [64–66]. The greatest value of the Risk Model is in identifying patients with low-stage OSCC who are at significantly elevated risk for treatment failure and may be offered multi-modality therapy based on the weight of evidence. These patients might otherwise have been treated with surgery alone. For patients with low-stage OSCC and “worst pattern of invasion type-5″ (WPOI-5) the positive predictive for developing locoregional recurrence is 42 % [66]. Low-stage OSCC classified as high-risk for a combination of features other than WPOI-5 is associated with 32 % likelihood for locoregional progression. One could argue that prediction based on odds of 1:2 or 1:3 are suboptimal. However, there is currently no better validated biomarker for patients with OSCC.
WPOI-5 tumors are recognized by their dispersed, discontiguous growth pattern; the degree of tumor dispersion exceeds that seen for WPOI-4 tumors with a defined cut-off of 1 mm. The tumor dispersion distance may be measured between the main tumor and “the first wave” of dispersed satellites, or between subsequent, distal waves of satellites. A conservative approach should be adopted when assessing WPOI. When identifying putative WPOI-5 tumor satellites, it is preferable to examine the sections immediately adjacent to the area of interest, to exclude any connecting tumor projections. There are a number of possible histological phenotypes for WPOI-5 tumors; most commonly WPOI-5 tumors are dispersed throughout soft tissue either as tumor strands, small rounded tumor satellites, or large tumor satellites. More rarely, tumors can be classified as WPOI-5 due to either dispersed perineural invasion or dispersed lymphovascular tumor emboli. A simple method for microscopic measurement is overlaying the pathology slides with millimeter rulers printed on acetate film (See http://www.vendian.org/mncharity/dir3/paper_rulers).
Vered and colleagues published a cohort of 50 patients with oral SCC, all stages, evaluating the performance of the Risk Model. Multivariable analysis demonstrated that high-risk classification was significantly predictive of local recurrence when adjusted for confounders (p = 0.022, HR 11.2, 95 % CI 1.4, 87.1) [67]. Rodrigues de Matos and colleagues applied the risk model to 62 patients with all stages of tongue carcinoma; they found that limited lymphocytic host response and perineural invasion correlated with positive cervical lymph nodes on presentation (p = 0.034, p = 0.035, respectively) [68]. This group found no association between WPOI-5 and positive lymph nodes; however they identified only one tumor with WPOI-5 (out of 62 OSCC, or 1.6 %), whereas we would have expected a higher frequency of WPOI-5 (13 % Stage I, 21 % Stage 2) [66].
We studied 71 patients with T1/T2 squamous carcinomas of the tongue and/or floor of mouth, all T1 cancers had depth-of invasion greater than 4 mm. Clinicoradiographically, all patients were deemed cN0, and underwent elective neck dissection. Table 1 demonstrates the rates of WPOI-3, WPOI-4, and WPOI-5 for patients per pathologic lymph node stage. WPOI-5 significantly predicts the presence of occult metastatic disease (p = 0.0001, Chi squared, 2 DF) [69]. Thus the Risk Model can also be used to make decisions regarding staged elective neck dissections. The 2013 publication provides practical details with respect to the application of the Risk Model [66]. However, we recommend that pathologists contact the senior author (MBG) for additional education materials. In summary, we recommend that the next WHO monograph include the Risk Model within the general introductory statements on oral squamous cell carcinoma, under a subheading of “Histological Prognosticators”. We believe that clinicians will ultimately drive the process by requesting this additional prognostic information added to the surgical reports.
