Abstract
Acute and chronic pain resulting from injury, surgery, or disease afflicts >100 million Americans each year, having a severe impact on mood, mental health, and quality of life. The lack of structural and functional information for most ion channels, many of which play key roles in the detection and transmission of noxious stimuli, means that there remain unidentified therapeutic targets for pain management. This study focuses on the TRPC4 ion channel, which is involved in the tissue-specific and stimulus-dependent regulation of intracellular Ca2+ signaling. Rats with a transposon-mediated TRPC4-knockout mutation displayed tolerance to visceral pain induced by colonic mustard oil (MO) exposure, but not somatic or neuropathic pain stimuli. Moreover, wild type rats treated with a selective TRPC4 antagonist (ML-204) prior to MO exposure mimicked the behavioral responses observed in TRPC4-knockout rats. Significantly, ML-204 inhibited visceral pain-related behavior in a dose-dependent manner without noticeable adverse effects. These data provide evidence that TRPC4 is required for detection and/or transmission of colonic mustard oil visceral pain sensation. In the future, inhibitors of TRPC4 signaling may provide a highly promising path for the development of first-in-class therapeutics for this visceral pain, which may have fewer side effects and less addictive potential than opioid derivatives.
Introduction
Visceral pain is associated with various acute and chronic disease states and does not respond adequately to current pain therapeutics. Visceral pain is often caused by distension, obstruction, or inflammation of the gastrointestinal tract. Nervous pathways involved in visceral pain transmission include the peripheral sensory fibers in the intestinal wall that pass through sympathetic chain ganglia to their spinal ganglia cell bodies, which then innervate neurons located in the layers I, II, V and X of the spinal cord (Ness and Gebhart, 1990). The elucidation of the molecular basis of pain is progressing and promises to deliver novel targets for the development of effective pain therapeutics as alternatives to morphine.
This study focuses on the role of the TRPC4 gene in a rat model of visceral pain induced by intra-colonic administration of mustard oil (MO). The TRPC4 channel, involved in the tissue-specific and stimulus-dependent regulation of intracellular Ca2+ signaling, belongs to a superfamily of plasma membrane transient receptor potential (TRP) channels, which are divided into 7 subfamilies (Nilius et al., 2007). The TRP Canonical subfamily (TRPC) family includes seven structurally related orthologs, TRPC1 to TRPC7 (Henley and Poo, 2004; Gomez and Zheng, 2006). TRP channels operate either as primary detectors of chemical and physical stimuli, as secondary transducers of ionotropic or metabotropic receptors, or as ion transport channels. Both TRPC4 expression and function have been documented in the brain (Mori et al., 1998; Riccio et al., 2002; Fowler et al., 2007). TRPC4 is also present in peripheral sensory neurons (Wu et al., 2008) as well as throughout the gastrointestinal tissue. TRPC4 mRNA and immunoreactivity was shown to be present in nerves innervating both the circular and the longitudinal muscles arising from the muscle-myenteric plexus, submucosal plexus and myenteric ganglia (Liu et al., 2008). Several TRP superfamily members play an important part in the control of GI motility and visceral sensation (Boesmans et al., 2011). Like other TRPCs, TRPC4 is postulated to play a role in the functional neurobiology of the enteric nervous system, including calcium homeostasis, membrane excitability, synaptic transmission and axon guidance. However, its role in sensory function, whether somatosensory or viscerosensory, including pain, has not been studied in vivo but will be addressed here.
In this study, behavioral tests and in situ hybridization (ISH) assays were performed to explore the role of TRPC4 in peripheral somatosensory and viscerosensory pain pathways. We utilized a novel transposon-mediated TRPC4 knockout (KO) model and wild type (WT) controls to examine the behavioral consequences of noxious stimulation with intracolonic MO. Data show that TRPC4 KO rats do not display the typical MO-induced effects seen in WT rats. Lastly, consistent with the notion that TRPC4 plays a key role in MO-induced pain behaviors, WT rats treated with ML-204, a selective TRPC4 channel antagonist (Miller et al., 2011), also displayed resistance to the noxious effects of intracolonic MO. Data presented in this study provides strong evidence that TRPC4 plays an essential role in the transmission of MO-induced visceral pain.
Methods
All procedures were consistent with the guidelines for Ethical Treatment of Research Animals published by the International Association for the Study of Pain and the National Institutes of Health Guide for Use of Experimental Animals to minimize animal use and discomfort. Procedures were approved by the Animal Care and Use Committee at the University of Kentucky. Animals received food and water ad libitum and were kept on a 12-h day-night cycle. Animals were raised and handled from birth by laboratory staff to facilitate acclimation to von Frey testing in order to minimize variability between animals within the experimental groups (outlined below).
Generation of TRPC4 knockout rats
Insertional mutant rats F344- TRPC4Tn(sb-T2/Bart3)2.192Mcwi (heterozygous, TRPC4+/−) were provided as “seed rats” by Transposagen Biopharmacueticals Inc. (www.transposagenbio.com). The KO rats were generated by random insertional gene trapping using the Sleeping Beauty transposon vector (T2/Bart3), which contains transcriptional termination elements (by Medical College of Wisconsin) (Lu et al., 2007). The transposon integrated within intron 1 of TRPC4 and resulted in the ablation of all isoforms containing exon 1. TRPC4−/− rats (250–400g) were bred in Dr. Westlund’s laboratory at the University of Kentucky. The TRPC4−/− rats were viable and bred equally well compared to inbred Fisher 344 wild type (WT) rats. Littermate TRPC4−/−, TRPC4+/−, WT male and female rats were used in experimental and control groups. Since the TRPC4−/− mutation is in the inbred Fisher 344 line, commercially available Fisher 344 rats represent appropriate WT controls for the studies.
PCR Genotyping
All rats bred at the University of Kentucky were genotyped by PCR. The genotype of each animal was confirmed using 3 primers designed by Transposagen to test WT, TRPC4+/− and TRPC4−/− rat pups: 5’-GTGTTGGTCTCCATTACTTCAGCT-3’, 5’-ATTCTTCCCTTTGAGCCCACT-3’, and transposon primer 5’-CTGACCTAAGACAGGGAATT-3’. PCR was carried out at 94°C for 7 min, and then 94°C for 30 s, 55°C for 30 s, and 72°C for 45 s for 25 cycles, followed by 7 min extension at 72°C using a PTC-100 programmable thermal controller (MJ Research Inc., Waltham, MA). The expected 905 bp product for the wild-type allele, which spans the transposon insertion site, and 510 bp product for the mutant allele, which corresponds to a unique junctional product consisting of host and transposon sequences, were separated on a 1% agarose gel.
