Abstract
One distinctive effect on T-cell development was analyzed by selectively increasing serum prolactin (PRL) concentration in thymus-grafted congenitally athymic nude mice and by neutralizing PRL in suspension cultures of thymus from 1-day-old neonatal mice. Flow cytometric analysis of single-positive CD4+ and CD8+ cells derived from inguinal lymph nodes revealed a CD4/CD8 cell ratio of 2.2 +/- 0.18 (mean +/- SEM) in thymus-grafted nude mice that is similar to the ratio for immune-competent BALB/c mice (2.0 +/- 0.06). Addition of the pituitary to thymus-grafted nude mice significantly elevated serum PRL (P < 0.005) and increased the CD4/CD8 cell ratio (2.8 +/- 0.12; P < 0.005), demonstrating preferential stimulation of CD4+ cell development. T cells in nude mice receiving sham (submandibular salivary gland) or pituitary grafts alone were below detectable levels. Suspension cultures of neonatal thymus treated with anti-mouse PRL antiserum resulted in 20% and 30% decreases in double-positive CD4+8+ thymocytes and thymocyte viability, respectively. A 10-fold increase in double-negative CD4-8- thymocytes expressing the interleukin 2 receptor alpha chain, CD25, was also observed concurrently. Our findings illustrate an important way in which PRL may participate in two interrelated mechanisms: the regulation of peripheral single-positive cells and the maintenance of thymocyte viability during the double-positive stage of intrathymic differentiation.
Full text
PDF
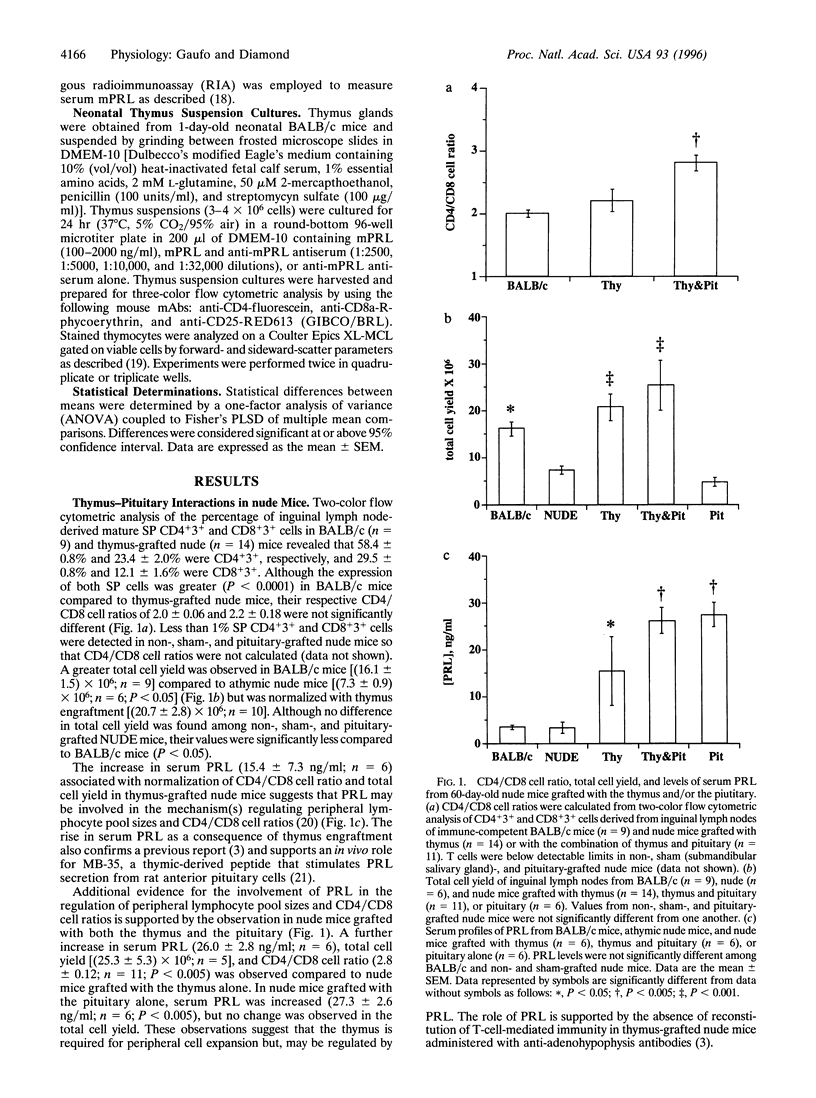
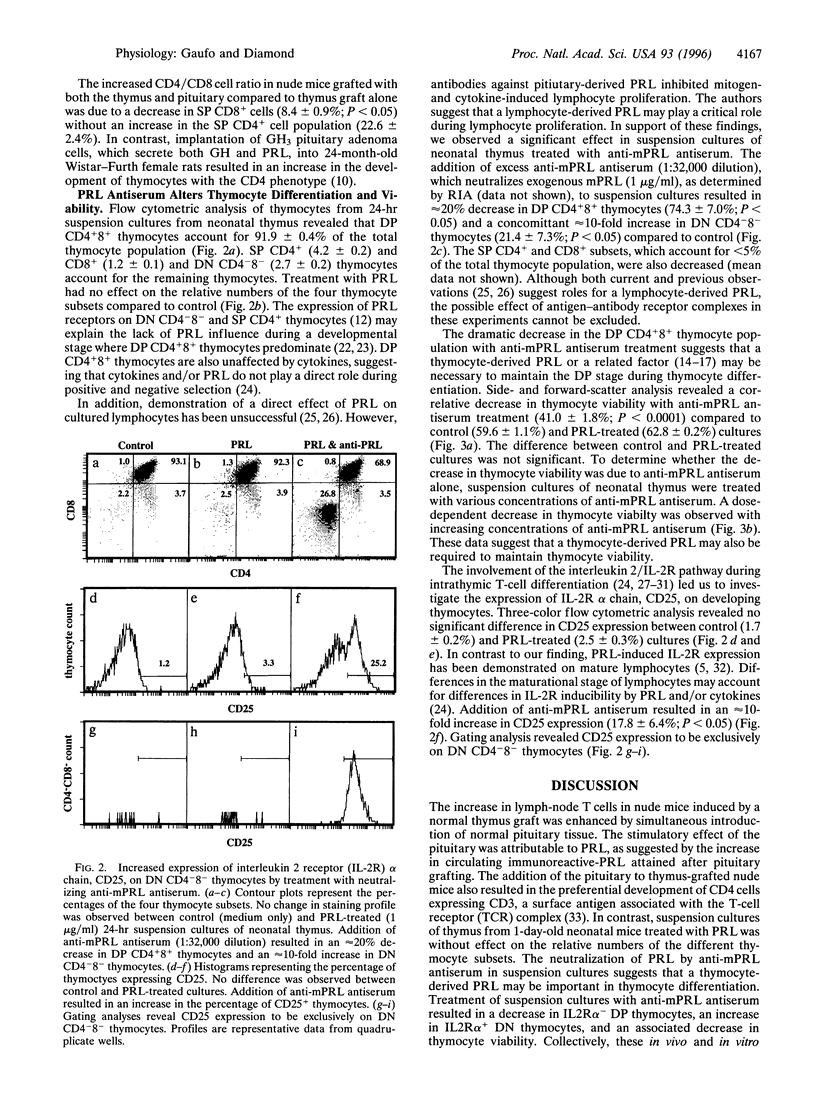
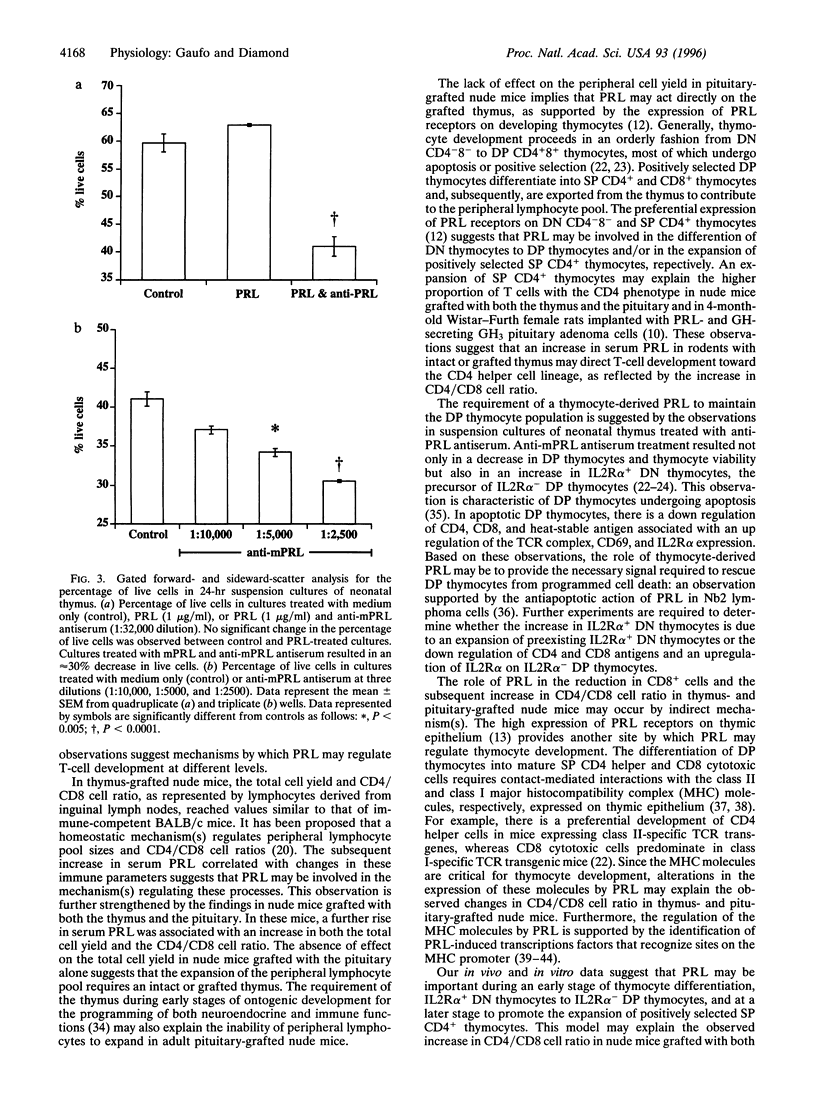

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson G., Jenkinson E. J., Moore N. C., Owen J. J. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993 Mar 4;362(6415):70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- Badamchian M., Spangelo B. L., Damavandy T., MacLeod R. M., Goldstein A. L. Complete amino acid sequence analysis of a peptide isolated from the thymus that enhances release of growth hormone and prolactin. Endocrinology. 1991 Mar;128(3):1580–1588. doi: 10.1210/endo-128-3-1580. [DOI] [PubMed] [Google Scholar]