References
- 1.Delgado R, Klimstra D, Albores-Saavedra J. Low grade salivary duct carcinoma. A distinctive variant with a low-grade histology and a predominant intraductal growth pattern. Cancer. 1996;78:958–967. doi: 10.1002/(SICI)1097-0142(19960901)78:5<958::AID-CNCR4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Brandwein-Gensler M, Hille J, Wang BY, Urken M, Gordon R, Wang LJ, Simpson JR, Simpson RH, Gnepp DR. Low-grade salivary duct carcinoma: description of 16 cases. Am J Surg Pathol. 2004;28:1040–1044. doi: 10.1097/01.pas.0000128662.66321.be. [DOI] [PubMed] [Google Scholar]
- 3.Brandwein-Gensler M, Gnepp DR, et al. Low-grade cribriform cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, et al., editors. Pathology and genetics head and neck tumors. Lyon: IARC Press; 2005. p. 233. [Google Scholar]
- 4.Kuo YJ, Weinreb I, Perez-Ordonez B. Low-grade salivary duct carcinoma or low-grade intraductal carcinoma? Review of the literature. Head Neck Pathol. 2013;7(Suppl 1):S59–S67. doi: 10.1007/s12105-013-0460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Liu Y, Lin X, Zhang D, Li Q, Qiu X, Wang EH. Low-grade cribriform cystadenocarcinoma of salivary glands: report of two cases and review of the literature. Diagn Pathol. 2013;8:28. doi: 10.1186/1746-1596-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen KT. Cytology of salivary duct carcinoma. Diagn Cytopath. 2000;22:132–135. doi: 10.1002/(SICI)1097-0339(200002)22:2<132::AID-DC17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb I. Intraductal carcinoma of salivary gland (so-called low-grade cribriform cystadenocarcinoma) arising in an intraparotid lymph node. Head Neck Pathol. 2011;5:321–325. doi: 10.1007/s12105-011-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusafuka K, Itoh H, Sugiyama C, et al. Low-grade salivary duct carcinoma of the parotid gland: report of a case with immunohistochemical analysis. Med Mol Morphol. 2010;43:178–184. doi: 10.1007/s00795-009-0479-2. [DOI] [PubMed] [Google Scholar]
- 9.Khurana KK, Pitman MB, Powers CN, et al. Diagnostic pitfalls of aspiration cytology of salivary duct carcinoma. Cancer. 1997;81:373–378. doi: 10.1002/(SICI)1097-0142(19971225)81:6<373::AID-CNCR12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Ide F, Mishima K, Saito I. Circumscribed salivary duct carcinoma of the palate: a non-threatening variant. Histopathology. 2004;45:89–91. doi: 10.1111/j.1365-2559.2004.01809.x. [DOI] [PubMed] [Google Scholar]
- 11.Tatemoto Y, Ohno A, Osaki T. Low malignant intraductal carcinoma on the hard palate: a variant of salivary duct carcinoma? Eur J Cancer B Oral Oncol. 1996;32B:275–277. doi: 10.1016/0964-1955(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 12.Weinreb I, Tabanda-Lichauco R, Van der Kwast T, Perez-Ordoñez B. Low-grade intraductal carcinoma of salivary gland: report of 3 cases with marked apocrine differentiation. Am J Surg Pathol. 2006;30:1014–1021. doi: 10.1097/00000478-200608000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Nakatsuka S, Harada H, Fujiyama H, Takeda K, Kitamura K, Kimura H, Nagano T, Ito M, Asada Y. An invasive adenocarcinoma of the accessory parotid gland: a rare example developing from a low-grade cribriform cystadenocarcinoma? Diagn Pathol. 2011;6:122. doi: 10.1186/1746-1596-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laco J, Podhola M, Dolezalova H. Low-grade cribriform cystadenocarcinoma of the parotid gland: a neoplasm with favorable prognosis, distinct from salivary duct carcinoma. Int J Surg Pathol. 2010;18:369–373. doi: 10.1177/1066896910367649. [DOI] [PubMed] [Google Scholar]
- 15.Anderson C, Muller R, Piorkowski R, Knibbs DR, Vignoti P. Intraductal carcinoma of major salivary gland. Cancer. 1992;69:609–614. doi: 10.1002/1097-0142(19920201)69:3<609::AID-CNCR2820690302>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Foss RD, Ellis GL, Auclair PL. Salivary gland cystadenocarcinomas. A Clinicopathologic study of 57 cases. Am J Surg Pathol. 1996;20:1440–1447. doi: 10.1097/00000478-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Ellis GL, Auclair PL. Armed Forces Institute of Pathology (AFIP) Atlas of Tumor Pathology. Tumors of the Salivary Glands (fourth series fascicle 9) Maryland: ARP Press; 2008. p. 283–288.
