Abstract
Some novel 6,8-diiodo-2-methyl-3-substituted-quinazolin-4(3H)-ones bearing sulfonamide derivatives (4–11) were synthesized in good yields and evaluated for their possible antibacterial, anti-inflammatory activities and acute toxicity. The structures of the synthesized compounds were confirmed on the basis of their spectral data and elemental analysis. Their antibacterial activities were evaluated by the agar well diffusion method while their anti-inflammatory activities were evaluated by the carrageenan-induced hind paw edema test. All the tested compounds showed considerable antibacterial activities and high to moderate anti-inflammatory activities that last for 12 h compared to ibuprofen. All the tested compounds showed no toxic symptoms or mortality rates 24 h post-administration at tested anti-inflammatory doses. In addition, LD50 for all tested compounds was higher than that for ibuprofen implying their good safety margin. The obtained results showed that the most active compounds could be useful as a template for future design, modification and investigation to produce more active analogs.
Keywords: Quinazolinone, Antibacterial, Anti-inflammatory, Sulfonamides, Diiodo, Synthesis
1. Introduction
There is a strong relationship between bacterial infection and inflammation (Sy et al., 2011). Bacterial infection often produces pain and inflammation. Inflammation remains a common with poorly controlled clinical problem which can be life threatening in extreme form of allergy, autoimmune diseases and rejection of transplanted organs (Gounon and Huerre, 1996). The treatment options which can be used for inflammatory diseases are unsatisfactory and complicated due to their lack of efficacy and adverse effect profile. It seemed worthwhile to look for candidates acting on more than one pathway involved in inflammatory conditions (Bot et al., 2011).
Quinazolin-4-one ring system has been consistently rewarded as a promising molecule because of its broad spectrum of pharmaceutical activities like antihistaminic (Lemura et al., 1989), anti inflammatory (Amin et al., 2010), antibacterial (Kini and Grover, 2006), antidiabetic (Ram et al., 2003), anticancer (Abbas et al., 2012), antifungal (Liu et al., 2006), anthelmintics (Connolly et al., 2005) and antiviral activities (Dinakaran et al., 2003). In addition to that, anti-inflammatory quinazolines possess remarkable anti-inflammatory activity through inhibition of tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (Rajan et al., 2010).
On the other hand, sulfonamide derivatives have been reported to possess significant antibacterial activities through competitive inhibition of dihydropteroate synthetase enzyme (DHPS) which is involved in folate synthesis (Skold, 2000). Moreover, some sulfonamides work as ant-inflammatory drugs like celecoxib which works as a COX-2 inhibitor (Gassani et al., 2010) and acetazolamide which works by diuretic mechanism (Jaiswal et al., 2004). On light of these findings, we planned to prepare the target compounds as hybrid molecules. These molecules contain the quinazolinone ring system and fused with sulfonamide derivatives to form a group of compounds resembling and collecting both features of nitrogen heterocyclic moiety and sulfonamide moiety. In addition, iodine atoms exist at 6th and 8th positions from quinazoline nucleus. Iodine was selected because it has received considerable attention in organic synthesis due to its high tolerance to air and moisture, low-cost, nontoxic nature and ready availability. Presence of iodine increases the lipophilicity of the molecules, the surface of contact, the absorption and the distribution (Laznicek et al., 1985; Yanming et al., 2003).
1.1. Rationale of the study
A literature survey revealed that the presence of quinazoline moiety, which can undergo substitution at the heteroatom or the distal aromatic ring, is a necessary requirement for the antibacterial and anti-inflammatory activities such as compounds [A] (Kini and Grover, 2006), [B] (Ali et al., 2010) and [C] (Panneerselvam et al., 2009). Moreover, quinazoline derivatives with the appropriate substituent mainly amine or substituted amine at 4th position and either halogen or electron rich substituent at 6th or 8th position are known to promote against bacteria and inflammation (Tiwari et al., 2006). In view of the previous rationale, it was thought worthwhile to study the effects of two pharmacophoric moieties like quinazolinone and sulfonamide in a single molecule on the antibacterial and anti-inflammatory activities. The target compounds have been designed to contain different substituents with different electronic environments. As shown in Scheme 1, these substituents joined to the fixed moiety (3) start with hydrogen from sulfanilamide in compound (4), acetamide from sulfacetamide in compound (5), benzamide from sulfabenzamide in compound (6), pyridine from sulfapyridine in compound (7), pyrimidine from sulfadiazine in compound (8), 5-methylisoxazole from sulfamethoxazole in compound (9), 4,6-dimethylpyrimidin from sulfamethazine in compound (10) and 3,4-dimethyl-1,2-oxazole from sulfafurazole in compound (11). These varied substituents allow us to study the effect of hydrophilic and hydrophobic changes on the biological activity of the target compounds. Fig. 1 represents the similarities between the reported antibacterial and anti-inflammatory quinazolinones and our designed compounds.