Probe Design and Synthesis
DNA templates (712 bp in length, 768–1478) were synthesized by standard PCR strategy. To enable synthesis of antisense and sense cRNA probes, T7 and SP6 RNA polymerase promoters were added at the extremities of the sense and antisense templates, respectively. The following primers were used:
-
Sense Probes
Rn_TRPC4-F1-T7ext: 5’-CGCTATAATACGACTCACTATAGGGAGACCTAGATCAGACACGGAGTTCCAGAGAGCT-3’
Rn_TRPC4-R1: 5’-CAGGCGGAGGGAACTGAAGATGTTT-3’
-
Anti-sense Probes
TRPC4-F1: 5’-CCTAGATCAGACACGGAGTTCCAGAGAGCT-3’
TRPC4-R1-SP6ext: 5’-GCATTAATTTAGGTGACACTATAGAAGCGCAGGCGGAGGGAACTGAAGATGTTT-3’
The cRNA transcripts were validated by cold RNA probe synthesis and agarose gel electrophoresis prior to radiolabeling with 35S-UTP (>1,000 Ci/mmol; Cat. #NEG039H, Perkin Elmer LAS Canada, Inc.).
Tissue Preparation, ISH and Autoradiography
Rat pups (postnatal day p5) were included in localization studies comparing Fisher WT (n=3), TRPC4+/− (n=6), and TRPC4−/− (n=6) rats. Adult rats were anesthetized (isoflurane), and decapitated. Spinal cord (thoracic, lumbar and sacral segments) and left and right dorsal root ganglia (DRG) including lumbar L6 and sacral S1, which both innervate the descending colon (Robinson et al., 2004), as well as the intestine (duodenum, jejunum and colon) were rapidly dissected, immersed in embedding media, and frozen in an isopentane and dry ice slurry cooled to −35°C. As controls, lumbar L4 and L5, which provide less innervation to the colon, were similarly prepared for ISH. Rat p5 pups were anaesthetized in CO2 gas, frozen in cooled isopentane and cut whole sagittally with a cryostat into 8 µm sections. These were mounted onto glass slides and prepared ISH by fixation in freshly prepared 4% formaldehyde treated with triethanolamine/acetic anhydride, buffer washed, and dehydrated through a graded ethanol series, as described previously (Biagini et al., 2001).
35S-labeled cRNA antisense and sense probes were prepared fresh just before use. Tissue sections were hybridized overnight (55°C) with 50–80,000 cpm/µl 35S-labeled probes in 50% deionized formamide buffered with Tris-HCl (20 mM, pH 7.4), 0.3 NaCl, and 10 nM NaPO4 with 5 mM EDTA, 10% dextran sulfate, 1× Denhardt’s, and 50 µg/ml total yeast RNA. The tissues were subjected to stringent washes (65°C) in 50% formamide, 2× SSC, and 10mM DTT, followed by PBS washes before treatment with 20 µg/ml RNase A for 30 min (37°C). After repeated washes in 2× SSC and 0.1× SSC for 10 min (55°C), the slides were dehydrated and exposed to X-ray film for 5 days. Slides were then coated with photographic emulsion (NTB) (Kodak), exposed for 15 days and developed with D19 photographic solution (Kodak). As a positive control for the assay, brain derived neurotrophic factor (BDNF) 35S-labeled cRNA probes were assayed as previously described (Biagini et al., 2001) under identical conditions, except with only 3 days exposure to X-ray film (data not shown).
Formalin injection in the foot pads
Each animal was placed in an empty see-through cage in an isolated room for a 10-min acclimatization period before the formalin injection. An injection of formalin (1.5%, 50 µl, subcutaneously) into the footpad with a 28G½ (0.36 × 13 mm) sterile needle mounted on a 0.5cc U-100 Insulin Syringe inserted into the footpad close to the border between hairy and glabrous skin. The 1.5% formalin is freshly prepared from stock (0.55% formaldehyde). Controls were injected with an equal volume of sterile saline at the same site and the same manner. Immediately after the injection, the animals were placed back in the same empty cage for a 45-min observation period recorded with a video camera linked to a computer recording program for offline data analysis). The number of seconds that the animals spend rubbing or flicking the injected hindpaw was recorded. These activities were present in the two phases typical of formalin test events.
Intracolonic Infusion of Mustard Oil (MO)
Animals were placed in the recording chamber 10 min before infusion of MO (0.5% allylisothiocyanate in peanut oil (Sigma-Aldrich, St. Louis, MO USA). Petroleum jelly (Vaseline) was applied in the perianal area previous to this to avoid the stimulation of somatic areas by contact with the irritant chemical. The oil (0.5 ml) was administered by introducing a fine cannula with a heat-rounded tip (PE90 tube; 7 cm long) into the colon via the anus.
Assessment of Pain-related Behaviors
Immediately after the MO infusion, the animals were placed back into the observation cage. Spontaneous pain-related behaviors were documented in videotaped recordings (25 min) and ratings performed by a blinded observer. Postures defined as pain-related behavior in this study included licking of the abdomen, stretching the abdomen or hindlimb, lowering the abdomen against the floor and abdomen retractions which were evaluated as previously described (Laird et al., 2001). These behaviors, while subjective, represent the standard assays used for assessing pain-related behaviors (Craft et al., 1995; Lanteri-Minet et al., 1995; Laird et al., 2001; Negus et al., 2006).
Paw Withdrawal Threshold Test
After videotaping of the MO-induced pain-related behaviors, mechanical withdrawal threshold testing was done on the plantar surface of both hindpaws using a set of eight von Frey monofilaments and ratings performed by a blinded observer. In order to minimize variability between animals within an experimental group, the animals were acclimated in the week before testing by visiting the adjacent testing room and being placed in the test cubicles several times. The day prior to testing, the animals participated in sham testing with the von Frey filaments. The von Frey filaments were applied perpendicularly to the plantar surface with sufficient force to bend the monofilament slightly and held for about 5 seconds. A positive response was defined as an abrupt withdrawal (flick response) of the foot during the stimulation or immediately after the removal of the stimulus. The up/down method by Chaplan et al. (Chaplan et al., 1994) was utilized, i.e. whenever there was a negative or positive response, the next stronger or weaker filament was applied, respectively. The pattern of positive and negative responses was converted into a 50% threshold value (in grams) using the formula given by Dixon (Dixon, 1980).
TRPC4 Antagonist ML-204
The pain-related behavioral assessments detailed above were also performed in WT rats after treatment with a small molecule TRPC4 antagonist, ML-204 (Evotec; South San Francisco, CA US). ML-204 (4-methyl-2-(piperidin-1-yl) quinoline) is the first potent and selective blocker of TRPC4 channels (Miller et al.; Miller et al., 2011). This agent was only characterized in vitro using cell cultures of peripheral nerves, intestinal smooth muscle myocytes and HEK cells transfected with mouse and guinea pig TRPC4 channels (Miller et al., 2011). Intraperitoneal (i.p.) injection doses of 0.03, 0.15, 0.75 mg/kg were administered in pre- and post-treatment trials. In a separate control group of WT animals oral ML-204 was given in pre- and post-treatment doses of 0.5 and 1mg/kg.
