Abstract
The current state in the field of classifying oral and laryngeal precursor lesions, as proposed in the WHO 2005 Blue Book is not ideal. The results of various inter-observer studies have shown that the currently used grading systems, with different basic concepts and different terminology, cannot continue to be reliably used in the future. The different etiology of cervical and head and neck precursor lesions requires a classification designed to cater to the specificities of the head and neck region. Trying to harmonize different classifications of the oral and laryngeal precursor lesions, we have proposed four crucial steps to set up a unified classification of squamous intraepithelial lesions (SILs): (a) the classification should contain two grades, low-grade and high-grade lesions and, specifically for the larynx, an additional grade—carcinoma in situ (CIS) which must be separated from high-grade laryngeal SILs; (b) the terminology should be unified; our preference is for the term SIL over squamous intraepithelial neoplasia; (c) all leading morphological criteria for low- and high-grade lesions, as well as for CIS, should be clearly defined; (d) agreement between clinicians and pathologists should be achieved on the most appropriate choice of treatment of different grades of SILs in separate head and neck areas.
Keywords: Head and neck, WHO Blue Book 2005, Squamous intraepithelial lesions, Classifications, Comparison with cervical intraepithelial neoplasia, Biological behavior, Perspectives
Introduction
Architectural and cytological changes of the squamous epithelium that follow a still not entirely recognized group of genetic changes in the process of head and neck carcinogenesis have been collectively designated squamous intraepithelial lesions (SILs). The term SILs has been proposed as an all embracing expression for the whole spectrum of epithelial changes, ranging from reactive lesions to carcinoma in situ (CIS). More traditionally, part of this spectrum has been designated dysplasia and later also squamous intraepithelial neoplasia (SIN) or, more specifically, laryngeal and oral SIN. However, these terms, which have been directly adopted from precursor lesions of the uterine cervix, do not include a spectrum of reactive epithelial changes, as does the system of the Ljubljana classification (LC) [1]. In view of this diversity in terminology and the disunity of morphological criteria for each individual grading system, it was only to be expected that the current World Health Organization (WHO) classification of head and neck tumours presented three different grading systems for the larynx and oral cavity. The dysplasia system (DS), most widely used in the world, was presented as the WHO classification 2005 (WHO-dysplasia system), accompanied by the system of SIN and the LC [2].
Clinically, head and neck SILs are most frequently visible as white, red or mixed red-whitish (speckled) patches, called leukoplakia, erythroplakia or erythroleukoplakia. Leukoplakias can be either sharply circumscribed and exophytic, or predominantly flat and diffuse, related in part to the amount of keratin. A speckled appearance of leukoplakias can also be present, caused by an unequal thickness of the surface keratin layer. Erythroplakias, which are the least frequent lesions, are characterized by a thinner epithelium and dilated subepithelial vessels. All these lesions are associated with different degrees of epithelial changes; in general, leukoplakias are thought to have a low risk of malignant transformation; pure red lesions have the highest risk of cancer development, especially in high-risk areas, such as the floor of the mouth, lateral borders of the tongue and soft palate/retromolar areas within the oral cavity, and anterior and posterior commissures within the larynx. However, none of these macroscopic features carry any microscopic connotation, which must always be determined by histological examination [1–4].
Eight years after the book’s publication, we would like to evaluate the advantages and disadvantages of the WHO 2005 classifications of head and neck precursor lesions. In addition, we will also discuss some grading systems, which, in spite of not being included in WHO 2005, are well established and in current use. Finally, we will add our vision of what the future holds within this important topic.
Review of Current Grading Systems: Reliability, Inter-Observer Agreement and Biological Behavior of Head and Neck SILs
All three WHO classifications of precursor lesions of the oral cavity and larynx have been variously used in different countries. Preference depends on a pathologist’s training, bias and local custom [5]. Numerous articles have reported attempts to evaluate the reliability and inter-observer agreement of these grading systems for oral and laryngeal SILs [6–13]. Some of them have also compared WHO grading systems with new proposals, such as a binary grading system and other classifications for oral lesions or, more widely, for the whole head and neck region (Table 1) [2, 7, 9, 15–17].
Table 1.
