Abstract
Spinal cord injury (SCI) is a serious disease of the center nervous system (CNS). It is a devastating injury with sudden loss of motor, sensory, and autonomic function distal to the level of trauma and produces great personal and societal costs. Currently, there are no remarkable effective therapies for the treatment of SCI. Compared to traditional treatment methods, stem cell transplantation therapy holds potential for repair and functional plasticity after SCI. However, the mechanism of stem cell therapy for SCI remains largely unknown and obscure partly due to the lack of efficient stem cell trafficking methods. Molecular imaging technology including positron emission tomography (PET), magnetic resonance imaging (MRI), optical imaging (i.e., bioluminescence imaging (BLI)) gives the hope to complete the knowledge concerning basic stem cell biology survival, migration, differentiation, and integration in real time when transplanted into damaged spinal cord. In this paper, we mainly review the molecular imaging technology in stem cell therapy for SCI.
1. Introduction
Spinal cord injury (SCI), which results from trauma or progressive neurodegeneration, is a devastating and life-altering injury. It often affects young and healthy individuals who are suffering from severe functional and sensory deficits. This debilitating condition not only creates enormous physical and emotional cost to individuals but also is a significant financial burden to the society. The annual incidence of SCI is 15–40 cases per million worldwidely [1].
SCI is mainly divided into two types: traumatic SCI and nontraumatic SCI. A global-incident rate (2007) of traumatic SCI is estimated at 23 traumatic SCI cases per million [2]. The most common causes of traumatic SCI are road traffic accidents, falls, occupational mishaps, and sports-related injuries [3]. Most SCI occurs at the cervical level (approximately 55%) with a mortality of 10% in the first year following injury and an expected lifespan of only 10 to 15 years after injury. Thoracic, thoracolumbar, and lumbosacral level injury each accounts for approximately 15% of SCI [3].
Despite the progress of medical and surgical management as well as rehabilitation approaches, many SCI patients still experience substantial neurological disabilities [4, 5]. Moreover, clinical trials of pharmacologic therapeutics within the last two decades have either failed to prove efficacy or provided only modest reductions in functional deficits [6–8].
Previous researches on SCI mainly focused on improving neurological manifestations of SCI while ignoring the pathological changes of spinal cord. According to the progress, SCI could be divided into primary injury phase which is the physical injury and secondary injury phase [9, 10]. The primary injury phase damages both upper and lower motor neurons and disrupts sensory, motor, and autonomic (including respiration, cardiac output, and vascular tone) functions. The secondary injury phase is the amplification of the original injury with a subsequent cascade of molecular and cellular events [11]. Pathophysiological processes occur after the primary injury phase and are rapidly instigated in response to the primary injury in order to control and minimize the damage. However, these are largely responsible for exacerbating the initial damage and creating an inhibitory microenvironment which prevent endogenous efforts of regeneration and remyelination. Such secondary processes include ischemia, inflammation, lipid peroxidation, disruption of ion channels, fluid and electrolyte disturbances, producing of free radicals, axonal demyelination, necrosis, glial scar formation, and apoptosis [12, 13]. Nevertheless, endogenous repair and regeneration happen during the secondary phase of SCI to minimize the extent of the lesion, reorganize blood supply through angiogenesis, clear cellular debris, reunite and remodel damaged neural circuits, and offer exploitable targets for therapeutic intervention [14, 15]. Thus, these secondary damages are crucial to SCI therapy.
Increasing interest has focused on the development of innovative therapeutic methods that aim to regenerate damaged CNS tissue by taking advantages of recent advances in stem cell and neuroscience research [25, 26]. Preclinical models demonstrated that stem cell transplantation could ameliorate some secondary events of SCI through neuroprotection and restore lost tissue through regeneration [27]. Cumulative researches have demonstrated the feasibility of stem cell therapy and various stem cells have been used to protect against the secondary damage with enhancing the regeneration of a damaged spinal cord. Thus, stem cell transplantation would be one of the promising approaches for the regeneration of an injured spinal cord [28].
Recent studies suggested that stem cell therapy could improve neural function in SCI by replacing damaged cells [29, 30]. Therefore, it becomes more and more important to explore the detailed mechanisms of stem cell therapy for SCI and monitor the fate of these cells in vivo, including survival, migration, distribution, rejection, integration, and differentiation.
Fortunately, molecular imaging gives an effective way for such research. Well-established imaging modalities used by researchers for the purpose of molecular imaging are positron emission tomography (PET), magnetic resonance imaging (MRI), whereas others have employed newer modalities based on the transmission of light through tissues, such as in vivo bioluminescence imaging (BLI) and fluorescence imaging (FLI). The label of stem cells requires labelling methods making grafted cells distinguishable from host cells to follow transplanted stem cells by the above methods.
PET is a nuclear imaging technique that produces a three-dimensional image or picture of functional processes in the body. The system detects pairs of gamma rays emitted indirectly by a positron-emitting radionuclide (tracer), which is introduced into the body on a biologically active molecule. It can provide metabolic changes and be used for tracing radioactively labeled stem cells in vivo. MRI can be used for noninvasive tracking of transplanted cells which are usually labeled with super-paramagnetic nanoparticles as contrast agents in longitudinal studies on living animals. Gadolinium and ferric oxide are two common contrast media used for cell labeling in MRI [31]. Recently, there were several studies using MRI to trace the transplanted stem cells in SCI models [32–34]. Their results showed that the transplanted stem cells could be readily detected in vivo using noninvasive MRI techniques. BLI is a newly developing technology for dynamically observing biological behavior. It is based on an enzymatic light production system. The enzyme in action is a luciferase which uses the substrate luciferin to produce light. Then the emitted light is subsequently captured by a highly sensitive CCD camera. Using this technology, cells should be transgenically modified to steadily express luciferase. Then, cells can be tracked in a long periods of time because luciferase expression is preserved during proliferation so that all cells and their descendants express luciferase without dilution. Thus, we can observe the stem cells' dynamic behavior in vivo through this method.
2. Stem/Progenitor Cell Therapy in SCI
2.1. Mechanism of Stem Cell Therapy
According to the pathophysiological targets of SCI, transplanted cells should satisfy the following requirements. Firstly, enable regenerating axons to cross any cysts or cavities; secondly, functionally replace dead cells; thirdly, create an environment to support axonal regeneration and myelination. Stem or progenitor cells are capable of modifying the lesion environment, providing structural support, myelination, increasing neurotrophic factors for neuroprotection, and endogenous activation [35–38]. As a result, stem cells have potential for remyelinating lesions and are an attractive cell source for cell therapy of SCI. And recent experimental studies suggested that stem cell therapy can improve neural function in SCI (Table 1). The opportunity to enhance endogenous adaptability through cell-based approaches has led to a great interest in developing stem cell transplantation therapies that could potentiate and synergise with other treatment modalities to maximise neuroplasticity and produce meaningful recovery.
Table 1.
