Abstract
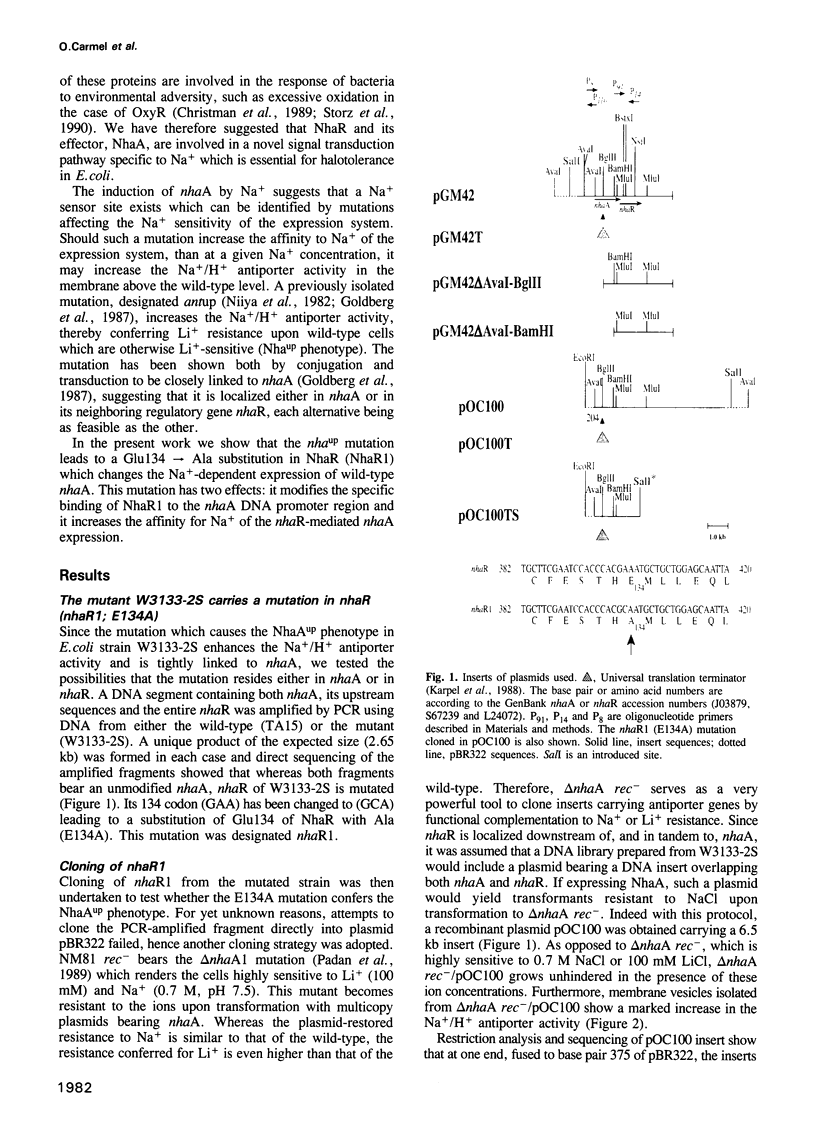
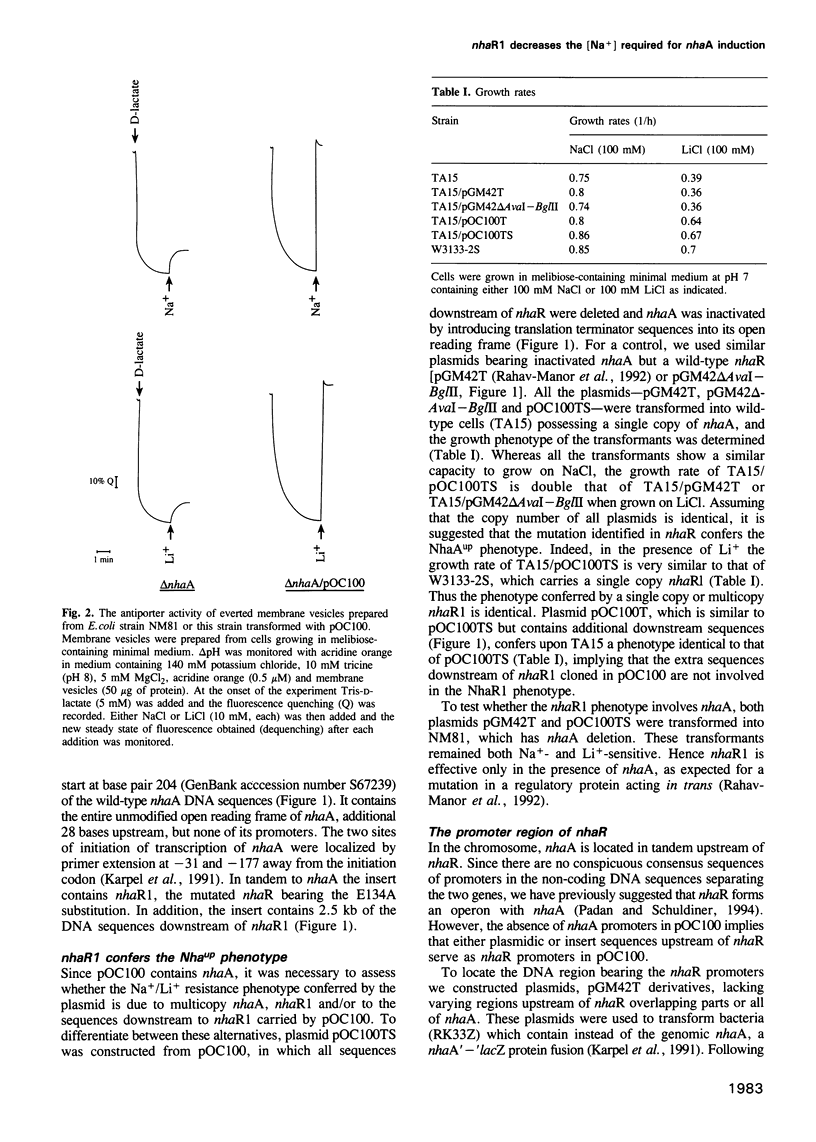
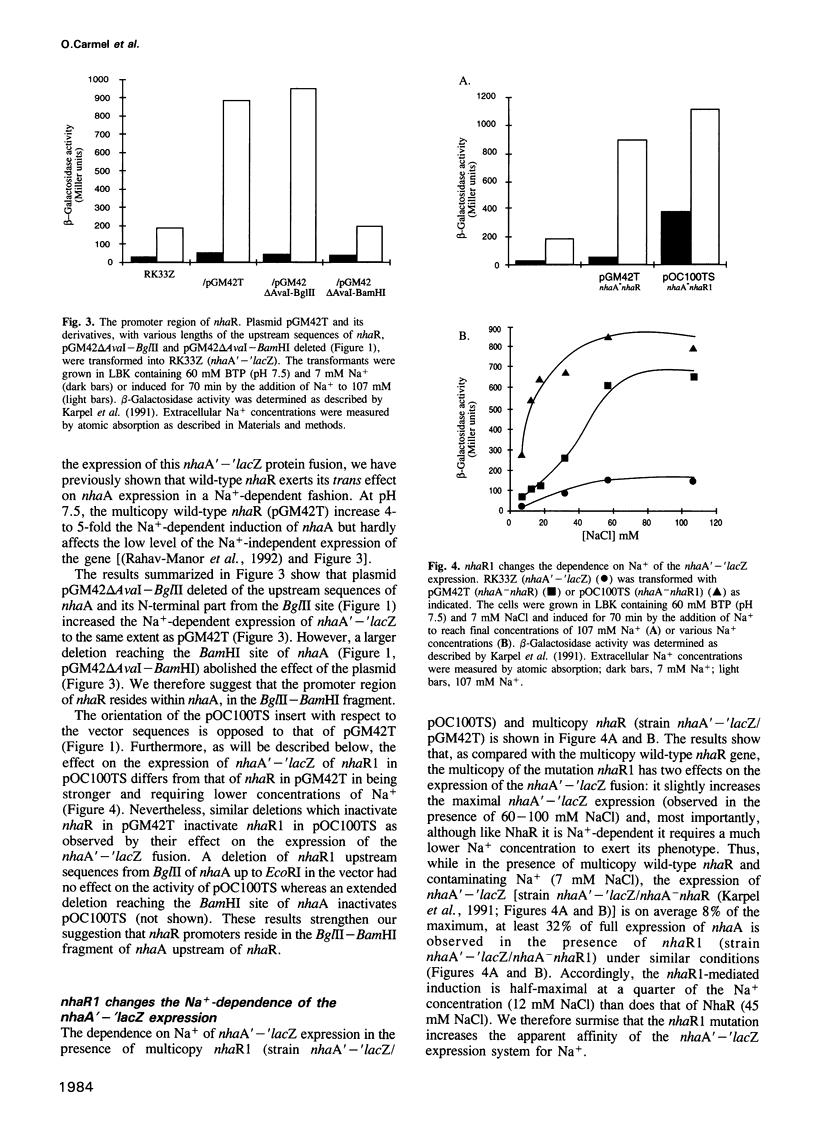
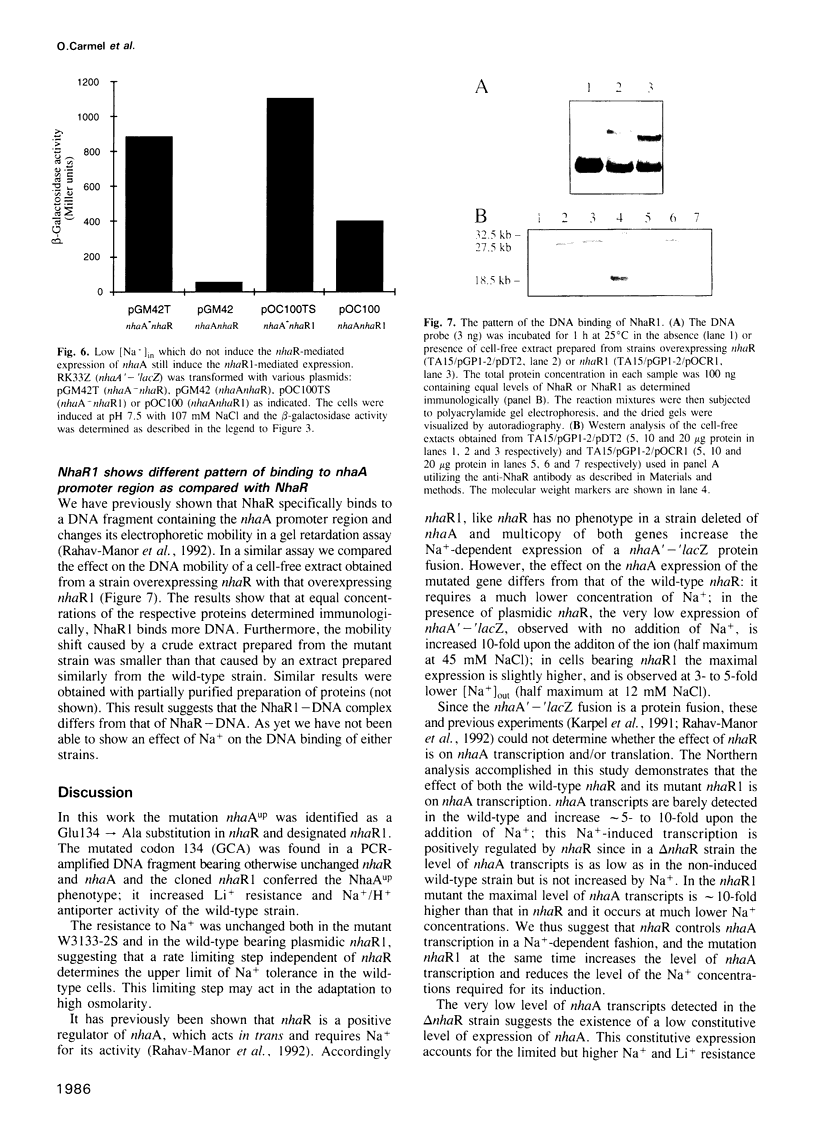
The mutation nhaAup (antup) has now been identified as a Glu134 to Ala substitution in NhaR and designated nhaR1. This was demonstrated by sequence analysis showing that the mutant contains a wild-type nhaA but nhaR1 instead of nhaR and by the finding that nhaR1 cloned in a plasmid confers the NhaAup phenotype. Na+ (107 mM) increases by 5- to 10-fold the level of nhaA transcripts, similar to the effect on the NhaR-mediated expression of a nhaA'-'lacZ fusion. These results are in agreement with the notion that nhaR is a positive regulator which controls Na(+)-dependent transcription of nhaA. The promoter region of nhaR and nhaR1 was found to reside within the BglII-BamHI fragment of the C-terminal sequences of nhaA. The mutation nhaR1, while increasing dramatically the level of transcription, reduces the requirement for Na+ by 3- to 5-fold both for nhaA transcription and for the nhaR1-mediated expression of nhaA'-'lacZ fusion. NhaR1, like NhaR, binds specifically to the promoter region of nhaA. However, at equal protein concentration NhaR1 binds more DNA and the NhaR1-DNA complex shows higher mobility than that of NhaR-DNA, suggesting the existence of two different binding complexes. Yet in this assay the DNA binding pattern of neither NhaR nor NhaR1 was affected by the addition of