Modified review of Izumo [14] presenting six classifications of oral and laryngeal precursor lesions [2, 7, 15–17]
WHO-DC World Health Organization dysplasia system; CIS carcinoma in situ; SIN squamous intraepithelial neoplasia; LC Ljubljana classification; SIL squamous intraepithelial lesions; OIN oral intraepithelial neoplasia; OED oral epithelial dysplasia; JSOP Japanese Society for Oral Pathology
Oral Cavity
The WHO-dysplasia system has been defined similarly for both the oral cavity and the larynx as architectural disturbance of the epithelium, accompanied by cytological atypia. The criteria for diagnosing dysplasia are presented in the WHO Blue Book 2005. In DS, there is difficulty in recognizing the earliest manifestations and in distinguishing consistently between hyperplasia and mild dysplasia, as well as in attempting rigidly to divide the spectrum of dysplasia into mild, moderate, and severe categories. CIS remains distinct from severe dysplasia [2]. The lack of defined criteria for grading dysplasia is an important source of inconsistent and poorly reproducible results [18]. Several studies have recently revealed major inter- and intra-observer variability in the assessment of the presence and absence, as well as the grade of oral dysplasia [6–9, 14, 17]. The WHO Collaborating Center for Oral Cancer and Precancer in the United Kingdom recommended DS among WHO 2005 classifications for routine use of oral lesions. However, due to frequent problems in providing reliable distinctions between the different grades, the Working Group considered a replacement of the four grades of DS with a two-grade DS, more applicable when reporting the presence or absence of epithelial dysplasia, such as: “no/questionable/mild”—low risk, and “moderate or severe”—implying high risk. This reduction of the number of grades has been shown to have merit, with an improvement in kappa agreements, as previously presented by Kujan and co-workers [6, 7, 14]. The authors compared the inter-observer agreement using the WHO-dysplasia system and the new binary grading system. For a more understandable reading of statistical data, Table 2 presents an interpretation of the kappa statistic, which is a commonly used method of measuring the agreement between two or more observers concerning qualitative items [19]. The overall inter-observer unweighted and weighted kappa agreements for the WHO-dysplasia system were κs = 0.50 (95 % CI 0.11–0.35), κw= 0.63 (CI 0.42–0.78 vs. κ = 0.50 (95 % CI 0.35–0.67) for the new binary system, which grades SILs into either “low risk” or “high risk” lesions. The sensitivity and specificity of the new binary grading system for predicting malignant transformation in oral dysplasia were 85 and 80 %, respectively, and the accuracy was 82 % [7]. The same authors additionally reviewed scores of agreement for individual architectural and cytological criteria of oral SILs. They found the highest degree of agreement for an increased number of mitotic figures and drop-shaped rete ridges in the architectural criteria and increased nuclear size and abnormal variation of cell shape in the cytological. Irregular epithelial stratification and loss of polarity of basal cells in addition to abnormal variation in the nuclear size, atypical mitotic figures and hyperchromatism, showed the lowest level of agreement among observers [14].
Table 2.
Modified table of Viera and Garrett [19] presenting kappa value interpretation
| Value of kappa | Agreement |
|---|---|
| <0.20 | Slight |
| 0.21–0.40 | Fair |
| 0.41–0.60 | Moderate |
| 0.61–0.80 | Good |
| 0.81–1.00 | Very good |
Tilakaratne and co-authors [8] correlated individual features of dysplasia with clinical parameters, such as the site of lesions and etiological factors. Their aim was to identify patterns of morphological features within dysplasia that might help in the recognition, grading and understanding of the biological processes. Their results show wide variation in the reproducibility of scoring individual morphological features, although many of them, such as epithelial thickness, some types of rete morphology, basaloid cell anisonucleosis, basal dyscohesion and dyskeratosis as deep single cells, correlated with oral subsites of SILs. Other morphological features, including rete morphology, type of keratinization, hyperchromatisms of the basaloid layer, prickle cell and anisonucleosis, correlated with smoking. In addition, the authors compared the conventional WHO-dysplasia system, without distinguishing severe dysplasia from CIS, with the oral intraepithelial neoplasia (OIN) system (number of thirds of the epithelial thickness affected by dysplasia). Both grading systems showed high correlation overall but the scores obtained with the OIN system gave a higher grade in all sites except the soft palate. Poor correlation was found in both systems for moderate dysplasia. The authors also concluded that individual morphological features could not be shown to form distinct patterns of dysplasia.
Nankivell et al. [9] observed that the binary system, proposed by Kujan et al. [7] has similar prognostic ability but superior reproducibility compared with the WHO-dysplasia system, and that prognostication is improved by using a two-grade.
Traditional light microscopic examination, in spite of a certain degree of subjectivity in interpretation and lack of reliable molecular prognostic markers, is the most accurate method for determining a particular grade of SILs and remains the main guidance for the clinician in decisions on how to treat patients.
The occurrence of the higher grades (moderate and severe dysplasia, atypical hyperplasia) of oral SILs is considered to be the most important risk factor for SCC development (12.3 %) [20]. Malignant transformation rates of oral leukoplakia (OL) range from 0.13 to 17.5 % [4]. Several locations of OL, together with histological abnormalities, are linked with higher risk of malignant transformation. The floor of the mouth is thus the highest risk site, followed by the tongue, retromolar region and lip. The clinical appearance of non-homogeneous or speckled OL may correlate with the likelihood that the lesion will show epithelial changes or malignant transformation. In a study by Silverman and co-workers, the overall malignant transformation of OL was 17.5 %; with the homogenous form only 6.6 % and with speckled OL 23.4 % [21]. Proliferative verrucous leukoplakia, a special subtype of OL that is defined based on a constellation of clinical and pathological findings, was found to develop into SCC in 70.3 % of patients [22]. Compared to OL, oral erythroplakia has significantly worse biological behavior, with up to 50 % of malignant transformation [23].
Larynx
Inter-observer studies of laryngeal SILs are not as frequent as those of their oral counterparts, although the results are also important guidelines for the current use of present or possible new grading systems in the near future.
