Abstract
A 59-year old man with idiopathic CD4 lymphopenia presented with extensive disseminated Cryptococcus neoformans infection including a large rib cryptoccocoma, vertebral spondylitis and pleural empyema. Complete resection of the affected part of the rib was necessary after failure of initial antifungal treatment. The vertebral spondylitis has been successfully managed at 3 years of follow-up by continuous itraconazole treatment and regular MRI combined with leucocyte scintigraphy assessment.
Keywords: Idiopathic CD4 penia, C. neoformans, Spondylitis, Cryptococcoma
1. Introduction
Idiopathic CD4 lymphopenia (ICL) is a rare condition with unknown natural history [1,2]. ICL is usually diagnosed on the basis of opportunistic infections including cryptococcosis, which usually presents as cryptococcal meningitis or pneumonia [3,4].
In contrast, skeletal infections are unusual manifestations of cryptococcosis. We report a case of rib and vertebral Cryptococcus neoformans infection due to ICL and discuss important aspects of the management of cryptococcal skeletal infection.
2. Case
A 59 year old male was admitted (day 0) with a non-fluctuant and tender mass related to the left posterolateral 10th rib, which had developed over an 8 week period (Fig. 1). There was no accompanying erythema, but the patient had experienced 4 weeks of low grade pyrexia and a moderate weight loss. No history of respiratory symptoms or back pain was reported, and the patient had no exposure to pigeons or other birds.
Fig. 1.
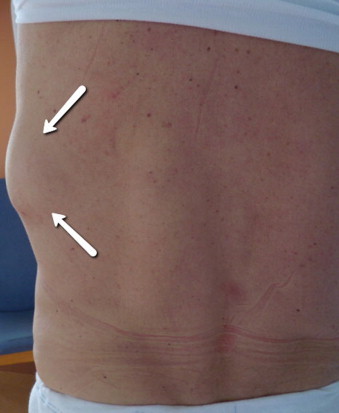
Non-fluctuant mass related to the left posterolateral thoracic (indicated by arrows).
Computed tomography (CT) (day 0) revealed a single 8×8 cm2 soft tissue tumour involving the left 10th rib, osteolytic destruction of Th6 and Th7 without any adjacent soft tissue involvement, minimal left pleural effusion, and a small pulmonary infiltrate (Fig. 2).
Fig. 2.
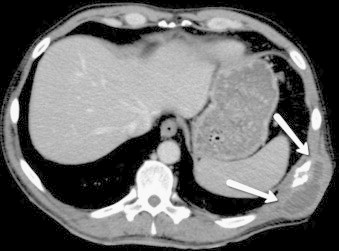
Transverse CT showing cryptoccoma involving the left 10th rib (indicated by arrows).
Initial blood (day 0) tests revealed C-reactive protein level of 130 mg/L (reference interval<10 mg/L). White blood counts, haemoglobin and immunoglobulin levels were all within normal range. Testing were negative human immunodeficiency virus (HIV) and Mycobacterium tuberculosis (day 10).
Open rib biopsy at day 6 revealed soft tissue with granulomatous inflammation and encapsulated yeast forms. C. neoformans was isolated from pleuratic fluid culture. Antimicrobial susceptibility test showed full susceptibility to voriconazole and amphotericin B, intermediate susceptibility to itraconazole, and resistance to fluconazole and caspofungin.
Antifungal treatment with AmBisome (3 mg/kg daily) was initiated at day 11, but was changed to oral voriconazole after 9 days because of azothemia. After 14 days of voriconazol treatment, during which time serum voriconazole levels were within therapeutic reference levels, control CT-scan revealed further progression of the cryptoccocoma, and surgical resections of the cryptoccocoma and the involved segment of the 10th rib was done (day +55).
After surgical resection the patient continued treatment with voriconazole for an additional 3 months and 15 days. Because of continuous photosensitivity and pruritus during voriconazole treatment, antifungal treatment was changed to fluconazole (800 mg daily) at day 155. However, the patient experienced continuous severe myalgia during fluconazole treatment, even after dosage tapering to 400 mg daily. Due to myalgia treatment was changed to itraconazole (400 mg daily) at day 216. Myalgia is an uncommon but recognised side effect of fluconazole, which have been noted in post-marketing safety studies and is included in the summary of product characteristics of fluconazole [5].
During continued antifungal treatment, the vertebral lesions were regularly assessed by magnetic resonance imaging (MRI) combined with bone- and leucocyte-scintigraphy. At day 225 slight progression of the osteolytic lesions at Th6 and Th7 was observed on MRI, but combined MRI and leucocyte scintigraphy at 6, 12, 24 and 36 months of follow-up showed stable osteolytic lesions without pathologic leucocyte accumulation (Fig. 3).
Fig. 3.