Na+. The possible relevance of these two DNA-binding complexes to the Na(+)-induced NhaR-mediated expression is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Storz G., Ames B. N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium, is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci U S A. 1989 May;86(10):3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sal G., Manfioletti G., Schneider C. A one-tube plasmid DNA mini-preparation suitable for sequencing. Nucleic Acids Res. 1988 Oct 25;16(20):9878–9878. doi: 10.1093/nar/16.20.9878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V., Wittel F. P. Effect of the RelA gene on the synthesis of individual proteins in vivo. Cell. 1976 May;8(1):115–122. doi: 10.1016/0092-8674(76)90192-6. [DOI] [PubMed] [Google Scholar]
- Gerchman Y., Olami Y., Rimon A., Taglicht D., Schuldiner S., Padan E. Histidine-226 is part of the pH sensor of NhaA, a Na+/H+ antiporter in Escherichia coli. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1212–1216. doi: 10.1073/pnas.90.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen J. R., Willis D. K., Clark A. J. Genetic analysis of the RecE pathway of genetic recombination in Escherichia coli K-12. J Bacteriol. 1981 Jan;145(1):521–532. doi: 10.1128/jb.145.1.521-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg E. B., Arbel T., Chen J., Karpel R., Mackie G. A., Schuldiner S., Padan E. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(9):2615–2619. doi: 10.1073/pnas.84.9.