Eversole [12] collected various studies in order to determine reliability for dysplasia grades in oral and laryngeal precancerous lesions; oral lesions were graded on a three- or four-part scale, while vocal cord lesions were graded by either the LC or the WHO-dysplasia system. Inter- and intra-rater reliability among pathologists revealed low correlation coefficients for four-part grading systems, whereas improved agreement was achieved (kappa correlation values) using the LC [12].
The results of inter-observer agreement in grading laryngeal pre-neoplastic lesions among Turkish pathologists were not promising. There were significant differences among the participants for the three classifications (WHO-dysplasia system, LC and SIN), with deviations in lower and higher scores. The mean linear-weighted kappa values of participants (0.42 + 0.10, 0.41 + 0.12 and 0.37 + 0.007) were not significantly different for the WHO-dysplasia system, LC and SIN, respectively [13].
A similar study was performed by Fleskens et al. [10]; three pathologists classified 110 laryngeal SILs according to WHO-dysplasia system, SIN and LC. The highest unweighted kappa values were noted for the SIN system (0.28, 95 % CI 0.23–0.33), followed by WHO-dysplasia system and LC. For the modified two-grade system provided by the authors, the LC showed the highest unweighted κ-values (0.50, 95 % confidence interval 0.39–0.61), followed by the WHO-dysplasia system and SIN. The authors did not in fact observe a clear trend in favor of any particular system and stated that, in the absence of reliable biological markers for progression, the grade of dysplasia is still the most often used parameter to guide treatment [10].
The histopathological degree of severity of laryngeal SILs is also used as the most reliable predictive factor [1, 11]. However, in addition to histological grade, the effect of different treatment modalities as well as the effect of risk factors such as smoking and excessive alcohol intake has to be considered in assessing risk and the interval to malignant transformation [24]. A recent meta-analysis, including 940 patients from 9 studies, showed that the overall malignant transformation rate was 14 % (95 % CI 8–22 %) and the mean time to malignant transformation was 5.8 years. The malignant transformation rate was higher with increased severity of dysplasia; the grade severe dysplasia/CIS was 30.4 % versus mild/moderate 10.6 % (p < 0.0002) [24].
Our experiences of laryngeal SILs are based on a study, presumed to be the largest study of SILs, with 1,268 patients who were followed up for 25 years. The results of the Ljubljana group showed 1.1 % of malignant progression in 12 of 1,089 patients with low-grade laryngeal SILs (squamous hyperplasia and basal-parabasal hyperplasia) and a mean time to invasive SCC of 7.9 years. High-grade SILs (atypical hyperplasia) progressed to malignancy (CIS or invasive SCC) in 9.5 % (17 of 179) of patients; the mean time to malignant transformation was 6.2 years [1]. However, there is an essential difference between the previous study [24] and that of the Ljubljana group. Patients with CIS are distinguished from those with high-risk SILs and are also treated more aggressively, including with radiotherapy if complete surgical removal is not possible [1]. Histologically, three important morphological criteria are required for distinguishing high-grade SILs from CIS: marked architectural disorder of the epithelium, conspicuous cellular atypias and increased mitotic activity with atypical mitoses. We also reviewed an additional series of laryngeal SILs and found a great variation of malignant alteration, ranging from 9.3 to 57 % in patients with severe dysplasia, SIN III and LIN III. These significant variations can undoubtedly be ascribed to the inconsistent use of morphological criteria, type of treatment, period of follow-up of patients, as well as to the fact that, in some studies, patients with severe dysplasia and CIS are combined into a single category [1]. In addition, a recently published study presented a considerable difference in progression to invasive SCC in relation to the severity of different grades of SILs, as follows: no malignant transformation in 22 patients with mild dysplasia, 5/25 (20 %) of patients with moderate dysplasia, 2/14 (15 %) with severe dysplasia and 10/25 (40 %) of patients with CIS progressed to invasive cancer (p = 0.001) [25].
Important Differences Between Cervical and Head and Neck SILs
Different views on a basic concept of classification of SILs, together with a clash of opinions on morphological criteria and the terminology of these lesions, creates great confusion within this important topic of head and neck pathology. From the very beginning, most classifications of head and neck SILs have been transferred directly from the grading of intraepithelial lesions of the uterine cervix, together with the appertaining terminology, such as dysplasia or cervical intraepithelial neoplasia systems. Bearing in mind important distinctions between normal squamous epithelium of the cervix and various forms of squamous epithelium of the oral cavity and larynx [26, 27], it is understandable that possibly comparable grades of SILs show different morphological characteristics; consequently, the definition of particular grades of SILs cannot be identical for the two regions, since it is a case of different views of the definition of CIS of cervical non-keratinizing and laryngeal keratinizing epithelium [1, 28].
Only a few grading systems, such as those of the Kleinsasser and Kambič and Lenart classifications were designed specifically for the head and neck and larynx, respectively. Kleinsasser’s classification distinguishes three grades: squamous epithelial hyperplasia, epithelial hyperplasia with occasional atypia and carcinoma in situ. The initial criteria and terminology of the Kambič-Lenart classification was supplemented and further formulated in 1997, and is now used as the LC, with four grades: squamous hyperplasia, basal-parabasal hyperplasia, atypical hyperplasia and carcinoma in situ [1].