Stem cell-based cell therapy in experimental SCI models.
| Cell type | Cell number | Route | Time after SCI | Weeks after cell injection | Host animal | Cotreatment method | Functional outcome | References |
|---|---|---|---|---|---|---|---|---|
| BMSCs | 5 × 106 | IL | 24 h | 3 | Rabbit | BMSCs were Ngb gene-modified | Significant functional improvement | [16] |
| OPCs | 5 × 105 | IL | 3 d | 4 | Rat | No | Functional improvements in SSEP amplitudes and latencies | [17] |
| iPSC-NS/PCs | 1 × 106 | IL | 9 d | 12 | Marmoset | No | Promoted functional recovery | [18] |
| NSCs + OECs | 3 × 105 | IL | Immediately | 4 | Rat | Cotransplantation of NSCs and OECs | Improve sensory function | [19] |
| ESCs | 5 × 105 | IV | 2 h | 4 | Mice | No | Promoted hind-limb recovery | [20] |
| iPSCs | 5 × 105 | IL | 9 d | 6 | Mice | No | Promoting locomotor function recovery | [21] |
| NS/PCs | 8 × 104 | IL | 9 d | 8 | Rat | Coinjected with HAMC | Enhanced tissue benefit and functional recovery | [22] |
| EMSCs | 5 × 104 | IL | 0.5 h | 12 | Rat | Coinjected with fibrin scaffolds | Improve the behavioral and histological recovery |
[23] |
| BMSCs | 1 × 107 | IL | 7 d | 4 | Dog | No | Improved functional recovery | [24] |
IL: intralesional injection, IT: intratheca injection, IV: intravenous injection, BMSCs: bone marrow mesenchymal stem cells, Ngb: neuroglobin, SSEPs: somatosensory evoked potential, iPSC-NS/PCs: induced pluripotent stem cell-derived neural stem/progenitor cells, HUCBCs: human umbilical cord blood cells, hES: transplanted human embryonic stem, OPCs: cell-derived oligodendrocyte progenitor cells, ESCs: embryonic stem cells, iPSCs: induced pluripotent stem cells, NSPCs: neural stem/progenitor cells, HAMC: hydrogel blend of hyaluronan and methyl cellulose, and EMSCs: ectomesenchymal stem cells.
Characteristics and purported mechanisms of action of stem cells will then be discussed, with specific attention paid to axonal regeneration and regrowth, growth factor release, guidance through inhibitory cues, remyelination, and induction of anatomical neuroplasticity.
2.2. Bone Marrow Stem Cells
Transplantation of bone marrow-derived mesenchymal stem cells (BMSCs) for SCI has been previously reviewed [39–43]. Many studies have examined BMSCs in SCI rodents and the results showed improved locomotor recovery [44–46].
There are two types of adult bone marrow stem cells: hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs). Zhu et al. compared various properties of human umbilical cord-derived MSCs (HUCMSCs) with human placenta-derived MSCs (HPDMSCs), including cell proliferation, apoptosis, cellular morphology, ultrastructure, and their ability to secrete various growth factors. Their findings indicate that different sources of MSCs have different properties and that care should be taken when choosing the appropriate sources of MSCs for stem cell transplantation [47]. In rodent studies, BMSCs was able to promote a certain degree of axonal regrowth and sprouting, at least in transection models [48]. Lee et al. have used human umbilical cord blood derived mesenchymal stem cells (hUCB-MSCs) to treat dogs with SCI and observed its long-term effects on dogs. It was found that hind-limb recovery in 4 dogs among the five transplanted dogs was significantly improved and the results suggest that transplantation of hUCB-MSCs may have beneficial therapeutic effects [49].
The grafting of MSCs to treat SCI has shown promising results in animals; however, less is known about the effects of autologous MSCs in human SCI. Park et al. explored 10 SCI patients who underwent intramedullary direct MSCs transplantation into injured spinal cords. All patients did not experience any permanent complication associated with MSC transplantation. Three patients showed gradual improvement in activities of daily living, changes on magnetic resonance imaging such as decreases in cavity size and the appearance of fiber-like low signal intensity streaks, and electrophysiological improvement [50].
Despite these potential benefits, there are reported adverse effects of MSCs, such as increased recurrence of hematological malignancies and enhanced tumor growth and metastases [41, 51, 52]. And more preclinical trials of MSC-based therapy need to be preformed.
2.3. Neural Stem Cells and Neural Progenitor Cells
Neural stem cells (NSCs) have been classified as a kind of neural lineage stem cell which is able to self-renew and to give rise to all types of mature neural cells including neurons, astrocytes, and oligodendrocytes [53, 54]. The isolation of adult neural stem cells in mammals was first reported in 1992 by Reynolds and Weiss [55]. NSCs for therapeutic applications are derived from ESCs and progenitor cells are isolated from fetal tissue. Neural progenitor cells (NPCs), like stem cells, have a tendency to differentiate into a specific type of cells.
NSCs and NPCs are found in both fetal and adult CNS [56]. Adult NPCs can be typically harvested from the subventricular zone of the brain or the spinal cord. Embryonic NPCs can be taken from the CNS of rodent embryos and expanded as neurospheres. They all contain precursors for neurons, astroglia, and oligodendrocytes plus stem cells capable of self-renewal [57]. Salazar and his colleagues injected human neural stem cells (hCNS-SCns) into immunodeficient NOD-scid mice 30 days after spinal cord contusion injury in order to test the ability of hCNS-SCns to survive, differentiate, migrate, and promote improved locomotor recovery [54]. They found that hCNS-SCns can survive, differentiate, and promote locomotor recovery when transplanted into an early chronic injury microenvironment. These results suggest that hCNS-SCns graft has efficacy in an early chronic SCI location and expands the “window of opportunity” for intervention.
The extent of glial scar formation and the characteristics of inflammation are the most remarkable difference in the injured spinal cord microenvironment between the subacute and chronic phases. By contrast, the distribution of chronically grafted NSPCs was restricted compared to NSPCs grafted at the subacute phase because a more prominent glial scar located around the lesion epicenter enclosed the grafted cells. Therefore, glial scar formation and inflammatory phenotype should be considered in order to achieve functional recovery by NSPCs transplantation in cases at the chronic phase [58]. Another study showed that grafted NSPCs mainly differentiate into astrocytes after transplantation at the acute phase [59]. Inflammatory cytokines, such as IL-6, TNF-α, and CXCL1, also increase remarkably in the injured spinal cord [60]. These cytokines might induce NSPCs to differentiate into astrocytes. Furthermore, growth factors like EGF and NT3 have demonstrated beneficial effects on the survival of NSPCs [61, 62].
When transplanted into mice in an animal model of SCI, human NSCs promoted locomotor recovery [63]. The study of Mothe indicated that multipotent NSPCs can be delivered from the adult human spinal cord of organ transplant donors and that these cells differentiate into both neurons and glia following transplantation into rats with SCI [64]. In addition, human fetal brain modified NSPCs transplanted subacutely into the contused cervical spinal cord of adult common marmosets produced significantly greater grip strength than controls [65]. Researchers have shown that these NSCs and NPCs primarily differentiate into oligodendrocytes in vitro [66] and in vivo [67, 68]. Recently, researchers demonstrated that self-renewing multipotent NSCs and NPCs could be acquired from the adult human spinal cord of organ transplant donors and that these cells differentiate into both neurons and glia following transplantation into rats with SCI [64].
Simultaneously, the aims of axonal regeneration through the injury area have been replaced preclinically by more realistic objectives of remyelination and provision of trophic support for endogenous precursors and axons. It makes NPCs much more promising candidates of cell therapy for SCI and probably heralds their increased use in clinical trials.
2.4. Embryonic Stem Cells
Embryonic stem cells (ESCs) are pluripotent cells derived from the inner cell mass of developing blastocyst embryos that can differentiate into nearly all cell types [69]. Notably, ESCs have great developmental plasticity and can be induced to become NSPCs with specific differentiation potentials, making them a major candidate of cell replacement therapies for SCI. Mcdonald et al. transplanted neural differentiated mouse embryonic stem cells into rats' spinal cord after traumatic injury. The cells that transplanted into the injured spinal cord differentiated into neurons, astrocytes, and oligodendrocytes and showed partial functional recovery [70]. As noted above, SCI causes extensive demyelination and oligodendrocytes are particularly vulnerable to apoptosis. ESCs predifferentiated into oligodendrocyte progenitor cells (OPCs) remyelinated spared axons and improved recovery when transplanted subacutely into the injured rat spinal cord [71, 72]. The studies document the feasibility of predifferentiating hESCs into functional OPCs and demonstrate their therapeutic potential after SCI.