- Benoist C., Mathis D. Regulation of major histocompatibility complex class-II genes: X, Y and other letters of the alphabet. Annu Rev Immunol. 1990;8:681–715. doi: 10.1146/annurev.iy.08.040190.003341. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Carding S. R., Hayday A. C., Bottomly K. Cytokines in T-cell development. Immunol Today. 1991 Jul;12(7):239–245. doi: 10.1016/0167-5699(91)90037-T. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Altmann S. W., Prystowsky M. B. Requirement of nuclear prolactin for interleukin-2--stimulated proliferation of T lymphocytes. Science. 1991 Jul 5;253(5015):77–79. doi: 10.1126/science.2063207. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Russell D. H., Appasamy P. M., Prystowsky M. B. Regulation of interleukin 2-driven T-lymphocyte proliferation by prolactin. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6460–6464. doi: 10.1073/pnas.87.16.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Dardenne M., Kelly P. A., Bach J. F., Savino W. Identification and functional activity of prolactin receptors in thymic epithelial cells. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9700–9704. doi: 10.1073/pnas.88.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardenne M., de Moraes M. do C., Kelly P. A., Gagnerault M. C. Prolactin receptor expression in human hematopoietic tissues analyzed by flow cytofluorometry. Endocrinology. 1994 May;134(5):2108–2114. doi: 10.1210/endo.134.5.8156910. [DOI] [PubMed] [Google Scholar]
- David M., Petricoin E. F., 3rd, Igarashi K., Feldman G. M., Finbloom D. S., Larner A. C. Prolactin activates the interferon-regulated p91 transcription factor and the Jak2 kinase by tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7174–7178. doi: 10.1073/pnas.91.15.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink P. J., Bevan M. J. Positive selection of thymocytes. Adv Immunol. 1995;59:99–133. doi: 10.1016/s0065-2776(08)60630-6. [DOI] [PubMed] [Google Scholar]
- Gala R. R., Shevach E. M. Influence of prolactin and growth hormone on the activation of dwarf mouse lymphocytes in vivo. Proc Soc Exp Biol Med. 1993 Nov;204(2):224–230. doi: 10.3181/00379727-204-43657. [DOI] [PubMed] [Google Scholar]