Scheme 1.
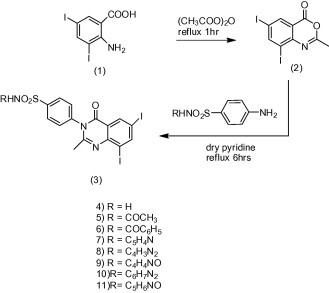
Synthesis of the target compounds (4–11).
Figure 1.
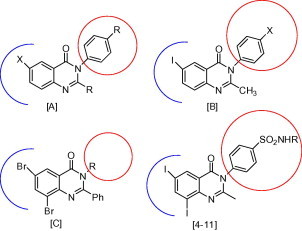
Similarities between reported compounds as antibacterial, anti-inflammatory and target compounds (4–11).
2. Materials and methods
2.1. Chemistry
The tested compounds were analyzed at the Analytical Center, College of Science, Cairo University, Egypt. All melting points were measured on a Griffin melting point apparatus (Griffin) and are uncorrected. The Infrared spectra were recorded as KBr disks on a Nicolet IR 200 (Thermo Fisher Scientific). The 1HNMR and 13CNMR spectra were run using TMS as an internal standard (Sigma–Aldrich) on Varian Mercury VXr-300 NMR (Varian). Mass spectra were obtained on a JEOL-SX-102 instrument using electron impact ionization. Elemental analyses (C, H, N) were performed on a Perkin–Elmer 240C analyzer (Perkin–Elmer). All compounds were within ±0.4% of the theoretical values. All chemicals used for synthesis were purchased from (Sigma–Aldrich).
2.2. Synthesis
The synthetic strategy to prepare the target compounds (4–11) is depicted in Scheme 1: it includes two simple reactions, first one is the acetylation/benzoylation followed by the ring closure reaction for 2-amino-3,5-diiodobenzoic acid (1). Compound (1) when refluxed with acetic anhydride for one hour converted into benzoxazinone. This reaction afforded quantitative yield of 6,8-diiodo-2-methyl-4H-benzo[d][1,3]oxazin-4-one (2). Second reaction is a nucleophilic displacement reaction for the oxygen of benzoxazinone with the nitrogen of the amino group upon treating with sulfonamides. The second reaction was done by refluxing compound (2) with the appropriate sulfonamide in dry conditions for six hours to give sulfonamide derivatives of 6,8-diiodo-2-methyl-quinazolinone (4–11) in variable yields between 62% and 76%.
2.3. Pharmacology
2.3.1. Animals
The animals were procured from the Animal House Center, College of Pharmacy, King Abdulaziz University, Saudi Arabia, and were maintained in a colony cages at 25 ± 2 °C, relative humidity of 45–55%, under 12 h light and dark cycles; they were fed standard animal feed. All animals were acclimatized for a week before use. The protocol adopted for the experimentation of animals was approved by the Institutional Animal Ethics Committee (approval No: 409/432). All the experiments were carried out according to the respective internationally valid guidelines.
2.3.2. Biological screening
All the newly synthesized compounds (4–11) were screened to evaluate their antibacterial and anti-inflammatory activities and acute toxicity. The antibacterial activity was performed by the agar well diffusion method while the anti-inflammatory activity was evaluated by the carrageen an induced rat Paw edema method using ibuprofen as a reference drug. The response of all the compounds to antibacterial activity is good however, some of the compounds showed promising anti-inflammatory activity. The other remaining compounds showed moderate anti-inflammatory activity. No toxic symptoms or mortality rates were observed 24 h post administration implying their good safety margin. The details of activity results are outlined below.
