Abstract
Dissolution profiles of four carbamazepine immediate-release generic products (200 mg tablets) and the reference product Tegretol® were evaluated using the USP paddles method and an alternative method with the flow-through cell system, USP Apparatus 4. Under official conditions all products met the Q specification, dissolution profiles of generic products were similar to the dissolution profile of the reference product (f2 > 50) and model-independent parameters showed non significant differences to the reference product except mean dissolution time for product A (p < 0.05). On the other hand, when the flow-through cell system was used, none of the products met the pharmacopeial specification at 15 min and product A did not reach dissolution criteria at 60 min, dissolution profiles of all generic products were not similar to the reference product profile (f2 < 50) and all model-independent parameters showed significant differences compared to the reference product (p < 0.05). Weibull’s model was more useful for adjusting the dissolution data of all products in both USP apparatuses and Td values showed significant differences compared to the reference product (p < 0.05) when USP Apparatus 4 was used. These results indicate that the proposed method, using the flow-through cell system, is more discriminative in evaluating both, rate and extent of carbamazepine dissolution process from immediate-release generic products.
Keywords: Antiepileptics, Carbamazepine, Dissolution rate, Flow-through cell system, Generic products
1. Introduction
In the development of a new formulation or as a measure of batch-to-batch quality control, the evaluation of rate and extent of dissolution of an active compound in a pharmaceutical dosage form is elemental. For generic products, the quality of excipients and the manufacturing process must allow full and timely release of the drug in the same way as the reference product does at predefined conditions. An adequate release evaluation of generic drugs promotes interchangeability of these products, to ensure the same pharmacological effect, with the benefit of a lower cost to the patient (Gidal and Tomson, 2008). Carbamazepine is a narrow therapeutic index drug that is widely used to treat epilepsy and other neurological disorders and is marketed as immediate-release generic products. Dissolution test for carbamazepine tablets is described in the United States Pharmacopeia (USP 30, 2007). The method indicates the use of USP Apparatus 2 (paddles) at 75 rpm and 900 ml of 1.0% sodium lauryl sulfate aqueous solution at 37.0 ± 0.5 °C as dissolution medium. Under these conditions and for products labeled as 200 mg tablets there are two tests with the following times and tolerances: Test 2 between 45% and 75% of the labeled amount of carbamazepine is dissolved in 15 min; not less than 75% (Q) of the labeled amount of carbamazepine is dissolved in 60 min and Test 3 between 60% and 85% of the labeled amount of carbamazepine is dissolved in 15 min; not less than 75% (Q) of the labeled amount of carbamazepine is dissolved in 60 min.
The Biopharmaceutics Classification System groups drugs into four classes (Amidon et al., 1995). Carbamazepine belongs to Class II (low solubility/high permeability), and its absorption in the gastrointestinal tract might be limited by the dissolution rate (Lindenberg et al., 2004). Compounds belonging to Class II are eligible to establish a significant in vitro/in vivo correlation (IVIVC), hence the appropriate selection for dissolution study’s conditions is essential to have a method able to discriminate between products with potential problems of bioavailability. The establishment of a significant IVIVC provides the basis for estimating in vivo performance predictions and waives the costly bioequivalence studies (FDA, 1999). Previous reports mentioned significant differences in dissolution profiles of carbamazepine commercial products (Lake et al.,1999; Mittapalli et al., 2008; Volonté et al., 2004), even within a single brand (Davidson, 1995); in plasma levels of healthy volunteers (Meyer et al., 1992) and in serum levels of patients undergoing therapy with this drug (Rentmeester et al., 1990) as well as loss of seizure control when one product is exchanged for another (Olling et al., 1999). Lack of correlation between in vitro dissolution data using the USP official method and in vivo data was also reported (Castro and Jung, 2000; Jung et al., 1997).
Since the introduction of the flow-through cell system (USP Apparatus 4), it was presented as an alternative dissolution apparatus to the conventional vessels methods USP Apparatus 1 and 2 because of several advantages over traditional dissolution systems (Chevalier et al., 2009; Greco et al., 2011; Shiko et al., 2011). The USP Apparatus 4 has a continuous extraction of the drug, simulating the absorption into the systemic circulation, generating intermittent flow of dissolution medium into the cell where the dosage form is placed (Qureshi et al., 1994). It is possible to use it as an open system that can work under sink conditions which facilitates the dissolution of poorly soluble drugs as well as changing the dissolution medium within a range of physiological pH values throughout the test (Zhang et al., 1994). Prior information shows that in vitro dissolution data obtained with the flow-through cell system better reflect the in vivo performance of some poorly soluble drugs (Jinno et al., 2008; Okumu et al., 2008). The flow-through cell system has proved to be useful in the development of a more discriminating dissolution method than the official one in the USP Apparatus 2 for the poorly soluble compound albendazole (Hurtado et al., 2003) and to establish an in vitro/in vivo relationship for paracetamol suppositories, a Class IV drug (Medina et al., 2009). Despite the advantages of the flow-through cell system over the USP Apparatus 1 and 2 information of the dissolution of carbamazepine immediate-release oral dosage forms under USP Apparatus 4 is scarce (Bønløkke et al., 1999).
