Abstract
Bacterial cell division occurs in conjunction with the formation of a cytokinetic Z-ring structure comprised of FtsZ subunits. Agents that disrupt Z-ring formation have the potential, through this unique mechanism, to be effective against several of the newly emerging multidrug-resistant strains of infectious bacteria. Several 1-phenylbenzo[c]phenanthridines exhibit notable antibacterial activity. Based upon their structural similarity to these compounds, a distinct series of substituted 1,6-diphenylnaphthalenes were synthesized and evaluated for antibacterial activity against Staphylococcus aureus and Enterococcus faecalis. In addition, the effect of select 1,6-diphenylnaphthalenes on the polymerization dynamics of S. aureus FtsZ and mammalian β-tubulin was also assessed. The presence of a basic functional group or a quaternary ammonium substituent on the 6-phenylnaphthalene was required for significant antibacterial activity. Diphenylnaphthalene derivatives that were active as antibiotics, did exert a pronounced effect on bacterial FtsZ polymerization and do not appear to cross-react with mammalian tubulin to any significant degree.
Keywords: Antibacterial, FtsZ-targeting, Staphylococcus aureus, Enterococcus faecalis, Diphenylnaphthalenes, FtsZ polymerization
Bacterial infections associated with drug-resistant pathogens such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) are recognized worldwide as a major health concern.1,2 Bacterial resistance to conventional antibacterial drugs has created a critical need for the identification of novel therapeutic antibacterial targets. In this connection, FtsZ has been identified as an appealing new target for antibacterial agents.10–17 FtsZ is an essential and highly conserved bacterial cytokinesis protein.3–8 Cell division in bacteria occurs at the site of formation of a cytokinetic Z-ring polymeric structure, which is comprised of FtsZ subunits.9 The potential for developing agents that target FtsZ and the recent advances in the development of small molecules that target FtsZ have been the subject of several recent reports.10–24
Sanguinarine 1 (Fig. 1) is a benzo[c]phenanthridine plant alkaloid that has been identified as small molecule that alters FtsZ Z-ring formation and has modest antibacterial activity.11,25 Studies in our laboratory have also shown that chelerythrine 2 (Fig. 1) has similar effects on S. aureus and Bacillus subtilis.26 In addition, the presence of a phenyl functionality at the 1-position of 2 (to yield 3) was shown to significantly enhance antibacterial activity relative to either 1 or 2.25,26 Within the cyclic core structure of 3, there is a scaffold consisting of a 1,6-diphenylnaphthalene. In light of these results, we examined the relative structure–activity relationships associated with the antistaphylococcal and antienterococcal activity of several 1,6-diphenylnaphthalene derivatives, including those with a constitutive cationic charge, those with basic functionalities that could be protonated to varying degrees at physiological pH, and those without a cationic charge.
Figure 1.
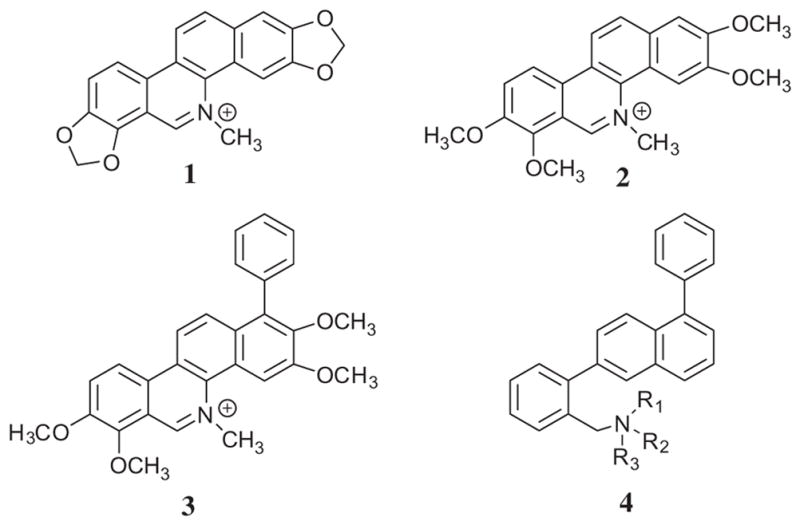
The structures of sanguinarine 1, chelerythrine 2 and 1-phenyl-5-methyl-2,3,7,8-tetramethoxybenzo[c]phenanthridine 3 relative to a generalized structure of a 1,6-diphenylnaphthalene 4.