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpel R., Alon T., Glaser G., Schuldiner S., Padan E. Expression of a sodium proton antiporter (NhaA) in Escherichia coli is induced by Na+ and Li+ ions. J Biol Chem. 1991 Nov 15;266(32):21753–21759. [PubMed] [Google Scholar]
- Karpel R., Olami Y., Taglicht D., Schuldiner S., Padan E. Sequencing of the gene ant which affects the Na+/H+ antiporter activity in Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10408–10414. [PubMed] [Google Scholar]
- King S. M., Otter T., Witman G. B. Characterization of monoclonal antibodies against Chlamydomonas flagellar dyneins by high-resolution protein blotting. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4717–4721. doi: 10.1073/pnas.82.14.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackie G. A. Structure of the DNA distal to the gene for ribosomal protein S20 in Escherichia coli K12: presence of a strong terminator and an IS1 element. Nucleic Acids Res. 1986 Sep 11;14(17):6965–6981. doi: 10.1093/nar/14.17.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachaliel N., Melnick J., Gafny R., Glaser G. Ribosome associated protein(s) specifically bind(s) to the upstream activator sequence of the E. coli rrnA P1 promoter. Nucleic Acids Res. 1989 Dec 11;17(23):9811–9822. doi: 10.1093/nar/17.23.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiya S., Yamasaki K., Wilson T. H., Tsuchiya T. Altered cation coupling to melibiose transport in mutants of Escherichia coli. J Biol Chem. 1982 Aug 10;257(15):8902–8906. [PubMed] [Google Scholar]