- 18.Auclair PL, et al. Cystadenocarcinoma. In: Barnes L, Eveson JW, Reichart P, et al., editors. Pathology and genetics head and neck tumors. Lyon: IARC Press; 2005. p. 232. [Google Scholar]
- 19.Hunter JB, Smith RV, Brandwein-Gensler M. Low-grade papillary adenocarcinoma of the palate: the significance of distinguishing it from polymorphous low-grade adenocarcinoma. Head Neck Pathol. 2008;2:316–323. doi: 10.1007/s12105-008-0082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans HL, Luna MA. Polymorphous low-grade adenocarcinoma: a study of 40 cases with long-term follow up and an evaluation of the importance of papillary areas. Am J Surg Pathol. 2000;24:1319–1328. doi: 10.1097/00000478-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Castle JT, Thompson LD, Frommelt RA, Wenig BM, Kessler HP. Polymorphous low grade adenocarcinoma: a clinicopathologic study of 164 cases. Cancer. 1999;86:207–219. doi: 10.1002/(SICI)1097-0142(19990715)86:2<207::AID-CNCR4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell DA, Eveson JW, Ord RA. Polymorphous low-grade adenocarcinoma of minor salivary glands—a report of three cases. Br J Oral Maxillofac Surg. 1989;27:494–500. doi: 10.1016/S0266-4356(89)80008-7. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Ordonez B, Linkov I, Huvos AG. Polymorphous low-grade adenocarcinoma of minor salivary glands: a study of 17 cases with emphasis on cell differentiation. Histopathology. 1998;32:521–529. doi: 10.1046/j.1365-2559.1998.t01-2-00410.x. [DOI] [PubMed] [Google Scholar]
- 24.Bishop JA, Yonescu R, Batista D, Begum S, Eisele DW, Westra WH. Utility of mammaglobin immunohistochemistry as a proxy marker for the ETV6-NTRK3 translocation in the diagnosis of salivary mammary analogue secretory carcinoma. Hum Pathol. 2013;44:1982–1988. doi: 10.1016/j.humpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel KR, Solomon IH, El-Mofty SK, Lewis JS, Jr, Chernock RD. Mammaglobin and S-100 immunoreactivity in salivary gland carcinomas other than mammary analogue secretory carcinoma. Hum Pathol. 2013;44:2501–2508. doi: 10.1016/j.humpath.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Simpson RH, Desai S, Di Palma S. Salivary duct carcinoma in situ of the parotid gland. Histopathology. 2008;53:416–425. doi: 10.1111/j.1365-2559.2008.03135.x. [DOI] [PubMed] [Google Scholar]
- 27.Simpson RH. Salivary duct carcinoma: new developments—morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol. 2013;7(Suppl 1):S48–S58. doi: 10.1007/s12105-013-0456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thackray AC, Lucas RB, Armed Forces Institute of Pathology (AFIP) Atlas of Tumor Pathology. Tumors of the Major Salivary Glands (second series fascicle 10); Maryland: ARP Press; 1974. p. 62–63.
- 29.Milchgrub S, Gnepp DR, Vuitch F, Delgado R, Albores-Saavedra J. Hyalinizing clear cell carcinoma of salivary gland. Am J Surg Pathol. 1994;18:74–82. doi: 10.1097/00000478-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Ellis G, et al. Clear cell carcinoma, not otherwise specified. In: Barnes L, Eveson JW, Reichart P, et al., editors. Pathology and genetics head and neck tumors. Lyon: IARC Press; 2005. p. 227. [Google Scholar]
- 31.Dardick I, Leong I. Clear cell carcinoma: review of its histomorphogenesis and classification as a squamous cell lesion. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:399–405. doi: 10.1016/j.tripleo.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 32.Ellis GL, Auclair PL, Armed Forces Institute of Pathology (AFIP) Atlas of Tumor Pathology. Tumors of the Salivary Glands (fourth series fascicle 9) Maryland: ARP Press; 2008. p. 301–309.