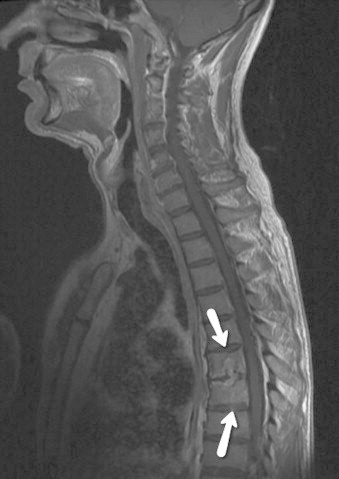
Whole spine MRI showing vertebral osteolytic lesions at Th6 and Th7 (indicated by arrows).
Immunologic investigations (day +11) showed low CD4 cell count 140 cells/µL (reference interval 390–1700 cells/µL), low CD8 cell count 92 cells/µL (reference interval 190–1030 cells/µL), low concentrations of B-lymphocytes 23 cells/µL (90–570 cells/µL), and depleted natural killer cells 69 cells/µL (80–560 cells/µL). Bone marrow biopsy (day 140) was normal. IL-12 and TNF-α response to Toll-like receptor 4/E. Coli LPS stimulation was within reference interval (day 246).
The patient is currently asymptomatic without any back pain and itraconazole treatment has been well tolerated without side-effects. CD4 and CD8 T-lymphocytes have remained suppressed with CD4 cell counts below 130 cells/µL and CD8 cell counts below 110 cells/µL.
3. Discussion
In this case report, we describe an unusual presentation of cryptococcosis confined to the skeletal system in a patient with Idiopathic CD4 lymphopenia. ICL is a rare syndrome characterised by depleted CD4 T-lymphocytes to a level less than 300 cells/µl or <20% of total lymphocytes over a period of minimum 6 weeks in patients without evidence of HIV-infection, history of primary immunodeficiency, or therapy induced immune suppression [6]. The incidence and prevalence of ICL is unknown, but Aledort et al. found a prevalence of continuous CD4 lymphocytopenia of 0.5% among 2.028 blood donors, transfusion recipients, and household contacts without evidence of HIV infection, immunodeficiency, or immunosuppression therapy [7]. Beside the CD4 lymphopenia patients with ICL may have low levels of other lymphocyte subsets, and patients with concomitant low levels of CD8 T-lymphocytes and B-lymphocytes at time of diagnosis might have a less favourable prognosis [1].
Cryptococcosis is found in one-third of patients with ICL, and is along with nontuberculous mycobacteriae the most frequent opportunistic infection in patients with ICL [1]. Cryptococcosis usually manifests as an opportunistic infection in patients with severely impaired cellular immunity, as the capsule of the C. neoformans has the ability to escape the complement system of the innate immune response, and cytotoxic eradication provided by T-lymphocytes and NK-cells is essential in effectively eliminating the infection [8–10].
In non-HIV infected patients predisposing factors for cryptococcosis include malignancy, solid organ transplant, connective tissue disease, and immunosuppressive therapy, which are estimated to cause 70–80% of all non-HIV related cryptococcal infections [3,11]. Estimates of the annual incidence of cryptococcosis in non-HIV infected individuals are 1.2–8 per 100,000 depending on geographic region [3,12].
In general, skeletal infections caused by fungi are uncommon and mostly confined to immunocompromised patients. Studies have found that 0.5–1.6% of all spinal infections are caused by fungi predominantly Aspergillus spp. and Candida albicans [13,14], and fungi to be the infectious agent in 10–11% of clinical reports on rib osteomyelitis [15].
In a study including 39 patients with skeletal infections caused by cryptococci, vertebrae and ribs were the most typical sites of infection. Soft tissue swelling and tenderness were the most frequent symptoms at time of diagnosis, and only 18% of the patients had experienced fewer. The median duration of symptoms before diagnosis was 3 months, and 10 patients (25%) had multifocal skeletal involvements at time of diagnosis [16]. Haematogenous dissemination with multifocal skeletal lesions is reported to occur in 5–10% of all cryptococcal disease often involving ribs, clavicle or pelvis [16–18].
In this patient, resection of the cryptoccocoma and adjacent rib was performed when the cryptoccocoma progressed during antifungal treatment. The vertebral lesions detected on CT were interpreted as part of a disseminated cryptococcal infection, as C. neoformans yeast was detected in both pleuritic fluid and in the resected rib. The vertebral lesions was monitored by MRI and bone- and leucocyte scintigraphy. MRI has high sensitivity of identifying spinal infections as well as visualising abscess formation, paravertebral, or epidural extension related to the vertebral infection, but may exhibit delay in detection of decreasing inflammation and osseous consolidation compared to CT [19].
Currently, no clear guidelines exists regarding antifungal prophylaxis, preferred antifungal treatment, or duration of antifungal treatment for patients with ICL, although anti-cryptococcal prophylaxis at CD4 cell count below 100 cells/µl has been suggested based on guidelines for HIV-infected patients [20,21].
In this patient, the vertebral infection has been stable during treatment with itraconazole, and as the CD4 cell count still remains low, the patients receives continuous antifungal treatment.
Conflict of interest statement
No conflicts of interest and no funding to declare.