2.5. Induced Pluripotent Stem Cells
The discovery of induced pluripotent stem cells (iPSCs) has opened a new potential therapeutic approach for regenerative neuroscience, although iPSCs have not yet been used clinically in SCI cell therapy. iPSCs were developed in 2006 by Takahashi and Yamanaka, who showed that mouse somatic cells, such as fibroblasts, could be reprogrammed to pluripotency with retroviral expression of four transcription factors OCT4, SOX2, KLF4, and c-MYC and achieve similar morphology, pluripotency, self-renewal, and gene expression as ESCs, without the requirement for an embryo [73].
The clinical use of ESCs is complicated; however, by ethical and immunological concerns, both of which might be overcome by using pluripotent stem cells which can be derived directly from a patient's own somatic cells [74]. Experimental studies using iPSCs-derived neurospheres transplanted subacutely after contusion SCI showed remyelination, axonal outgrowth of serotonergic fibers, and promotion of locomotor recovery.
Different kinds of iPSCs have different performance in SCI treating. Transplantation of “unsafe” iPS-derived neurospheres may result in teratoma formation and sudden loss of locomotor function [21]. Kramer's review highlights emerging evidence that suggests that iPSCs are not necessarily indistinguishable from ESCs and may occupy a different “state” of pluripotency with differences in gene expression, methylation patterns, and genomic aberrations which may reflect incomplete reprogramming and may therefore impact on the regenerative potential of these donor cells in therapies [75]. Furthermore, the first study on iPSC-derived chimeric mice demonstrated that they were prone to cancer and attributed this property to the reexpression of the c-myc reprogramming factor [76]. In brief, iPSCs are likely to carry a higher risk of tumorigenicity than ESCs, due to the inappropriate reprogramming of these somatic cells, the activation of exogenous transcription factors [77, 78]. Thus, safe iPSC-derived clones would need to be screened and selected [21, 78].
Undifferentiated IPSCs, like ESCs, have high tumourigenicity in pathological conditions [79]. Therefore, the safety of IPSCs should be evaluated before clinical IPSCs-based therapy. Of note, Tsuji et al. recently derived “safe” mouse iPSCs and observed trilineage neural differentiation and functional recovery in a contusion model of SCI without teratoma formation [21].
3. Molecular Imaging in Stem Cell Therapy for Spinal Cord Injury
Transplantation of stem cells has a good prospect of clinical application. However, the challenges in the field of molecular imaging are to develop effective imaging strategies with reporter systems and probes which should firstly reveal cellular and molecular processes throughout an entire study period. Secondly, probes should be highly sensitive to small changes in cell function and distribution. Finally, they do not alter the labeled biological process itself significantly. Molecular imaging technologies will greatly facilitate the functional monitoring and evaluation of a wide range from genes to organs for their roles in health and disease. Herein, molecular imaging has served as a platform to test stem cell therapy for SCI.
3.1. Stem Cell Labelling
Cell labeling can be divided into two types: physical cell labeling and reporter gene imaging. Physical cell labeling is completed before cell administration and can be accomplished with superparamagnetic iron oxide (SPIO) particles for MRI [83, 84], radionuclide labeling for SPECT [85], and PET, nanoparticle labeling for fluorescent imaging [86]. In reporter gene imaging, a gene coding for the synthesis of a detectable protein is introduced into a target cell line or tissue via viral or nonviral vectors. Because the reporter gene integrates into the host cell's chromosome following stable transfection or transduction, the reporter gene is expressed by progeny. As a result, changes in signals following cell administration can be used as indicators of cell proliferation and cell death [87].
3.2. Positron Emission Tomography
Positron emission tomography (PET) imaging, especially 18FDG PET imaging, has been used mainly in cancer [88–90]. PET uses positron emitting radioisotopes as probes for imaging cells in vivo to monitor labeled stem cells and it also has been applied widely to detect and quantify subtle abnormalities in CNS diseases. PET has served as a platform to test stem cell therapy for neurological disease allowing more rapid progression in both preclinical and clinical studies. In order to explore the effect of in vivo PET in tracking the stem cells transplanted into spinal cord, Bai et al. transplanted human neural progenitor cells into rabbits' injured spinal cord; rabbits were injected with 11C-raclopride intravenously and then underwent PET imaging [91]. 11C-raclopride PET imaging of the live rabbits showed accumulation of radioactivity at the hNPC-TERT cell injection site with a standard uptake value significantly higher than that of control group (the HeLa cell transplantation group) (P < 0.01). 11C-raclopride PET imaging of the isolated spinal cords showed rounded focal image of increased radioactivity in the hNPC-TERT cell transplantation group and linear image of radioactivity without clear border in the HeLa cell transplantation group. Meanwhile, fluorescent microscopy showed the same results. These results suggested that PET with radioisotope labeled tracer is useful for functional studies in developing cell-based therapies.
3.3. Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) is a widely used and powerful imaging technique which can provide high resolution and 3D anatomical imaging. It can be used to evaluate the reparation of the injured spinal cord [92]. For monitoring the efficiency of cell transplantation, cellular homing, or targeting, grafted cells can be labeled with superparamagnetic iron oxide nanoparticles (SPION) and detected by means of MRI. In a study of NPC transplantation, SPIO labeling does not affect NPCs' survival and differentiation potential in vitro and labeled NPCs were found migrating along white matter tracts as demonstrated by MRI [93]. In addition, cells labeled with SPION can be manipulated in a magnetic field. In research of Vanecek et al., intrathecally transplanted cells labeled with SPION were guided by a magnetic field and successfully targeted near the lesion site in the rat spinal cord. The results showed that targeting efficiency could be increased by using magnets that produce spatially modulated stray fields [84]. Such magnetic systems with tunable geometric parameters may provide the additional level of control needed to enhance the efficiency of stem cell delivery in injured spinal cord. Hu et al. used labeled human umbilical cord mesenchymal stem cells (hUC-MSCs) in vivo for tracking hUC-MSCs' fate with noninvasive MRI [94]. SPIO was added to cultures at concentrations equivalent to 0, 7, 14, 28, and 56 mg Fe/ml and incubated for 16 h. In vivo MRI 1 and 3 weeks after injection showed a large reduction in signal intensity in the region transplanted with SPIO-labeled hUC-MSCs. The results showed that noninvasive imaging of transplanted SPIO-labeled hUC-MSCs is feasible. Recently, Amemori et al. used neural stem cell line derived from human fetal spinal cord tissue (SPC-01) to treat a balloon-induced SCI [80]. SPC-01 cells labeled with poly-L-lysine-coated SPION were implanted into the lesion 1 week after SCI. Then T2-weighted images were obtained (Figure 1). The transplanted animals displayed significant motor and sensory improvement 2 months after SCI, when the cells robustly survived in the lesion and partially filled the lesion cavity.
Figure 1.