The objective of this study was to evaluate the dissolution characteristics of four carbamazepine generic products sold in the local market and the reference product Tegretol® under the hydrodynamic environment generated by the flow-through cell system and to compare it with the results obtained with the USP paddles method.
2. Materials and methods
2.1. Materials
Five carbamazepine commercial products (200 mg) were used in this study. Generic products (codified as: A, B, C and D products) were compared with Tegretol® as the reference product (codified as: R product). Sodium lauryl sulfate was purchased from Distribuidora Química Lufra-México. Carbamazepine standard was purchased from Sigma–Aldrich Co. (St. Louis MO, USA).
2.2. Content uniformity and assay
Content uniformity and assay tests were performed with all products, according to the procedures described in the United States Pharmacopeia (USP 30, 2007).
2.3. Analytical method validation
The analytical method used was validated according to Mexican regulations (Norma Oficial Mexicana, 1999). To demonstrate the linearity of the spectrophotometric system, six calibration curves with five different carbamazepine concentrations (range 2.5–20 μg/ml) prepared in the dissolution medium (1.0% sodium lauryl sulfate aqueous solution) were analyzed at 285 nm. Data obtained were fitted by linear regression and the coefficients of regression, regression analysis of variance (ANOVA) and 95% confidence interval (CI95%) for the value of the intercept were calculated. The system precision was demonstrated by calculating the percentage coefficients of variation (CV%) at each concentration level.
The method linearity (drug with excipients) was determined by the added standard method by separately dissolving, in 900 ml of dissolution medium, quantities of powder from all products equivalent to 20, 40, 60, 80, 100 and 120% of carbamazepine dose plus seven mg of carbamazepine standard in each vessel. The USP Apparatus 2 at 75 rpm was used. At 60 min the amount of carbamazepine dissolved in each sample was calculated with reference to a calibration curve prepared on the day of the experiment. Each determination was performed in triplicate. In order to evaluate the linearity of the method, data were plotted (dissolved amount vs added amount) and determination coefficient (R2), CI95% for the slope and intercept and regression ANOVA was calculated. The accuracy was evaluated by calculating the CI95% of the average percentage of carbamazepine recovered from the known added amount of the drug. The precision was determined by calculating the CV for the percentage of the drug dissolved (repeatability). To evaluate the random events effect on the analytical method precision a homogeneous sample of tablets powder, equivalent to 50% of the dose plus 10 mg of carbamazepine standard was analyzed in triplicate, by two analysts in two different days (reproducibility); results obtained were analyzed by a two-way ANOVA. Differences were considered significant if p < 0.05.
2.4. USP paddles method (pharmacopeial method)
Carbamazepine dissolution profiles were determined according to the United States Pharmacopeia (USP 30, 2007) in an automated dissolution USP Apparatus 2 (Vankel VK 7000, Erweka, Germany) with an auto-controlled multi-channel peristaltic pump (Vankel VK 810, England), an UV/VIS spectrophotometer (Varian Cary 50 Tablet, USA) with 1 mm flow cells and Vankel software. Carbamazepine intact tablets were added on 900 ml of 1.0% sodium lauryl sulfate aqueous solution as dissolution medium at 37.0 ± 0.5 °C. Rotational speed of 75 rpm was tested. Sequential sampling using filter probes occurred over 60 min at regular five min intervals using 12 replicates. The amount of carbamazepine dissolved was determined with a known concentration of the standard solution at 285 nm.
2.5. Flow-through cell system (USP Apparatus 4)
Carbamazepine dissolution profiles were obtained with an automated flow-through cell system, USP Apparatus 4 (Sotax CE6, Sotax AG, Switzerland) with 22.6 mm cells (i.d.) and a piston pump (Sotax CY7–50, Sotax AG, Switzerland). In all experiments laminar flow (with a bed of 6 g of glass beads) was used. The degassed dissolution medium, 1.0% sodium lauryl sulfate aqueous solution at 37.0 ± 0.5 °C, was pumped at a flow rate of 16 ml/min. An open system was used, without recycling the dissolution media. Sequential sampling using 0.45 μm nitrocellulose membranes (Millipore®) occurred over 60 min at regular five min intervals using 12 replicates. The amount of carbamazepine dissolved was determined in an UV/VIS spectrophotometer (Perkin Elmer Lambda 10, USA) with 1 mm flow cells at 285 nm. For every trial, a standard curve was prepared.
