Abstract
Context:
Under regional anesthesia, geriatric patients are prone to shivering induced perioperative complications that Anesthesiologists should prevent rather than treat.
Aim:
We investigated the prophylactic efficacy of oral tramadol 50 mg to prevent the perioperative shivering after transurethral resection of prostate (TURP) surgery under subarachnoid blockade (SAB). Shivering is usually overlooked in patients undergoing urological surgery under spinal anesthesia and may result in morbidity, prolonged hospital stay and increased financial burden. Use of prophylactic measures to reduce shivering in geriatric patients who undergo urological procedures could circumvent this. Oral formulation of tramadol is a universally available cost-effective drug with the minimal side-effects.
Settings and Design:
Prospective, randomized, double-blinded, placebo-controlled study.
Patients and Methods:
A total of 80 patients who were scheduled for TURP surgery under subarachnoid block were randomly selected. Group I and II (n = 40 each) received oral tramadol 50 mg and placebo tablet respectively. After achieving subarachnoid block, the shivering, body temperature (tympanic membrane, axillary and forehead), hemodynamic parameters and arterial saturation were recorded at regular intervals.
Statistical Analysis Used:
T-test, analysis of variance test, Z-test and Fisher exact test were utilized while Statistical Product and Service Solutions, IBM, Chicago (SPSS statistics (version 16.0)), software was used for analysis.
Results:
Incidence of shivering was significantly less in patients who received tramadol (7.5% vs. 40%; P < 0.01). The use of tramadol was associated with clinically inconsequential side-effects.
Conclusion:
We conclude that the use of oral tramadol 50 mg is effective as a prophylactic agent to reduce the incidence, severity and duration of perioperative shivering in patients undergoing TURP surgery under SAB.
Keywords: Shivering, subarachnoid blockade, tramadol, transurethral resection of prostate, urology
INTRODUCTION
Patients undergoing transurethral resection of prostate (TURP) own a noteworthy cardiac risk due to concomitant medical problems.[1] Hypothermia can complicate TURP procedures and geriatric patients especially are predisposed to the risk of hypothermia induced shivering under anesthesia as their lowered core temperature does not initiate protective autonomic response. Neuraxial blockade ominously impairs the regulation of body temperature by inhibition of vasomotor and shivering responses and affiliated redistribution of heat from the core of the body to the peripheral tissue.[2] Under neuraxial blockade hypothermia may not be perceived by patients who typically feel less cold after induction of the block or sometimes is not duly appreciated by the Anesthesiologist.[3] The shivering threshold is consequently breached soon and more shivering is requisite to avert further hypothermia. Shivering is both morbid and uncomfortable for patients and may interfere with monitoring of electrocardiogram, blood pressure and pulse oxygen saturation. It escalates oxygen consumption, lactic acidosis and carbon dioxide production; thus, predisposing a patient with a low cardiopulmonary reserve to potential harm.[4]
Maintaining strict normothermia may prevent shivering during the regional anesthesia.[5] Various drugs have undergone investigative trial to reduce perioperative shivering with more or less similar efficacy, but the search for the ideal drug that can prevent perioperative shivering still continues.[4,6,7,8,9,10,11] Oral formulation of tramadol is a universally available cost-effective drug with the minimal side-effects [Table 1].
Table 1.