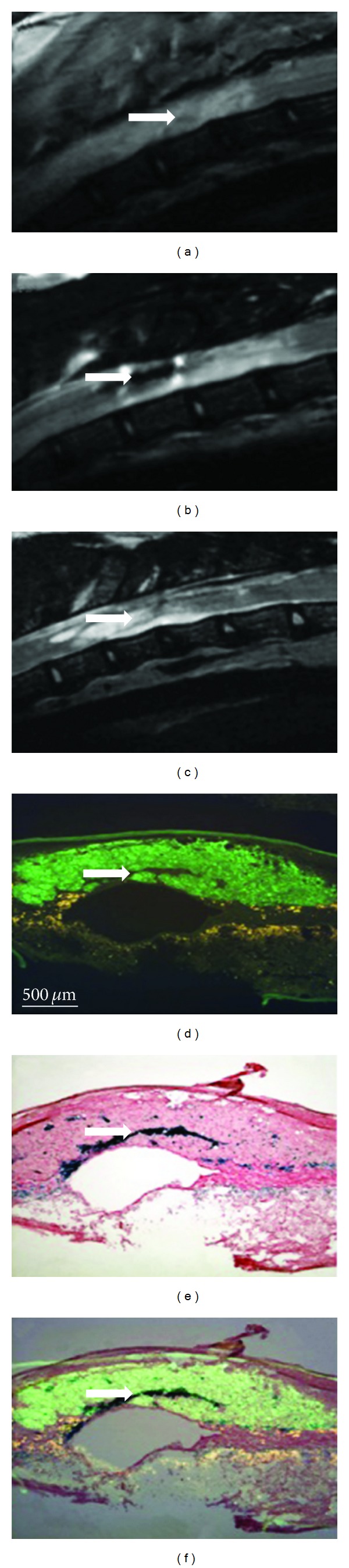
The T2-weighted MR images of the injured rat's spinal cord before and after SPC-01 cell transplantation. The white arrows show labeled transplanted cells and lesion site. (a) The T2-weighted MR images of a spinal cord lesion 5 days after lesion induction before transplantation. (b) Spinal cord with a cell graft 8 weeks after cell transplantation. (c) Control spinal cord lesion 8 weeks after saline injection. (d) Two serial sections were stained with the human mitochondrial marker (MTCO2). (e) The same sections of D were stained with iron. (f) Overlay of MTC02 and iron staining [80].
3.4. Optical Imaging
Bioluminescence imaging (BLI) uses luciferase-transduced transplanted cells that can be detected noninvasively in vivo by virtue of their reporter gene, which is expressed only when cells are alive [95, 96]. Luciferase (Luc) gene is the most commonly used gene for in vivo luminescence which is obtained from the North American firefly. This Luc gene encodes a 550 amino acid protein, Luc. Considering the near absence of endogenous light from mammalian cells and tissues, luciferases have a significant advantage as optical indicators in live mammalian cells and tissues, the inherently low background [97]. It is a powerful tool for the detection of exclusively living grafted cells that stably express luciferase in living animals after administration of luciferin (the substrate of luciferase) in the presence of oxygen and ATP as a source of energy [98]. Using the luciferase reaction in vivo as a marker of gene expression requires that the substrate is nontoxic and well distributed to the animals' tissues after exogenously adding (usually via intraperitoneal injection). At present, no humoral immune responses, hepatic toxicity, or germ-line integration were observed in models of luciferase reaction. Studies have also suggested that luciferin could be distributed throughout the entire animal and even could not be restricted by the blood-brain or placental barriers [98]. Previous studies demonstrated that the number of photons which emitted from the labeled cells and transmitted through murine tissues was sufficient to detect 1–2.5 × 103 cells in the peritoneal cavity, 1 × 104 cells at subcutaneous sites, and 1 × 106 circulating cells immediately following injection [99, 100]. Recently, noninvasive bioluminescent imaging was successfully applied to investigate the survival of neural stem/progenitor cells following transplantation into the lesioned mouse spinal cord. It was found that the intralesional application of NSPCs among 3 different procedures (intralesional, intrathecal, and intravenous injection) is the most effective and feasible method for transplanting NSPCs into the SCI site [101]. Okada et al.'s study is a well example of stem cell transplantation study [59]. The third-generation lentiviral vectors enabled efficient transduction and stable expression of both luciferase and a variant of green fluorescent protein in primary cultured NSPCs. Signals from these cells were detectable for up to 10 months or more after transplantation into the injured spinal cords of C57BL/6J mice. Analysis of both acute and delayed transplantation groups revealed drastic reductions in signal intensity within the first 4 days after transplantation, which was followed by a relatively stable bioluminescent signal for 6 wk (Figure 2).
Figure 2.
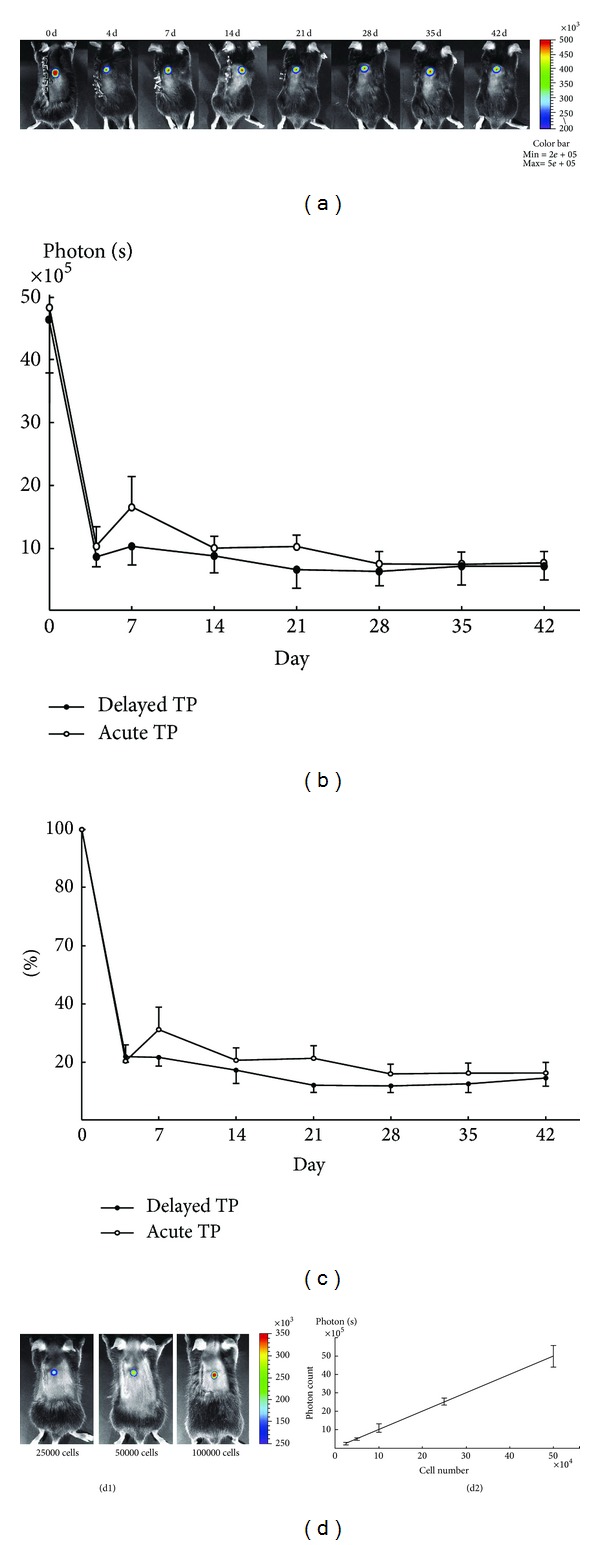
The time course of viability of transplanted NSPCs for SCI. (a) Images of a representative mouse that received acute transplantation (TP) of luciferase-expressing NSPCs confirmed long-term cell viability. Drastic reductions in signal intensity within the first 4 days after transplantation and then relatively stable bioluminescent signals for the following 6 wk were observed in both acute and delayed transplantation groups. There were no differences between acute and delayed transplantation groups in both value of signal intensity (b) and the rate to initial value (c) at each time point. Values are means ± SE (n = 8). (d) The correlation between grafted cell numbers and luminescent intensity was confirmed in vivo. Values are means ± SE (n = 4) [59].
3.5. Multimodality Imaging
No single imaging modality can provide all the information required to track transplanted stem cells and monitor their functional effects. Every imaging modality for stem cell tracing has its own advantages and disadvantages [81, 82] (Table 2). PET has a high sensitivity in tracking biomarkers in vivo but lacks the ability of providing detailed anatomic structures. SPIO-labeled stem cells can be detected by means of MRI and get high resolution and 3D anatomical imaging; however, the low sensitivity limits its application on cell tracing. Furthermore, cell division may dilute intracellular markers and then the signals may fail to reflect cells' number and location due to shedding of iron particles [102]. Hence, it is necessary to combine complementary imaging methodologies. Multimodality imaging approaches may minimize the potential drawbacks of using each imaging modality alone and a tailored combination of 2 or more techniques may be the best approach for a given experiment (Figure 3).