- Gilmour K. C., Reich N. C. Receptor to nucleus signaling by prolactin and interleukin 2 via activation of latent DNA-binding factors. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6850–6854. doi: 10.1073/pnas.91.15.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpur A. G., Andres A. C., Ziemiecki A., Aston R. R., Wilks A. F. JAK2, a third member of the JAK family of protein tyrosine kinases. Oncogene. 1992 Jul;7(7):1347–1353. [PubMed] [Google Scholar]
- Hartmann D. P., Holaday J. W., Bernton E. W. Inhibition of lymphocyte proliferation by antibodies to prolactin. FASEB J. 1989 Aug;3(10):2194–2202. doi: 10.1096/fasebj.3.10.2787766. [DOI] [PubMed] [Google Scholar]
- Hiestand P. C., Mekler P., Nordmann R., Grieder A., Permmongkol C. Prolactin as a modulator of lymphocyte responsiveness provides a possible mechanism of action for cyclosporine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2599–2603. doi: 10.1073/pnas.83.8.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson E. J., Kingston R., Owen J. J. Importance of IL-2 receptors in intra-thymic generation of cells expressing T-cell receptors. Nature. 1987 Sep 10;329(6135):160–162. doi: 10.1038/329160a0. [DOI] [PubMed] [Google Scholar]
- Kelley K. W., Brief S., Westly H. J., Novakofski J., Bechtel P. J., Simon J., Walker E. B. GH3 pituitary adenoma cells can reverse thymic aging in rats. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5663–5667. doi: 10.1073/pnas.83.15.5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. A., Djiane J., Postel-Vinay M. C., Edery M. The prolactin/growth hormone receptor family. Endocr Rev. 1991 Aug;12(3):235–251. doi: 10.1210/edrv-12-3-235. [DOI] [PubMed] [Google Scholar]
- Kishimoto H., Surh C. D., Sprent J. Upregulation of surface markers on dying thymocytes. J Exp Med. 1995 Feb 1;181(2):649–655. doi: 10.1084/jem.181.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVoie H. A., Witorsch R. J. Investigation of intracellular signals mediating the anti-apoptotic action of prolactin in Nb2 lymphoma cells. Proc Soc Exp Biol Med. 1995 Jul;209(3):257–269. doi: 10.3181/00379727-209-43901. [DOI] [PubMed] [Google Scholar]