Comparison of cost of a few drugs available for treatment of perioperative shivering in India
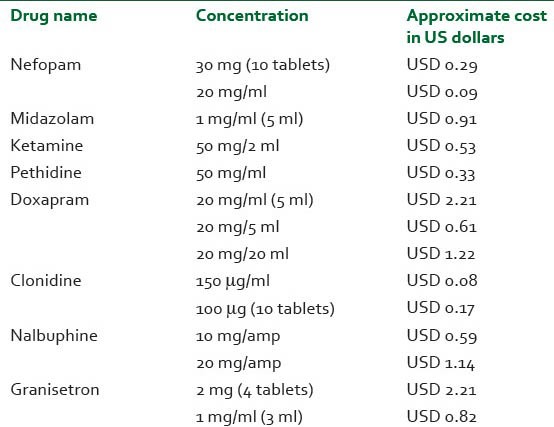
Tramadol, a cyclohexanol derivative, has μ agonist activity as well as acts as an inhibitor of serotonin and norepinephrine uptake.[12] Metabolized in the liver, it is mainly consumed as an analgesic. Oral tramadol is economical ($0.15/tablet) when compared with its intravenous formulation ($0.28/injection). Intravenous tramadol has been used for treatment of shivering.[13,14,15,16] In this study, we evaluated the prophylactic efficacy of tramadol's oral formulation for prevention of perioperative shivering in geriatric patients undergoing TRUP as most of the previous studies have concentrated on the intravenous form.
PATIENTS AND METHODS
After Institutional Ethical Committee approval and written informed consent, 80 randomly chosen geriatric patients (American Society of Anesthesiologists Grade I, II, III) scheduled to undergo elective TURP surgery under subarachnoid blockade (SAB) were enrolled into the study. Patients who were obese (body mass index >30%) or febrile, patients who had any history suggestive of allergy to tramadol, ischemic heart disease, cerebrovascular disease, thyroid dysfunction, severe diabetic/autonomic neuropathy, infection of the urinary tract or the external auditory meatus were excluded. Patients who were taking vasodilators/vasoconstrictors were not included in the study as those drugs could interfere with the body thermoregulation.
Anesthesiology department technologists, who were not involved in the study, prepared the trial preparations and recorded the group randomization separately. The Anesthesiologist conducting the case and recording the data was unaware of the preparation administered. Patients were randomly assigned into two groups (by random number table), each receiving a sealed envelope of the oral formulation 2 h prior to the surgery. Group I received oral tramadol 50 mg while the Group II received starch tablet.
Spinal anesthesia, with a sensory blockade up to T9-10 level was achieved using heavy (80 mg dextrose) bupivacaine (0.5%) 2-3 ml. All operations were performed in the same operation theater, which was maintained at a constant humidity and an ambient temperature of around 22 ± 2°C. No means of active rewarming was used in the study, unless deemed essential for rescue measures. The operating room was not equipped to provide laminar flow. Pre-warmed (up to the body temperature of 37 ± 1°C) intravenous and irrigating fluids were used. Heart rate, noninvasive blood pressure, respiratory rate, SpO2, skin temperature (axillary and forehead, using the Excel 210 temperature probes and tympanic membrane temperature using the Braun Thermoscan®) were recorded every 5 min from the baseline (when SAB was given) for 1 h and thereafter every 15 min for the rest of the observation period. Shivering was recorded by the same attending Anesthesiologist at a period of 0, 1, 5, 10, 15, 30, 45, 60 and 90 min from the baseline as per grades given by Wrench et al.[8] [Table 2], in all cases. Perioperatively if shivering occurred; it was treated in the same manner in both groups with reassurance, warming blanket or meperidine. Forced air warming blankets were available when necessary. Associated conditions such as nausea, vomiting, bradycardia (heart rate <50/min) and hypotension (systolic blood pressure <30% of baseline) were recorded. Bradycardia and hypotension were appropriately treated with atropine and mephentermine respectively in titrated doses when required. Nausea and vomiting was treated with metoclopramide or ondansetron in titrated doses. The sedative side-effects of tramadol were assessed with a four-point sedation score as per Filos et al.[17] [Table 3].
Table 2.
Grading of shivering as per Wrench et al.[8]

Table 3.
The sedation score assessed with afour-point scale as per Filos et al.[17]

Statistical analysis
No power analysis could be performed before choosing the number of geriatric patients for the study as the exact incidence of perioperative shivering in geriatric patients during TURP surgery under spinal anesthesia was not available despite the extensive literature search. However, the power of our study is the large number of patients in comparison with other studies.