2.6. Data analysis
Dissolution profiles of generic products (both apparatuses) were compared to dissolution profile of the reference product using f2 similarity factor according to Eq. (1) (Moore and Flanner, 1996).
| (1) |
where n is the number of time points used to evaluate the amount of carbamazepine dissolved, Rj and Tj are the average percentage of carbamazepine dissolved at a j specific time from the reference and test products, respectively.
Additionally, carbamazepine dissolution data of each product were used to calculate model-independent parameters: mean dissolution time (MDT) (Podczeck, 1993) and dissolution efficiency (DE) (Khan, 1975). Generic products values were compared with reference product values by a univariate one-way ANOVA, followed by Dunnett’s or Dunnett’s T3 multiple comparisons test as appropriate. Data analysis was carried out using SPSS software (Version 17.0). Differences were considered significant if p < 0.05.
In order to evaluate the kinetics of the release process of carbamazepine from the used products, under hydrodynamic environments generated by the USP paddles method and the flow-through cell system, dissolution data were fitted to different kinetic models: First-order, Higuchi, Korsmeyer-Peppas, Hixson-Crowell, Makoid-Banakar, Weibull and Logistic. The model with the highest determination coefficient () and the minimum Akaike information criterion (AIC) was chosen as the best fit (Yuksel et al., 2000). Data analysis was carried out using the Excel add-in DDSolver program (Zhang et al., 2010).
Finally, to evaluate the dissolution profiles of the generic products with respect to the reference product with model-dependent methods a parameter derived from the best fit model was compared with a univariate one-way ANOVA followed by Dunnett’s or Dunnett’s T3 multiple comparisons test. Differences were considered significant if p < 0.05.
3. Results
3.1. Content uniformity and assay
All products met the content uniformity and assay tests specified in the United States Pharmacopeia. The percentages of carbamazepine on the content uniformity test ranged from 100.20 to 101.34% and the assay test was between 101.42 and 105.11%.
3.2. Analytical method validation
The mean regression equation from six standard calibration curves was: y = 0.0435x + 0.0005. Linear regression was significant (R2 = 0.999; p < 0.05). The CI95% estimated for the value of the intercept was −0.0149 to 0.0158. The highest CV value was 0.93% for the five concentration levels evaluated.
The analytical method validation was done with all products used in the present study however, as an example and in order that method validation is not the main objective of this work, only the reference product data are shown. The regression equation to assess the method linearity was y = 0.9896x − 1.1244 (R2 = 0.998; p < 0.05). The CI95% estimated for the slope was 0.9445 to 1.0346 and −8.4289 to 6.1801 for the intercept. The method accuracy was 98.19% with a CI95% of 95.39 to 100.99%. The CV value calculated to assess the method precision was 1.82% and the two-way ANOVA showed no significant differences in drug dissolved between days and analysts (p > 0.05). All generic products met national standard validation criteria too.
3.3. Dissolution profiles
Carbamazepine dissolution profiles obtained with the USP paddles method and the flow-through cell system are shown in Fig. 1a and b, respectively.
Figure 1.
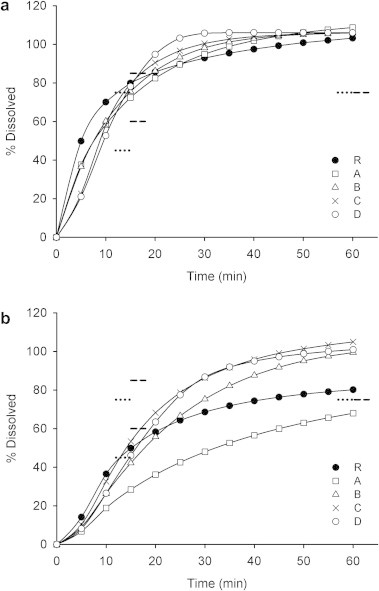
Dissolution profiles of carbamazepine reference (R) and generic products (A−D) with (a) USP paddles method and (b) flow-through cell system. Mean, n = 12. Error bars were omitted for clarity. The dotted and dashed lines close to 15 and 60 min show limits for USP Test 2 and Test 3, respectively.
Only product A met the USP Test 2 and all products met the USP Test 3 using the USP paddles method. Taken the same official dissolution criteria (involving the use of USP Apparatus 2) products R, C and D met the USP Test 2 and none of the products met the USP Test 3 using the flow-through cell system. Dissolution profiles of all generic products were similar to dissolution profile of the reference product when USP paddles method was used (f2 > 50), while opposite results were found with the flow-through cell system (f2 < 50), Fig. 2.