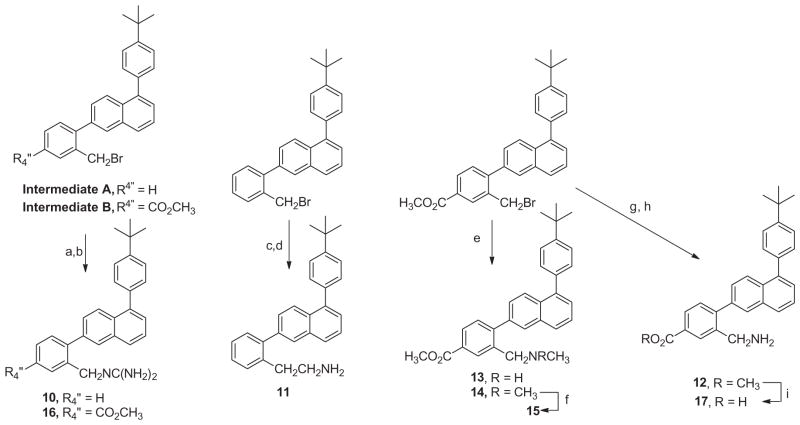
The critical intermediate for the preparation of the various 1,6-diphenylnaphthalenes that were synthesized was 5-bromo-2-naphthol, which was prepared from 6-hydroxy-1-naphthylamine as described in the literature (19).27 Suzuki coupling of 4-tert-butyphenylboronic acid to this naphthylbromide provided 5-(4-t-butylphenyl)-2-naphthol, which was then converted to its triflate derivative with Tf2O. This triflate derivative could then be used in a second Suzuki coupling reaction for the preparation of 5, 6, and 18 (see Scheme 1).
Scheme 1.
Methods used for the preparation of 1,6-diphenylnaphthalenes. Reagents and conditions: (a) 4-(t-butyl)phenylboronic acid, Pd(PPh3)4, K2CO3, dioxane/H2O, 100 °C; (b) Tf2O, Et3N, DCM, −78 °C; (c) 2-NO2-phenylboronic acid, Pd(OAc)2, XPhos, K2CO3, CH3CN/H2O, 100 °C; (d)H2, Pd/C (10%), EtOH; (e) 2-methylphenylboronic acid-B(OH)2 or 2-methyl-4-carboxymethylphenylboronic acid, Pd(OAc)2, XPhos, K2CO3, CH3CN/H2O, 100 °C; (f) 2-formylphenylboronic acid, Pd(OAc)2, XPhos, K2CO3, CH3CN/H2O, 100 °C; (g) 3-aminophenylboronic acid, Pd(OAc)2, XPhos, K2CO3, CH3CH/H2O (1:1), 100 °C; (h) NBS, AIBN, CCl4, 85 °C; (i) NaBH4, EtOH; (j) MsCl, Et3N, CH2Cl2, rt; see Ref. 28; (k) 7, CH3NH2 in THF or 8, (CH3)2NH in THF, 60 °C, sealed tube; (l) MeI, sealed tube 100 °C.
The triflate of 1-[(4-t-butyl)phenyl]-6-hydroxynaphthalene was also used in a Suzuki coupling reaction with 2-formylphenylboronic acid. The resulting aldehyde was reduced with NaBH4 and the hydroxymethyl derivative converted to its benzyl chloride. Treatment of this chloride with either methylamine or dimethylamine provided 7 and 8, respectively. Treatment of 8 with methyl iodide provided the quaternary ammonium derivative, 9. The triflate of 1-[(4-t-butyl)phenyl]-6-hydroxynaphthalene was also coupled with 4-carboxymethyl-2-methylphenylboronic acid. NBS bromination of 5 and this 6-(2-methyl-4-carboxymethyl)-1-(4-t-butylphenylphenyl) naphthalene provided the bromomethyl intermediates A and B that were employed for the preparation of 10–17, as outlined in Scheme 2.
Scheme 2.
Methods used for the preparation of 1,6-diphenylnaphthalenes. Reagents and conditions: (a) 1,3-bis(tert-butoxycarbonyl)guanidine, K2CO3, DMF, 50 °C, acetonitrile; (b) TFA, DCM, 50 °C, sealed vial, (c) KCN, DMF, rt; (d) LAH, toluene/THF(1:1), 100 °C; (e) 13, CH3NH2 in THFor14, (CH3)2NH in THF, 60 °C, sealed tube; (f) MeI, sealed tube 100 °C; (g) NaN3, DMF, rt; (h) PPh3 polymer bound, THF/H2O, rt; (i) LiOH, THF-H2O.