- Padan E., Maisler N., Taglicht D., Karpel R., Schuldiner S. Deletion of ant in Escherichia coli reveals its function in adaptation to high salinity and an alternative Na+/H+ antiporter system(s). J Biol Chem. 1989 Dec 5;264(34):20297–20302. [PubMed] [Google Scholar]
- Pan J. W., Macnab R. M. Steady-state measurements of Escherichia coli sodium and proton potentials at alkaline pH support the hypothesis of electrogenic antiport. J Biol Chem. 1990 Jun 5;265(16):9247–9250. [PubMed] [Google Scholar]
- Pinner E., Carmel O., Bercovier H., Sela S., Padan E., Schuldiner S. Cloning, sequencing and expression of the nhaA and nhaR genes from Salmonella enteritidis. Arch Microbiol. 1992;157(4):323–328. doi: 10.1007/BF00248676. [DOI] [PubMed] [Google Scholar]
- Pinner E., Kotler Y., Padan E., Schuldiner S. Physiological role of nhaB, a specific Na+/H+ antiporter in Escherichia coli. J Biol Chem. 1993 Jan 25;268(3):1729–1734. [PubMed] [Google Scholar]
- Pinner E., Padan E., Schuldiner S. Cloning, sequencing, and expression of the nhaB gene, encoding a Na+/H+ antiporter in Escherichia coli. J Biol Chem. 1992 Jun 5;267(16):11064–11068. [PubMed] [Google Scholar]
- Rahav-Manor O., Carmel O., Karpel R., Taglicht D., Glaser G., Schuldiner S., Padan E. NhaR, a protein homologous to a family of bacterial regulatory proteins (LysR), regulates nhaA, the sodium proton antiporter gene in Escherichia coli. J Biol Chem. 1992 May 25;267(15):10433–10438. [PubMed] [Google Scholar]