2.3.3. Antibacterial activity
Antibacterial activity of all the test compounds was determined by agar well diffusion method (Waynae, 1997) which is recommended by the National Committee for Clinical Laboratory Standards (NCCLS) against two kinds of Gram positive micro organisms Staphylococcus aureus (MTCC 96) and Staphylococcus epidermis (MTCC 435) and two kinds of Gram negative micro organisms Pseudomonas aeruginosa (MTCC 741), and Escherichia coli (MTCC443) at 100 μg/mL concentration using dimethylsulfoxide (DMSO) as a solvent. The bacteria were sub-cultured on Mueller Hinton agar medium. Streptomycin was used as a standard antibacterial under similar conditions at a concentration of 50 μg/mL for comparison while solvent control was also maintained under similar conditions. The results of antibacterial screening are outlined in Table 1.
Table 1.
Antibacterial activity of tested compounds (4–11).
| Compound ID | Gram positive strains |
Gram negative strains |
||
|---|---|---|---|---|
| S. aureus | S. epidermis | P. aeruginosa | E. coli | |
| 4 | 20 | 21 | 18 | 22 |
| 5 | 17 | 19 | 20 | 20 |
| 6 | 14 | 18 | 14 | 17 |
| 7 | 18 | 20 | 15 | 20 |
| 8 | 19 | 17 | 21 | 19 |
| 9 | 15 | 16 | 21 | 16 |
| 10 | 13 | 15 | 20 | 21 |
| 11 | 19 | 18 | 21 | 19 |
| Streptomycin (standard) | 21 | 23 | 24 | 29 |
Inhibitory zone diameters in mm; conc of standard 50 μg/ml, compounds 100 μg/ml.
2.3.4. Acute toxicity
Toxicological studies of the synthesized compounds were performed using (LD50) which is the dose that will kill 50% of the animal population within 24 h post treatment with the test substance. The toxicity test was performed using standard method in mice and rats (Ishii and Yoshikawa, 1993; Ecobichon, 1997; Upmanyu et al., 2011). When there is no information on a substance to be tested, for animal welfare reasons, it is recommended to use the starting dose of 300 mg/kg body weight. The animals were given the lowest dose of 300 mg/kg of the compounds at the first instance. Then the animals were observed for three days. They were treated orally with different doses of tested compounds (400, 600, 800, 1000, and 2000 mg/kg). The animals were then observed for 24 h for any behavioral effects such as nervousness, excitement, dullness, in-coordination or even death. Results for LD50 were calculated and are reported in Table 2.
Table 2.
Acute toxicity studies in mice and rats.
| Compound No | LD50(mg/kg) |
|
|---|---|---|
| Mice | Rats | |
| 4 | 1015 | 950 |
| 5 | 974 | 844 |
| 6 | 1300 | 1245 |
| 7 | 1136 | 1020 |
| 8 | 1035 | 985 |
| 9 | 1554 | 1470 |
| 10 | 1633 | 1580 |
| 11 | 1670 | 1513 |
| Ibuprofen (standard) | 750 | 650 |
LD50: dose that kills 50% of animals within 24 h after drug administration.
2.3.5. Anti-inflammatory activity
Anti-inflammatory activity of all the test compounds was determined according to the reported method (Winter et al., 1962; Vogel, 2007) by carrageenan-induced hind paw edema test using ibuprofen as a standard drug. Briefly, Male or female rats are starved overnight. To insure uniform hydration, the rats received 5 ml of water by stomach tube (controls), standard ibuprofen (25, 50 and 100 mg/kg), or test compounds (25, 50, 100 and 200 mg/kg) suspended in the same volume. One hour later, the rats were challenged by a subcutaneous injection of 0.1 ml of 1% solution of carrageenan (Sigma–Aldrich) into the plantar side of the left hind paw. The thickness of dorso-ventral diameter of each rat was measured using a pair of dial thickness gauge calipers accurate to 0.001 cm3 (Progressive Trading Corporation) 3, 6 and 12 h after induction of inflammation. The increase of paw volume after 3, 6 and 12 h was calculated as percentage compared with the volume measured immediately after injection of the irritant for each animal (0 h). Effectively treated animals show much less edema. The percentage of anti-inflammatory activity (% inhibition of inflammation) was calculated according to the following equation:
Lt is the mean increase in paw thickness in rats treated with the tested compounds and Lc is the mean increase in paw thickness in control group.
Evaluation: The difference at the various time intervals gives some hints for the duration of the anti-inflammatory effect for each compound as reported in Table 3. Doses that exhibited 50% protection in addition to the relative potencies of the test compounds to ibuprofen were recorded for comparisons and are shown in Table 4.