- 33.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, Perez-Ordoñez B, Have C, Asa SL, Leong IT, Bradley G, Klieb H, Weinreb I. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer. 2011;50:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 34.Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, Edelman M, Rosenberg AE, Nielsen GP, Dal Cin P, Fletcher CD. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosom Cancer. 2010;49:1114–1124. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol. 2012;36:e1–e11. doi: 10.1097/PAS.0b013e31825485c5. [DOI] [PubMed] [Google Scholar]
- 36.Kauzman A, Tabet JC, Stiharu TI. Hyalinizing clear cell carcinoma: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e26–e34. doi: 10.1016/j.tripleo.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 37.Solar AA, Schmidt BL, Jordan RC. Hyalinizing clear cell carcinoma: case series and comprehensive review of the literature. Cancer. 2009;115:75–83. doi: 10.1002/cncr.23974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinreb I. Hyalinizing clear cell carcinoma of salivary gland: a review and update. Head Neck Pathol. 2013;7(Suppl 1):S20–S29. doi: 10.1007/s12105-013-0466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Sullivan-Mejia ED, Massey HD, Faquin WC, Powers CN. Hyalinizing clear cell carcinoma: report of eight cases and a review of literature. Head Neck Pathol. 2009;3:179–185. doi: 10.1007/s12105-009-0124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanguay J, Weinreb I. What the EWSR1-ATF1 fusion has taught us about hyalinizing clear cell carcinoma. Head Neck Pathol. 2013;7:28–34. doi: 10.1007/s12105-013-0427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Regan E, Shandilya M, Gnepp DR, Timon C, Toner M. Hyalinizing clear cell carcinoma of salivary gland: an aggressive variant. Oral Oncol. 2004;40:348–352. doi: 10.1016/j.oraloncology.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 42.Fulciniti F, Pia Curcio M, Liguori G, Aquino G, Botti G, Campanile AC, DeCecio R, Pavone E, Aversa C, Perri F, Caponigro F, Ionna F. Hyalinizing clear cell carcinoma of the parotid gland: Report of a recurrent case with aggressive cytomorphology and behavior diagnosed on fine-needle cytology sample. Diagn Cytopathol. 2013; doi:10.1002/dc.22956. [DOI] [PubMed]
- 43.Wang B, Brandwein M, Gordon R, Robinson R, Urken M, Zarbo RJ. Primary salivary clear cell tumors–a diagnostic approach: a clinicopathologic and immunohistochemical study of 20 patients with clear cell carcinoma, clear cell myoepithelial carcinoma, and epithelial–myoepithelial carcinoma. Arch Pathol Lab Med. 2002;126:676–685. doi: 10.5858/2002-126-0676-PSCCTA. [DOI] [PubMed] [Google Scholar]
- 44.Jin R, Craddock KJ, Irish JC, Perez-Ordonez B, Weinreb I. Recurrent hyalinizing clear cell carcinoma of the base of tongue with high-grade transformation and EWSR1 gene rearrangement by FISH. Head Neck Pathol. 2012;6:389–394. doi: 10.1007/s12105-012-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamashita K, Kawakami F, Nakashima Y, Murakami K. Clear cell carcinoma of the minor salivary gland: an autopsy case with multiple metastases 29 years after the initial surgery and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:819–825. doi: 10.1016/j.tripleo.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 46.Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, Barker B, Seethala RR. Clear cell odontogenic carcinomas show EWSR1 rearrangements: a novel finding and a biological link to salivary clear cell carcinomas. Am J Surg Pathol. 2013;37:1001–1005. doi: 10.1097/PAS.0b013e31828a6727. [DOI] [PubMed] [Google Scholar]
- 47.Kumar M, Fasanmade A, Barrett AW, Mack G, Newman L, Hyde NC. Metastasizing clear cell odontogenic carcinoma: a case report and review of the literature. Oral Oncol. 2003;39:190–194. doi: 10.1016/S1368-8375(02)00012-X. [DOI] [PubMed] [Google Scholar]
- 48.Brandwein M, Said-Al-Naief N, Gordon R, Urken M. Clear cell odontogenic carcinoma: report of a case and analysis of the literature. Arch Otolaryngol Head Neck Surg. 2002;128:1089–1095. doi: 10.1001/archotol.128.9.1089. [DOI] [PubMed] [Google Scholar]
- 49.Mosqueda-Taylor A, Meneses-García A, Ruíz-Godoy Rivera LM, de Lourdes Suárez-Roa M. Clear cell odontogenic carcinoma of the mandible. J Oral Pathol Med. 2002;31:439–441. doi: 10.1034/j.1600-0714.2002.00133.x. [DOI] [PubMed] [Google Scholar]