Treatment of these bromomethyl derivatives with 1,3-bis(t-butoxycarbonyl) guanidine followed by removal of the N-Boc protecting groups provided the guanidinomethyl derivatives 10 and 16. Treatment of 1-(4-t-butyl)phenyl-6-(2-bromomethylphenyl) naphthalene with potassium cyanide followed by reduction of the resulting nitrile with LAH yielded the 2-aminoethyl derivative 11. 1-(4-t-Butyl)phenyl-6-(2-bromomethyl-4-carboxymethylphenyl) naphthalene when treated with either methylamine or dimethylamine provided 13 and 14, respectively. Compound 14 was methylated using methyl iodide to provide the quaternary ammonium derivative, 15. Treatment of 1-(4-t-butyl)phenyl-6-(2-bromomethyl-4-carboxymethylphenyl)naphthalene with NaN3 followed by reduction with triphenylphosphine provide the methylamino derivative, 12. The methyl ester of 12 was hydrolyzed using LiOH in aqueous THF to yield its carboxy derivative, 17.
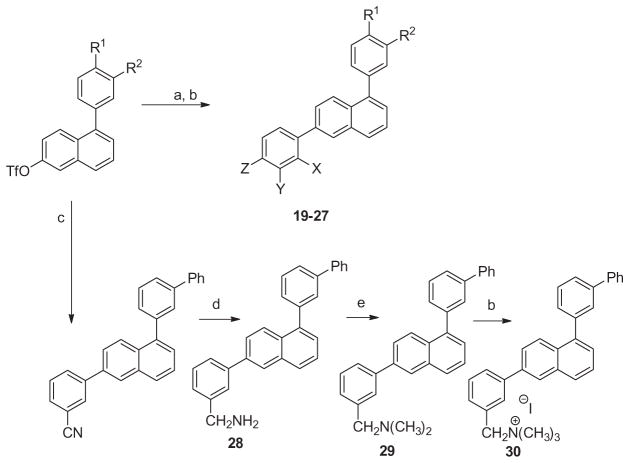
Using the appropriate triflate of 6-hydroxynaphthalene, various 1,6-diphenylnaphthalenes could be synthesized (Scheme 3). Treatment of the requisite N,N-dimethylamino and N,N-dimethylanilino derivatives with methyl iodide provided the quaternary ammonium salts, 19–27. The triflate of 1-(3-[1,1′]-biphenyl)-6-hydroxynaphthalene served as an intermediate for Suzuki coupling with 3-aminophenylboronic acid to give 18. Alternatively, this triflate intermediate was treated with 3-cyanophenylboronic acid, which was reduced with LAH to give 28, which in the presence of formaldehyde and formic acid provided the N,N-dimethyl derivative, 29. Treatment of 29 with methyl iodide resulted in the formation of the quaternary ammonium derivative, 30.
Scheme 3.
Reagents and conditions: (a) R–B(OH)2, Pd(OAc)2, XPhos, K2CO3, CH3CN/H2O, 100 °C; (b) MeI, sealed tube 100 °C; (c) 3-cyanophenylboronic acid, Pd(OAc)2, XPhos, K2CO3, CH3CN/H2O, 100 °C; (d) LAH, THF/toluene, 100 °C; (e) formaldehyde, NaBH3CN, pH 6
Several 1,6-diphenylnaphthalenes (DPNs) were evaluated for antibiotic activity against methicillin-sensitive and methicillin-resistant S. aureus (MSSA and MRSA, respectively) and vancomycin-sensitive and vancomycin-resistant Enterococcus faecalis (VSE and VRE, respectively), as summarized in Table 1.
Table 1.