Table 3.
Anti-inflammatory activity and duration of tested compounds at 50 mg/kg using carrageen an induced rat paw edema method.
| Compound ID | Percent protection |
||
|---|---|---|---|
| 3 h | 6 h | 12 h | |
| 4 | 39.14 ± 0.02⁎,⁎⁎ | 56.26 ± 0.04⁎,⁎⁎ | 51.41 ± 0.24⁎,⁎⁎ |
| 5 | 41.75 ± 0.65⁎,⁎⁎ | 62.83 ± 0.61⁎,⁎⁎ | 58.15 ± 0.06⁎,⁎⁎ |
| 6 | 27.2 ± 0.01⁎,⁎⁎ | 38.15 ± 0.04⁎,⁎⁎ | 36.41 ± 0.25⁎,⁎⁎ |
| 7 | 36.58 ± 0.47⁎,⁎⁎ | 44.62 ± 0.09⁎,⁎⁎ | 49.71 ± 0.34⁎,⁎⁎ |
| 8 | 27.92 ± 0.3⁎,⁎⁎ | 41.14 ± 0.07⁎,⁎⁎ | 39.61 ± 0.67⁎,⁎⁎ |
| 9 | 23.27 ± 0.71⁎,⁎⁎ | 31.34 ± 0.06⁎,⁎⁎ | 35.78 ± 0.86⁎,⁎⁎ |
| 10 | 20.56±.03⁎,⁎⁎ | 27.47 ± 0.03⁎,⁎⁎ | 34.58 ± 0.43⁎,⁎⁎ |
| 11 | 19.25 ± 0.71⁎,⁎⁎ | 28.16 ± 0.02⁎,⁎⁎ | 25.52 ± 0.6⁎,⁎⁎ |
| Ibuprofen (standard) | 68.12 ± 0.61⁎ | 69.91 ± 0.74⁎ | 67.54 ± 0.07⁎ |
Significant difference from negative control and ibuprofen (standard), respectively at P < 0.0001, using Tukey’s test as post ANOVA test.
Table 4.
ED50 and Relative potency of tested compounds to Ibuprofen at 6 h.
| Compound ID | ED50 (mg/kg) | Relative potency |
|---|---|---|
| 4 | 60 | 0.66 |
| 5 | 54 | 0.74 |
| 6 | 90 | 0.44 |
| 7 | 69 | 0.58 |
| 8 | 75 | 0.53 |
| 9 | 105 | 0.38 |
| 10 | 120 | 0.33 |
| 11 | 117 | 0.34 |
| Ibuprofen (standard) | 40 | 1.00 |
ED50 is the dose required to induce 50% inhibition of rat paw edema (50% anti-inflammatory effect).
ED50 was calculated using instate program by plotting results at all doses levels; Ibuprofen was tested at a dose range from 25 to 100 mg/kg and tested compounds from 25 to 200 mg/kg.
3. Results
Antibacterial assay of all the test compounds (4–11) showed good activities against both of Gram positive bacteria and Gram negative bacteria. These activities were ranged from 61.91% up to 95.23% from the activity of the standard. The activity data generated are tabulated in Table 1. The anti-inflammatory screening showed that compounds (6, 7, 8, 9, 10 and 11) were considered to have moderate anti-inflammatory activity however, compounds (4 and 5) showed considerable inhibition as shown in Tables 3 and 4. No toxic symptoms or mortality rates were observed 24 h post-administration at all suggested therapeutic doses however, the LD50 for most of tested compounds is much higher than that reported for ibuprofen. Concerning other actions, compounds 7, 8 and 11 induced urination while compounds 4, 5 and 6 induced sedation, calmness, muscle relaxation and decreased respiration.
4. Discussion
Inspection of the chemical structure of the target compounds suggested that target compounds could be divided into two subunits: the quinazolinone part and the sulfonamide part (Fig. 1). The two parts have been reported to have significant broad spectrum of antibacterial activities which might contribute to the good results obtained from testing them as antibacterial agents. Moreover, substitution of the distal aromatic ring from quinazolinone moiety with iodine at 6th and 8th positions might also helped in obtaining such good results.