- Li Y. M., Brunke D. L., Dantzer R., Kelley K. W. Pituitary epithelial cell implants reverse the accumulation of CD4-CD8- lymphocytes in thymus glands of aged rats. Endocrinology. 1992 May;130(5):2703–2709. doi: 10.1210/endo.130.5.1572290. [DOI] [PubMed] [Google Scholar]
- Markoff E., Colosi P., Talamantes F. Homologous radioimmunoassay for secreted mouse prolactin. Life Sci. 1981 Jan 12;28(2):203–211. doi: 10.1016/0024-3205(81)90554-3. [DOI] [PubMed] [Google Scholar]
- Maruŝić-Galesić S., Stephany D. A., Longo D. L., Kruisbeek A. M. Development of CD4-CD8+ cytotoxic T cells requires interactions with class I MHC determinants. Nature. 1988 May 12;333(6169):180–183. doi: 10.1038/333180a0. [DOI] [PubMed] [Google Scholar]
- Montgomery D. W., LeFevre J. A., Ulrich E. D., Adamson C. R., Zukoski C. F. Identification of prolactin-like proteins synthesized by normal murine lymphocytes. Endocrinology. 1990 Nov;127(5):2601–2603. doi: 10.1210/endo-127-5-2601. [DOI] [PubMed] [Google Scholar]
- Moreno J., Vicente A., Heijnen I., Zapata A. G. Prolactin and early T-cell development in embryonic chicken. Immunol Today. 1994 Nov;15(11):524–526. doi: 10.1016/0167-5699(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Mukherjee P., Mastro A. M., Hymer W. C. Prolactin induction of interleukin-2 receptors on rat splenic lymphocytes. Endocrinology. 1990 Jan;126(1):88–94. doi: 10.1210/endo-126-1-88. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Longo D. L. Differential effects of growth hormone and prolactin on murine T cell development and function. J Exp Med. 1993 Jul 1;178(1):231–236. doi: 10.1084/jem.178.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Longo D. L. Role of neuroendocrine hormones in murine T cell development. Growth hormone exerts thymopoietic effects in vivo. J Immunol. 1992 Dec 15;149(12):3851–3857. [PubMed] [Google Scholar]