Antistaphylococcal and antienterococcal activities of substituted 1,6-diphenylnaphthalenes
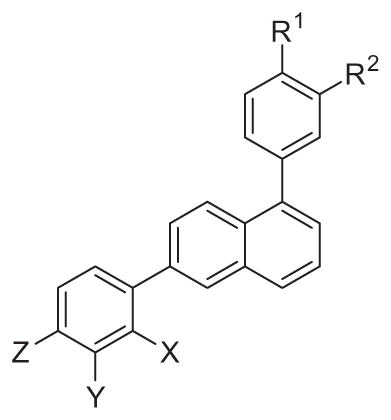
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | R2 | R1 | MIC (μg/mL)
|
||||
|
S. aureus 8325-4 (MSSA) |
S. aureus ATCC 33591 (MRSA) |
E. faecalis ATCC 19433 (VSE) |
E. faecalis ATCC 51575 (VRE) |
||||||
| 5 | CH3 | H | H | H | t-Butyl | >64 | >64 | >64 | >64 |
| 6 | NH2 | H | H | H | t-Butyl | >64 | >64 | >64 | >64 |
| 7 | CH2NH(CH3) | H | H | H | t-Butyl | 2 | 2 | 2 | 2 |
| 8 | CH2N(CH3)2 | H | H | H | t-Butyl | 32 | >64 | >64 | >64 |
| 9 | CH2N(CH3)3 | H | H | H | t-Butyl | 0.5 | 0.5 | 4 | 4 |
| 10 | CH2NC(NH2)2 | H | H | H | t-Butyl | 1 | 1 | 2 | 2 |
| 11 | CH2CH2NH2 | H | H | H | t-Butyl | 2 | 2 | 4 | 4 |
| 12 | CH2NH2 | H | CO2CH3 | H | t-Butyl | 2 | 2 | 16 | 16 |
| 13 | CH2NHCH3 | H | CO2CH3 | H | t-Butyl | 4 | 4 | 8 | 4 |
| 14 | CH2N(CH3)2 | H | CO2CH3 | H | t-Butyl | >64 | >64 | >64 | >64 |
| 15 | CH2N(CH3)3 | H | CO2CH3 | H | t-Butyl | 1 | 2 | 4 | 8 |
| 16 | CH2NC(NH2)2 | H | CO2CH3 | H | t-Butyl | 1 | 1 | 2 | 4 |
| 17 | CH2NH2 | H | CO2H | H | t-Butyl | >64 | >64 | >64 | >64 |
| 18 | H | NH2 | H | H | t-Butyl | >64 | >64 | >64 | >64 |
| 19 | H | N(CH3)3 | H | H | t-Butyl | 0.5 | 1 | 2 | 2 |
| 20 | H | N(CH3)3 | H | H | Phenyl | 0.5 | 1 | 2 | 4 |
| 21 | H | N(CH3)3 | H | H | CF3 | 1 | 4 | 8 | 8 |
| 22 | H | N(CH3)3 | H | H | F | 1 | 4 | 8 | 8 |
| 23 | H | N(CH3)3 | H | phenyl | H | 1 | 1 | 2 | 2 |
| 24 | H | H | N(CH3)3 | H | t-Butyl | 1 | 2 | 4 | 4 |
| 25 | H | H | N(CH3)3 | H | Phenyl | 1 | 1 | 2 | 2 |
| 26 | H | H | N(CH3)3 | H | F | 4 | 4 | 4 | 4 |
| 27 | H | H | N(CH3)3 | phenyl | H | 1 | 1 | 2 | 2 |
| 28 | H | CH2NH2 | H | phenyl | H | 2 | 4 | 4 | 8 |
| 29 | H | CH2N(CH3)2 | H | phenyl | H | 4 | 8 | 16 | 16 |
| 30 | H | CH2N(CH3)3 | H | phenyl | H | 0.5 | 1 | 4 | 4 |
| 1 | Sanguinarine | 2 | 2 | 8 | 16 | ||||
| 2 | Chelerythrine | 4 | 4 | 32 | 32 | ||||
| Oxacillin | 0.063 | >64 | 8 | >64 | |||||
| Vancomycin | 1 | 2 | 1 | >64 | |||||
| Erythromycin | 0.13 | >64 | 1 | >64 | |||||
| Tetracycline | 0.063 | 64 | 0.5 | >64 | |||||
| Clindamycin | 0.031 | >64 | 2 | >64 | |||||
Minimum inhibitory concentration (MIC) assays were conducted in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines for broth microdilution.29 MIC is defined as the lowest compound concentration at which bacterial growth is ≥90% inhibited.