The essential difference between cervical intraepithelial lesions and invasive squamous cell carcinoma (SCC) and comparable oral and laryngeal lesions derives from different etiology. Persistent infection with 14 high risk human papillomaviruses (HPV), mainly types 16 and 18, is widely accepted as the main causative, although not a sufficient factor for the development of cervical SCC, with well recognized molecular pathways in the process of carcinogenesis [29]. Data from a recent international European study including 17 countries showed that, of a total of 3,103 women with high-grade CIN and a total of 3,162 with invasive cervical SCC, 98.5 and 91.8 % respectively, were HPV positive [30]. In addition, the comparative epidemiology of HPV infection and associated cancers of the head and neck and cervix, revealed that the global incidence of head and neck SCC and cervical SCC is similar; however, a minority of HNSCC, in comparison to virtually all cervical SCC, are caused by HPV. HPV prevalence is considerably lower in the oral than anogenital region for reasons that are still unclear, in spite of the fact that HPV infection in both sites is strongly associated with sexual behavior [31]. Only a significant fraction of oropharyngeal HNSCC is strongly etiologically associated with HPV infection. Prevalence in HPV positive oropharyngeal SCC has increased significantly in North America and Europe, and the significant gap that existed before 2000 between both continents (50.7 % vs. 35.3 %, p = 0.008) has disappeared (now 69.7 vs. 73 %, respectively, p = .8). Prevalence in non-oropharyngeal SCC (21.8 %, 95 % CI 18.9–25.1 %) has not increased over time (p = .97) [32]. In contrast to the high percentage of HPV related tumors of the oropharyngeal region, HPV-positive premalignant lesions are extremely rare findings in tonsillectomy specimens [33, 34]. The development of oral and laryngeal SILs and SCC, on the other hand, is mainly related to tobacco and alcohol consumption. About 70–90 % of OL is related to smoking and areca nut use, either alone or in combination. Although there appears to be some link between HPV infection and OL, there is little evidence to support a causal relationship either between HPV infection and OL or between HPV infected leukoplakic keratinocytes and their malignant transformation [35]. Woo et al. [36] recently published a subset of oral epithelial dysplasia, mainly located on the lateral or ventral tongue of 17 men and 3 women, all adult, and with transcriptionally active high-risk HPV. In these cases, epithelial hyperplasia with marked karyorrhexis and apoptosis were histologically detected, together with features of conventional dysplasia. The authors propose the use of the term HPV associated oral intraepithelial neoplasia for such lesions. Another case has been published of an HPV related lesion, designated non-keratinizing CIS, typical of HPV related SCC, involving the surface epithelium of the oral cavity, oropharynx and larynx. The lesion was strongly p16 positive and harbored transcriptionally active HPV, as demonstrated by E6/E7 RNA in situ hybridization [37].
In contrast to anogenital SCC, the prevalence of HPV in laryngeal SCC varies considerably, from 3 to 60 % [38, 39]. Li and co-workers [40] recently published a meta-analysis with 55 studies, addressing HPV prevalence in laryngeal SCC. The overall prevalence of HPV infection was found to be 28.0 % (95 % CI, 23.5–32.9 %). HPV 16 was the most frequently observed subtype, with a prevalence of 19.8 % (95 % CI 15.7–24.6 %). A significant association was therefore exposed between HPV infection and laryngeal SCC risk, with OR of 5.39 (95 % CI, 3.25–8.94). However, the authors pointed out the important limitations of the present meta-analysis. First, potential bias cannot be excluded due to different sensitivity and accuracy of the HPV DNA detection method and HPV types targeted by the method. Second, the study considered only articles published in English. Third, evident heterogeneity was observed between the included studies. Fourth, the crude division of study population by geographic regions made the analyses prone to misclassification bias [40]. In an additional study, Halec and co-workers revealed that HPV 16 had a causative role only in a small subset of laryngeal SCC (<5 % of 102 tumors) [39]. Although the authors found at least one HPV DNA positive sample in 35 % of patients, they stressed that the mere detection of HPV DNA is not sufficient to identify HPV- driven cancers, since high-risk HPV has frequently been found in benign laryngeal lesions and in normal mucosa [39, 41]. In this study, the authors looked for the presence of 51 HPV types, viral load, HPV mRNA expression and expression of p16, pRb, p53 and cyclinD1 to provide reliable findings of transcriptionally active HPV [39].
In the same fashion as laryngeal cancer, the prevalence of HPV infection in laryngeal SILs varies widely, between 0 and 56 %, with an overall prevalence of HPV infection of 12.4 % [1]. HPV positive SILs harbor mainly high-risk HPV types, with HPV 16 being the most frequent. In addition to laryngeal carcinoma and SILs, HPV DNA has also been detected in a substantial proportion, 12–25 %, of individuals with clinically and histologically normal laryngeal mucosa [1]. A final answer on the role of HPV infection in the etiopathogenesis of laryngeal SILs can thus only be reliably provided by additional studies, in which biological evidence of the existence of truly HPV- driven SILs is presented [39].