Table 2.
Advantages and limitations of different imaging methods for detection of grafted stem cells (modified from Modo et al. [81] and Spiriev et al. [82]).
| Imaging modality | PET | MRI | BLI | FLI |
|---|---|---|---|---|
| Depth of penetration | No limit | No limit | 1-2 mm | <1 mm |
| Spatial resolution | 1-2 mm | 10–100 μm | Several mm | 2-3 mm |
| Temporal resolution | sec–min | min–hrs | sec–min | sec–min |
| Imaging agents | Radionuclide labeled compound | Gadolinium, dysprosium iron oxide particles |
Luciferins | Fluorescent protein |
| Toxicity | No | Yes | No | No |
| Time range of detection | 6–12 months | 1-2 months | 2–8 weeks | Long-term |
| Detection limits in terms of cell numbers in vivo | 1 × 104–1 × 105 | 5 × 105–1 × 106 | 1 × 103–1 × 106 | 2 × 104–5 × 105 |
Sec: second, min: minute, and hrs: hours.
Figure 3.
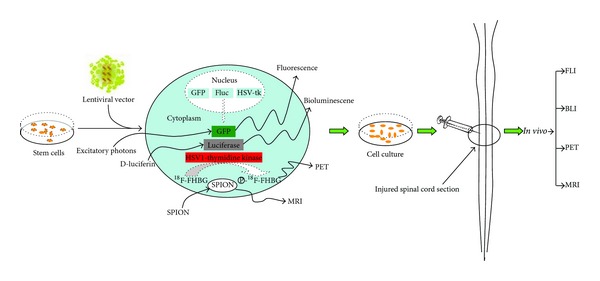
Multimodality of imaging can be applied for tracking stem cell behavior. A work flow chart for labeling cells and introducing labeled cells in vivo: firstly, cells are labeled using a marker for positron emission tomography (PET), magnetic resonance imaging (MRI), bioluminescence imaging (BLI), or fluorescence imaging (FLI). Secondly, cells are cultured in vitro and injected into the injured spinal cord. Finally, stem cells are then tracked in vivo with a camera or scanner.
Computed tomography (CT) scanners can overcome the lacks of anatomic structures. PET and CT scanners can be used in conjunction to get more detailed, fused images. In a research of Nandoe Tewarie RD, PET was used in combination with CT imaging techniques for longitudinal monitoring of the injured spinal cord. With PET-CT, combined with a simulation-based partial-volume compensation (PVC) method, they can serially measure standardized uptake values of the T9 and T6 spinal cord segments and reveal small, but significant, differences [103]. Because MRI has limitations in determining the viability of labeled transplanted cells, another imaging modality is required. The combination of MRI and PET also allows the acquisition of anatomical, physiological, and metabolic information, all from the same subject [104, 105]. By utilizing CT information acquired by an X-ray detector, the 3-dimensional location can be reconstructed using some bioluminescent tomography (BLT) reconstruction methods such as the adaptive finite element method and Bayesian method [82]. Researchers combined MRI with BLI to simultaneously monitor the location and viability of transplanted cells in vivo within the same animal. In recent study, Kim et al. evaluated the therapeutic effects of transplanted human glial precursor cells (hGPs) together with the in vivo fate of these cells using MRI and BLI [106]. In order to determine their potential for therapy of multiple sclerosis (MS), they used MRI and BLI side-by-side as complementary imaging techniques to evaluate the effects of transplanted hGPs on experimental autoimmune encephalomyelitis (EAE) pathogenesis. The results demonstrated that intracerebroventricularly (ICV) transplantation of short-lived hGPs can have a remote therapeutic effect through immunomodulation from within the ventricle, without cells directly participating in remyelination.
There are also some problems of nonspecific signals obtained with the different imaging modalities as a result of grafted cells that have died. For example, grafted cells prelabeled with iron oxide nanoparticles that have died postgrafting may result in MRI signals representing macrophage phagocytosis of labeled cell debris. However, PET can use report gene system to avoid such disadvantage, and the high detection sensitivity of PET imaging techniques could offset nonspecific signals of MRI. Hybrid PET/MR imaging might present a formidable technical and suggest potential spinal cord applications exploiting unique properties of the combined instrumentation.
4. Perspective and Summary
The potential of stem cells to reconnect the injured spinal cord and repopulate the area of injury has fascinated SCI researchers. Although some authors believe that endogenous remyelination is effective albeit somewhat slower. The field has learned a great deal about evaluating the fate of stem cells once they are implanted into the cord. How many survive? Do they integrate and migrate? How do they influence the host microenvironment? More and more evidence suggest that the stem cells themselves may be the source of growth factors and have remarkable influence on the injured microenvironment.
The interest in stem cell transplantation for SCI will remain high. This field will obviously continue to evolve, with hopes that further refinement, and understanding will increase the chances that cell transplantation will someday emerge as a fruitful treatment for patients. The complexities of attenuating the tissue damage and secondary complications due to trauma and reconstructing the cytoarchitecture of the injured spinal cord are very challenging. Hopefully, the rapid advances being made in stem cell biology will result in effective experimental and clinical trials of stem cell therapy for SCI.
Molecular imaging is a new discipline which makes it possible in vivo of cellular and molecular aspects of pathophysiological processes and therapeutic interventions. PET has high flexibility for the production of specific probes for the detection of different processes in the living subject. However, the production of PET needs advanced chemistry and tight quality control. MRI is also a contending and complementing modality in molecular imaging essential for stem cell studies. It can be used to evaluate the reparation of the injured spinal cord as well as tracing labeled stem cell in vivo. Optical imaging has a high molecularly sensitivity, but it provides lesser anatomical localization and is mainly used in small animals.
In summary, the usage of stem cell treatment might restore axonal continuity, connect the area for axonal regeneration, and promote axonal growth back to its distal targets. And the use of a noninvasive imaging method would have the advantage that stem cells transplanting in individual animals can be monitored longitudinally. The multimodality imaging technique allows for studying acute pathological events following a spinal cord lesion, and the development of implanted spinal chamber enables long-term imaging for chronic spinal cord preparations. Therefore, in vivo imaging allows the direct observation of dynamic regenerative events of individual stem cells after traumatic injury in the living subject [107]. We predict that future improvement in molecular imaging will make important contributions to our understanding of stem cells' transplantation and allow us to assess the therapeutic effect on a molecular scale.
Acknowledgments
This work is partly sponsored by Grants from the National Key Basic Research Program of China (2013CB329506), Zhejiang Provincial Natural Science Foundation of China (Z2110230), Health Bureau of Zhejiang Province (2010ZA075, 2011ZDA013), Science and Technology Bureau of Zhejiang Province (2012R10040), National Science Foundation of China (NSFC) (no. 81101023, 81173468, 81271601), and Ministry of Science and Technology of China (2011CB504400).