It is evident from the lack of antimicrobial activity for 5 and 6 that the presence of a sufficiently basic functional group, which would be significantly charged at physiological pH, is critical for activity. The first series of DPNs that was studied consisted of 1-[4-(t-butyl)phenyl]naphthalene derivatives that had an o-aminomethyl substituent on the 6-phenyl moiety. Among the analogs related to 6-(o-aminomethyl)phenyl, the presence of a single N-methyl group as in 7 is associated with significant activity against all S. aureus and E. faecalis strains. However, the N,N-dimethyl derivative, 8, is substantially less active. Conversion of this tertiary amine to its N,N,N,-trimethyl ammonium derivative, 9, resulted in a significant enhancement of its antibacterial activity against both the MSSA and MRSA strains, with MICs of 0.5 μg/ mL. With regard to E. faecalis, 9 is slightly less active than 7, with MICs of 4.0 μg/mL against both VSE and VRE. The guanidinomethyl derivative, 10, and the 2-aminoethyl analog, 11, also have similar antibacterial activity to 7.
The structure–activity relationship among the 6-[(2-aminomethyl)-4-(carboxymethyl)]phenylnaphthalene analogs, 12–16, was similar to that associated with 6-(o-aminomethyl)phenylnaphthalene derivatives, 7–11. The 6-[(2-aminomethyl)-4-(carboxymethyl)] phenylnaphthalene 12 has good antibacterial activity against both MSSA and MRSA, but was notably less active against E. faecalis. In contrast, the 6-[(2-aminomethyl)-4-(carboxy)]phenylnaphthalene 17 did not exhibit appreciable antibacterial activity. Within this group of compounds, the most remarkable result, as was observed for 8, is the loss of antibacterial activity for the N,N-dimethyl derivative, 14.
Several DPNs were evaluated that incorporated into their structure a 1-[4-(t-butyl)phenyl]naphthalene moiety and either an m-amino or m-aminomethyl substituent on the 6-phenyl group. While the aniline derivative, 18, is inactive, its N,N,N-trimethyl ammonium derivative 19 had potent antibacterial activity against all the S. aureus and E. faecalis strains.
Those DPNs that incorporated within their structure a 6-[3-(trimethylammonium)phenyl]naphthalene and either a 1-[4-biphenyl], 1-(4-fluorophenyl), 1-(4-trifluoromethylphenyl), or 1-[3-biphenyl] moiety (20–23) had significant antibacterial activity. Unexpectedly, similar activity was observed for 6-[4-(trimethylammonium) phenyl]naphthalenes that had incorporated in their structure a 1-[4-(t-butyl)phenyl], 1-[4-biphenyl], 1-(4-fluorophenyl), or 1-[3-biphenyl] moiety (24–27). The 6-[4-(trimethylammonium) phenyl]naphthalene analog, 24, is only slightly less active than 19 against all the S. aureus and E. faecalis strains.
In a comparison of the 1-[3-biphenyl]-6-(3-aminomethylphenyl) naphthalene derivatives, 28–30, the primary amine 28 was significantly more active than the tertiary amine 29. The N,N,N-trimethylammonium derivative, 30, was as or more potent than 28 against all the S. aureus and E. faecalis strains.
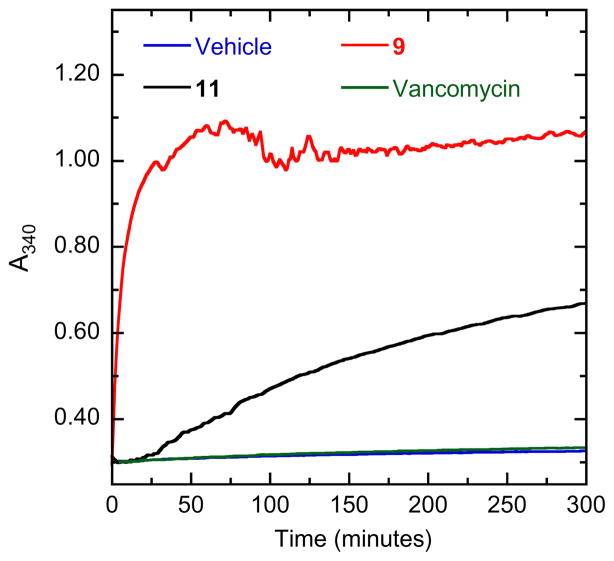
Stimulation of FtsZ self-polymerization and the concomitant stabilization of FtsZ polymers has been implicated in the antibacterial actions of several distinct classes of small molecules, including substituted benzamides,10,13,14,20 dibenzoquinoliziniums,30 isoquinolines, 31 and biaryls.32 We used a light scattering (turbidity) assay to monitor the impact, if any, of select diphenylnaphthalene compounds on the dynamics of self-polymerization by S. aureus FtsZ (SaFtsZ), which was expressed in Escherichia coli and purified as previously described.32 In this assay, FtsZ polymerization is detected in solution by a time-dependent increase in light scattering, as reflected by a corresponding increase in solution absorbance at 340 nm (A340). As illustrative examples, Figure 2 shows the time-dependent A340 profiles of SaFtsZ in the presence of vehicle (DMSO) only or compounds 9 and 11 at a concentration of 40 μg/mL. Vancomycin was also included as a non-FtsZ-targeting control antibiotic. Note that vancomycin exerts a negligible impact on SaFtsZ polymerization, an expected result given that the antibacterial target of vancomycin is the bacterial cell wall and not FtsZ. By contrast, compounds 9 and 11 increase both the kinetics and extent of SaFtsZ polymerization, with the relative magnitude of these increases correlating with the relative antistaphylococcal potencies of the compounds (see Table 1). Thus, as we have previously indicated for the dibenzoquinoliziniums,30 isoquinolines,31 and biaryls,32 the antibacterial activities of the diphenylnaphthalenes appear to be related to the impact the compounds have on the polymerization dynamics of FtsZ.