Figure 2.
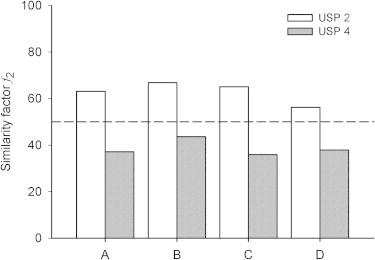
Similarity factor f2 calculated with dissolution data of reference (R) and generic products (A−D).
MDT and DE mean values ± standard error medium (SEM) for products under study in both USP apparatuses are shown in Table 1.
Table 1.
Dissolution parameters calculated with model-independent methods. Mean dissolution time (MDT) and dissolution efficiency (DE). Mean ± SEM, n = 12.
| Product | MDT (min) | DE (%) |
|---|---|---|
| USP paddles method | ||
| R | 10.89 ± 0.43 | 84.54 ± 2.08 |
| A | 13.59 ± 0.20a | 84.04 ± 0.75 |
| B | 11.74 ± 0.08 | 85.19 ± 0.58 |
| C | 11.75 ± 0.18 | 85.21 ± 0.66 |
| D | 11.00 ± 0.28 | 86.55 ± 0.60 |
| Flow-through cell system | ||
| R | 15.64 ± 0.15 | 59.28 ± 1.61 |
| A | 22.30 ± 0.18a | 42.71 ± 0.41a |
| B | 20.80 ± 0.30a | 64.91 ± 0.74a |
| C | 18.35 ± 0.36a | 72.80 ± 0.86a |
| D | 18.31 ± 0.12a | 70.13 ± 0.75a |
p < 0.05 vs R, Dunnett’s or Dunnett’s T3 multiple comparisons test.
No significant differences were found in data generated by USP paddles method, excepting MDT of product A (p < 0.05), while significant differences were found in all values from generic products when compared to the reference product (p < 0.05) using the flow-through cell system.
According to the previously established criteria to chose the best kinetic model the dissolution data of all products with both USP apparatuses were well fitted by Weibull’s model, Eq. (2) and Table 2.
| (2) |
where F is the percentage of drug dissolved at t time, Fmax is the maximal percentage of drug dissolved at infinite time, α is the scale parameter, β is the shape parameter and Ti is the location parameter.
Table 2.
Criteria used for the selection of the best kinetic model. Mean, n = 12.
| Product | First-order | Higuchi | Korsmeyer-Peppas | Hixson-Crowell | Makoid-Banakar | Weibull | Logistic |
|---|---|---|---|---|---|---|---|
| USP paddles method | |||||||
| R | 0.8537 | 0.8851 | 0.9361 | 0.6181 | 0.9885 | 0.9996 | 0.9191 |
| A | 0.9398 | 0.8331 | 0.9459 | 0.9646 | 0.9981 | 1.0000 | 0.8911 |
| B | 0.9551 | 0.7336 | 0.8927 | 0.9771 | 0.9961 | 0.9999 | 0.9199 |
| C | 0.9124 | 0.7147 | 0.7956 | 0.9581 | 0.9639 | 0.9997 | 0.9572 |
| D | 0.8647 | 0.6818 | 0.7423 | 0.9276 | 0.9656 | 0.9996 | 0.9305 |
| Flow-through cell system | |||||||
| R | 0.9151 | 0.9023 | 0.9036 | 0.8171 | 0.9837 | 0.9999 | 0.9857 |
| A | 0.9885 | 0.9336 | 0.9732 | 0.9679 | 0.9970 | 1.0000 | 0.9981 |
| B | 0.9525 | 0.9162 | 0.9484 | 0.9844 | 0.9978 | 0.9999 | 0.9863 |
| C | 0.9314 | 0.9107 | 0.9120 | 0.9712 | 0.9912 | 0.9994 | 0.9761 |
| D | 0.9222 | 0.8827 | 0.8901 | 0.9642 | 0.9939 | 0.9996 | 0.9894 |
| AIC | |||||||
| USP paddles method | |||||||
| R | 66.68 | 70.40 | 62.89 | 82.83 | 43.99 | −10.48 | 64.30 |
| A | 66.97 | 83.17 | 70.93 | 64.94 | 30.59 | −30.88 | 77.99 |
| B | 64.91 | 88.90 | 78.88 | 58.00 | 39.71 | −9.90 | 74.57 |
| C | 78.67 | 93.12 | 90.24 | 68.87 | 70.10 | 2.57 | 69.20 |
| D | 85.92 | 96.14 | 94.74 | 78.08 | 70.84 | 17.35 | 78.01 |
| Flow-through cell system | |||||||
| R | 71.10 | 74.72 | 75.48 | 81.51 | 54.86 | −12.59 | 50.05 |
| A | 47.54 | 69.52 | 59.39 | 60.18 | 33.60 | −25.70 | 26.29 |
| B | 74.77 | 82.48 | 77.41 | 61.25 | 38.84 | −6.16 | 58.97 |
| C | 79.70 | 83.70 | 84.22 | 68.86 | 57.37 | 22.15 | 64.65 |
| D | 82.65 | 87.90 | 87.96 | 73.41 | 53.94 | 22.65 | 55.68 |
The comparison of the dissolution profiles was made analyzing the derived time parameter (Td) from Weibull’s function. Td value can be calculated with α and β values and is equivalent to the MDT value calculated with statistical moments (Langenbucher, 1972). Significant differences were found in Td values between product A and the reference product using the USP paddle method while significant differences were found in all Td values from generic products with respect to the reference product using the flow-through cell system (p < 0.05), Table 3.
