Abstract
Background:
Spinal anesthesia is an established mode of anesthesia for lower limb orthopedic surgeries. The limitations of the technique are short duration of action and limited post-operative analgesia. Concomitant use of intravenous infusion of magnesium sulfate may have an effect on the block characteristics and duration of action of intrathecal bupivacaine.
Methods:
A total of 80 American Society of Anesthesiologists I and II patients, either sex, 20-60 years of age scheduled for elective orthopedic fixation of fracture of long bones of lower limbs under spinal anesthesia were included. Spinal anesthesia administered with 2.5 ml heavy bupivacaine mixed with 10 mcg fentanyl. The groups were then divided to receive an infusion of injection magnesium sulfate 50 mg/kg/h over 15 min followed by 15 mg/kg/h until the end of the surgery (Group M) and 15 ml of Normal Saline over 15 min followed by 100 ml/h until the end of surgery (Group S). Onset, duration of sensory and motor block and amount of post-operative analgesic were noted.
Results:
A total of 6 patients (Group M) and seven patients (Group S) had inadequate block and excluded from the study. Mean block height was T6. Time required to achieve block height was 8.82 min versus 7.42 min in Groups M and S respectively (P = 0.04). Mean duration of motor block was longer in group M (160.63 ± 17.76 min) compared with Group S (130.12 ± 20.70 min) (P = 0.000). Time for regression of sensory block to T12/L1was 206.88 ± 20.96 min (Group M) and 163.88 ± 15.46 min (Group S) (P = 0.000). Hemodynamic parameters were similar and statistically not significant. Need for first analgesic requirement was after 262.88 ± 21.11 min in group M and 193.25 ± 17.74 min in the group S (P = 0.000). Mean dosage of tramadol needed in first 24 h was less in group M (190 ± 30.38 mg vs. 265 ± 48.30 mg, P = 0.000).
Conclusion:
Use of intravenous magnesium with spinal anesthesia reduces post-operative pain and analgesic consumption.
Keywords: Intravenous magnesium, post-operative pain, spinal anesthesia
INTRODUCTION
Fundamental of regional anesthesia is pharmacologically interrupting transmission of sensation in the specific nerve fiber. The sensory signals generated by tissue damage triggers a state of increased excitability, leading to prolonged post-operative pain or sensitization to such pain. The optimal pain treatment pre-empts the establishment of pain hypersensitivity during and after surgery by minimizing the patient discomfort while leaving physiologic nociceptive mechanisms intact so as to function as an early warning symptom.[1,2]
Spinal anesthesia is an established mode of anesthesia for lower limb orthopedic surgeries because of its simplicity, ease of administration and absence of side-effects of general anesthesia. The limitations of the technique are short duration of action and limited postoperative analgesia. Additives to local anesthetic agent's solutions increases the duration of the spinal block and modulate post-operative analgesia.
Magnesium (Mg) is the fourth common cation in body. Anti-nociceptive effects of Mg are due to regulation of calcium influx into the cell and antagonism of the N-methyl D-aspartate NMDA receptors. Numerous clinical investigations have demonstrated that Mg infusion during general anesthesia reduced anesthetic requirement and post-operative analgesic consumption.[3,4] The addition of intrathecal Mg to bupivacaine prolonged the time of two segment regression of spinal block height but did not affect maximum sensory level or the time to reach the highest level of sensory block.[5,6]
With this background, we hypothesized that concomitant use of intravenous infusion of magnesium sulfate may have an effect on the block characteristics and duration of action of intrathecal bupivacaine. This study was planned to evaluate and observe the effect of concomitant intravenous infusion of magnesium sulfate in the patients undergoing lower limb orthopedic surgery under subarachnoid block with Bupivacaine.
METHODS
This prospective randomized trial was conducted after institutional ethics approval and written consent from the patients. A total of 80 American Society of Anesthesiologists (ASA) I and II patients, either sex, 20-60 years of age scheduled for elective orthopedic fixation of fracture of long bones of lower limbs under spinal anesthesia were included. Exclusion criteria's included patient's refusal for spinal anesthesia, ASA III and IV patients, age > 60 years, Body weight > 120 kg or height < 150 cm, known allergy to study drug and known contraindications to spinal anesthesia. Patients fulfilling inclusion criteria were randomized by computer generated randomization into two groups to receive injection magnesium sulfate 50 mg/kg/h over 15 min followed by infusion 15 mg/kg/h until the end of the surgery (Group M) and 15 ml of normal saline over 15 min followed by infusion at the rate of 100 ml/h till the end of surgery (Group S).