Figure 2.
Impact of select diphenylnaphthalenes on the polymerization of S. aureus FtsZ (SaFtsZ), as determined by monitoring time-dependent changes in absorbance at 340 nm (A340). Polymerization profiles of SaFtsZ (10 μM) in the presence of DMSO vehicle (blue) or 40 μg/mL of either 9 (red), 11 (black), or the comparator antibiotic vancomycin (green) are depicted. Experiments were conducted at 25 °C in solution containing 50 mM Tris·HCl (pH 7.4), 50 mM KCl, 2 mM magnesium acetate, 1 mM CaCl2, and 1 mM GTP. The reactions (100 μL total volume) were assembled in half-volume, flat-bottom 96-well microtiter plates, and their A340 values were continuously monitored using a VersaMax® (Molecular Devices, Inc.) plate reader.
Tubulin is the closest mammalian functional homolog to bacterial FtsZ. We therefore sought to determine whether 9, which is a potent stimulator of FtsZ polymerization (Fig. 2), would exert a similar effect on mammalian tubulin. To this end, we monitored the impact of 9 on porcine β-tubulin polymerization using a light scattering assay similar to that described above for FtsZ polymerization. We used the antineoplastic drugs paclitaxel (taxol) and nocodazole as positive controls in these assays, the former drug being a known stimulator of tubulin polymerization33,34 and the latter drug being a known inhibitor of tubulin polymerization.35 Figure 3 shows the time-dependent A340 profiles of porcine β-tubulin in the presence of DMSO vehicle, 9 (at 40 μg/mL), paclitaxel (at 25 μg/mL), or nocodazole (at 10 μg/mL). Both paclitaxel and nocodazole produce their expected impacts on tubulin polymerization dynamics. By contrast, 9 exerts little or no impact. Thus, a diphenylnaphthalene compound that exerts a profound impact on bacterial FtsZ polymerization does not appear to cross-react with mammalian tubulin to any significant degree. This degree of target specificity bodes well for the potential of desirable toxicological properties on the part of the diphenylnaphthalenes.
Figure 3.
Impact of 9 on the polymerization of microtubule associated protein (MAP)-rich porcine β-tubulin (70% tubulin, 30% MAPs), as determined by monitoring time-dependent changes in A340. Polymerization profiles of tubulin (2 mg/mL) in the presence of DMSO vehicle (blue), 40 μg/mL 9 (black), 25 μg/mL paclitaxel (red), or 10 μg/mL nocodazole (green) are depicted. Experiments were conducted at 37 °C in solution containing 80 mM PIPES·NaOH (pH 7.0), 2 mM MgCl2, 1 mM EGTA, and 1 mMGTP. The reactions (100 μL total volume) were assembled in half-volume, flat-bottom 96-well microtiter plates, and their A340 values were continuously monitored using a VersaMax® plate reader.