Table 3.
Weibull’s parameters and Td values derived from the data adjustment to this kinetic model. Mean, n = 12.
| Product | α | β | Ti | Fmax | Td (±SEM) |
|---|---|---|---|---|---|
| USP paddles method | |||||
| R | 2.63 | 0.43 | 2.70 | 114.69 | 10.22 ± 0.68 |
| A | 9.26 | 0.83 | 0.16 | 112.97 | 14.54 ± 0.36a |
| B | 14.94 | 1.06 | −0.65 | 106.57 | 12.12 ± 0.09 |
| C | 8.57 | 0.98 | 3.02 | 105.89 | 11.71 ± 0.26 |
| D | 210.27 | 1.99 | −1.72 | 106.20 | 12.64 ± 0.31 |
| Flow-through cell system | |||||
| R | 7.31 | 0.77 | 3.54 | 84.16 | 16.93 ± 0.30 |
| A | 22.09 | 0.88 | 3.06 | 86.32 | 37.82 ± 2.48a |
| B | 40.67 | 1.17 | 2.10 | 104.66 | 24.79 ± 0.53a |
| C | 23.58 | 1.09 | 2.97 | 108.07 | 20.55 ± 0.81a |
| D | 97.49 | 1.56 | 1.21 | 100.26 | 19.93 ± 0.15a |
p < 0.05 vs R, Dunnett’s or Dunnett’s T3 multiple comparisons test.
4. Discussion
The dissolution test helps both in characterizing in vitro release rate and in arriving at single-time-point specifications that are appropriate for a conventional (immediate-release) product. For a dissolution test to be useful as a quality control tool, it must be sensitive to process and/or manufacturing changes. Uznović et al. (2010) using the dissolution official method with the USP Apparatus 2, reported drug percent dissolved differences at 15 and 60 min from two carbamazepine commercial products and regardless of similar composition of the products it could be assumed that the technological process of tablet production significantly affected the dissolution rate. In the present study, carbamazepine official dissolution test was not discriminating enough, all commercial products used reached the dissolution criteria (Test 3) and the dissolution profiles of the generic products were similar to the dissolution profile of the reference product. For different carbamazepine formulations Girad et al. (1996) reported a faster dissolution when 1.0% sodium lauryl sulfate aqueous solution was used together with the USP paddles method however, simulated intestinal fluid pH 7.5 was more discriminative between products; Castro and Jung (2000) demonstrated that the USP dissolution test cannot be used to predict the bioavailability of commercial products and Jung et al. (1997) reported that no correlation was found between in vitro dissolution data, obtained with the USP paddles method, and in vivo parameters. On the other hand, Hamlin et al. (1962) hypothesized and later proved that products differing significantly in in vivo performance would not show in vitro differences if the dissolution test was conducted at a high agitation rate. They concluded that in vitro test with relatively low agitation rates correlated better with the in vivo performance of drug products.
In vitro carbamazepine dissolution performance from all generic products differed from the reference product (f2 < 50) when the proposed flow-through cell method was used. Products A and B did not reach the dissolution criteria of USP Test 2. Furthermore, all products showed a slower dissolution rate than the one found with the USP paddles method. Langenbucher et al. (1989) expressed that this kind of behavior can be explained by the hydrodynamic conditions that characterize the flow-through cell system, where no agitation mechanisms exist and the dosage form and the drug particles are continuously exposed to a uniform laminar flow, similar to the natural environment of the gastrointestinal tract, causing different dissolution pattern. We used 16 ml/min as flow rate because this rate is one of the three suggested by the European and United States Pharmacopeias (others are 4 and 8 ml/min). Fotaki et al. (2005) reported that the intestinal fluid axial velocity has been estimated to be approximately of 1.5 cm/min and the fluid flow inside the 22.6 mm cells is 4 cm/min when the flow rate of dissolution medium is 16 ml/min.