Classification of Head and Neck SILs: Perspectives
The current situation with the three different classifications for oral and laryngeal SILs of the WHO Blue Book 2005 is not very promising. The general approach to this important problem should thus be considerably altered. Various basic concepts, different morphological criteria and disunited terminology should be replaced by a basically harmonized classification with a unified terminology for oral and laryngeal SILs.
The first step towards general agreement in this field could be achieved by following the trend of a reduced number of grades. Leaving behind the old concepts of classifications, it seems logical, and probably also acceptable for most pathologists, to divide SILs into two main categories: low-grade and high-grade lesions. If this proposal proves to be acceptable, a considerable step forward will be achieved. It is also important to bear in mind specificity of individual organs, particularly from the therapeutic point of view. It is therefore necessary to distinguish CIS from high-grade laryngeal SILs. This approach can assure more advanced treatment of patients, including the application of radiotherapy.
The second step is unified terminology, which is also of the utmost importance for general agreement. We believe that the terms low-grade and high-grade SILs are more acceptable than low-grade and high-grade neoplasia. This problem has been frequently discussed and we are in agreement that low-grade lesions cannot be classified as neoplastic by definition.
The third step requires definitions for all leading morphological criteria for low- and high-grade lesions, as well as for CIS.
Finally, the fourth step needs to be agreement between clinicians and pathologists, which should represent the advantages of the three graded unified system with a correlation of the expected biological behavior for each grade of SILs. The choice of treatment should be determined by clinicians in accordance with the expected risk factors and anatomical and clinical specificities of different head and neck areas.
Following these four crucial steps, we could obtain an objective framework for our final aspiration—a basically single classification with a unified terminology for the head and neck region.
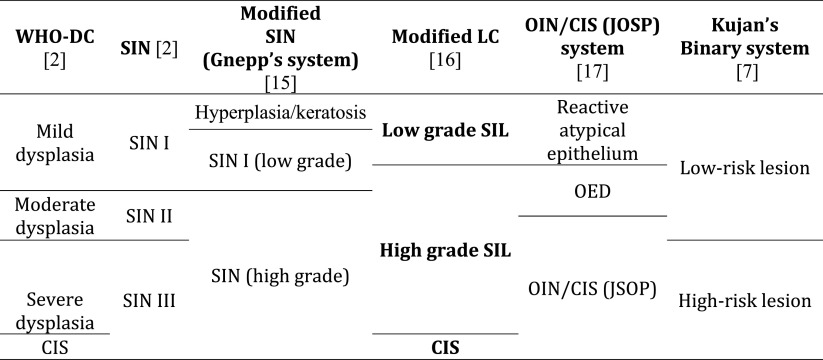
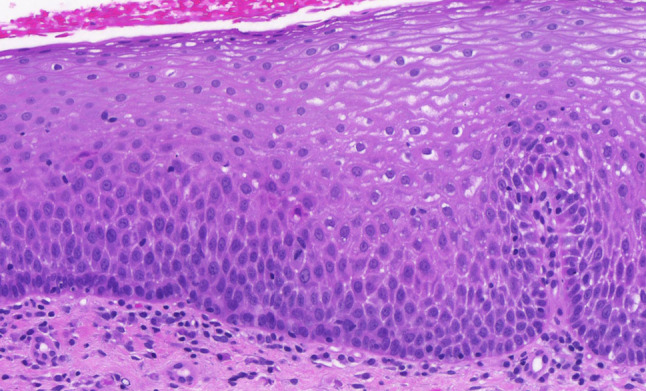
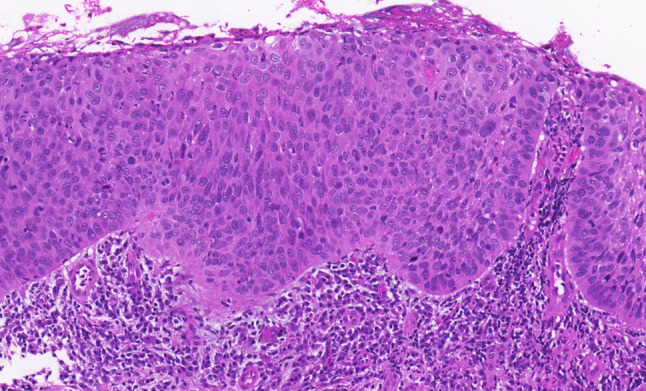
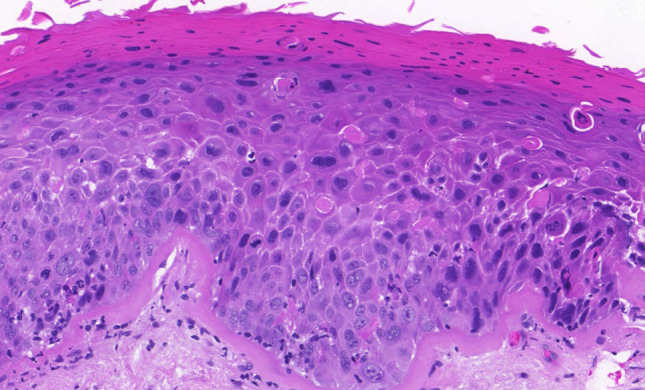
We have already made a contribution in the proposed direction by introducing a modification of the LC. This proposal can be therefore considered as a test model for a unified grading system of the head and neck region. The proposed modification, with low-and high-grade SILs, and the retained grade of CIS, is objectively based on the results of a previous follow-up study, which showed the possibility of combining reactive lesions (squamous hyperplasia and basal–parabasal hyperplasia) into a single category of low-grade SILs, and transforming atypical hyperplasia into high-grade SIL. CIS remains as within the LC, the separate grade (Figs. 1,2, 3, 4, 5) [1, 16].