For the anti-inflammatory activity, compounds with aliphatic side chain (4) and (5) were more active than that with aromatic one. Compound (5), the most active compound among all the test compounds, contains aliphatic side chain. The relative potency of this compound was 74% of the reference’s potency, as shown in Table 4. Pyridine containing compound (7) was more active than those with pyrimidine or oxazole instead. Within the pyrimidine and oxazole derivatives, dimethylated derivatives (10) and (11) were less active than monomethylated one (9) and methyl free one (8). The results of acute toxicity test which have been done to all test compounds indicated to their good safety margin. Although the title compounds exhibited potent antibacterial and anti-inflammatory actions, moderate anti-inflammatory activity was found. Hence, necessary structural modifications are planned in the future study to increase the anti-inflammatory activity. In general, the present study showed that compound (5) was the most active compound with combined ability to inhibit bacterial infection and inflammation. This compound could therefore serve as a lead molecule for further modification to obtain clinically useful antibacterial and anti-inflammatory agents.
5. Conclusion
We have synthesized and tested some novel 6,8-diiodo-2-methyl-3-substituted-quinazolin-4(3H)-ones derivatives for their antibacterial and anti-inflammatory activities. All compounds induced significant antibacterial activity. Two compounds showed promising anti-inflammatory activity while the other five compounds showed moderate anti-inflammatory activity. All Compounds were tested for acute toxicity and showed good safety margin.
6. Experimental
6.1. Chemistry
6.1.1. 6,8-Diiodo-2-methyl-4H-benzo[d][1,3]oxazin-4-one (2)
It was prepared by refluxing (3,88 gm, 0.01 mol) of 2-amino-3,5-diiodobenzoic acid (1) with the appropriate amount of acetic anhydride for one hour. The residue obtained was evaporated till complete dryness, left to cool, washed with petroleum ether many times, collected, filtered and dried well in the absence of moisture.
Yield 85%, mp: 185–187 °C; 1HNMR (DMSO-d6): δ 1.21 (s, 3H, CH3) 7.21–8.19 (m, 2H, Ar–H). 13C NMR (DMSO-d6): δ 24.23, 82, 90.5, 118.5, 138.2, 149.6, 150, 154.1, 158. Anal. Calcd. for C9H5I2NO2 (412.84): C, 26.18; H, 1.22; N, 3.39. Found C, 26.32; H, 1.01; N, 3.74. MS (EI) m/z 413.8 [M + 1].
6.1.2. General method for preparation of test compounds (4–11)
They were prepared in a conical flask by mixing (0.01 mol, 4.13 gm) of compound (2) with the appropriate amount (0.01 mol) of sulfonamide derivatives in 100 ml dry pyridine, refluxed for six hours, cooled then treated with few amounts of 10% hydrochloric acid and poured onto crushed ice. The obtained crystals were collected by filtration and re-crystallized from ethanol or glacial acetic acid. The melting points for all synthesized compounds were above 300 °C.
6.1.3. 4-(6,8-Diiodo-7-methyl-4-oxoquinazolin-3(4H)-yl)benzenesulfonamide (4)
Yield 73%, 1HNMR (DMSO-d6): δ 2.26 (s, 3H, CH3), 5.3 (s, 2H, NH2), 6.96–7.77 (m, 6H, Ar–H). 13C NMR (DMSO-d6): δ 48.2, 82.3, 91.4, 120.5, 121.9, 125, 192.7, 134.5, 142.7, 145.1, 149.4, 157.2, 164. Anal. Calcd. for C15H11I2N3O3S (566.88): C, 31.77; H, 1.95; N, 7.41. Found C, 31.61; H, 1.81; N, 7.29. MS (EI) m/z 567.8 [M + 1].
6.1.4. N-(4-(6,8-diiodo-7-methyl-4-oxoquinazolin-3(4H)-yl)phenylsulfonyl)acetamide (5)
Yield 70%, 1HNMR (DMSO-d6): δ 2.21 (s, 3H, CH3), 2.41(s, 3H, CH3), 7.01–8.12 (m, 6H, Ar–H), 8.4 (s, 1H, NH) 13C NMR (DMSO-d6): δ 24.5, 48, 85.4, 93.7, 123.5, 128, 135, 137.5, 141.7, 149.2, 159.2, 166, 175.4. Anal. Calcd. for C17H13I2N3O4S (608.87): C, 33.52; H, 2.15; N, 6.90. Found C, 33.43; H, 1.98; N, 7.12. MS (EI) m/z 609.8 [M + 1].