Morphine (Boehringer Ingelheim, Roxane Laboratories, Columbus, OH) was administered subcutaneously (5 and 10 mg/kg) as a benchmark for in vivo efficacy in animal pain models.
Electrocardiogram (ECG) Recording of Blood Pressure and Heart Rate
ML-204 was gavaged with 1mg/kg in 10% sucrose 30min prior to taking ECG and blood pressure measurements. Animals were placed onto conductive electrode lead plates on an ECG recording platform (ECGenie™), which was enclosed on three sides with acrylic panels. Animals were allowed to acclimate for 3–10 minutes. After the acclimation period, ECG signals were recorded for 2–10 minutes to provide heart rate and ECG indices (P, Q, R, S, and T points). The procedure performed by a blinded observer was non-invasive and required no sedation or anesthesia (Chu et al., 2001).
Statistics
Data with multiple groups were analyzed with one-way analysis of variance (ANOVA) followed by Tukey post hoc tests. The time cause among groups was performed a two-way ANOVA with Bonferroni posttest using GraphPad Prism version 4.0 (GraphPad Software, San Diego California USA. Data compared between two groups was analyzed using two-tailed Student’s t-tests. A p value of ≤0.05 was considered statistically significant. All values are reported as mean +/− standard error (SE) of the mean (x ± SEM).
Results
TRPC4 mRNA in situ hybridization in control and TRPC4−/− neonate (p5) rat tissues
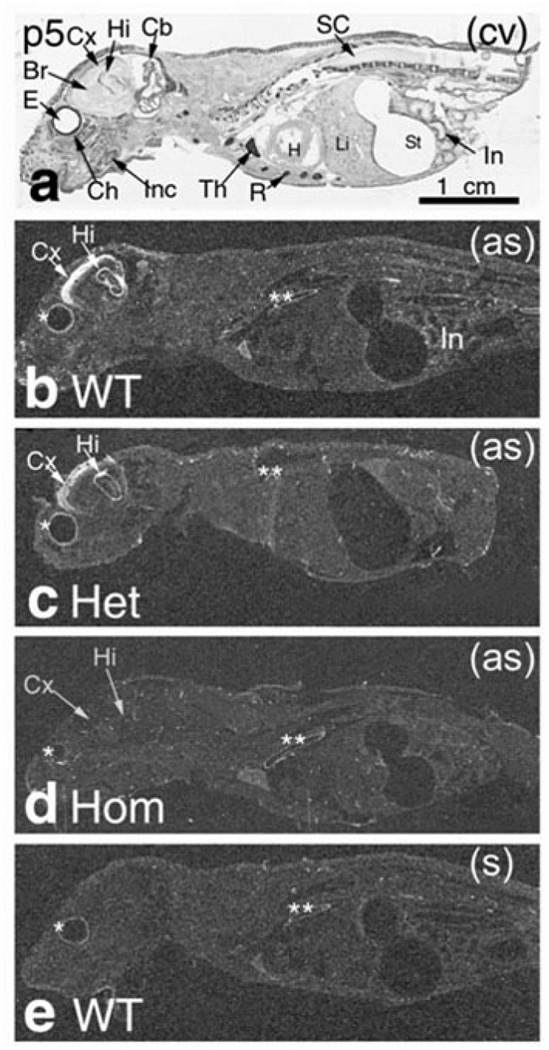
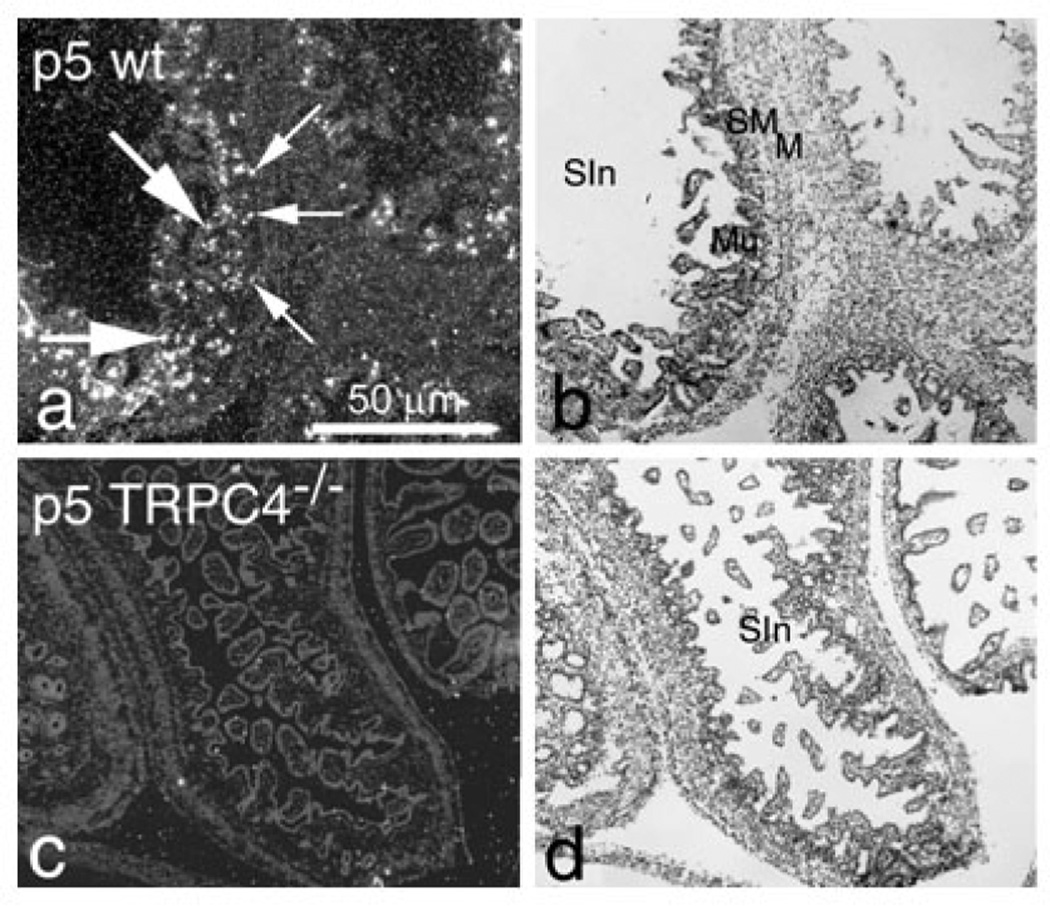
The TRPC4 knockout efficiency was assessed by ISH in day 5 postnatal rats (p5) from WT, heterozygous (TRPC4+/−) and homozygous (TRPC4−/−) animals (photomicrographs obtained from various tissues are shown in Figures 1 and 2. The ISH signal was localized mainly in the brain cortex and hippocampus, but was also detected in a number of other organs including the intestinal wall, tooth dentine, ganglion VIII, spinal dorsal root ganglia (DRG), bone marrow, liver erythropoietic centers and skin sweat cells (data not shown). When compared to WT animals, the TRPC4 mRNA levels decreased by approximately 50% in TRPC4+/− tissues and were undetectable in TRPC4−/− tissues (Fig. 1). In the gut, TRPC4 mRNA was expressed in lamina propria cells and the submucosa plexus in p5 and in adult rats (Fig. 2a). TRPC4 mRNA was detected in the intestine of WT rats (Figs. 1b and 2a) but was undetectable in TRPC4−/− rat intestinal tissue (Figs. 1d and 2c).