- 50.Bilodeau EA, Hoschar AP, Barnes EL, Hunt JL, Seethala RR. Clear cell carcinoma and clear cell odontogenic carcinoma: a comparative clinicopathologic and immunohistochemical study. Head Neck Pathol. 2011;5:101–107. doi: 10.1007/s12105-011-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berho M, Huvos AG. Central hyalinizing clear cell carcinoma of the mandible and the maxilla a clinicopathologic study of two cases with an analysis of the literature. Hum Pathol. 1999;30:101–105. doi: 10.1016/S0046-8177(99)90308-8. [DOI] [PubMed] [Google Scholar]
- 52.Zur KB, Brandwein M, Wang B, Som P, Gordon R, Urken ML. Primary description of a new entity, renal cell-like carcinoma of the nasal cavity: van Meegeren in the house of Vermeer. Arch Otolaryngol Head Neck Surg. 2002;128:441–447. doi: 10.1001/archotol.128.4.441. [DOI] [PubMed] [Google Scholar]
- 53.Moh’d Hadi U, Kahwaji GJ, Mufarrij AA, Tawil A, Noureddine B. Low grade primary clear cell carcinoma of the sinonasal tract. Rhinology. 2002;40:44–47. [PubMed] [Google Scholar]
- 54.Storck K, Hadi UM, Simpson R, Ramer M, Brandwein-Gensler M. Sinonasal renal cell-like adenocarcinoma: a report on four patients. Head Neck Pathol. 2008;2:75–80. doi: 10.1007/s12105-008-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng LH, Lin YS, Lee JC. Primary clear cell carcinoma of the nasopharynx. Otolaryngol Head Neck Surg. 2008;139:592–593. doi: 10.1016/j.otohns.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 56.Negahban S, Daneshbod Y, Khademi B, Rasekhi AR, Soleimanpour H. Sinonasal clear cell adenocarcinoma: a case report. Acta Cytol. 2009;53:597–600. doi: 10.1159/000325393. [DOI] [PubMed] [Google Scholar]
- 57.Huang XJ, Chen JD, Shi QF. One case with primary clear-cell carcinoma of nasal cavity. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:245–246. [PubMed] [Google Scholar]
- 58.Hong J, Bi Y, Li P, Fang L. Primary nasal clear cell carcinoma: a case report and literature review. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013;27:239–240. [PubMed] [Google Scholar]
- 59.Li P, Yin WH, Yao XJ, Wan L, Chen GR. Primary clear cell carcinoma of nasal cavity: report of a case. Zhonghua Bing Li Xue Za Zhi. 2011;40:52–53. [PubMed] [Google Scholar]
- 60.Suzuki K, Wanibuchi M, Akiyama Y, Ikeda J, Minamida Y, Hasegawa T, Houkin K, Mikuni N. Primary clear cell carcinoma of the skull base and paranasal cavity: a case report. No Shinkei Geka. 2012;40:617–621. [PubMed] [Google Scholar]
- 61.Moran CA, Wenig BM, Mullick FG. Primary adenocarcinoma of the nasal cavity and paranasal sinuses. Ear Nose Throat J. 1991;70(12):821–828. [PubMed] [Google Scholar]
- 62.Newman JP, Funkhouser WK. Pathologic quiz case 1. Clear cell carcinoma of the nasal cavity. Arch Otolaryngol Head Neck Surg. 1993;119:1046–1049. doi: 10.1001/archotol.1993.01880210142022. [DOI] [PubMed] [Google Scholar]
- 63.Heffner DK, Hyams VJ, Hauck KW, Lingeman C. Low-grade adenocarcinoma of the nasal cavity and paranasal sinuses. Cancer. 1982;50:312–322. doi: 10.1002/1097-0142(19820715)50:2<312::AID-CNCR2820500225>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 64.Brandwein-Gensler M, Teixeira MS, Lewis CM, Lee B, Rolnitzky L, Hille JJ, Genden E, Urken ML, Wang BY. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am J Surg Pathol. 2005;29:167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 65.Brandwein-Gensler M, Smith RV, Wang B, Penner C, Theilken A, Broughel D, Schiff B, Owen RP, Smith J, Sarta C, Hebert T, Nason R, Ramer M, DeLacure M, Hirsch D, Myssiorek D, Heller K, Prystowsky M, Schlecht NF, Negassa A. Validation of the histologic risk model in a new cohort of patients with head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676–688. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Bai S, Carroll W, Dayan D, Dort JC, Heller K, Jour G, Lau H, Penner C, Prystowsky M, Rosenthal E, Schlecht NF, Smith RV, Urken M, Vered M, Wang B, Wenig B, Negassa A, Brandwein-Gensler M. Validation of the risk model: high-risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7:211–223. doi: 10.1007/s12105-012-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vered M, Dayan D, Dobriyan A, Yahalom R, Shalmon B, Barshack I, Bedrin L, Talmi YP, Taicher S. Oral tongue squamous cell carcinoma: recurrent disease is associated with histopathologic risk score and young age. J Cancer Res Clin Oncol. 2010;136:1039–1048. doi: 10.1007/s00432-009-0749-3. [DOI] [PubMed] [Google Scholar]
- 68.de Matos FR, Lima E, Queiroz LM, da Silveira EJ. Analysis of inflammatory infiltrate, perineural invasion, and risk score can indicate concurrent metastasis in squamous cell carcinoma of the tongue. J Oral Maxillofac Surg. 2012;70:1703–1710. doi: 10.1016/j.joms.2011.08.023. [DOI] [PubMed] [Google Scholar]
- 69.Brandwein-Gensler M, Chiosea S, unpublished data.