Fig. 1.
Low-grade squamous intraepithelial lesion. Hyperplasic squamous epithelium with augmented parabasal cells extend up to the midportion of the epithelial thickness. The upper part of the epithelium is unchanged
Fig. 2.
High-grade squamous intraepithelial lesion. The thickened epithelium is entirely occupied by moderately polymorphic epithelial cells, which show preserved perpendicular orientation to the basement membrane
Fig. 3.
Carcinoma in situ. Pronounced architectural disorder of the epithelium with severe cellular and nuclear atypias and increased number of mitoses and dyskeratotic cells are evident in the lower two-thirds of the epithelium. The upper third shows partially preserved epithelial maturation
Fig. 4.
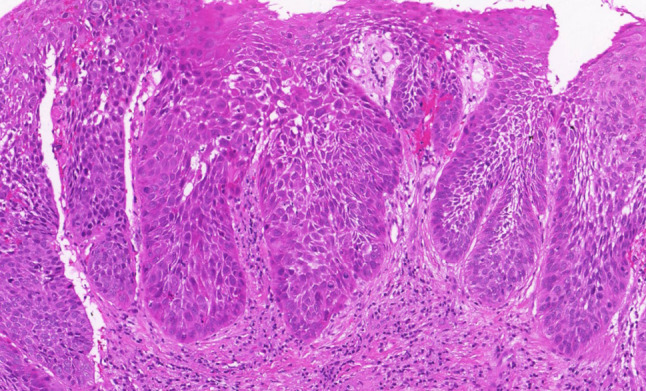
High-grade squamous intraepithelial lesion. Thickened epithelium with drop-shaped rete pegs, two-thirds of the epithelium is occupied by moderately atypical epithelial cells with partially preserved perpendicular orientation to the basement membrane. Mitotic activity is evident in the lower part of the epithelium
Fig. 5.
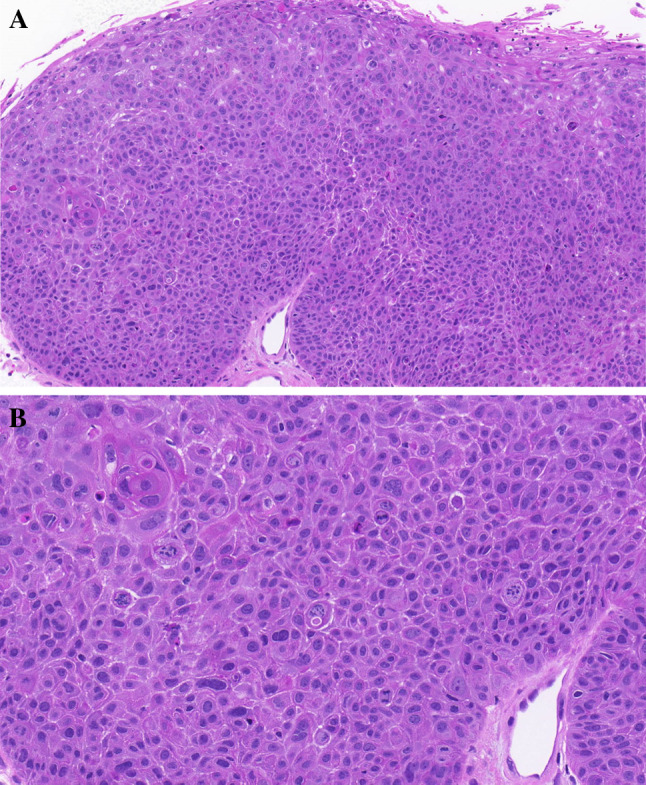
Carcinoma in situ. a Thickened epithelium with entire architectural disorder over the whole epithelial thickness. Epithelial atypias are evident. b Higher magnification of Fig. 5a. Complete architectural disorder of atypical epithelial cell; a pronounced number of atypical mitoses are present
Conclusions
The current state in the field of classifying oral and laryngeal precursor lesions, as proposed in the WHO 2005 Blue Book, has not proved to be optimal. The results of various inter-observer studies have shown that the currently used grading systems, with different basic concepts and different terminology, cannot continue to be reliably used in the future. The different etiology of cervical and head and neck precursor lesions requires a classification designed to cater for the specificities of the head and neck region.