Figure 1. TRPC4 mRNA is absent in TRPC4−/− rats.
In situ Hybridization. ISH in p5 postnatal rat whole-body sections stained with cresyl violet (a). Emulsion autoradiography bright ISH labeling is seen in (b) WT rat and (c) TRPC4+/− (Het) but not (d) TRPC4−/− (Hom). Control (sense) ISH is in (e).
Abbreviations: Br – brain; Cb – cerebellum; Ch – choroid; Cx – brain cortex; E – eye; H – heart; Hi – hippocampus; In –intestine, Inc – incisor; Het – heterozygous (TRPC4+/−); Hom – homozygous (TRPC4−/−); KO – knockout (TRPC4−/−); Li – liver, erythropoietic centers; R – ribs; SC – spinal cord; St – Stomach; Th – thymus; (as) – antisense; (cv) – cresyl violet; (s) – sense. The asterisks (*) indicate non-specific hybridization in the eye choroid (*) and dorsal aorta (**).
Figure 2. TRPC4 expression in wild type but not in TRPC4−/− p5 rat intestine.
(a) ISH in p5 wild type (WT) rat small intestine mucosa. Bright labeling is seen in lamina propria cells in the intestinal wall (large arrow) and submucosal Meissner’s plexus (small arrows); cresyl violet staining is in (b).
(c) Undetectable ISH labeling in p5 TRPC4−/− rat intestine; cresyl violet staining is in (d).
Abbreviations: M – muscle layers; Mu – mucosa; SIn – small intestine; SM – submucosa.
Magnification ×50.
Pain-related behavior
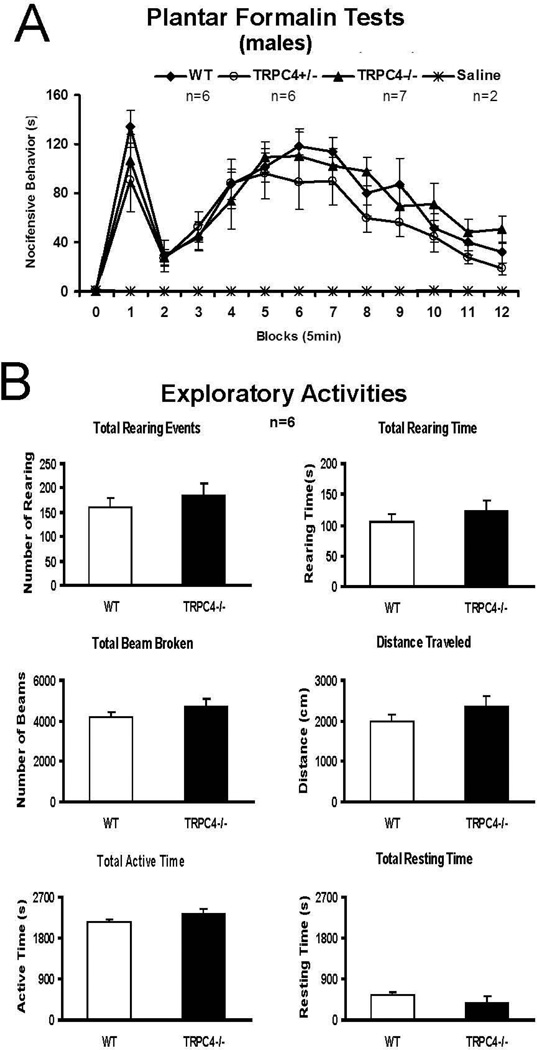
The effects of TRPC4 gene disruption was assessed using several animal models of somatic, neuropathic and visceral pain. Negligible differences occurred between WT control and TRPC4−/− rat groups for several conventional assessments of somatic and neuropathic pain, including hindpaw formalin irritation (see Fig. 3A), heat stimulation of the tail, temporo-mandibular or knee joint inflammation with complete Freund’s adjuvant, and sciatic or infraorbital (trigeminal) nerve constriction injury (data not shown).Exploratory behaviors adapting to a novel environment in an open field test box were normal on the six parameters collected: rearing events, rearing duration, total beam broken, distance traveled; total active and resting times (see Fig. 3B). These data imply that unprovoked higher brain level functions are normal in TRPC4-deficient rats and that there does not appear to be any evident physical or locomotor anomaly that could interfere with the pain assays.
Figure 3. TRPC4−/− rats resemble WT rats in plantar formalin and open field tests.
(A) Plantar Formalin Test
After hindpaw intraplantar injection of formalin, rats immediately showed typical formalin-induced nocifensive behaviors in a biphasic pattern, including: hindlimb favoring, lifting, biting/licking, and increased rearing and locomotion. The phase I begins immediately after the formalin injection and lasts about 10 min (2 blocks). Phase II typically begins 15 to 20 min after the formalin injection and continues for ≥45 min. There were no significant differences among the groups. (two-way ANOVA with Bonferroni post-test, P>0.05).