6.1.5. N-(4-(6,8-diiodo-7-methyl-4-oxoquinazolin-3(4H)-yl)phenylsulfonyl)benzamide (6)
Yield 76%, 1HNMR (DMSO-d6): δ 2.33 (s, 3H, CH3), 6.71–8.26 (m, 11H, Ar–H), 8.37 (s, 1H, NH) 13C NMR (DMSO-d6): δ 48.51, 84.9, 95.3, 121.5, 123.2, 127.5, 128.3, 129, 132.6, 134.3, 135, 137.6, 140.7, 146.2, 154.9, 163, 172.2. Anal. Calcd. for C22H15I2N3O4S (670.89): C, 39.36; H, 2.25; N, 6.26. Found C, 39.51; H, 2.47; N, 6.47. MS (EI) m/z 671.8 [M + 1].
6.1.6. 4-(6,8-Diiodo-7-methyl-4-oxoquinazolin-3(4H)-yl)-N-(pyridin-2-yl)benzenesulfonamide (7)
Yield 68%, 1HNMR (DMSO-d6): δ 2.42 (s, 3H, CH3), 6.92–8.15 (m, 10H, Ar–H), 8.27 (s, 1H, NH) 13C NMR (DMSO-d6): δ 48.71, 82.9, 93.2, 112.2, 120.4, 122.2, 128.3, 129, 136.6, 139, 140.7, 141.2, 147.1, 148, 152.4, 155.9, 162. Anal. Calcd. for C20H14I2N4O3S (643.89): C, 37.29; H, 2.19; N, 8.70. Found C, 37.12; H, 2.37; N, 8.93. MS (EI) m/z 644.8 [M + 1].
6.1.7. 4-(6,8-Diiodo-7-methyl-4-oxoquinazolin-3(4H)-yl)-N-(pyrimidin-2-yl)benzenesulfonamide (8)
Yield 65%, 1HNMR (DMSO-d6): δ 2.35 (s, 3H, CH3), 7.02–8.35 (m, 9H, Ar–H), 8.41 (s, 1H, NH) 13C NMR (DMSO-d6): δ 48.15, 81.2, 114, 122, 126.4, 128, 129.4, 138, 140.7, 146.2, 154, 158, 160.7, 170. Anal. Calcd. for C19H13I2N5O3S (644.88): C, 35.37; H, 2.03; N, 10.85. Found C, 35.64; H, 2.33; N, 10.75. MS (EI) m/z 645.8 [M + 1].
6.1.8. 4-(6,8-Diiodo-7-methyl-4-oxoquinazolin-3(4H)-yl)-N-(5-methylisoxazol-3-yl)benzenesulfonamide (9)
Yield 70%, 1HNMR (DMSO-d6): δ 2.32 (s, 3H, CH3), 2.46 (s, 3H, CH3), 7.21–8.14 (m, 7H, Ar–H), 8.31 (s, 1H, NH) 13C NMR (DMSO-d6): δ 14.5, 48.57, 85.2, 92, 96, 122, 124.6, 129.3, 136, 136.2, 140, 146.8, 150, 154, 156.5, 160, 171.3. Anal. Calcd. for C19H14I2N4O4S (647.88): C, 35.20; H, 2.18; N, 8.64. Found C, 35.16; H, 2.41; N, 8.54. MS (EI) m/z 648.8 [M + 1].
6.1.9. 4-(6,8-Diiodo-7-methyl-4-oxoquinazolin-3(4H)-yl)-N-(4,6-dimethlpyrimidin-2-yl)benzenesulfonamide (10)
Yield 66%, 1HNMR (DMSO-d6): δ 2.41 (s, 3H, CH3), 2.52 (s, 6H, 2CH3), 6.91–7.98 (m, 7H, Ar–H), 8.21 (s, 1H, NH) 13C NMR (DMSO-d6): δ 28, 49, 86.4, 95.1, 112, 121, 126.6, 130, 138, 138.2, 144, 148.2, 158, 162.4, 164, 175.3. Anal. Calcd. for C21H17I2N5O3S (672.91): C, 37.46; H, 2.55; N, 10.40. Found C, 37.29; H, 2.77; N, 10.54. MS (EI) m/z 673.9 [M + 1].