In order to compare the dissolution data of carbamazepine from the generic products to the reference product, model-independent parameters MDT and DE were calculated. Cardot et al. (2007) mentioned that these parameters have been proposed as adequate parameters for some IVIVC levels. IVIVC Level B is based on the comparison of parameters calculated by statistical moments as MDT is (time necessary to dissolve 63.2% of the drug) and IVIVC Level C requires the calculation of an in vitro parameter that expresses a global drug dissolution performance as is the case of DE. This parameter relates the area under the curve of the dissolution profile of the product to the total area of the rectangle formed by the theoretical dissolution of 100% of the dose and the time interval of the test. For an in vitro study of carbamazepine controlled-release products Barakat et al. (2009) reported MDT and DE data using the USP paddles method. Values for Tegretol® CR product were 1.91 h and 71.32%, respectively. Dissolution studies were conducted with three determinations for a period of seven h and this was done only for comparative purposes of the proposed formulations.
All products used in the present study were well fitted with Weibull’s model and comparisons with the Td parameter showed a better discriminatory capacity of the flow-through cell system to differentiate between products. Release kinetics described by Weibull’s equation was reported by Koester et al. (2004) for dissolution profiles of hydroxypropylmethylcellulose matrix tablets containing carbamazepine associated to β-cyclodextrin.
The use of generic products is important in the health system of any country to benefit patients, hospitals and pharmaceutical industry. Recently and according to Gidal and Tomson (2008) the substitution of antiepileptic drugs in patients with epilepsy has gained increased attention however, Shaw and Krauss (2008) reported that the FDA has not shown safety in generic-to-generic switches, which could potentially cause drug concentration changes of up to 40%. The present study with the flow-through cell system reveals significant differences in the rate and extent of carbamazepine dissolution from Mexican immediate-release generic products used and this could be replicated in other countries. Previous studies with the flow-through cell method demonstrated a good IVIVC between the dissolution profiles obtained with the USP Apparatus 4 and in vivo data, Jinno et al. (2008) for cilostazol immediate-release tablets and Emara et al. (2000) for vincamine prolonged-release products, both poorly soluble drugs.
As previously mentioned, a significant IVIVC has not been found when the USP paddles method has been used to evaluate the in vitro performance of carbamazepine tablets. On the other hand, it might be expected that the results obtained with a more discriminative dissolution method, which has been proved to simulate more adequately the in vivo performance of poorly soluble drugs, could better reflect the bioavailability of carbamazepine from immediate-release tablets.
5. Conclusion
Data with the flow-through cell system confirm that the dissolution method proposed has a greater discriminating ability than the USP paddles method to identify significant differences between rate and extent dissolution of carbamazepine immediate-release tablets. It is possible to mention that carbamazepine products with differences in dissolution performance are candidates to show bioavailability differences so it is suggested to evaluate their in vivo performance to confirm the predictability of the in vitro proposed method.
Footnotes
Peer review under responsibility of King Saud University.
References
- Amidon G.L., Lennernäs H., Shah V.P., Crison J.R. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharmaceutical Research. 1995;12:413–420. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- Barakat N.S., Elbagory I.M., Almurshedi A.S. Controlled-release carbamazepine matrix granules and tablets comprising lipophilic and hydrophilic components. Drug Delivery. 2009;16:57–65. doi: 10.1080/10717540802518157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bønløkke L., Hovgaard L., Kristensen H.G., Knutson L., Lindahl A., Lennernäs H.A. Comparison between direct determination of in vivo dissolution and the deconvolution technique in humans. European Journal of Pharmaceutical Sciences. 1999;8:19–27. doi: 10.1016/s0928-0987(98)00055-4. [DOI] [PubMed] [Google Scholar]
- Cardot J.M., Beyssac E., Alrici M. In vitro–in vivo correlation: importance of dissolution in IVIVC. Dissolution Technologies. 2007;14:15–19. [Google Scholar]
- Castro N., Jung H. Determination of absorption profiles of carbamazepine products by the deconvolution method and its correlation with in vitro data. Revista Mexicana de Ciencias Farmacéuticas. 2000;31:42–46. [Google Scholar]
- Chevalier E., Viana M., Artaud A., Chomette L., Haddouchi S., Devidts G., Chulia D. Comparison of three dissolution apparatuses for testing calcium phosphate pellets used as ibuprofen delivery systems. AAPS PharmSciTech. 2009;10:597–605. doi: 10.1208/s12249-009-9252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A.G. A multinational survey of the quality of carbamazepine tablets. Drug Development and Industrial Pharmacy. 1995;21:2167–2186. [Google Scholar]
- Emara L.H., El-Menshawi B.S., Estefan M.Y. In vitro-in vivo correlation and comparative bioavailability of vincamine in prolonged-release preparations. Drug Development and Industrial Pharmacy. 2000;26:243–251. doi: 10.1081/ddc-100100352. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration, 1999. Guidance for industry waiver of in vivo bioavailability and bioequivalence studies for immediate release solid oral dosage forms containing certain active moieties/active ingredients based on a biopharmaceutics classification system. Center for Drug Evaluation and Research (CDER), U.S. Department of Health and Human Services.