Trying to realize our aims in the forthcoming edition of the WHO Blue Book Head and Neck Tumours in the field of oral and laryngeal precursor lesions, we have proposed four crucial steps to setting up a unified classification of SILs: (a) the classification should contain two grades, low-grade and high-grade lesions and, specifically for the larynx, also a distinction of CIS from high-grade laryngeal SILs; (b) the terminology should be unified; our preference is for the term SIL over squamous intraepithelial neoplasia; (c) All leading morphological criteria for low- and high-grade lesions, as well as for CIS, should be clearly defined; (d) agreement between clinicians and pathologists on the most appropriate choice of treatment of different grades of SILs in separate head and neck areas.
Abbreviations
- SILs
Squamous intraepithelial lesions
- CIN
Cervical intraepithelial neoplasia
- WHO
World Health Organization
- DS
Dysplasia system
- SIN
Squamous intraepithelial neoplasia
- LC
Ljubljana classification
- OIN
Oral intraepithelial neoplasia
- OL
Oral leukoplakia
- HPV
Human papillomavirus
- HN
Head and neck
- SCC
Squamous cell carcinoma
- JSOP
Japanese Society for Oral Pathology
References
- 1.Gale N, Michaels L, Luzar B, et al. Current review on squamous intraepithelial lesions of the larynx. Histopathology. 2009;54(6):639–656. doi: 10.1111/j.1365-2559.2008.03111.x. [DOI] [PubMed] [Google Scholar]
- 2.Gale N, Pilch BZ, Sidransky D, et al. Epithelial precursor lesions. In: Barnes L, Eveson JW, Reichart P, Sidransky D, et al., editors. World Health Organization classification of tumours—pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. pp. 143–153. [Google Scholar]
- 3.Boy S. Leukoplakia and erythroplakia of oral mucosa—a brief overview. SADJ. 2012;67(10):558–560. [PubMed] [Google Scholar]
- 4.Amagasa T, Yamashiro M, Uzawa N. Oral premalignant lesions: from a clinical perspective. Int J Clin Oncol. 2011;16(1):5–14. doi: 10.1007/s10147-010-0157-3. [DOI] [PubMed] [Google Scholar]
- 5.Barnes L. Diseases of the larynx, hypopharynx, and trachea. In: Barnes L, editor. Surgical pathology of the head and neck. 3. New York: Informa Healthcare; 2009. pp. 109–200. [Google Scholar]
- 6.Warnakulasuriya S, Reibel J, Bouquot J, et al. Oral epithelial dysplasia classification systems: predictive value, utility, weaknesses and scope for improvement. J Pathol Med. 2008;37(3):127–133. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 7.Kujan O, Oliver RJ, Khattab A, et al. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42(3):987–993. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Tilakaratne WM, Sherriff M, Morgan PR, et al. Grading oral epithelial dysplasia: analysis of individual features. J Oral Pathol Med. 2011;40(7):533–540. doi: 10.1111/j.1600-0714.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 9.Nankivell P, Williams H, Matthews P, et al. The binary oral dysplasia grading system: validity testing and suggested improvement. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(1):87–94. doi: 10.1016/j.oooo.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Fleskens SA, Bergshoeff VE, Voogd AC, et al. Interobserver variability of laryngeal mucosal premalignant lesions: a histopathological evaluation. Mod Pathol. 2011;24(7):889–892. doi: 10.1038/modpathol.2011.50. [DOI] [PubMed] [Google Scholar]
- 11.McLaren KM, Burnett RA, Goodlad JR, et al. Consistency of histopathological reporting of laryngeal dysplasia. The Scottish Pathology Consistency Group. Histopathology. 2000;37(5):460–463. doi: 10.1046/j.1365-2559.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- 12.Eversole LR. Dysplasia of the upper aerodigestive tract squamous epithelium. Head Neck Pathol. 2009;3(1):63–68. doi: 10.1007/s12105-009-0103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarioglu S, Cakalagaoglu F, Elagoz S, et al. Inter-observer agreement in laryngeal pre-neoplastic lesions. Head Neck Pathol. 2010;4(4):276–280. doi: 10.1007/s12105-010-0208-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kujan O, Khattab A, Oliver RJ, Roberts SA, Thakker N, Sloan P. Why oral histopathology suffers inter-observer variability on grading oral epithelial dysplasia: an attempt to understand the sources of variation. Oral Oncol. 2007;43(3):224–231. doi: 10.1016/j.oraloncology.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Sakr W, Gale N, Gnepp DR, et al. Squamous intraepithelial neoplasia of the upper aerodigestive tract. In: Gnepp DR, et al., editors. Diagnostic surgical pathology of the head and neck. Philadelphia: Saunders Elsevier; 2009. pp. 1–44. [Google Scholar]
- 16.Gale N. A suggested consensus classification of laryngeal preneoplastic lesions. 25th European Congress of Pathology, Lisbon 2013; SC-04. Abstract.