The SPSS version 16.0 was used for statistical analysis. All data was tabulated and expressed as mean ± standard deviation. Parametric data was analyzed using Student t-test and analysis of variance (ANOVA) test while the nonparametric values were analyzed using the test of proportions, i.e., Z-test. We used the Fisher exact test to compare the incidences between the two groups. A P < 0.05 was considered statistically significant.
RESULTS
The demographic data, duration of surgery and the time required to achieve Bromage score of 4 was similar amongst both the groups [Table 4]. Incidence of shivering (7.5% vs. 40%, P < 0.05) was significantly less in Group I patients (who were administered oral tramadol) than that in Group II [Figure 1]. In the study group, 37 patients (92.5%) did not experience shivering while 3 (7.5%) patients did. These three patients experienced shivering ranging from Grade 1-3 during the observation period. None had Grade 4 of shivering. In the placebo group, 24 patients (60%) did not shiver. Sixteen patients (40%) experienced shivering ranging from the grade of 1-4. It was seen that shivering started earlier (within 5 min) and persisted for a longer duration in the placebo group (range = 5-90 min). While in tramadol group the onset was delayed (15 min) and the duration was also significantly less (range = 15-60 min). Many patients progressed from low to high grades of shivering. During the whole study period, 15 (37.5%), 11 (27.5%), 10 (25%) and 5 (12.5%) patients in the control group experienced Grades 1, 2, 3 and 4 of shivering respectively. On comparison, a P value <0.01 was observed, thus implying a statistically significant variance regarding the incidence among them. The variation was significant throughout the period of study, especially at 30, 45 and 60 min intervals where the P value was <0.01 and while at 5, 10, 15 and 90 min interval the P value was <0.05. At the end of the observation period, i.e., 90 min none of the patients who were given tramadol were shivering whereas 4 (10%) patients were still shivering in the control group.
Table 4.
Demographic profile and characteristic of surgery and anesthesia
Figure 1.
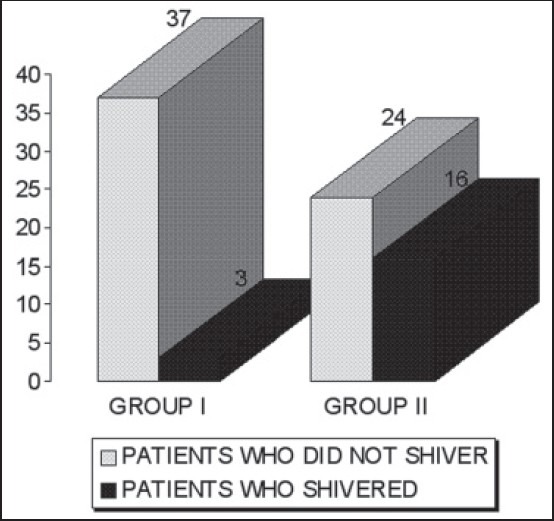
Incidence of shivering among the two groups
All the patients who experienced Grade 3-4 of shivering had clinically insignificant tachycardia, increased blood pressure recording and decreased arterial oxygen saturation when compared with the baseline values. These patient were made normothermic on demand by the patient using the warming blankets in 14 (1 in tramadol group and 13 in the control group) and meperidine in two (both control group). Two patients in the control group who experienced Grade 4 of shivering had premature supraventricular contraction, which were successfully treated with intravenous xylocard 2% 1.5 mg/kg.
The tympanic membrane temperature [Figure 2] showed statistically significant variation especially in the later part of the study (P < 0.01) while trends in axillary and forehead temperatures were similar throughout the study [Figures 3 and 4]. There was also no statistical variance when the mean heart rate, systolic blood pressure, respiratory rate and SpO2 were compared. Sedation of Grade 2 was observed in 3 (7.5%) and 5 (12.5%) patients in tramadol and control group respectively. No patient of the either group experienced Grade 3 or 4 sedation. The sedation had no statistically significant difference between the two groups and was also clinically insignificant. No patient in either group had nausea, vomiting or pruritus.