- Fotaki N., Symillides M., Reppas C. In vitro versus canine data for predicting input profiles of isosorbide-5-mononitrate from oral extended release products on a confidence interval basis. European Journal of Pharmaceutical Sciences. 2005;24:115–122. doi: 10.1016/j.ejps.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Gidal B.E., Tomson T. Debate: substitution of generic drugs in epilepsy: is there cause for concern? Epilepsia. 2008;49:56–62. doi: 10.1111/j.1528-1167.2008.01927.x. [DOI] [PubMed] [Google Scholar]
- Girad M.E., Millán R., Jung H., Torres S., Montoya M.A. Dissolution study of carbamazepine tablets. Revista Mexicana de Ciencias Farmacéuticas. 1996;27:13–17. [Google Scholar]
- Greco K., Bergman T.L., Bogner R. Design and characterization of a laminar flow-through dissolution apparatus: comparison of hydrodynamic conditions to those of common dissolution techniques. Pharmaceutical Development and Technology. 2011;16:75–87. doi: 10.3109/10837450903499341. [DOI] [PubMed] [Google Scholar]
- Hamlin W.E., Nelson E., Ballard B.E., Wagner J.G. Loss of sensitivity in distinguishing real differences in dissolution rates due to increasing intensity of agitation. Journal of Pharmaceutical Sciences. 1962;51:432–435. doi: 10.1002/jps.2600510509. [DOI] [PubMed] [Google Scholar]
- Hurtado M., Vargas Y., Domínguez-Ramírez A.M., Cortés A.R. Comparison of dissolution profiles for albendazole tablets using USP Apparatus 2 and 4. Drug Development and Industrial Pharmacy. 2003;29:777–784. doi: 10.1081/ddc-120021777. [DOI] [PubMed] [Google Scholar]
- Jinno J., Kamada N., Miyake M., Yamada K., Mukai T., Odomi M., Toguchi H., Liversidge G.G., Higaki K., Kimura T. In vitro-in vivo correlation for wet-milled tablet of poorly water-soluble cilostazol. Journal of Controlled Release. 2008;130:29–37. doi: 10.1016/j.jconrel.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Jung H., Milán R.C., Girard M.E., León F., Montoya M.A. Bioequivalence study of carbamazepine tablets: in vitro/in vivo correlation. International Journal of Pharmaceutics. 1997;152:37–44. [Google Scholar]
- Khan K.A. The concept of dissolution efficiency. Journal of Pharmacy and Pharmacology. 1975;27:48–49. doi: 10.1111/j.2042-7158.1975.tb09378.x. [DOI] [PubMed] [Google Scholar]
- Koester L.S., Ortega G.G., Mayorga P., Bassani V.L. Mathematical evaluation of in vitro profiles of hydroxypropylmethylcellulose matrix tablets containing carbamazepine associated to β-cyclodextrin. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58:177–179. doi: 10.1016/j.ejpb.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Lake O.A., Olling M., Barends D.M. In vitro/in vivo correlations of dissolution data of carbamazepine immediate release tablets with pharmacokinetic data obtained in healthy volunteers. European Journal of Pharmaceutics and Biopharmaceutics. 1999;48:13–19. doi: 10.1016/s0939-6411(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Langenbucher F., Benz D., Kurth W., Moller H., Otz M. Standardized flow-cell method as an alternative to existing pharmacopoeial dissolution testing. Pharmaceutical Industry. 1989;51:1276–1281. [Google Scholar]
- Langenbucher F. Linearization of dissolution rate curves by the Weibull distribution. Journal of Pharmacy and Pharmacology. 1972;24:979–981. doi: 10.1111/j.2042-7158.1972.tb08930.x. [DOI] [PubMed] [Google Scholar]
- Lindenberg M., Kopp S., Dressman J.B. Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. European Journal of Pharmaceutics and Biopharmaceutics. 2004;58:265–278. doi: 10.1016/j.ejpb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Medina J.R., Hurtado M., Cortés A.R., Aoki K., Domínguez A.M. Dissolution of paracetamol suppositories using the flow-through cell system and their absorption in an animal model. Revista Mexicana de Ciencias Farmacéuticas. 2009;40:11–18. [Google Scholar]
- Meyer M.C., Straughn A.B., Jarvi E.J., Wood G.C., Pelsor F.R., Shah V.P. The bioinequivalence of carbamazepine tablets with a history of clinical failures. Pharmaceutical Research. 1992;9:1612–1616. doi: 10.1023/a:1015872626887. [DOI] [PubMed] [Google Scholar]
- Mittapalli P.K., Suresh B., Hussaini S.S.Q., Rao Y.M., Apte S. Comparative in vitro study of six carbamazepine products. AAPS PharmSciTech. 2008;9:357–365. doi: 10.1208/s12249-008-9035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.W., Flanner H.H. Mathematical comparison of dissolution profiles. Pharmaceutical Technology. 1996;20:64–74. [Google Scholar]
- Norma Oficial Mexicana, 1999. NOM-177-SSA1-1998 Diario Oficial de la Federación, México DF, p. 44–67.