(B) Exploratory activity comparisons between wild type and TRPC4−/− animals During a 45 min-session of open field testing, both WT and TRPC4 −/− groups showed the same adaptive exploratory activity on the six parameters collected: rearing events, rearing duration, total beam broken, distance traveled; total active time and resting time. (T-test P>0.05)
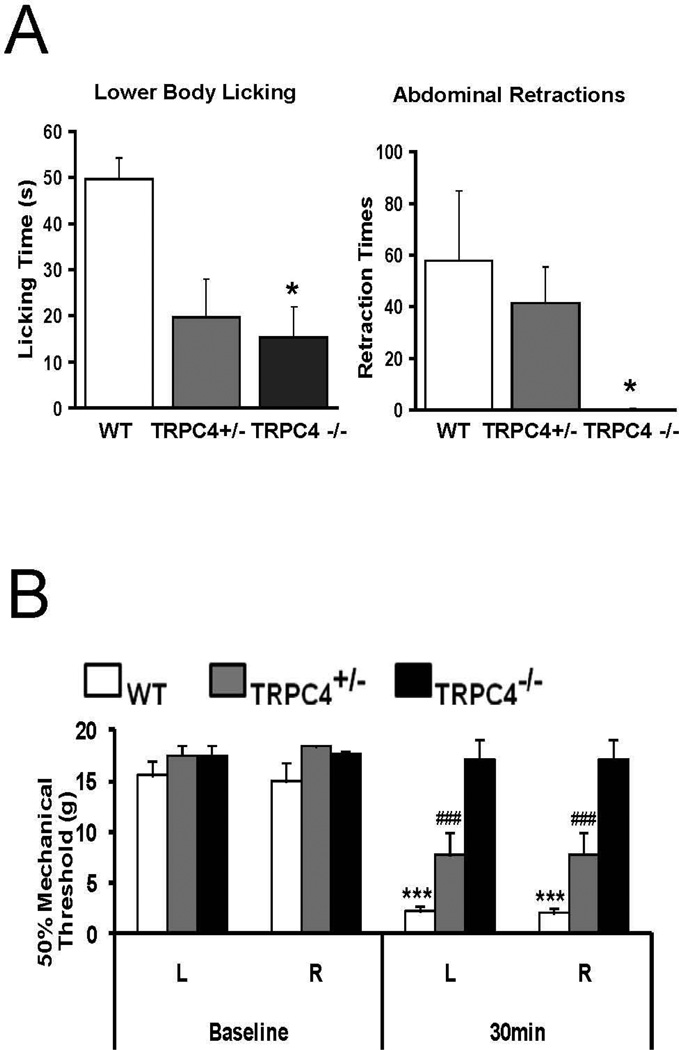
In contrast, a marked difference was observed between WT and TRPC4 KO rats following intra-colonic infusion of MO, an established model of visceral pain (Laird et al., 2001). Following exposure to MO, WT rats displayed complex spontaneous pain-related behaviors, such as licking of the lower abdomen and abdominal retractions/stretching, clearly identifiable during the 25-min videotaping session. In genetically modified rats, licking of the lower abdomen was significantly reduced (p<0.05) by approximately 60% and 69% respectively in TRPC4+/− and TRPC4−/− rats (WT 49.75±4.5, TRPC4+/− 19.57±6.6; TRPC4−/− 15.28±6.6 events) while abdominal retractions/stretching was inhibited by 28% and 99% (WT 57.75±27, TRPC4+/− 41.43±13.91; TRPC4−/− 0.43±0.42 events) (Fig. 4A).
Figure 4. TRPC4 is required for intracolonic mustard oil (MO) induced visceral pain-related responses and sensitized hindpaw plantar mechanical allodynia.
(A) Spontaneous pain behaviors. Bar graphs show increases in pain-related behaviors during a subsequent 25 min observation period after intracolonic infusion of mustard oil in male rats (n=7). Lower body licking and abdominal retractions were greatly increased in WT and TRPC4+/− rats. Responses were significantly less or did not appear in the TRPC4−/− rats, except some lower body licking. (TRPC4−/− vs. WT *p<0.05)
(B) Paw withdrawal threshold for mechanical stimulation. Paw withdrawal thresholds for mechanical stimulation with von Frey fibers were significantly reduced for WT and TRPC4+/− rats. TRPC4−/− rats maintained the same mechanical threshold 30 min after intracolonic mustard oil infusion (n=7). (***p<0.005 TRPC4+/− and TRPC4−/− vs. WT, ###p<0.001 TRPC4+/− vs. TRPC4−/−)
Rats exposed to intracolonic MO typically display a primary visceral hyperalgesia and a secondary mechanical allodynia. The “referred” secondary hypersensitivity is evident as an increased sensitivity of the hind paws to stimulation by von Frey filaments. However, the paw withdrawal threshold in response to mechanical stimuli remained unchanged from baseline in TRPC4−/− rats following MO exposure, providing additional evidence of insensitivity to the visceral pain in these rats. The TRPC4−/− rats had a consistent 50% mechanical threshold of 17–18 g both before and after intracolonic MO infusion (TRPC4−/− vs. WT p<0.001). In contrast, mechanical threshold responses to von Frey fiber stimuli after exposure to MO revealed a sharp reduction in the WT rats from a baseline of 17–18 g to 2 g (Fig. 4B). The mechanical threshold of the TRPC4+/− rats was decreased to levels intermediate between the WT and KO groups (TRPC4+/− vs. WT p<0.001; TRPC4+/− vs. TRPC4−/− p<0.001), displaying a 50% mechanical threshold of 7–8g.
Visceral pain-related behaviors following potent selective TRPC4 antagonist treatment
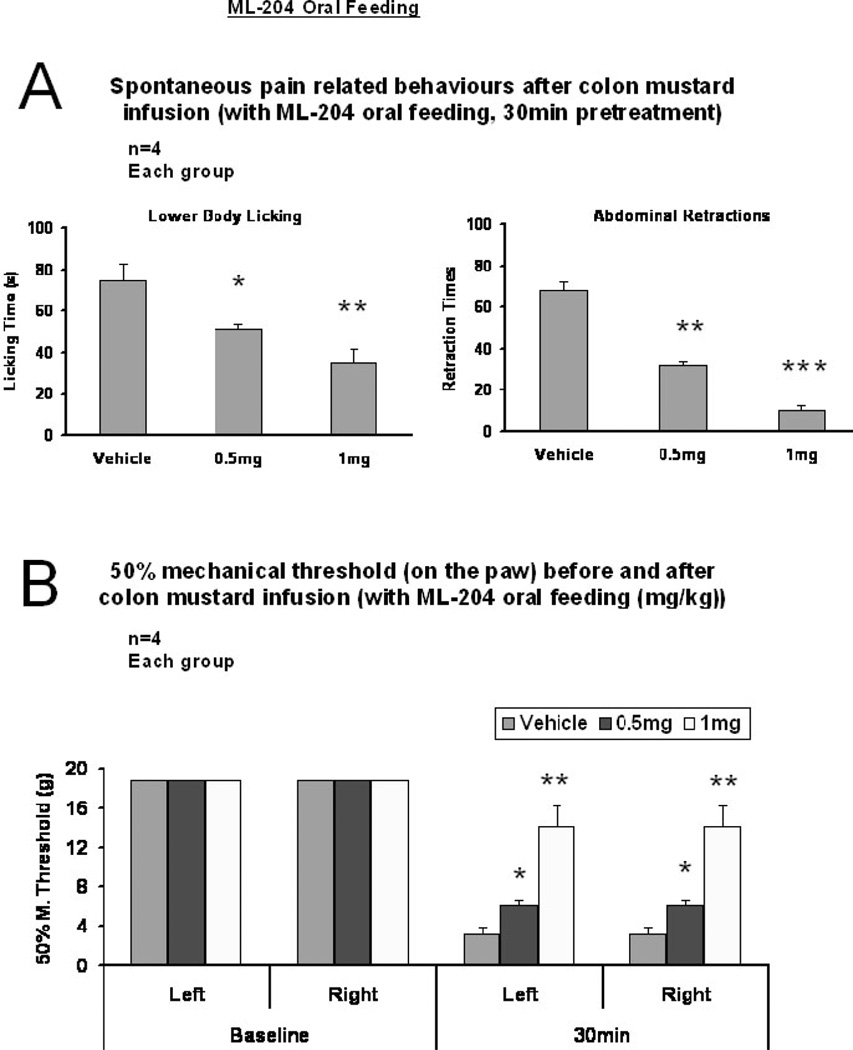
Further evidence of the involvement of TRPC4 in visceral pain transmission was provided by treatment of WT rats with ML-204, a novel small molecule identified as a potent selective TRPC4 channel antagonist (Figs. 5, 6). Oral administration of ML-204 in pre-treatment trials effectively reduced spontaneous pain-related behaviors induced by colon MO infusion, i.e. fewer body licking responses compared to the vehicle treatment group (52.14±7.27; 21.67±4.9 (p<0.05) and 2±2 (p<0.01) responses respectively for the vehicle control group and the 0.5 mg/kg and 1.0 mg/kg treatment groups) and fewer abdominal retractions (vehicle 68.28±16.67, 0.5 mg/kg 31.67±2.18 p<0.01, 1.0 mg/kg 10.66±5.4) (Fig. 5A). A statistically significant dose dependent reduction in the mechanical allodynia response was also observed for the two doses tested (Fig. 5B; 0.5 and 1 mg/kg, p<0.05 and 0.01, n=4).