Abbreviations
- SCI:
Spinal cord injury
- CNS:
Center nervous system
- PET:
Positron emission tomography
- MRI:
Magnetic resonance imaging
- BLI:
Bioluminescence imaging
- FLI:
Fluorescence imaging
- CCD:
Charge-coupled device
- BMSCs:
Bone marrow-derived mesenchymal stem cells
- HSCs:
Hematopoietic stem cells
- MSCs:
Mesenchymal stem cells
- HUCMSCs:
Human umbilical cord-derived MSCs
- HPDMSCs:
Human placenta-derived MSCs
- hUCB-MSCs:
Human umbilical cord blood derived mesenchymal stem cells
- NSCs:
Neural stem cells
- NPCs:
Neural progenitor cells
- hCNS-SCns:
Human neural stem cells
- NSPCs:
Neural stem or progenitor cells
- NOD-scid:
Nonobese diabetic-severe combined immunodeficiency disease
- IL-6:
Interleukin-6
- TNF-α:
Tumor necrosis factor-alpha
- CXCL1:
Chemokine (C-X-C motif) ligand 1
- EGF:
Epidermal growth factor
- NT3:
Neurotrophin-3
- ESCs:
Embryonic stem cells
- OPCs:
Oligodendrocyte progenitor cells
- iPSCs:
Induced pluripotent stem cells
- OCT4:
Octamer-binding transcription factor-4
- SOX2:
SRY-related high-mobility-group (HMG)-box protein-2
- Klf4:
Kruppel-like factor-4
- c-MYC:
Cancer-MYC
- SPIO:
Superparamagnetic iron oxide
- SPECT:
Single photon emission tomography
- hNPC-TERT:
Telomerase-immortalized human neural progenitor cells
- SPION:
Superparamagnetic iron oxide nanoparticles
- hUC-MSCs:
Human umbilical cord mesenchymal stem cells
- SPC-01:
Neural stem cell line derived from human fetal spinal cord tissue
- CT:
Computed tomography
- PVC:
Partial-volume compensation
- BLT:
Bioluminescent tomography
- hGPs:
Human glial precursor cells
- MS:
Multiple sclerosis
- EAE:
Experimental autoimmune encephalomyelitis
- ICV:
Intracerebroventricularly
- IL:
Intralesional injection
- IT:
Intratheca injection
- IV:
Intravenous injection
- Ngb:
Neuroglobin
- SSEPs:
Somatosensory evoked potential
- iPSC-NS/PCs:
Induced pluripotent stem cell-derived neural stem/progenitor cells
- HUCBCs:
Human umbilical cord blood cells
- hES:
Transplanted human embryonic stem
- OPCs:
Cell-derived oligodendrocyte progenitor cells
- HAMC:
Hydrogel blend of hyaluronan and methyl cellulose
- EMSCs:
Ectomesenchymal stem cells
- sec:
Second
- min:
Minute
- hrs:
Hours.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. Journal of Neurotrauma. 2004;21(10):1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 2.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2013 doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 3.Sekhon LHS, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26(24, supplement):S2–S12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 4.Morawietz C, Moffat FL. The effects of locomotor training after incomplete spinal cord injury: a systematic review. Archives of Physical Medicine and Rehabilitation. 2013;94(11):2297–2308. doi: 10.1016/j.apmr.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 5.Michailidou C, Marston L, De Souza LH, et al. A systematic review of the prevalence of musculoskeletal pain, back and low back pain in people with spinal cord injury. Disability and Rehabilitation. 2013 doi: 10.3109/09638288.2013.808708. [DOI] [PubMed] [Google Scholar]
- 6.Bracken MB. Steroids for acute spinal cord injury. Cochrane Database of Systematic Reviews. 2002;(3) doi: 10.1002/14651858.CD001046.CD001046 [DOI] [PubMed] [Google Scholar]
- 7.Esposito E, Cuzzocrea S. Anti-TNF therapy in the injured spinal cord. Trends in Pharmacological Sciences. 2011;32(2):107–115. doi: 10.1016/j.tips.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Current Opinion in Neurology. 2009;22(5):467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- 9.Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathology. 1995;5(4):407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 10.McDonald JW, Sadowsky C. Spinal-cord injury. The Lancet. 2002;359(9304):417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 11.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. Journal of Neurosurgery. 1991;75(1):15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Lepski G. Cell transplantation for spinal cord injury: a systematic review. BioMed Research International. 2013;2013:32 pages. doi: 10.1155/2013/786475.786475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang N, Yin Y, Xu S-J, Wu Y-P, Chen W-S. Inflammation & apoptosis in spinal cord injury. Indian Journal of Medical Research. 2012;135(3):287–296. [PMC free article] [PubMed] [Google Scholar]
- 14.Benowitz LI, Popovich PG. Inflammation and axon regeneration. Current Opinion in Neurology. 2011;24(6):577–583. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- 15.Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nature Reviews Neuroscience. 2006;7(8):628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- 16.Lin WP, Chen XW, Zhang LQ, et al. Effect of neuroglobin genetically modified bone marrow mesenchymal stem cells transplantation on spinal cord injury in rabbits. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0063444.e63444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.All AH, Bazley FA, Gupta S, et al. Human embryonic stem cell-derived oligodendrocyte progenitors aid in functional recovery of sensory pathways following contusive spinal cord injury. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0047645.e47645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi Y, Okada Y, Itakura G, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052787.e52787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo Y, Zou Y, Yang L, et al. Transplantation of NSCs with OECs alleviates neuropathic pain associated with NGF downregulation in rats following spinal cord injury. Neuroscience Letters. 2013;549:103–108. doi: 10.1016/j.neulet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Bottai D, Cigognini D, Madaschi L, et al. Embryonic stem cells promote motor recovery and affect inflammatory cell infiltration in spinal cord injured mice. Experimental Neurology. 2010;223(2):452–463. doi: 10.1016/j.expneurol.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji O, Miura K, Okada Y, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(28):12704–12709. doi: 10.1073/pnas.0910106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 2013;34(15):3775–3783. doi: 10.1016/j.biomaterials.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Chen Q, Zhang Z, et al. Fibrin scaffolds containing ectomesenchymal stem cells enhance behavioral and histological improvement in a rat model of spinal cord injury. Cells, Tissues, Organs. 2013;198(1):35–46. doi: 10.1159/000351665. [DOI] [PubMed] [Google Scholar]
- 24.Jung D-I, Ha J, Kang B-T, et al. A comparison of autologous and allogenic bone marrow-derived mesenchymal stem cell transplantation in canine spinal cord injury. Journal of the Neurological Sciences. 2009;285(1-2):67–77. doi: 10.1016/j.jns.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 25.Drago D, Cossetti C, Iraci N, et al. The stem cell secretome and its role in brain repair. Biochimie. 2013;95(12):2271–2285. doi: 10.1016/j.biochi.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker RA. Stem cells and neurodegenerative diseases: where is it all going? Regenerative Medicine. 2012;7(6, supplement):26–31. doi: 10.2217/rme.12.64. [DOI] [PubMed] [Google Scholar]
- 27.Cao HQ, Dong ED. An update on spinal cord injury research. Neuroscience Bulletin. 2013;29(1):94–102. doi: 10.1007/s12264-012-1277-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández J, Torres-Espín A, Navarro X. Adult stem cell transplants for spinal cord injury repair: current state in preclinical research. Current Stem Cell Research and Therapy. 2011;6(3):273–287. doi: 10.2174/157488811796575323. [DOI] [PubMed] [Google Scholar]
- 29.Kumagai G, Tsoulfas P, Toh S, McNiece I, Bramlett HM, Dietrich WD. Genetically modified mesenchymal stem cells (MSCs) promote axonal regeneration and prevent hypersensitivity after spinal cord injury. Experimental Neurology. 2013;248:369–380. doi: 10.1016/j.expneurol.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Han D, Wang Z, et al. Clinical analysis of the treatment of spinal cord injury with umbilical cord mesenchymal stem cells. Cytotherapy. 2013;15(2):185–191. doi: 10.1016/j.jcyt.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Yan L, Han Y, He Y, et al. Cell tracing techniques in stem cell transplantation. Stem Cell Reviews. 2007;3(4):265–269. doi: 10.1007/s12015-007-9004-y. [DOI] [PubMed] [Google Scholar]