- Okumu A., Dimaso M., Löbenberg R. Dynamic dissolution testing to establish in vitro/in vivo correlations for montelukast sodium, a poorly soluble drug. Pharmaceutical Research. 2008;25:2778–2785. doi: 10.1007/s11095-008-9642-z. [DOI] [PubMed] [Google Scholar]
- Olling M., Mensinga T.T., Barends D.M., Groen C., Lake O.A., Meulenbelt J. Bioavailability of carbamazepine from four different products and the occurrence of side effects. Biopharmaceutics and Drug Disposition. 1999;20:19–28. doi: 10.1002/(sici)1099-081x(199901)20:1<19::aid-bdd152>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Podczeck F. Comparison of in vitro dissolution profiles by calculating mean dissolution time (MDT) or mean residence time (MRT) International Journal of Pharmaceutics. 1993;97:93–100. [Google Scholar]
- Qureshi S.A., Caillé G., Brien R., Piccirilli G., Yu V., Mc Gilveray I.J. Application of flow-through dissolution method for the evaluation of oral formulations of nifedipine. Drug Development and Industrial Pharmacy. 1994;20:1869–1882. [Google Scholar]
- Rentmeester T.W., Doelman J.C., Hulsman J.A. Carbamazepine: branded formulation versus generic formulation. Pharma Weekblad. 1990;125:1108–1110. [Google Scholar]
- Shaw S.J., Krauss G.L. Generic antiepileptic drugs. Current Treatment Options in Neurology. 2008;10:260–268. doi: 10.1007/s11940-008-0029-6. [DOI] [PubMed] [Google Scholar]
- Shiko G., Gladden L.F., Sederman A.J., Connolly P.C., Butler J.M. MRI studies of the hydrodynamics in a USP 4 dissolution testing cell. Journal of Pharmaceutical Sciences. 2011;100:976–991. doi: 10.1002/jps.22343. [DOI] [PubMed] [Google Scholar]
- United States Pharmacopeia (USP 30) 2007. United States Pharmacopeial Convention.
- Uznović A., Vranić E., Hadžidedić Š. Impairment of the in vitro release of carbamazepine from tablets. Bosnian Journal of Basic Medical Sciences. 2010;10:234–238. doi: 10.17305/bjbms.2010.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonté M.G., Viñas M.A., De Buschiazzo P.M., Piersante M.V., Escales M.C., Gorriti C.E. Comparative study of carbamazepine 200 mg tablets for pharmaceutical equivalence determination. Acta Farmacéutica Bonaerense. 2004;23:391–397. [Google Scholar]
- Yuksel N., Kanik A.E., Baykara T. Comparison of in vitro dissolution profiles by ANOVA-based, model-dependent and independent-methods. International Journal of Pharmaceutics. 2000;209:57–67. doi: 10.1016/s0378-5173(00)00554-8. [DOI] [PubMed] [Google Scholar]
- Zhang G.H., Vadinno W.A., Yang T.T., Cho W.P., Chaudry I.A. Evaluation of the flow-through cell dissolution apparatus: effects of flow rate, glass beads and tablet position on drug release from different type of tablets. Drug Development and Industrial Pharmacy. 1994;20:2063–2078. [Google Scholar]
- Zhang Y., Huo M., Zhou J., Zou A., Li W., Yao C., Xie S. DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS Journal. 2010;12:263–271. doi: 10.1208/s12248-010-9185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]