Figure 5. Visceral pain-related behaviors evoked by intracolonic mustard oil are blocked by oral pre-treatment with TRPC4 antagonist, ML-204.
(A) Spontaneous pain behavior. TRPC4 antagonist ML-204 administered orally inhibits MO-induced pain-related behaviors dose-dependently in wild type rats (n=4) (*p<0.05, **p<0.01, ***p>0.005 vs. vehicle).
(B) Mechanical hypersensitivity. ML-204 inhibits mechanical allodynia induced in WT rats by intracolonic MO infusion. The variation in baseline sample measurements was negligible. Values represent mean + SE of ~20 readings in rats (n=4, 1 hr after ML-204 administration).
(*p<0.05, **p<0.01 vs. vehicle)
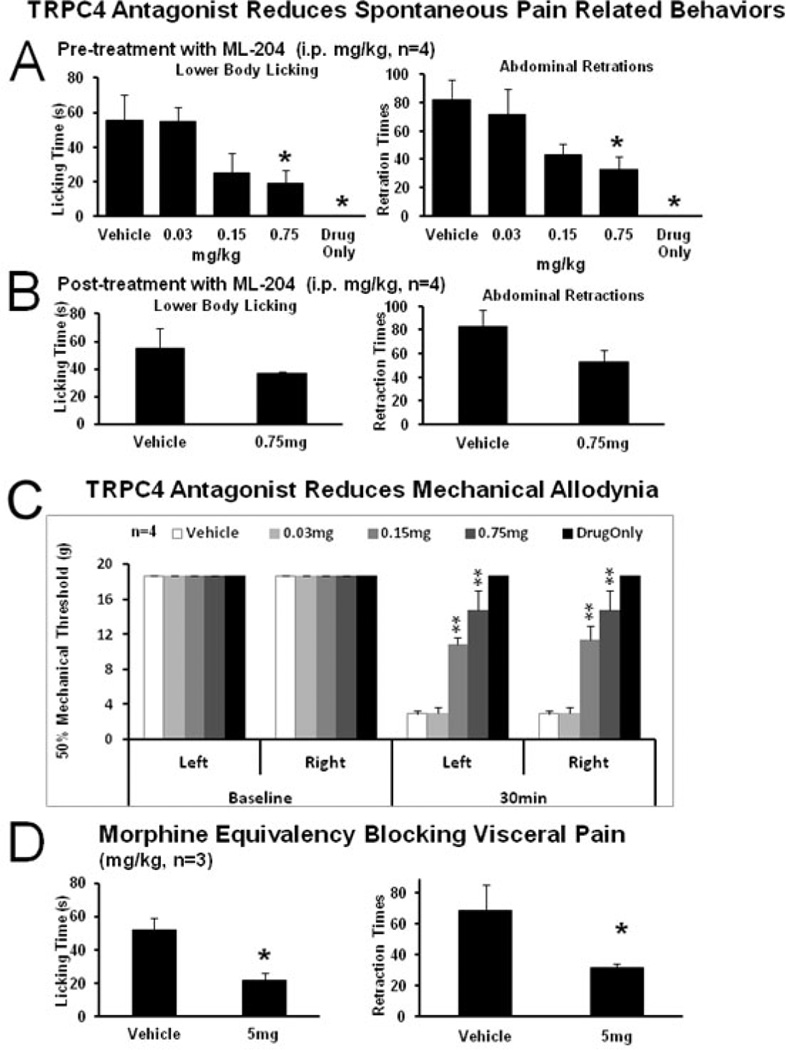
Figure 6. Visceral pain-related behaviors evoked by intracolonic mustard oil are blocked by intraperitoneal application of TRPC4 antagonist, ML-204.
(A) Pre-treatment. TRPC4 antagonist ML-204, dose-dependently inhibited MO-induced pain-related behaviors in wild type rats injected intraperitoneally.
(B) Post-treatment. ML-204 inhibited pain-related behaviors induced by intracolonic MO infusion. Values represent mean ± SED (n=4, 1 hr).
(C) Pre-treatment. ML-204 inhibited mechanical allodynia induced in WT rats 30 minutes after intracolonic MO infusion.
(D) Responses to morphine. Inhibition of pain-related behaviors induced by intracolonic MO infusion after subcutaneous pre-treatment with morphine.
(*p<0.05, **p<0.01 vs. vehicle)
Abbreviations: PO vehicle–peanut oil; MO- mustard oil irritant
The results showed that i.p. administration was more effective (Fig. 6). A statistically significant diminution was noted in spontaneous pain-related behaviors (Fig. 6A) in pre-treatment trials. In the 0.75 mg/kg group, the number of lower body licking events decreased to 19.25±7.19 from in the vehicle treatment group 58±12.92, p<0.05. The number of abdominal retractions was 32.5±9.26 vs. 82.5±12.22 in the vehicle group (p<0.05, n=4 each group). There was also a reduction trend for single dose, post-treatment injections (Fig. 6B, 0.75 mg/kg). Mechanical allodynia responses demonstrated a statistically significant dose-dependent reduction for both doses tested at 30 min (Fig. 6C). In the 0.15 mg/kg group the 50% mechanical threshold was 10.85±0.78 g for left paw and 11.35±1.62 g for right paw, and in the 0.75 mg/kg group the 50% mechanical threshold was 14.77±2.3 g for both paws while it was 2.88±0.44 g for the vehicle treatment group, (p<0.01 and p<0.05, n=4 each group). The maximally effective dose of 0.75 mg/kg, is about 50 times the IC50 in vitro (Miller et al. 2011). ML-204 blocked visceral pain-related behaviors in rats with colon MO infusion, including both primary visceral hyperalgesia and/or secondary mechanical allodynia. By comparison, a ten-fold higher dose of morphine (5 mg/kg, subcutaneous) gave an equivalently effective block (Fig. 6D). The number of lower body licking events was 21.67±4.9 vs. 52.14±7.27 for the vehicle group, (P<0.05, n=3). The number of abdominal retractions was 31.67±2.18 vs. 68.29±16.67 compared to the vehicle group (p<0.05, n=3). ML-204 administration had no effect on blood pressure, heart rate or ECG parameters (Table 1).