6.1.10. 4-(6,8-Diiodo-7-methyl-4-oxoquinazolin-3(4H)-yl)-N-(3,4-dimethlisoxazol-5-yl)benzenesulfonamide (11)
Yield 62%, 1HNMR (DMSO-d6): δ 2.31 (s, 6H, 2CH3), 2.43 (s, 3H, CH3) 7.03–8.17 (m, 6H, Ar–H), 8.36 (s, 1H, NH) 13C NMR (DMSO-d6): δ 7.5, 10.8, 48.92, 86, 93.1, 102, 123, 125.2, 129.5, 136, 136.4, 140, 146.2, 149, 156.1, 157, 159.4, 162. Anal. Calcd. for C20H16I2N4O4S (661.90): C, 36.27; H, 2.44; N, 8.46. Found C, 36.49; H, 2.65; N, 8.61. MS (EI) m/z 662.9 [M + 1].
6.2. Statistical analysis
Data obtained were expressed as means. Statistical difference between the treated and the control groups was evaluated by One Way Analysis of Varian (ANOVA) followed by the Tukey’s test as a post ANOVA multiple comparison test (Sigma Stat version 3; SPSS Inc.) to determine the statistical significance. A P value <0.05 was considered statistically significant.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at Taibah University for funding the work through the research group project No (409/432).
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbas E.S., Awadallah M.F., Ibrahim A.N., Said G.E., Kamel M.G. New quinazolinone–pyrimidine hybrids: synthesis, anti-inflammatory, and ulcerogenicity studies. Eur. J. Med. Chem. 2012;53:141–149. doi: 10.1016/j.ejmech.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Ali M.M., Mohamed A.Yahia, El-Bayouki M.A.K., Basyouni M.W., Abbas Y.S. Synthesis of some new 4(3H)-quinazolinone-2-carboxaldehyde thiosemicarbazones and their metal complexes and a study on their anticonvulsant, analgesic, cytotoxic and antimicrobial activities. Eur. J. Med. Chem. 2010;45:3365–3373. doi: 10.1016/j.ejmech.2010.04.020. [DOI] [PubMed] [Google Scholar]
- Amin M.K., Kamel M.M., Anwar M.M., Khedr M., Syam M.Y. Synthesis, biological evaluation and molecular docking of novel series of spiro [(2H,3H) quinazoline-2,1′-cyclohexan]-4(1H)-one derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010;45:2117–2131. doi: 10.1016/j.ejmech.2009.12.078. [DOI] [PubMed] [Google Scholar]
- Bot M., Carney M.R., Freedland E.K., Rubin H.E., Rich W.M., Steinmeyer C.B., Mann L.D. Inflammation and treatment response to sertraline in patients with coronary heart disease and comorbid major depression. J. Psychosom. Res. 2011;71:13–17. doi: 10.1016/j.jpsychores.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly J.D., Cusack D., Sullivan P.T., Guiry J.P. Synthesis of quinazolinones and quinazolines. Tetrahedron. 2005;61:10153–10202. [Google Scholar]
- Dinakaran M., Selvam P., Declercq E., Sridhar S.K. Synthesis, antiviral and cytotoxic activity of 6-bromo-2,3-disubstituted-4(3H)-quinazolinones. Biol. Pharm. Bull. 2003;26:1278–1282. doi: 10.1248/bpb.26.1278. [DOI] [PubMed] [Google Scholar]
- Ecobichon D.J. CRC Press; New York: 1997. The Basis of Toxicology Testing. (pp. 43–86) [Google Scholar]
- Gassani A.B., Rezende M.R., Lima P.P., Alves L.D., dosreis G.W., Bakhle S.Y., Francischi N.J. Is the sulphonamide radical in the celecoxib molecule essential for its analgesic activity? Pharmacol. Res. 2010;62:439–443. doi: 10.1016/j.phrs.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Gounon P., Huerre R.M. Inflammation: patterns and new concepts. Res. Immunol. 1996;147:417–434. doi: 10.1016/S0923-2494(97)84407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii R., Yoshikawa K. Microbial bioassay of acute toxicity by the pH inhibition method and comparison of IC50 (pHI) with LD50 for rats and mice. J. Biosci. Bioeng. 1993;76:361–366. [Google Scholar]
- Jaiswal M., Khadikar V.P., Supuran T.C. Topological modeling of lipophilicity, diuretic activity, and carbonic inhibition activity of benzenesulfonamides: a molecular connectivity approach. Bioorg. Med. Chem. Lett. 2004;14:5661–5666. doi: 10.1016/j.bmcl.2004.08.051. [DOI] [PubMed] [Google Scholar]