All patients were kept nil per oral for 6 h and received tablet alprazolam 0.25 mg night before and on the day of surgery. Intravenous (i.v) access was established and preloading with 500 ml Ringer Lactate was carried out. Routine monitors such as non-invasive blood pressure, Pulse oximeter (SpO2), Electrocardiogram were attached and baseline parameters recorded. Spinal anesthesia was administered in L3-L4 intervertebral space in sitting position using 23G Quincke's needle and 12.50 mg of injection Bupivacaine mixed with 10 μg Fentanyl was administered in both groups. The patients were turned to supine position and i.v infusion of the study drug was started as per group allotted. Onset of spinal block, level of sensory and motor blockade was evaluated by pin prick method and modified Bromage scale respectively.
Surgery was started after achieving a sensory block of T10 or above and Bromage score of 3. Time taken to achieve these conditions was recorded. Blood pressure (BP), heart rate (HR), SpO2 were recorded every 2 min for first 15 min and thereafter every 10 min until the end of surgery.
A fall in BP of more than 20% of baseline was treated with fast i.v infusion of Ringer Lactate and (injection) Mephentermine-5 mg i.v bolus. Fall in HR less than 45 beats/min or more than 20% of baseline and accompanied by hemodynamic variability was treated with i.v bolus of injection Atropine-0.6 mg intravenously. At the end of the surgery, respective infusions were stopped, level of sensory and motor blockade checked and patients shifted to post-anesthesia care unit. Pain assessments were performed utilizing visual analogue scale (VAS) at 0, 2, 4, 12 and 24 h post-operatively. VAS ≥4 was treated with i.v slow bolus of injection Tramadol 100 mg. Further boluses of tramadol 100 mg were administered as per need of the patient. Total consumption over the first 24 h post-operative was noted. Time for demand of first bolus of tramadol was also noted. All these parameters were recorded by an independent investigator blinded to the group allotment of patients.
Sample size calculation was based on a previous study. With the level of significance of 95%, power of study 80%, α error of 0.05 and β error of 0.2, to show a 20% delay in the requirement of 1st rescue analgesic, at least 35 patients per group were needed. We took 40 patients per group for our study to compensate for any drop outs.
Data was analyzed statistically using the software Microsoft office Excel 2007 and SPSS version 19 (SPSS software IBM Corporation, Amrock, New York). Quantitative data was expressed as mean and standard deviation while qualitative data was expressed in terms of median and range or frequencies and percentages. The means of the continuous variables (Time to achieve effect, Time to achieve Bromage 0/1, Sensory Regression to T12/L1) were compared by using independent sample t-test. P < 0.05 was considered to be significant.
RESULTS
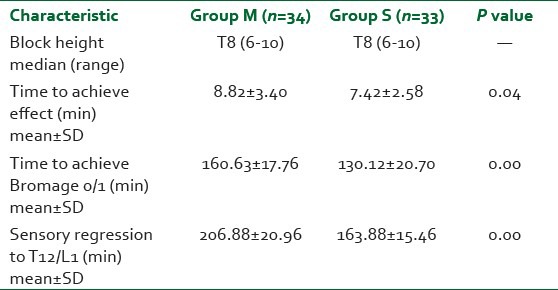
The demographic profile and type of surgeries are depicted in Tables 1 and 2 respectively. Six patients in group M and seven patients in Group S had inadequate block and were excluded from the study so total of 67 patients were taken for final analysis. The mean block height was T6 in both groups; however, the time needed to achieving it was different 8.82 min versus 7.42 min in Group M and S respectively (P = 0.04). The mean duration of motor block was longer in Group M (160.63 ± 17.76 min) compared with Group S (130.12 ± 20.70 min) (P = 0.000). Time for regression of sensory block to T12/L1was 206.88 ± 20.96 min (Group M) and 163.88 ± 15.46 min (Group S) (P = 0.000) [Table 3].
Table 1.
Demographic profile
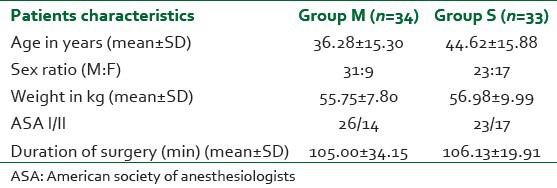
Table 2.