The structure-activity relationships associated with the antistaphylococcal and antienterococcal activity of several 1,6-diphenylnaphthalenes, including those with a constitutive cationic charge and those with basic functionalities that could be protonated to varying degrees at physiological pH, reveal several derivatives with significant antibacterial activity against S. aureus and E. faecalis. The presence on the 6-phenyl moiety of a methylaminomethyl-, 2-methylaminoethyl, guanidinomethyl, or (trimethylammonium) methyl at the o-position was associated with significant antibacterial activity within a series of 6-phenyl-1-(4-t-butylphenyl) naphthalenes. In addition, the presence of a trimethylammonium group at either the m- or p-position of the 6-phenyl moiety was associated with antibacterial activity for 6-phenyl-1-(4-t-butylphenyl)naphthalenes, 6-phenyl-1-(3-[1,1′-phenyl)naphthalenes and 6-phenyl-1-(4-[1,1′-biphenyl)naphthalenes. As was observed with benzo[c]phenanthridinium derivatives 1 or 2, it appears that for the 1,6-diphenylnaphthalenes 9 and 11, that there antibacterial activity is associated with their ability to effect FtsZ polymerization. While having a pronounced impact on bacterial FtsZ polymerization, they do not affect mammalian tubulin.
Supplementary Material
Acknowledgments
This study was supported by research agreements between TAXIS Pharmaceuticals, Inc. and both Rutgers, The State University of New Jersey (E.J.L.) and the University of Medicine and Dentistry of New Jersey (D.S.P). We would like to thank Drs. Steve Tuske and Eddy Arnold (Center for Advanced Biotechnology and Medicine, Rutgers University) for their assistance with the expression and purification of S. aureus FtsZ. S. aureus 8325-4 was the generous gift of Dr. Glenn W. Kaatz (John D. Dingell VA Medical Center, Detroit, MI). The Brucker Avance III 400 MHz NMR spectrometer used in this study was purchased with funds from NCRR Grant No. 1S10RR23698-1A1. Mass spectrometry was provided by the Washington University Mass Spectrometry Resource with support from the NIH National Center for Research Resources Grant No. P41RR0954.
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2013.02.016.
References and notes
- 1.Leavis HL, Willems RJL, Top J, Spalburg E, Mascini EM, Fluit AC, Hoepelman A, de Neeling AJ, Bonten MJM. Emerg Infect Dis. 2003;9:1108. doi: 10.3201/eid0909.020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. J Am Med Assoc. 2007;298:1763. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Addinall SG, Holland B. J Mol Biol. 2002;318:219. doi: 10.1016/S0022-2836(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 4.Margolin W. Nat Rev Mol Cell Biol. 2005;6:862. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addinall SG, Bi E, Lutkenhaus J. J Bacteriol. 1996;178:3877. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinho MG, Errington J. Mol Microbiol. 2003;50:871. doi: 10.1046/j.1365-2958.2003.03719.x. [DOI] [PubMed] [Google Scholar]
- 7.Lutkenhaus J, Addinall SG. Annu Rev Biochem. 1997;66:93. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Lowe J, van den Ent F, Amos LA. Annu Rev Biophys Biomol Struct. 2004;33:177. doi: 10.1146/annurev.biophys.33.110502.132647. [DOI] [PubMed] [Google Scholar]
- 9.Lock RL, Harry EJ. Nat Rev. 2008;7:324. doi: 10.1038/nrd2510. [DOI] [PubMed] [Google Scholar]
- 10.Andreu JM, Schaffner-Barbero C, Huecas S, Alonso D, Lopez-Rodriguez ML, Ruiz-Avila LB, Núñez-Ramírez R, Llorca O, Martín-Galiano AJ. J Biol Chem. 2010;285:14239. doi: 10.1074/jbc.M109.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beuria TK, Santra MK, Panda D. Biochemistry. 2005;44:16584. doi: 10.1021/bi050767+. [DOI] [PubMed] [Google Scholar]
- 12.Domadia PN, Bhunia A, Sivaraman J, Swarup S, Dasgupta D. Biochemistry. 2008;47:3225. doi: 10.1021/bi7018546. [DOI] [PubMed] [Google Scholar]