Table 1. Effect of ML-204 on Blood Pressure, Heart Rate and ECG Parameters.
ML-204 was gavaged with 1mg/kg in 10% sucrose 30min prior to taking ECG and blood pressure measurements. ML-204 administration had no effect on blood pressure, heart rate or ECG parameters.
| Mean Blood Pressure | ||||
|---|---|---|---|---|
| Baseline | 30 min | 1 hour | ||
| Control | n=2 | 111.0429 | 123.5556 | 127.7 |
| ML-204 | n=3 | 113.4222 | 120.9714 | 98.1553 |
| ECG Parameters |
HR (bpm) | CV (%) (LVP) | PQ (ms) | QRS (ms) | ST (ms) | QT (ms) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal Group n= |
Before | After | Before | After | Before | After | Before | After | Before | After | Before | After |
| WT +ML204 n=3 | 449.89±16.8 | 424.89±2.65 | 1.289±0.14 | 0.86±0.21 | 28.92±1.54 | 29.6±0.28 | 15.4±0.92 | 15.41±0.56 | 53.73±3.65 | 57.59±1.1 | 68.11±4.56 | 72.02±1.68 |
| TRPC4−/− +ML204 n=3 | 471.78±6.62 | 448.89±14.80 | 1.137±0.20 | 0.95±0.03 | 30.14±0.85 | 30.14±0.64 | 17.49±0.83 | 17.49±0.83 | 53.72±2.14 | 56.77±3.76 | 69.9±2.74 | 73.36±4.43 |
All of the TRPCs are reportedly expressed in blood vessels, especially in endothelial cells and vascular smooth muscle cells. Blood pressure and ECG measurement were carried out in healthy WT rats to determine if block of TRPC4 alone with ML-204 would produce cardiovascular effects. ML-204 administration had no effect on blood pressure, heart rate or ECG parameters (Table 1).
Discussion
This study focuses on the role of the TRPC4 gene in responses to noxious colon irritation. The TRPC4 channel has been shown to have a physiological role in microvascular permeability, vasorelaxation, gastrointestinal motility, and GABA release. This study provides direct evidence that TRPC4 is required for MO-related chemical sensing in the colon and suggests its involvement in transmission of visceral pain, associated with various acute and chronic states. Most often, visceral pain is caused by distension, obstruction, or inflammation of the gastrointestinal tract, which activates the normally ‘silent’ visceral afferents. Instillation of MO into the descending colon induces an acute irritation identified as a cross-organ reflex sensitization with evoked activity in the spinal cord (Peng et al., 2009). Neuronal pathways involved in visceral pain include the peripheral sensory fibers present along hollow internal organ walls and vasculature. The sensory nerves innervate neurons located in spinal cord laminae I, II, V and X that transmit information about pain (Ness and Gebhart, 1990).
When noxious MO is administered to the colon of rats deficient in TRPC4, insensitivity is noted (Fig. 4), in contrast to the robust pain-related responses seen in WT rats (Laird et al., 2001). Hence, inactivating the TRPC4 channel, likely involving sensory pathways, is sufficient to block MO-induced-behavior. Consistent with our observations, TRPC4 was localized by immunocytochemistry in adult DRG sensory neurons (Wu et al., 2008). DRG sensory fibers may, likely, play a role in transmission of MO-induced sensations to the central nervous system. In addition, the ISH data, showing expression in lamina propria cells and submucosa plexus (Fig 2), have provided insight into the local structures that may specifically require TRPC4 for MO-related visceral pain transmission. This is consistent with immunocytochemical localization of TRPC4 in intestinal myenteric and submucosal plexuses, where it has been observed in the varicose nerve fibers in myenteric ganglia and interganglionic fibers bundles (Liu et al., 2008).
Regarding primary sensory fibers, TRPC4 was localized by immunohistochemistry to non-cholinergic sensory neuronal fibers (Liu et al., 2008) and additional TRP superfamily members may also have a role in activation of nociceptive nerve endings in response to mechanical stretch, low pH and noxious chemicals (Caterina et al., 2000; Caterina and Julius, 2001). Furthermore, TRPC4 could form complexes with other TRP channel family members, such as TRPA1, which are present in sensory fibers, TRPA1 mRNA was also detected in human and mouse mucosa (Purhonen et al., 2008) and are responsive to MO (McMahon and Wood, 2006; McNamara et al., 2007; Bessac et al., 2009). It is also possible that TRPC4 supports chemosensory functions other than pain in the intestinal wall, for example, monitoring and controlling extracellular pH (Semtner et al., 2007) and alerting the autonomic nervous system to low oxygen levels (Liu et al., 2008). Responses to pH shift, mechanical stretch, and smooth muscle contractility important in intestinal pacemaking currents reportedly involve TRPC4 and TRPC6 (Walker et al., 2002), with TRPC4 mediating more than 80% of muscarinic receptor-induced cation current in intestinal smooth muscle cells important for accelerating gastrointestinal motility (Tsvilovskyy et al., 2009).
The insensitivity of TRPC4 KO rats was similar to the response profile of control rats after the noxious visceral insult if given a TRPC4 antagonist or morphine in the studies here or in studies reported by others testing various chemical models of inflammatory bowel syndrome after injection of gabapentin, pregabalin or morphine (Shannon et al., 2005; Gale and Houghton, 2011). While the TRPC4−/− rats were highly tolerant to noxious chemical stimuli applied to the colon, their responses to several other conventional somatic and neuropathic pain assessments were typical, indicating that TRPC4 is specifically relevant to visceral nociception.
The present study has identified the TRPC4 ion channel as a potential therapeutic target for visceral pain that may be more suitable than opioids and non-steroidal anti-inflammatory agents for GI pain management and potentially for other types of pain. Visceral pain does not respond adequately to the current pain therapeutics, giving a rationale to search for novel potent analgesics. In this respect, we report here for the first time that the small molecule TRPC4 antagonist, ML-204, has analgesic properties and is effective in vivo either orally or i.p. (i.e., for exposure to abdominal cavity celiac ganglion or systemic absorption) in doses 10-fold lower than effective doses of morphine and does not result in changes in the animal’s cardiovascular profile or other apparent side effects. ML-204 is the first selective blocker for TRPC4 channels with low micromolar potencies in several in vitro cell models (Miller et al.; Miller et al., 2011). Because of its selectivity for TRPC4, which is unique among this family of ion channels with high sequence homology, ML-204 could become the basis for a drug discovery program to seek innovative and targeted therapeutics for visceral pain. It is not clear whether ML-204 can cross the blood-brain-barrier and impact TRPC4 activity in the central nervous system. Future studies should address this question.