Type of surgeries
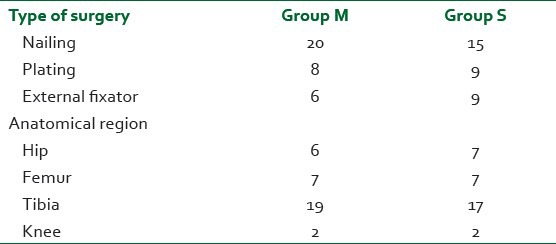
Table 3.
Block characteristics
Hemodynamic parameters such as HR and mean BP were similar in both groups and statistically not significant [Figures 1 and 2].
Figure 1.
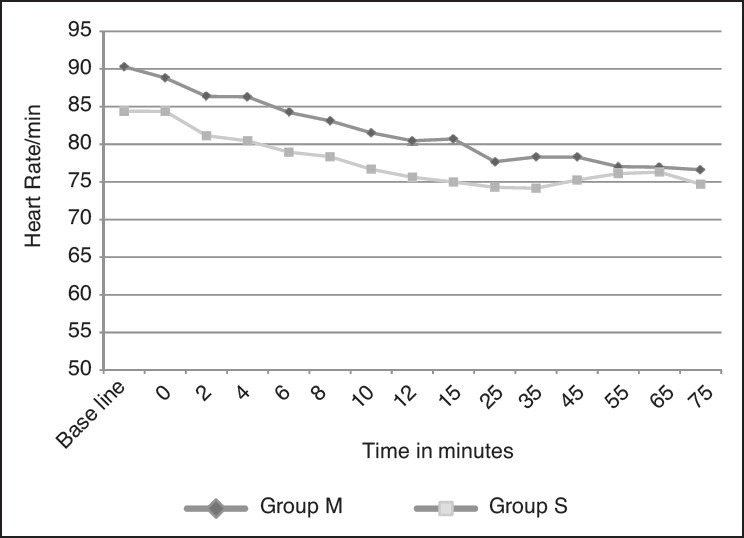
Heart rate at different intervals
Figure 2.
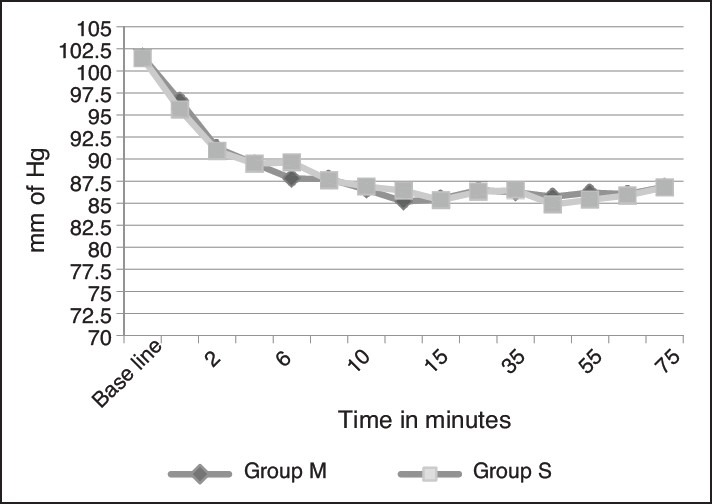
Mean blood pressure at different interval
Need for first analgesic requirement was present after 262.88 ± 21.11 min in group M and 193.25 ± 17.74 min in group S (P = 0.000). Mean dosage of tramadol needed in first 24 h was also less in group M compared with group S (190 ± 30.38 mg versus 265 ± 48.30 mg, P = 0.000).
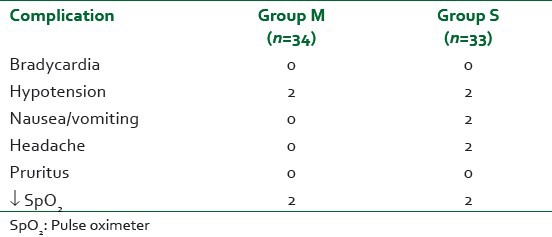
Incidences of intraoperative complications are depicted in Table 4. Hypotension in both groups responded to i.v administration of injection Mephentermine 5 mg bolus.
Table 4.
Incidences of intraoperative complications
DISCUSSION
Our study demonstrates the effectiveness of perioperative infusion of Mg in prolonging the spinal sensory block as well as reducing the post-operative analgesic requirements without significant hemodynamic variability or side effects.