Figure 2.
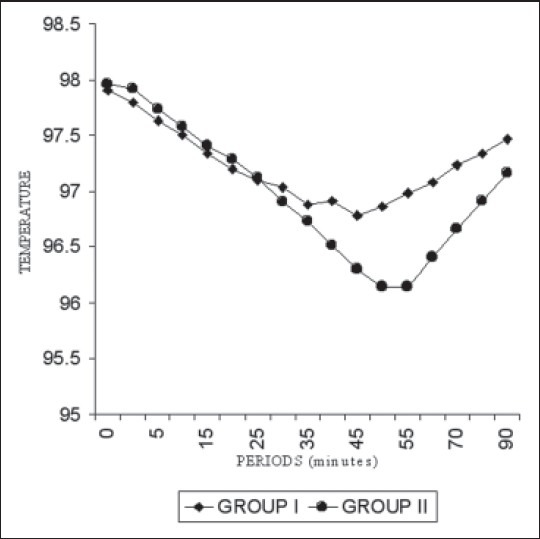
Trends in mean tympanic membrane temperature among the two groups
Figure 3.
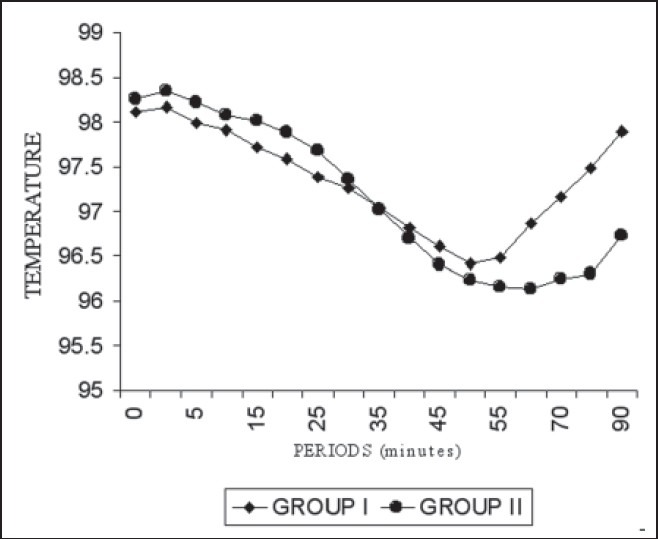
Trends in mean axillary temperature
Figure 4.
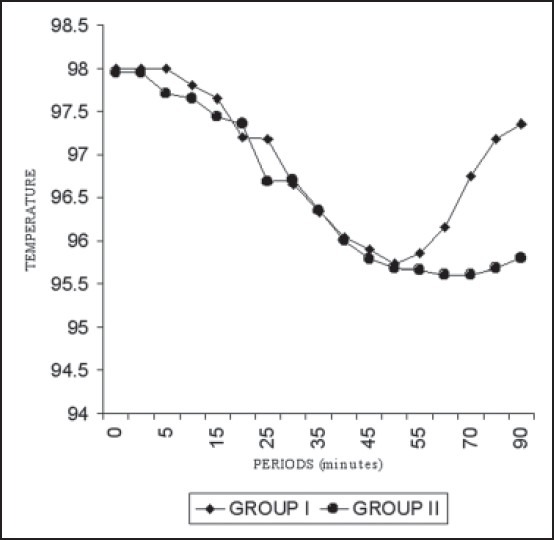
Trends in mean forehead temperature
DISCUSSION
In our study, we found that oral tramadol 50 mg was saliently effective in providing prophylaxis against shivering in TURP surgery conducted under SAB. The incidence of shivering in patients who were given oral tramadol was only 7.5% as compared with 40% in the control group (P < 0.01). The patient in tramadol group, who shivered, experienced only Grade 1-3 of shivering while the patients in the control group experienced Grade 1-4 of shivering. It was observed that shivering had early onset and persisted longer in the control group, while in the tramadol group its onset was delayed and with a shorter duration. This is in concordance with various studies, which showed that tramadol reduces the incidence, severity and duration of perioperative shivering.[13,14,15,16] We observed a significant fall in the tympanic membrane temperature in the control group as compared with tramadol group (P < 0.001). Tramadol as an analgesic is effective at the opiate receptors in the central nervous system. Its action is similar to that of nefopam that acts as an inhibitor of reuptake of serotonin and nor epinephrine in the spinal cord.[12] Thus, the release of 5-hydroxytryptamine is facilitated, which activates μ-receptors and thereby influences the thermoregulatory control. The dose of 1 mg/kg body weight is safe and does not cause respiratory depression. Tramadol has been used to treat shivering at its onset. Thermoregulatory effects of tramadol resemble those of midazolam, as it reduces the “setpoint” rather than produce a generalized impairment of thermoregulatory control.[13] Thus tramadol slightly decreases the precision of thermoregulatory control in addition to reducing the setpoint.