- 17.Izumo T. Oral premalignant lesions: from the pathological viepoint . Int J Clin Oncol. 2011;16(1):15–26. doi: 10.1007/s10147-010-0169-z. [DOI] [PubMed] [Google Scholar]
- 18.Bosman FT. Dysplasia classification: pathology in disgrace? J Pathol. 2001;194(2):143–144. doi: 10.1002/1096-9896(200106)194:2<143::AID-PATH883>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 20.Mehanna HM, Rattay T, Smith J, et al. Treatment and follow-up of oral dysplasia—a systematic review and meta-analysis. Head Neck. 2009;31(12):1600–1609. doi: 10.1002/hed.21131. [DOI] [PubMed] [Google Scholar]
- 21.Silverman S, Jr, Gorsky M. Proliferative verrucous leukoplakia: a follow-up study of 54 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(2):154–157. doi: 10.1016/S1079-2104(97)90062-7. [DOI] [PubMed] [Google Scholar]
- 22.Batsakis JG, Suarez P, El-Naggar AK. Proliferative verrucous leukoplakia and its related lesions. Oral Oncol. 1999;35(4):354–359. doi: 10.1016/S1368-8375(99)00007-X. [DOI] [PubMed] [Google Scholar]
- 23.Reichart PA, Philipsen HP. Oral erythroplakia–a review. Oral Oncol. 2005;41(6):551–561. doi: 10.1016/j.oraloncology.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Weller MD, Nankivell PC, McConkey C, et al. The risk and interval to malignancy of patients with laryngeal dysplasia; a systematic review of case series and meta-analysis. Clin Otolaryngol. 2010;35(5):364–372. doi: 10.1111/j.1749-4486.2010.02181.x. [DOI] [PubMed] [Google Scholar]
- 25.Zhang HK, Liu HG. Is severe dysplasia the same lesion as carcinoma in situ? 10-year follow-up of laryngeal precursor lesions. Acta Otolaryngol. 2012;132(3):325–328. doi: 10.3109/00016489.2011.642812. [DOI] [PubMed] [Google Scholar]
- 26.Kambič V, Gale N. Epithelial hyperplastic lesions of the larynx. Amsterdam: Elsevier; 1995. pp. 1–265. [Google Scholar]
- 27.Milles S. Histology for pathologists. 4. Philadelphia: Wolters Kluwer/Lippincot Williams&Wilkins; 2012. pp. 3–1331. [Google Scholar]
- 28.Wenig BM. Squamous cell carcinoma of the upper aerodigestive tract: precursors and problematic variants. Mod Pathol. 2002;15(3):229–254. doi: 10.1038/modpathol.3880520. [DOI] [PubMed] [Google Scholar]
- 29.Hausen HZ. Papillomavirus infections-a major cause of human cancers. Biochim Biophys Acta. 1996;1288(2):F55–F78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- 30.Tjalma WA, Fiander A, Reich O, et al. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int J Cancer. 2013;132(4):854–867. doi: 10.1002/ijc.27713. [DOI] [PubMed] [Google Scholar]
- 31.Gillison ML. Current topics in the epidemiology of oral cavity and oropharyngeal cancers. Head Neck. 2007;29(8):779–792. doi: 10.1002/hed.20573. [DOI] [PubMed] [Google Scholar]
- 32.Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35(5):747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 33.Begum S, Cao D, Gillison M, Zahurak M, et al. Tissue distribution of human papillomavirus 16 DNA integration in patients with tonsillar carcinoma. Clin Cancer Res. 2005;11(16):5694–5699. doi: 10.1158/1078-0432.CCR-05-0587. [DOI] [PubMed] [Google Scholar]
- 34.Begum S, Gillison ML, Nicol TL, et al. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13(4):1186–1191. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 35.Feller L, Lemmer J. Oral Leukoplakia as it relates to HPV infection: a review. Int J Dent. 2012; ID 540561 (in press). [DOI] [PMC free article] [PubMed]
- 36.Woo SB, Cashman EC, Lerman MA. Human papillomavirus-associated oral intraepithelial neoplasia. Mod Pathol. 2013;26(10):1288–1297. doi: 10.1038/modpathol.2013.70. [DOI] [PubMed] [Google Scholar]
- 37.Chernock RD, Nussenbaum B, Thorstad WL, et al. Extensive HPV-Related Carcinoma In Situ of the Upper Aerodigestive Tract with ‘Nonkeratinizing’ Histologic Features. Head Neck Pathol. 2013; (epub ahead of print). [DOI] [PMC free article] [PubMed]
- 38.Torrente MC, Ojeda JM. Exploring the relation between human papilloma virus and larynx cancer. Acta Otolaryngol. 2007;127(9):900–906. doi: 10.1080/00016480601110238. [DOI] [PubMed] [Google Scholar]
- 39.Halec G, Holzinger D, Schmitt M, et al. Biological evidence for a causal role of HPV16 in a small fraction of laryngeal squamous cell carcinoma. Br J Cancer. 2013;109(1):172–183. doi: 10.1038/bjc.2013.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X, Gao L, Li H, Gao J, et al. Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis. J Infect Dis. 2013;207(3):479–488. doi: 10.1093/infdis/jis698. [DOI] [PubMed] [Google Scholar]
- 41.Duray A, Descamps G, Arafa M, et al. High incidence of high-risk HPV in benign and malignant lesions of the larynx. Int J Oncol. 2011;39(1):51–59. doi: 10.3892/ijo.2011.1031. [DOI] [PubMed] [Google Scholar]