- 32.Guenoun J, Ruggiero A, Doeswijk G, et al. In vivo quantitative assessment of cell viability of gadolinium or iron-labeled cells using MRI and bioluminescence imaging. Contrast Media & Molecular Imaging. 2013;8(2):165–174. doi: 10.1002/cmmi.1513. [DOI] [PubMed] [Google Scholar]
- 33.Shen J, Zhong X-M, Duan X-H, et al. Magnetic resonance imaging of mesenchymal stem cells labeled with dual (MR and fluorescence) agents in rat spinal cord injury. Academic Radiology. 2009;16(9):1142–1154. doi: 10.1016/j.acra.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Lara LE, Xu X, Hofstetrova K, Pniak A, Brown A, Foster PJ. In vivo magnetic resonance imaging of spinal cord injury in the mouse. Journal of Neurotrauma. 2009;26(5):753–762. doi: 10.1089/neu.2008.0704. [DOI] [PubMed] [Google Scholar]
- 35.Mothe AJ, Tator CH. Review of transplantation of neural stem/progenitor cells for spinal cord injury. International Journal of Developmental Neuroscience. 2013;31(7):701–713. doi: 10.1016/j.ijdevneu.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Forostyak S, Jendelova P, Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie. 2013;95(12):2257–2270. doi: 10.1016/j.biochi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto A, Sakai K, Matsubara K, et al. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neuroscience Research. 2013 doi: 10.1016/j.neures.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Research. 2013;23(1):70–80. doi: 10.1038/cr.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplantation. 2007;40(7):609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 40.Vaquero J, Zurita M. Bone marrow stromal cells for spinal cord repair: a challenge for contemporary neurobiology. Histology and Histopathology. 2009;24(1):107–116. doi: 10.14670/HH-24.107. [DOI] [PubMed] [Google Scholar]
- 41.Wong RSY. Mesenchymal stem cells: angels or demons? Journal of Biomedicine and Biotechnology. 2011;2011:8 pages. doi: 10.1155/2011/459510.459510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fehlings MG, Vawda R. Cellular treatments for spinal cord injury: the time is right for clinical trials. Neurotherapeutics. 2011;8(4):704–720. doi: 10.1007/s13311-011-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright KT, El Masri W, Osman A, Chowdhury J, Johnson WEB. Concise review: bone marrow for the treatment of spinal cord injury: mechanisms and clinical applications. Stem Cells. 2011;29(2):169–178. doi: 10.1002/stem.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chopp M, Zhang XH, Li Y, et al. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. NeuroReport. 2000;11(13):3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 45.Hofstetter CP, Schwarz EJ, Hess D, et al. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Himes BT, Neuhuber B, Coleman C, et al. Recovery of function following grafting of human bone marrow-derived stromal cells into the injured spinal cord. Neurorehabilitation and Neural Repair. 2006;20(2):278–296. doi: 10.1177/1545968306286976. [DOI] [PubMed] [Google Scholar]
- 47.Zhu SF, Zhong ZN, Fu XF, et al. Comparison of cell proliferation, apoptosis, cellular morphology and ultrastructure between human umbilical cord and placenta-derived mesenchymal stem cells. Neuroscience Letters. 2013;541:77–82. doi: 10.1016/j.neulet.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 48.Kang KN, Lee JY, Kim DY, et al. Regeneration of completely transected spinal cord using scaffold of poly(D,L-Lactide-co-Glycolide)/small intestinal submucosa seeded with rat bone marrow stem cells. Tissue Engineering A. 2011;17(17-18):2143–2152. doi: 10.1089/ten.TEA.2011.0122. [DOI] [PubMed] [Google Scholar]
- 49.Lee J-H, Chung W-H, Kang E-H, et al. Schwann cell-like remyelination following transplantation of human umbilical cord blood (hUCB)-derived mesenchymal stem cells in dogs with acute spinal cord injury. Journal of the Neurological Sciences. 2011;300(1-2):86–96. doi: 10.1016/j.jns.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 50.Park JH, Kim DY, Sung IY, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 2012;70(5):1238–1247. doi: 10.1227/NEU.0b013e31824387f9. [DOI] [PubMed] [Google Scholar]
- 51.Ramasamy R, Lam EW-F, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21(2):304–310. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Zhang Z, Yao C. Survivin is upregulated in myeloma cell lines cocultured with mesenchymal stem cells. Leukemia Research. 2010;34(10):1325–1329. doi: 10.1016/j.leukres.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96(1):25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 54.Salazar DL, Uchida N, Hamers FPT, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS ONE. 2010;5(8) doi: 10.1371/journal.pone.0012272.e12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255(5052):1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 56.Gage FH. Mammalian neural stem cells. Science. 2000;287(5457):1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 57.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, et al. A systematic review of cellular transplantation therapies for spinal cord injury. Journal of Neurotrauma. 2011;28(8):1611–1682. doi: 10.1089/neu.2009.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishimura S, Yasuda A, Iwai H, et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Molecular Brain. 2013;6, article 3 doi: 10.1186/1756-6606-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okada S, Ishii K, Yamane J, et al. In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. The FASEB Journal. 2005;19(13):1839–1841. doi: 10.1096/fj.05-4082fje. [DOI] [PubMed] [Google Scholar]
- 60.Kumamaru H, Saiwai H, Ohkawa Y, Yamada H, Iwamoto Y, Okada S. Age-related differences in cellular and molecular profiles of inflammatory responses after spinal cord injury. Journal of Cellular Physiology. 2012;227(4):1335–1346. doi: 10.1002/jcp.22845. [DOI] [PubMed] [Google Scholar]
- 61.Kusano K, Enomoto M, Hirai T, et al. Transplanted neural progenitor cells expressing mutant NT3 promote myelination and partial hindlimb recovery in the chronic phase after spinal cord injury. Biochemical and Biophysical Research Communications. 2010;393(4):812–817. doi: 10.1016/j.bbrc.2010.02.088. [DOI] [PubMed] [Google Scholar]
- 62.Kumar S, Kahn MA, Dinh L, et al. NT-3-mediated TrkC receptor activation promotes proliferation and cell survival of rodent progenitor oligodendrocyte cells in vitro and in vivo. Journal of Neuroscience Research. 1998;54(6):754–765. doi: 10.1002/(SICI)1097-4547(19981215)54:6<754::AID-JNR3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 63.Cummings BJ, Uchida N, Tamaki SJ, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mothe AJ, Zahir T, Santaguida C, Cook D, Tator CH. Neural stem/progenitor cells from the adult human spinal cord are multipotent and self-renewing and differentiate after transplantation. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027079.e27079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamane J, Nakamura M, Iwanami A, et al. Transplantation of galectin-1-expressing human neural stem cells into the injured spinal cord of adult common marmosets. Journal of Neuroscience Research. 2010;88(7):1394–1405. doi: 10.1002/jnr.22322. [DOI] [PubMed] [Google Scholar]
- 66.Kulbatski I, Mothe AJ, Keating A, Hakamata Y, Kobayashi E, Tator CH. Oligodendrocytes and radial glia derived from adult rat spinal cord progenitors: morphological and immunocytochemical characterization. Journal of Histochemistry and Cytochemistry. 2007;55(3):209–222. doi: 10.1369/jhc.6A7020.2006. [DOI] [PubMed] [Google Scholar]
- 67.Mothe AJ, Tator CH. Transplanted neural stem/progenitor cells generate myelinating oligodendrocytes and Schwann cells in spinal cord demyelination and dysmyelination. Experimental Neurology. 2008;213(1):176–190. doi: 10.1016/j.expneurol.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 68.Mothe AJ, Kulbatski I, Parr A, Mohareb M, Tator CH. Adult spinal cord stem/progenitor cells transplanted as neurospheres preferentially differentiate into oligodendrocytes in the adult rat spinal cord. Cell Transplantation. 2008;17(7):735–751. doi: 10.3727/096368908786516756. [DOI] [PubMed] [Google Scholar]