Sajedi et al. found that the prophylactic use of granisetron 40 μg/kg is as effective as meperidine (0.4 mg/kg) and tramadol (0.1 mg/kg) in preventing post-anesthetic shivering without prolonging the emergence time from anesthesia.[11] Nefopam is a non-opiate analgesic and also known to have an anti-shivering effect and can be a good substitute for meperidine for prevention of shivering during the spinal anesthesia with more stable hemodynamics, if injection pain is effectively controlled.[6] Tsai and Chu compared the anti-shivering potency and side-effects among tramadol, amitriptyline and meperidine for the treatment of post-epidural anesthetic shivering in parturients. Both tramadol and meperidine showed a significantly faster response rate in the “treatment” of shivering when compared with amitriptyline. Tramadol had less frequent incidence of somnolence than meperidine.[4] Both tramadol and meperidine have similar shivering reducing effects, whereas tramadol decreased the incidence of central depressive effects, hence could be considered superior to meperidine for the treatment of shivering in parturients.[4]
All the above studies support the use of tramadol either as equally effective or superior to various drugs studied. Tramadol is safe with lesser side-effects.
Oral tramadol is easily available and economical (Rs. 8/tablet). Tramadol's salient attributes are its high safety profile and weak sedative properties, particularly in patients with poor cardiorespiratory reserves. Tramadol hydrochloride is readily absorbed following oral administration. Oral bioavailability is approximately 68% after a single dose and increases to 90% at steady state. The bioavailability of tramadol hydrochloride after intramuscular injection or intravenous administration is the same. Its intravenous formulation is around Rs. 15 and another widely studied and proved equally effective injection. Clonidine costs Rs. 40. Thus, making tablet form of tramadol an economical, readily available, safe drug for prophylactic use with proven efficacy (among the various drugs studied).