- 13.Haydon DJ, Bennett JM, Brown D, Collins I, Galbraith G, Lancett P, Macdonald R, Stokes NR, Chauhan PK, Sutariya JK, Nayal N, Srivastava A, Beanland J, Hall R, Henstock V, Noula C, Rockley C, Czaplewski L. J Med Chem. 2010;53:3927. doi: 10.1021/jm9016366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haydon DJ, Stokes NR, Ure R, Galbraith G, Bennett JM, Brown DR, Baker PJ, Barynin VV, Rice DW, Sedelnikova SE, Heal JR, Sheridan JM, Aiwale ST, Chauhan PK, Srivastava A, Taneja A, Collins I, Errington J, Czaplewski LG. Science. 2008;321:1673. doi: 10.1126/science.1159961. [DOI] [PubMed] [Google Scholar]
- 15.Kumar K, Awasthi D, Lee SY, Zanardi I, Ruzsicska B, Knudson S, Tonge PJ, Slayden RA, Ojima I. J Med Chem. 2011;54:374. doi: 10.1021/jm1012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Läppchen T, Hartog AF, Pinas VA, Koomen GJ, den Blaauwen T. Biochemistry. 2005;44:7879. doi: 10.1021/bi047297o. [DOI] [PubMed] [Google Scholar]
- 17.Urgaonkar S, La Pierre HS, Meir I, Lund H, RayChaudhuri D, Shaw JT. Org Lett. 2005;7:5609. doi: 10.1021/ol052269z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapoor S, Panda D. Expert Opin Ther Targets. 2009;1037:13. doi: 10.1517/14728220903173257. [DOI] [PubMed] [Google Scholar]
- 19.Foss MH, Eun YJ, Weibel DB. Biochemistry. 2011;50:7719. doi: 10.1021/bi200940d. [DOI] [PubMed] [Google Scholar]
- 20.Schaffner-Barbero C, Martin-Fontecha M, Chacon P, Andreu JM. ACS Chem Biol. 2012;7:269. doi: 10.1021/cb2003626. [DOI] [PubMed] [Google Scholar]
- 21.Margalit DN, Romberg L, Mets RB, Hebert AM, Mitchision TJ, Kirschner MW, RayChaudhuri D. PNAS, USA. 2004;101:11821. doi: 10.1073/pnas.0404439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awasthi D, Kumar K, Ojima I. Expert Opin Ther Pat. 2011;21:657. doi: 10.1517/13543776.2011.568483. [DOI] [PubMed] [Google Scholar]
- 23.Huang Q, Tonge PJ, Slayden RA, Kirikae T, Ojima I. Curr Top Med Chem. 2007;7:527. doi: 10.2174/156802607780059790. [DOI] [PubMed] [Google Scholar]
- 24.Kumar K, Awasthi D, Berger WT, Tonge PJ, Slayden RA, Ojima I. Future Med Chem. 2010;2:1305. doi: 10.4155/fmc.10.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parhi A, Kelley C, Kaul M, Pilch DS, LaVoie EJ. Bioorg Med Chem Lett. 2012;22:7080. doi: 10.1016/j.bmcl.2012.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaVoie EJ, Parhi A, Pilch DS, Kaul M. BENZO[c]PHENANTHRIDINES AS ANTIMICROBIAL AGENTS. 20120022061 United States. 2012
- 27.Everett R, Hamilton J, Abelt C. Preparation of 5-Bromo-2-naphthol: The Use of a Sulfonic Acid as a Protecting and Activating Group. Molbank; 2009. p. M602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai G, Tan PZ, Ghoshal P. Synth Commun. 2003;33:1727. [Google Scholar]
- 29.CLSI Methods for Dilution Antimicrobial Sucseptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Eighth Edition. Clinical and Laboratory Standards Institute; Wayne, PA: 2009. [Google Scholar]
- 30.Parhi A, Lu S, Kelley C, Kaul M, Pilch DS, LaVoie EJ. Bioorg Med Chem Lett. 2012;22:6962. doi: 10.1016/j.bmcl.2012.08.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelley C, Zhang Y, Parhi A, Kaul M, Pilch DS, LaVoie EJ. Bioorg Med Chem. 2012;20:7012. doi: 10.1016/j.bmc.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaul M, Parhi AK, Zhang Y, LaVoie EJ, Tuske S, Arnold E, Kerrigan JE, Pilch DS. J Med Chem. 2012;55:10160. doi: 10.1021/jm3012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CM, Hamel E. J Biol Chem. 1981;256:9242. [PubMed] [Google Scholar]
- 34.Kumar N. J Biol Chem. 1981;256:10435. [PubMed] [Google Scholar]
- 35.Carlier MF, Pantaloni D. Biochemistry. 1983;22:4814. doi: 10.1021/bi00289a031. 2006. [DOI] [PubMed] [Google Scholar]; Zhang Y, et al. Bioorg Med Chem Lett. 2013;23:2001–2006. doi: 10.1016/j.bmcl.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.