Use of Mg is associated with beneficial effects in perioperative period, intensive care and postoperatively.[7,8,9] Mg has been found to have multisystemic effects in the body involving the cardiovascular,[10,11] central nervous systems,[12,13] analgesia,[14,15] decrease in catecholamine surges,[16,17,18,19] management of asthma,[20] eclampsia,[21,22] tetanus[23] and intensive care.[24,25] Use of Mg infusion with spinal anesthesia has been sparingly tested before.[26,27]
In our study, the onset of the block was slower with Mg infusion. Few studies have commented on the onset of block, most of them relates to intrathecal or epidural administration of Mg. Use of the intrathecal Mg has been demonstrated to delay the onset of block[28,6] though Ghatak et al.[29] has found that the onset of the block was faster with epidural Mg. Dayioğlu et al.[5] however, in their study did not find any difference in time to reach the maximum sensory block. Ozalevli et al.[30] and Malleswaran et al.[31] have postulated the changes in pH and baricity of local anesthetic solution with the addition of Mg as the cause of delayed onset of block. The same mechanism however cannot work with intravenous administration of Mg and further research is needed to assess this aspect.
Sensory block has been found to be increased with the use of Mg-mechanism postulated being its action as calcium channel as well as NMDA receptors antagonists. In humans, calcium channel antagonists, mediate anti-nociception by its action of decreasing calcium mediated release of neurotransmitter implicated in nociception and inflammation. Blocking of NMDA receptors has been implicated with a decrease in post nociception central sensitization of pain.[4] Motor blockade produced by Mg is a result of reduction of release of acetylcholine from motor nerve terminals as well as direct depression of muscle fiber membrane excitability leading to increase motor block with its use.[26]
The dosage of Mg chosen by us was in accordance to the study of Hwang et al.[26] The difference in our study was in the dosage of bupivacaine as well as an addition of fentanyl 10 mcg to bupivacaine. Hwang et al. administered the volume of local anesthetic solution according to the height of the patient while we administered a fixed dose of bupivacaine mixed with fentanyl. This may have been the reason for increased duration of analgesia in our case (266.88 ± 21.11 min vs. 249 ± 41 min in Hwang study). Various other dosages and regimes have been investigated by different authors for evaluation of Mg for post-operative analgesia with varied results.[32,33,34,35,36]
Hemodynamic depression was not seen in our study and hemodynamic variability did not differ from the control group. This may have been attributed to preloading with ringer lactate as well as slow infusion of Mg. Mg causes a dose dependent negative ionotropic effect due to primarily arteriolar vasodilatation.[37] In our study, only two patients developed hypotension and it responded to a single bolus of injection Mephentermine 5 mg.
Side-effects reported in our study were minor and included hypotension (5%), decreased SpO2 (5%) with the use of Mg. This is slightly less than previously reported studies.[26]
In a recent study Mercieri et al.[38] investigated whether intravenous infusion of Mg along with spinal anesthesia has any effect on the cerebrospinal fluid (CSF) Mg level corresponding to activation of central NMDA receptors responsible for analgesia. They concluded that use of spinal anesthesia is responsible for decrease in CSF Mg level for the time local anesthetic effect persists as Increasing serum Mg concentration over 80% of the baseline value left CSF Mg levels unchanged. They thus postulated that central NMDA receptors are not responsible for the analgesic effects of Mg and a peripheral mechanism is responsible for the same.
The major drawback of our study was the inability to measure the serum Mg level as well as the inability of use of patient control analgesia for control of pain.