The average age of patients undergoing TURP is 69 years.[18] Transurethral prostatectomy is considered the best treatment for relieving obstruction of the urinary bladder in patients of benign or malignant prostatic disease.[19] The high incidence of shivering in TURP may be due to decreased core temperature secondary to peripheral vasodilatation from sympathetic blockade, cold operation theatre temperature and/or cold irrigating fluids. Elderly patients are not able to initiate autonomic protective responses.[3] An important factor could be the rapid decrease in body temperature, which accompanies normal irrigation. Previous studies have reported a reduction in core temperature during the transurethral prostatectomy.[14,15,16] Rapid reduction in core temperature starts with bladder irrigation during transurethral prostatectomy as unheated irrigant is used and could be a significant etiological factor in the production of the hemodynamic responses observed.[2]
The reported hospital mortality for TURP ranges from 0.2% to 2.5%.[21] Most of morbidity and mortality is associated with the cardiovascular system, with myocardial infarction, cardiac arrest, heart failure or cardiac dysrhythmias occurring in up to 2.5% of patients.[21,22] The incidence of cardiovascular complications is higher than expected for a non-invasive procedure. This could be due to the medical state of the patients or TURP related adverse effect including hypervolemia, hypovolemia, hypothermia, hypotension, glycine absorption, hyperammonia, hyponatremia and sepsis.[20] The possibilities that the cardiovascular system could be adversely affected by perioperative reduction in core temperature and metabolic demands have also been noted. Hypothermia causes bradycardia, reduced cardiac output and peripheral vasoconstriction. There are additional adverse effects on every other body system.[8] The cardiovascular system is also stressed as a result of increased after load, effects on myocardial excitability and conduction and the metabolic demands of rewarming.[23] The metabolic cost of shivering is an increase in oxygen consumption from 300% to 800%.[24] We therefore undertook the study in TURP surgery as these patients are predisposed to shivering and any therapeutic effect of our study might prove beneficial for such patients.
No power analysis could be performed before choosing the number of patients for the study, as the exact incidence of perioperative shivering during TURP surgery under spinal anesthesia was not available to the best of our efforts. However the power of our study is the high number of patients in comparison with other studies.
CONCLUSION
To conclude we suggest that oral tramadol should be used prophylactically as a part of premedication to prevent the shivering in patients undergoing TURP surgery under SAB in this group of patients, since it is easily available and cheap. This will prevent perioperative morbidity and lead to shorter hospital stay for geriatric patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chen SC, Tang CS, Chen YT, Ko CJ, Yu KL, Tseng CK, et al. The evaluation of the anti-shivering effect of tramadol during epidural anesthesia. Gaoxiong Yi Xue Ke Xue Za Zhi. 1994;10:632–9. [PubMed] [Google Scholar]
- 2.De Witte J, Rietman GW, Vandenbroucke G, Deloof T. Post-operative effects of tramadol administered at wound closure. Eur J Anaesthesiol. 1998;15:190–5. [PubMed] [Google Scholar]
- 3.Pausawasdi S, Jirasirithum S, Phanarai C. The use of tramadol hydrochloride in the treatment of post-anesthetic shivering. J Med Assoc Thai. 1990;73:16–20. [PubMed] [Google Scholar]
- 4.Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001;93:1288–92. doi: 10.1097/00000539-200111000-00052. [DOI] [PubMed] [Google Scholar]
- 5.Chan AM, Ng KF, Tong EW, Jan GS. Control of shivering under regional anesthesia in obstetric patients with tramadol. Can J Anaesth. 1999;46:253–8. doi: 10.1007/BF03012605. [DOI] [PubMed] [Google Scholar]