- Ricca E., Aker D. A., Calvo J. M. A protein that binds to the regulatory region of the Escherichia coli ilvIH operon. J Bacteriol. 1989 Mar;171(3):1658–1664. doi: 10.1128/jb.171.3.1658-1664.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen B. P. Ion extrusion systems in Escherichia coli. Methods Enzymol. 1986;125:328–336. doi: 10.1016/s0076-6879(86)25028-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Padan E. Molecular analysis of the role of Na+/H+ antiporters in bacterial cell physiology. Int Rev Cytol. 1993;137C:229–266. [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Ames B. N. Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science. 1990 Apr 13;248(4952):189–194. doi: 10.1126/science.2183352. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taglicht D., Padan E., Schuldiner S. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. J Biol Chem. 1991 Jun 15;266(17):11289–11294. [PubMed] [Google Scholar]
- Taglicht D., Padan E., Schuldiner S. Proton-sodium stoichiometry of NhaA, an electrogenic antiporter from Escherichia coli. J Biol Chem. 1993 Mar 15;268(8):5382–5387. [PubMed] [Google Scholar]
- Willins D. A., Ryan C. W., Platko J. V., Calvo J. M. Characterization of Lrp, and Escherichia coli regulatory protein that mediates a global response to leucine. J Biol Chem. 1991 Jun 15;266(17):10768–10774. [PubMed] [Google Scholar]