In conclusion, use of intravenous Mg with spinal anesthesia reduces post-operative pain and analgesic consumption in moderate to severe pain associated with lower limb orthopedic surgeries.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Woolf CJ, Chong MS. Preemptive analgesia – Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 2.Thompson SW, King AE, Woolf CJ. Activity-dependent changes in rat ventral horn neurons in vitro; summation of prolonged afferent evoked postsynaptic depolarizations produce a d-2-Amino-5-Phosphonovaleric acid sensitive windup. Eur J Neurosci. 1990;2:638–49. doi: 10.1111/j.1460-9568.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 3.Telci L, Esen F, Akcora D, Erden T, Canbolat AT, Akpir K. Evaluation of effects of magnesium sulphate in reducing intraoperative anesthetic requirements. Br J Anaesth. 2002;89:594–8. doi: 10.1093/bja/aef238. [DOI] [PubMed] [Google Scholar]
- 4.Koinig H, Wallner T, Marhofer P, Andel H, Hörauf K, Mayer N. Magnesium sulfate reduces intra-and postoperative analgesic requirements. Anesth Analg. 1998;87:206–10. doi: 10.1097/00000539-199807000-00042. [DOI] [PubMed] [Google Scholar]
- 5.Dayioðlu H, Baykara ZN, Salbes A, Solak M, Toker K. Effects of adding magnesium to bupivacaine and fentanyl for spinal anesthesia in knee arthroscopy. J Anesth. 2009;23:19–25. doi: 10.1007/s00540-008-0677-4. [DOI] [PubMed] [Google Scholar]
- 6.Khalili G, Janghorbani M, Sajedi P, Ahmadi G. Effects of adjunct intrathecal magnesium sulfate to bupivacaine for spinal anesthesia: A randomized, double-blind trial in patients undergoing lower extremity surgery. J Anesth. 2011;25:892–7. doi: 10.1007/s00540-011-1227-z. [DOI] [PubMed] [Google Scholar]
- 7.Dubé L, Granry JC. The therapeutic use of magnesium in anesthesiology, intensive care and emergency medicine: A review. Can J Anaesth. 2003;50:732–46. doi: 10.1007/BF03018719. [DOI] [PubMed] [Google Scholar]
- 8.James MF. Magnesium: An emerging drug in anesthesia. Br J Anaesth. 2009;103:465–7. doi: 10.1093/bja/aep242. [DOI] [PubMed] [Google Scholar]
- 9.Delhumeau A, Granry JC, Monrigal JP, Costerousse F. Indications for the use of magnesium in anesthesia and intensive care. Ann Fr Anesth Reanim. 1995;14:406–16. doi: 10.1016/s0750-7658(05)80393-4. [DOI] [PubMed] [Google Scholar]
- 10.Reinhart RA. Clinical correlates of the molecular and cellular actions of magnesium on the cardiovascular system. Am Heart J. 1991;121:1513–21. doi: 10.1016/0002-8703(91)90160-j. [DOI] [PubMed] [Google Scholar]
- 11.Vigorito C, Giordano A, Ferraro P, Acanfora D, De Caprio L, Naddeo C, et al. Hemodynamic effects of magnesium sulfate on the normal human heart. Am J Cardiol. 1991;67:1435–7. doi: 10.1016/0002-9149(91)90478-4. [DOI] [PubMed] [Google Scholar]
- 12.Meloni BP, Campbell K, Zhu H, Knuckey NW. In search of clinical neuroprotection after brain ischemia: The case for mild hypothermia (35 degrees C) and magnesium. Stroke. 2009;40:2236–40. doi: 10.1161/STROKEAHA.108.542381. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009 Jan;21(1):CD004661. doi: 10.1002/14651858.CD004661.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Brill S, Sedgwick PM, Hamann W, Di Vadi PP. Efficacy of intravenous magnesium in neuropathic pain. Br J Anaesth. 2002;89:711–4. [PubMed] [Google Scholar]
- 15.Ozcan PE, Tugrul S, Senturk NM, Uludag E, Cakar N, Telci L, et al. Role of magnesium sulfate in postoperative pain management for patients undergoing thoracotomy. J Cardiothorac Vasc Anesth. 2007;21:827–31. doi: 10.1053/j.jvca.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 16.James MF, Beer RE, Esser JD. Intravenous magnesium sulfate inhibits catecholamine release associated with tracheal intubation. Anesth Analg. 1989;68:772–6. [PubMed] [Google Scholar]
- 17.Jee D, Lee D, Yun S, Lee C. Magnesium sulphate attenuates arterial pressure increase during laparoscopic cholecystectomy. Br J Anaesth. 2009;103:484–9. doi: 10.1093/bja/aep196. [DOI] [PubMed] [Google Scholar]
- 18.James MF. Magnesium sulphate in phoechromocytoma. Anesthesiol. 1985;62:188–9. [Google Scholar]
- 19.Van Braeckel P, Carlier S, Steelant PJ, Weyne L, Vanfleteren L. Perioperative management of phaeochromocytoma. Acta Anaesthesiol Belg. 2009;60:55–66. [PubMed] [Google Scholar]