- 6.Kim YA, Kweon TD, Kim M, Lee HI, Lee YJ, Lee KY. Comparison of meperidine and nefopam for prevention of shivering during spinal anesthesia. Korean J Anesthesiol. 2013;64:229–33. doi: 10.4097/kjae.2013.64.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelrahman RS. Prevention of shivering during regional anesthesia: Comparison of midazolam, midazolam plus ketamine, tramadol, and tramadol plus ketamine. Life Sci J. 2012;9:132–9. [Google Scholar]
- 8.Wrench IJ, Singh P, Dennis AR, Mahajan RP, Crossley AW. The minimum effective doses of pethidine and doxapram in the treatment of post-anesthetic shivering. Anesthesia. 1997;52:32–6. doi: 10.1111/j.1365-2044.1997.006-az006.x. [DOI] [PubMed] [Google Scholar]
- 9.Usta B, Gozdemir M, Demircioglu RI, Muslu B, Sert H, Yaldiz A. Dexmedetomidine for the prevention of shivering during spinal anesthesia. Clinics (Sao Paulo) 2011;66:1187–91. doi: 10.1590/S1807-59322011000700011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oranuch K, Tamdeeb D, Charuluxananana S. Comparison of the efficacy of nalbuphine, tramadol, ondansetron and placebo in the treatment of postanesthetic shivering after spinal anesthesia for cesarean delivery. Asian Biomed. 2007;1:189–94. [Google Scholar]
- 11.Sajedi P, Yaraghi A, Moseli HA. Efficacy of granisetron in preventing postanesthetic shivering. Acta Anaesthesiol Taiwan. 2008;46:166–70. doi: 10.1016/S1875-4597(09)60004-7. [DOI] [PubMed] [Google Scholar]
- 12.Bilotta F, Pietropaoli P, Sanita’ R, Liberatori G, Rosa G. Nefopam and tramadol for the prevention of shivering during neuraxial anesthesia. Reg Anesth Pain Med. 2002;27:380–4. doi: 10.1053/rapm.2002.33563. [DOI] [PubMed] [Google Scholar]
- 13.Mathews S, Al Mulla A, Varghese PK, Radim K, Mumtaz S. Postanesthetic shivering – A new look at tramadol. Anesthesia. 2002;57:394–8. doi: 10.1046/j.1365-2044.2002.2457_3.x. [DOI] [PubMed] [Google Scholar]
- 14.Kranke P, Eberhart LH, Roewer N, Tramèr MR. Pharmacological treatment of postoperative shivering: A quantitative systematic review of randomized controlled trials. Anesth Analg. 2002;94:453–60. doi: 10.1097/00000539-200202000-00043. [DOI] [PubMed] [Google Scholar]
- 15.Alfonsi P. Postanesthetic shivering: Epidemiology, pathophysiology, and approaches to prevention and management. Drugs. 2001;61:2193–205. doi: 10.2165/00003495-200161150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001;93:1288–92. doi: 10.1097/00000539-200111000-00052. [DOI] [PubMed] [Google Scholar]
- 17.Filos KS, Goudas LC, Patroni O, Polyzou V. Hemodynamic and analgesic profile after intrathecal clonidine in humans. A dose-response study. Anesthesiology. 1994;81:591–60. doi: 10.1097/00000542-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Mebust WK, Holtgrewe HL, Cockett AT, Peters PC. Transurethral prostatectomy: Immediate and postoperative complications. A cooperative study of 13 participating institutions evaluating 3,885 patients. J Urol. 1989;141:243–7. doi: 10.1016/s0022-5347(17)40731-2. [DOI] [PubMed] [Google Scholar]
- 19.Chisholm GD. Benign prostatic hyperplasia: The best treatment. BMJ. 1989;299:215–6. doi: 10.1136/bmj.299.6693.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neal DE. Prostatectomy – An open or closed case. Br J Urol. 1990;66:449–54. doi: 10.1111/j.1464-410x.1990.tb14986.x. [DOI] [PubMed] [Google Scholar]
- 21.Holtgrewe HL, Valk WL. Factors influencing the mortality and morbidity of transurethral prostatectomy: A study of 2,015 cases. J Urol. 1962;87:450–9. doi: 10.1016/S0022-5347(17)64980-2. [DOI] [PubMed] [Google Scholar]
- 22.Melchior J, Valk WL, Foret JD, Mebust WK. Transurethral prostatectomy: Computerized analysis of 2,223 consecutive cases. J Urol. 1974;112:634–42. doi: 10.1016/s0022-5347(17)59817-1. [DOI] [PubMed] [Google Scholar]
- 23.Carli F. Metabolic disturbances of hypothermia. Clin Anaesthesiol. 1989;3:405–21. [Google Scholar]
- 24.Horvath SM, Spurr GB, Hutt BK, Hamilton LH. Metabolic cost of shivering. J Appl Physiol. 1956;8:595–602. doi: 10.1152/jappl.1956.8.6.595. [DOI] [PubMed] [Google Scholar]