In summary, this study using a single gene knockout rat model, demonstrates the role of TRPC4 in the transmission of MO-induced visceral pain-like behaviors. While causing robust pain-related responses in control rats, application of irritative MO to the colon provokes no behavioral responses in TRPC4−/− rats. Likewise, a small molecule TRPC4 antagonist significantly reduces behavioral responses to the noxious visceral stimulus without adverse cardiovascular or other side effects when given orally or i.p. Taken together, our results demonstrate that TRPC4 is a novel pain target and suggest that inhibition of this target by administration of ML204 may suppress colonic visceral pain without the side effects associated with morphine and provide a clinically relevant benefit.
Highlights.
We show that TRPC4 plays a role in visceral pain transmission pathways
TRPC4 knockout rats show no behavioral responses to mustard oil intracolonic exposure
A TRPC4 small molecule antagonist ML-204, mimics the responses seen in knockout rats
Acknowledgements
This study was funded by the grant from NIH NS039041 (KNW) and start-up Dean’s funds from the University of Kentucky (KNW). Partial support was by R&D funds program of Government of Canada to Cytochem Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 2009;23:1102–1114. doi: 10.1096/fj.08-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, Avoli M, Marcinkiewicz J, Marcinkiewicz M. Brain-derived neurotrophic factor superinduction parallels anti-epileptic--neuroprotective treatment in the pilocarpine epilepsy model. J Neurochem. 2001;76:1814–1822. doi: 10.1046/j.1471-4159.2001.00163.x. [DOI] [PubMed] [Google Scholar]
- Boesmans W, Owsianik G, Tack J, Voets T, Vanden Berghe P. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. Br J Pharmacol. 2011;162:18–37. doi: 10.1111/j.1476-5381.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chu V, Otero JM, Lopez O, Morgan JP, Amende I, Hampton TG. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 2001;1:6. doi: 10.1186/1472-6793-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Henley SR, Haaseth RC, Hruby VJ, Porreca F. Opioid antinociception in a rat model of visceral pain: systemic versus local drug administration. J Pharmacol Exp Ther. 1995;275:1535–1542. [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS One. 2007;2:e573. doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JD, Houghton LA. Alpha 2 Delta (alpha(2)delta) Ligands, Gabapentin and Pregabalin: What is the Evidence for Potential Use of These Ligands in Irritable Bowel Syndrome. Front Pharmacol. 2011;2:28. doi: 10.3389/fphar.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Henley J, Poo MM. Guiding neuronal growth cones using Ca2+ signals. Trends Cell Biol. 2004;14:320–330. doi: 10.1016/j.tcb.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Lanteri-Minet M, Bon K, de Pommery J, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats: model elaboration and spinal structures involved as revealed by the expression of c-Fos and Krox 24 proteins. Exp Brain Res. 1995;105:220–232. doi: 10.1007/BF00240958. [DOI] [PubMed] [Google Scholar]
- Liu S, Qu MH, Ren W, Hu HZ, Gao N, Wang GD, Wang XY, Fei G, Zuo F, Xia Y, Wood JD. Differential expression of canonical (classical) transient receptor potential channels in guinea pig enteric nervous system. J Comp Neurol. 2008;511:847–862. doi: 10.1002/cne.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Geurts AM, Poirier C, Petit DC, Harrison W, Overbeek PA, Bishop CE. Generation of rat mutants using a coat color-tagged Sleeping Beauty transposon system. Mamm Genome. 2007;18:338–346. doi: 10.1007/s00335-007-9025-5. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Wood JN. Increasingly irritable and close to tears: TRPA1 in inflammatory pain. Cell. 2006;124:1123–1125. doi: 10.1016/j.cell.2006.03.006. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Shi J, Zhu Y, Kustov M, Tian JB, Stevens A, Wu M, Xu J, Long S, Yang P, Zholos AV, Salovich JM, Weaver CD, Hopkins CR, Lindsley CW, McManus O, Li M, Zhu MX. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J Biol Chem. 2011;286:33436–33446. doi: 10.1074/jbc.M111.274167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Shi J, Wu M, Engers J, Hopkins CR, Lindsley CW, Salovich JM, Zhu Y, Tian JB, Zhu MX, McManus OB, Li M. Novel Chemical Inhibitor of TRPC4 Channels. 2010 [PubMed] [Google Scholar]
- Mori Y, Takada N, Okada T, Wakamori M, Imoto K, Wanifuchi H, Oka H, Oba A, Ikenaka K, Kurosaki T. Differential distribution of TRP Ca2+ channel isoforms in mouse brain. Neuroreport. 1998;9:507–515. [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther. 2006;319:507–514. doi: 10.1124/jpet.106.106377. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Peng HY, Chen GD, Tung KC, Lai CY, Hsien MC, Chiu CH, Lu HT, Liao JM, Lee SD, Lin TB. Colon mustard oil instillation induced cross-organ reflex sensitization on the pelvic-urethra reflex activity in rats. Pain. 2009;142:75–88. doi: 10.1016/j.pain.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Purhonen AK, Louhivuori LM, Kiehne K, Kerman KE, Herzig KH. TRPA1 channel activation induces cholecystokinin release via extracellular calcium. FEBS Lett. 2008;582:229–232. doi: 10.1016/j.febslet.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Riccio A, Medhurst AD, Mattei C, Kelsell RE, Calver AR, Randall AD, Benham CD, Pangalos MN. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res Mol Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- Robinson DR, McNaughton PA, Evans ML, Hicks GA. Characterization of the primary spinal afferent innervation of the mouse colon using retrograde labelling. Neurogastroenterol Motil. 2004;16:113–124. doi: 10.1046/j.1365-2982.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Semtner M, Schaefer M, Pinkenburg O, Plant TD. Potentiation of TRPC5 by protons. J Biol Chem. 2007;282:33868–33878. doi: 10.1074/jbc.M702577200. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Eberle EL, Peters SC. Comparison of the effects of anticonvulsant drugs with diverse mechanisms of action in the formalin test in rats. Neuropharmacology. 2005;48:1012–1020. doi: 10.1016/j.neuropharm.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 2009;137:1415–1424. doi: 10.1053/j.gastro.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RL, Koh SD, Sergeant GP, Sanders KM, Horowitz B. TRPC4 currents have properties similar to the pacemaker current in interstitial cells of Cajal. Am J Physiol Cell Physiol. 2002;283:C1637–C1645. doi: 10.1152/ajpcell.00266.2002. [DOI] [PubMed] [Google Scholar]
- Wu D, Huang W, Richardson PM, Priestley JV, Liu M. TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J Biol Chem. 2008;283:416–426. doi: 10.1074/jbc.M703177200. [DOI] [PubMed] [Google Scholar]