- 20.Rodrigo G, Rodrigo C, Burschtin O. Efficacy of magnesium sulfate in acute adult asthma: A meta-analysis of randomized trials. Am J Emerg Med. 2000;18:216–21. doi: 10.1016/s0735-6757(00)90024-x. [DOI] [PubMed] [Google Scholar]
- 21.Duley L, Gülmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2003;2:CD000025. doi: 10.1002/14651858.CD000025. [DOI] [PubMed] [Google Scholar]
- 22.Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: A brief review. Stroke. 2009;40:1169–75. doi: 10.1161/STROKEAHA.108.527788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James MF, Manson ED. The use of magnesium sulphate infusions in the management of very severe tetanus. Intensive Care Med. 1985;11:5–12. doi: 10.1007/BF00256058. [DOI] [PubMed] [Google Scholar]
- 24.Dacey MJ. Hypomagnesemic disorders. Crit Care Clin. 2001;17:155–73. doi: 10.1016/s0749-0704(05)70157-3. [DOI] [PubMed] [Google Scholar]
- 25.Jones NA, Jones SD. Management of life-threatening autonomic hyper-reflexia using magnesium sulphate in a patient with a high spinal cord injury in the intensive care unit. Br J Anaesth. 2002;88:434–8. doi: 10.1093/bja/88.3.434. [DOI] [PubMed] [Google Scholar]
- 26.Hwang JY, Na HS, Jeon YT, Ro YJ, Kim CS, Do SH. I.V. infusion of magnesium sulphate during spinal anesthesia improves postoperative analgesia. Br J Anaesth. 2010;104:89–93. doi: 10.1093/bja/aep334. [DOI] [PubMed] [Google Scholar]
- 27.Dabbagh A, Elyasi H, Razavi SS, Fathi M, Rajaei S. Intravenous magnesium sulfate for post-operative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Scand. 2009;53:1088–91. doi: 10.1111/j.1399-6576.2009.02025.x. [DOI] [PubMed] [Google Scholar]
- 28.Shukla D, Verma A, Agrawal A, Pandey HD, Tyagi C. Comparitive study of intrathecal dexmedetomidine with intrathecal magnesium sulfate used as a adjuvant to bupivacaine. J Anesthesiol Clin Pharmacol. 2011;27:495–9. doi: 10.4103/0970-9185.86594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghatak T, Chandra G, Malik A, Singh D, Bhatia VK. Evaluation of the effect of magnesium sulphate vs. clonidine as adjunct to epidural bupivacaine. Indian J Anaesth. 2010;54:308–13. doi: 10.4103/0019-5049.68373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozalevli M, Cetin TO, Unlugenc H, Guler T, Isik G. The effect of adding intrathecal magnesium sulphate to bupivacaine-fentanyl spinal anesthesia. Acta Anaesthesiol Scand. 2005;49:1514–9. doi: 10.1111/j.1399-6576.2005.00793.x. [DOI] [PubMed] [Google Scholar]
- 31.Malleswaran S, Panda N, Mathew P, Bagga R. Magnesium as an intrathecal adjuvant in mild precclampsia. Int J Obstet Anesth. 2010;19:161–6. doi: 10.1016/j.ijoa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Tramer MR, Glynn CJ. An evaluation of single dose of magnesium to supplement analgesia after ambulatory surgery: Randomized controlled trial. Anesth Analg. 2007;104:1374–9. doi: 10.1213/01.ane.0000263416.14948.dc. [DOI] [PubMed] [Google Scholar]
- 33.Karaaslan D, Arslan G. The role of nitric oxide on the potentiating effect of magnesium on morphine analgesia. Acta Anaesthesiol Scand. 2005;49:129. doi: 10.1111/j.1399-6576.2005.00544.x. [DOI] [PubMed] [Google Scholar]
- 34.Levaux Ch, Bonhomme V, Dewandre PY, Brichant JF, Hans P. Effect of intra-operative magnesium sulphate on pain relief and patient comfort after major lumbar orthopaedic surgery. Anesthesia. 2003;58:131–5. doi: 10.1046/j.1365-2044.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- 35.Kiran S, Gupta R, Verma D. Evaluation of a single-dose of intravenous magnesium sulphate for prevention of postoperative pain after inguinal surgery. Indian J Anaesth. 2011;55:31–5. doi: 10.4103/0019-5049.76605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS. Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiology. 2001;95:640–6. doi: 10.1097/00000542-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 37.James MF, Cork RC, Dennett JE. Cardiovascular effects of magnesium sulphate in the baboon. Magnesium. 1987;6:314–24. [PubMed] [Google Scholar]
- 38.Mercieri M, De Blasi RA, Palmisani S, Forte S, Cardelli P, Romano R, et al. Changes in cerebrospinal fluid magnesium levels in patients undergoing spinal anesthesia for hip arthroplasty: Does intravenous infusion of magnesium sulphate make any difference? A prospective, randomized, controlled study. Br J Anaesth. 2012;109:208–15. doi: 10.1093/bja/aes146. [DOI] [PubMed] [Google Scholar]