- Kini G.S., Grover G. Synthesis and evaluation of new quinazolone derivatives of nalidixic acid as potential antibacterial and antifungal agents. Eur. J. Med. Chem. 2006;41(2):256–262. doi: 10.1016/j.ejmech.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Laznicek M., Beno P., Waisser K., Kvetina J. Quantitative chemical structure-pharmacokinetic data relationships. IV. Relationships between pharmacokinetic data and lipophilicity of iodine-substituted aromatic and arylaliphatic compounds. Cesko. Farm. 1985;34:353–358. [Google Scholar]
- Lemura R., Manabe H., Todayaki S. Bioisosteric transformation of H1-antihistaminic benzimidazole derivatives. Chem. Pharm. Bull. 1989;37:2723–2726. doi: 10.1248/cpb.37.2723. [DOI] [PubMed] [Google Scholar]
- Liu J., Wilson J.C., Ye P., Sprague K., Sargent K., Si Y., Beletsky G., Yohannes D., Chung S. Privileged structure based quinazolinone natural product-templated libraries: identification of novel tubulin polymerization inhibitors. Bioorg. Med. Chem. Lett. 2006;16:686–690. doi: 10.1016/j.bmcl.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Panneerselvam P., Rather A.B., Reddy S.R.D., Kumar R.N. Synthesis and anti-microbial screening of some Schiff bases of 3-amino-6,8-dibromo-2-phenylquinazolin-4(3H)-ones. Eur. J. Med. Chem. 2009;44:2328–2333. doi: 10.1016/j.ejmech.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Rajan S.G., Hardik M.T., Tony G., Jill W., Donna R., Kamala K.V., Vasudevan S. Design, synthesis and evaluation of novel 2-thiophen-5-yl-3H-quinazolin-4-one analogues as inhibitors of transcription factors NF-кB and AP-1 mediated transcriptional activation: their possible utilization as anti-inflammatory and anti-cancer agents. Bioorg. Med. Chem. Lett. 2010;18:2796–2808. doi: 10.1016/j.bmc.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Ram V.J., Farhanullah, Tripathi B.K., Srivastava A.K. Synthesis and antihyperglycemic activity of suitably functionalized 3H-quinazolin-4-ones. Bioorg. Med. Chem. 2003;1:2439–2444. doi: 10.1016/s0968-0896(03)00142-1. [DOI] [PubMed] [Google Scholar]
- Skold O. Sulfonamide resistance: mechanisms and trends. Drug Resist. Update. 2000;3:155–160. doi: 10.1054/drup.2000.0146. [DOI] [PubMed] [Google Scholar]
- Sy M., Kitazawa M., Medeiros R., Whitman L., Cheng D., Lane E.T., Laferla M.F. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am. J. Pathol. 2011;178:2811–2822. doi: 10.1016/j.ajpath.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A.K., Mishra A.K., Bajpai A., Mishra P., Sharma R.K., Pandey V.K., Singh V.K. Synthesis and pharmacological study of novel pyrido-quinazolone analogues as anti-fungal, antibacterial, and anticancer agents. Bioorg. Med. Chem. Lett. 2006;16:4581–4585. doi: 10.1016/j.bmcl.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Upmanyu N., Gupta J.K., Shah K., Mishra P. Anti-inflammatory and antinociceptive evaluation of newly synthesized 4-(substituted ethanoyl) amino-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazoles. J. Pharm. Bioall. Sci. 2011;3(2):259–265. doi: 10.4103/0975-7406.80783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G.H. Drug Discovery and Evaluation Pharmacological Assays. second ed. Springer-Verlag; New York: 2007. [Google Scholar]
- Waynae, P.A., 1997. National Committee for Clinical Laboratory Standards approved Standards M2–A6. Performance Standards for Antimicrobial Disc Susceptibility Testing, sixth ed. Perseus, Cambridge.
- Winter C.A., Risely E.A., Nuss G.W. Carrageenin-induced edema in hind paw of the rat an assay for anti-inflammatory drugs. Proc. Soc. Exp. Biol. Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- Yanming W., Chester A.M., Guo-Feng H., Manik L.D., Daniel P.H., Li S. Effects of lipophilicity on the affinity and nonspecific binding of iodinated benzothiazole derivatives. J. Mol. Neurosci. 2003;20:255–260. doi: 10.1385/JMN:20:3:255. [DOI] [PubMed] [Google Scholar]