- Pierpaoli W., Besedovsky H. O. Role of the thymus in programming of neuroendocrine functions. Clin Exp Immunol. 1975 May;20(2):323–338. [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Kopp H. G., Bianchi E. Interdependence of thymic and neuroendocrine functions in ontogeny. Clin Exp Immunol. 1976 Jun;24(3):501–506. [PMC free article] [PubMed] [Google Scholar]
- Robey E., Fowlkes B. J. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- Rocha B., Dautigny N., Pereira P. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur J Immunol. 1989 May;19(5):905–911. doi: 10.1002/eji.1830190518. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Mills K. T., Talamantes F. J., Bern H. A. Neonatal administration of prolactin antiserum alters the developmental pattern of T- and B-lymphocytes in the thymus and spleen of BALB/c female mice. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7404–7407. doi: 10.1073/pnas.85.19.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabharwal P., Glaser R., Lafuse W., Varma S., Liu Q., Arkins S., Kooijman R., Kutz L., Kelley K. W., Malarkey W. B. Prolactin synthesized and secreted by human peripheral blood mononuclear cells: an autocrine growth factor for lymphoproliferation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7713–7716. doi: 10.1073/pnas.89.16.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M., Le Gros G., Marbrook J., Watson J. D. Development of fetal thymocytes in organ cultures. Effect of interleukin 2. J Exp Med. 1987 Jun 1;165(6):1481–1493. doi: 10.1084/jem.165.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A. M., Wang Y. F., Sieger K. A., Lu H. F., Yu-Lee L. Y. Biphasic transcriptional regulation of the interferon regulatory factor-1 gene by prolactin: involvement of gamma-interferon-activated sequence and Stat-related proteins. Mol Endocrinol. 1995 Apr;9(4):513–525. doi: 10.1210/mend.9.4.7659094. [DOI] [PubMed] [Google Scholar]
- Ting J. P., Baldwin A. S. Regulation of MHC gene expression. Curr Opin Immunol. 1993 Feb;5(1):8–16. doi: 10.1016/0952-7915(93)90074-3. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Moore T. A. Cytokine production and requirements during T-cell development. Curr Opin Immunol. 1995 Apr;7(2):206–213. doi: 10.1016/0952-7915(95)80005-0. [DOI] [PubMed] [Google Scholar]