- 69.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 70.Mcdonald JW, Liu X-Z, Qu Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nature Medicine. 1999;5(12):1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 71.Keirstead HS, Nistor G, Bernal G, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. Journal of Neuroscience. 2005;25(19):4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28(1):152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 74.Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006;441(7097):1061–1067. doi: 10.1038/nature04955. [DOI] [PubMed] [Google Scholar]
- 75.Kramer AS, Harvey AR, Plant GW, Hodgetts SI. Systematic review of induced pluripotent stem cell technology as a potential clinical therapy for spinal cord injury. Cell Transplantation. 2013;22(4):571–617. doi: 10.3727/096368912X655208. [DOI] [PubMed] [Google Scholar]
- 76.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 77.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 78.Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nature Biotechnology. 2009;27(8):743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- 79.Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplantation. 2011;20(6):883–891. doi: 10.3727/096368910X539092. [DOI] [PubMed] [Google Scholar]
- 80.Amemori T, Romanyuk N, Jendelova P, et al. Human conditionally immortalized neural stem cells improve locomotor function after spinal cord injury in the rat. Stem Cell Research & Therapy. 2013;4(3):p. 68. doi: 10.1186/scrt219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Modo M, Hoehn M, Bulte JWM. Cellular MR imaging. Molecular Imaging. 2005;4(3):143–164. doi: 10.1162/15353500200505145. [DOI] [PubMed] [Google Scholar]
- 82.Spiriev T, Sandu N, Schaller B. Molecular imaging and tracking stem cells in neurosciences. Methods in Molecular Biology. 2013;1052:195–201. doi: 10.1007/7651_2013_27. [DOI] [PubMed] [Google Scholar]
- 83.Syková E, Jendelová P. Magnetic resonance tracking of transplanted stem cells in rat brain and spinal cord. Neurodegenerative Diseases. 2006;3(1-2):62–67. doi: 10.1159/000092095. [DOI] [PubMed] [Google Scholar]
- 84.Vanecek V, Zablotskii V, Forostyak S, et al. Highly efficient magnetic targeting of mesenchymal stem cells in spinal cord injury. International Journal of Nanomedicine. 2012;7:3719–3730. doi: 10.2147/IJN.S32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arbab AS, Thiffault C, Navia B, et al. Tracking of In-111-labeled human umbilical tissue-derived cells (hUTC) in a rat model of cerebral ischemia using SPECT imaging. BMC Medical Imaging. 2012;12, article 33 doi: 10.1186/1471-2342-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaiswal JK, Mattoussi H, Mauro JM, Simon SM. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nature Biotechnology. 2003;21(1):47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 87.Sun N, Lee A, Wu JC. Long term non-invasive imaging of embryonic stem cells using reporter genes. Nature Protocols. 2009;4(8):1192–1201. doi: 10.1038/nprot.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trinkaus ME, Blum R, Rischin D, et al. Imaging of hypoxia with (18) F-FAZA PET in patients with locally advanced non-small cell lung cancer treated with definitive chemoradiotherapy. Journal of Medical Imaging and Radiation Oncology. 2013;57(4):475–481. doi: 10.1111/1754-9485.12086. [DOI] [PubMed] [Google Scholar]
- 89.Quartuccio N, Treglia G, Salsano M, et al. The role of Fluorine-18-Fluorodeoxyglucose positron emission tomography in staging and restaging of patients with osteosarcoma. Radiology and Oncology. 2013;47(2):97–102. doi: 10.2478/raon-2013-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patel K, Hadar N, Lee J, Siegel BA, Hillner BE, Lau J. The lack of evidence for PET or PET/CT surveillance of patients with treated lymphoma, colorectal cancer, and head and neck cancer: a systematic review. Journal of Nuclear Medicine. 2013;54(9):1518–1527. doi: 10.2967/jnumed.112.119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bai J-Z, Liu Z-J, Ding W-M, et al. Tracking neural progenitor cells tranplanted into rabbit spinal cord by detection of dopamine receptors 2 with positron emission computed tomography. National Medical Journal of China. 2006;86(29):2060–2064. [PubMed] [Google Scholar]
- 92.Han X, Yang N, Cui Y, Xu Y, Dang G, Song C. Simvastatin mobilizes bone marrow stromal cells migrating to injured areas and promotes functional recovery after spinal cord injury in the rat. Neuroscience Letters. 2012;521(2):136–141. doi: 10.1016/j.neulet.2012.05.071. [DOI] [PubMed] [Google Scholar]
- 93.Cohen ME, Muja N, Fainstein N, Bulte JWM, Ben-Hur T. Conserved fate and function of ferumoxides-labeled neural precursor cells in vitro and in vivo. Journal of Neuroscience Research. 2010;88(5):936–944. doi: 10.1002/jnr.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu S-L, Lu P-G, Zhang L-J, et al. In Vivo magnetic resonance imaging tracking of SPIO-labeled human umbilical cord mesenchymal stem cells. Journal of Cellular Biochemistry. 2012;113(3):1005–1012. doi: 10.1002/jcb.23432. [DOI] [PubMed] [Google Scholar]
- 95.Berman SC, Galpoththawela C, Gilad AA, Bulte JWM, Walczak P. Long-term MR cell tracking of neural stem cells grafted in immunocompetent versus immunodeficient mice reveals distinct differences in contrast between live and dead cells. Magnetic Resonance in Medicine. 2011;65(2):564–574. doi: 10.1002/mrm.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li Z, Suzuki Y, Huang M, et al. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26(4):864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Molecular and Cellular Biology. 1987;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annual Review of Biomedical Engineering. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 99.Edinger M, Sweeney TJ, Tucker AA, Olomu AB, Negrin RS, Contag CH. Noninvasive assessment of tumor cell proliferation in animal models. Neoplasia. 1999;1(4):303–310. doi: 10.1038/sj.neo.7900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sweeney TJ, Mailander V, Tucker AA, et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(21):12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takahashi Y, Tsuji O, Kumagai G, et al. Comparative study of methods for administering neural stem/progenitor cells to treat spinal cord injury in mice. Cell Transplantation. 2011;20(5):727–739. doi: 10.3727/096368910X536554. [DOI] [PubMed] [Google Scholar]
- 102.Mani V, Adler E, Briley-Saebo KC, et al. Serial in vivo positive contrast MRI of iron oxide-labeled embryonic stem cell-derived cardiac precursor cells in a mouse model of myocardial infarction. Magnetic Resonance in Medicine. 2008;60(1):73–81. doi: 10.1002/mrm.21642. [DOI] [PubMed] [Google Scholar]
- 103.Tewarie RDSN, Yu J, Seidel J, et al. Positron emission tomography for serial imaging of the contused adult rat spinal cord. Molecular Imaging. 2010;9(2):108–116. [PubMed] [Google Scholar]
- 104.Jackson J, Chapon C, Jones W, Hirani E, Qassim A, Bhakoo K. In vivo multimodal imaging of stem cell transplantation in a rodent model of Parkinson’s disease. Journal of Neuroscience Methods. 2009;183(2):141–148. doi: 10.1016/j.jneumeth.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 105.Chapon C, Jackson JS, Aboagye EO, Herlihy AH, Jones WA, Bhakoo KK. An in vivo multimodal imaging study using MRI and pet of stem cell transplantation after myocardial infarction in rats. Molecular Imaging and Biology. 2009;11(1):31–38. doi: 10.1007/s11307-008-0174-z. [DOI] [PubMed] [Google Scholar]
- 106.Kim H, Walczak P, Muja N, Campanelli JT, Bulte JWM. ICV-transplanted human glial precursor cells are short-lived yet exert immunomodulatory effects in mice with EAE. GLIA. 2012;60(7):1117–1129. doi: 10.1002/glia.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laskowski CJ, Bradke F. In vivo imaging: a dynamic imaging approach to study spinal cord regeneration. Experimental Neurology. 2013;242:11–17. doi: 10.1016/j.expneurol.2012.07.007. [DOI] [PubMed] [Google Scholar]