References
- 1.Zonios D.I., Falloon J., Bennett J.E., Shaw P.A., Chaitt D., Baseler M.W. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112(2):287–294. doi: 10.1182/blood-2007-12-127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zonios D., Sheikh V., Sereti I. Idiopathic CD4 lymphocytopenia: a case of missing, wandering or ineffective T cells. Arthritis Res Ther. 2012;14(4):222. doi: 10.1186/ar4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speed B., Dunt D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin Infect Dis. 1995;21(1):28–34. doi: 10.1093/clinids/21.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Chayakulkeeree M., Perfect J.R. Cryptococcosis. Infect Dis Clin N Am. 2006;20(3) doi: 10.1016/j.idc.2006.07.001. 507-44, v-vi. [DOI] [PubMed] [Google Scholar]
- 5.〈http://www.medicines.org.uk/emc/medicine〉
- 6.Smith D.K., Neal J.J., Holmberg S.D. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. An investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328(6):373–379. doi: 10.1056/NEJM199302113280601. [DOI] [PubMed] [Google Scholar]
- 7.Aledort L.M., Operskalski E.A., Dietrich S.L., Koerper M.A., Gjerset G.F., Lusher J.M. Low CD4+ counts in a study of transfusion safety. The Transfusion Safety Study Group. N Engl J Med. 1993;328(6):441–442. doi: 10.1056/NEJM199302113280614. [DOI] [PubMed] [Google Scholar]
- 8.Li S.S., Mody C.H. Cryptococcus. Proc Am Thorac Soc. 2010;7(3):186–196. doi: 10.1513/pats.200907-063AL. [DOI] [PubMed] [Google Scholar]
- 9.Wozniak K.L., Hardison S., Olszewski M., Wormley F.L., Jr. Induction of protective immunity against cryptococcosis. Mycopathologia. 2012;173(5–6):387–394. doi: 10.1007/s11046-011-9505-8. [DOI] [PubMed] [Google Scholar]
- 10.Price M.S., Perfect J.R. Host defenses against cryptococcosis. Immunol Investig. 2011;40(7–8):786–808. doi: 10.3109/08820139.2011.605196. [DOI] [PubMed] [Google Scholar]
- 11.Pappas P.G., Perfect J.R., Cloud G.A., Larsen R.A., Pankey G.A., Lancaster D.J. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33(5):690–699. doi: 10.1086/322597. [DOI] [PubMed] [Google Scholar]
- 12.Mirza S.A., Phelan M., Rimland D., Graviss E., Hamill R., Brandt M.E. The changing epidemiology of cryptococcosis: an update from population-based active surveillance in 2 large metropolitan areas, 1992–2000. Clin Infect Dis. 2003;36(6):789–794. doi: 10.1086/368091. [DOI] [PubMed] [Google Scholar]
- 13.Cottle L., Riordan T. Infectious spondylodiscitis. J Infect. 2008;56(6):401–412. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Gouliouris T., Aliyu S.H., Brown N.M. Spondylodiscitis: update on diagnosis and management. J Antimicrob Chemother. 2010;65(3):iii11–iii24. doi: 10.1093/jac/dkq303. [DOI] [PubMed] [Google Scholar]
- 15.Bishara J., Gartman-Israel D., Weinberger M., Maimon S., Tamir G., Pitlik S. Osteomyelitis of the ribs in the antibiotic era. Scand J Infect Dis. 2000;32(3):223–227. doi: 10.1080/00365540050165839. [DOI] [PubMed] [Google Scholar]
- 16.Liu P.Y. Cryptococcal osteomyelitis: case report and review. Diagn Microbiol Infect Dis. 1998;30(1):33–35. doi: 10.1016/s0732-8893(97)00190-9. [DOI] [PubMed] [Google Scholar]
- 17.Behrman R.E., Masci J.R., Nicholas P. Cryptococcal skeletal infections: case report and review. Rev Infect Dis. 1990;12(2):181–190. doi: 10.1093/clinids/12.2.181. [DOI] [PubMed] [Google Scholar]
- 18.Corr P.D. Musculoskeletal fungal infections. Semin Musculoskelet Radiol. 2011;15(5):506–510. doi: 10.1055/s-0031-1293496. [DOI] [PubMed] [Google Scholar]
- 19.Wirtz D.C., Genius I., Wildberger J.E., Adam G., Zilkens K.W., Niethard F.U. Diagnostic and therapeutic management of lumbar and thoracic spondylodiscitis—an evaluation of 59 cases. Arch Orthop Trauma Surg. 2000;120(5–6):245–251. doi: 10.1007/s004020050457. [DOI] [PubMed] [Google Scholar]
- 20.Luo L., Li T. Idiopathic CD4 lymphocytopenia and opportunistic infection—an update. FEMS Immunol Med Microbiol. 2008;54(3):283–289. doi: 10.1111/j.1574-695X.2008.00490.x. [DOI] [PubMed] [Google Scholar]
- 21.Perfect J.R., Dismukes W.E., Dromer F., Goldman D.L., Graybill J.R., Hamill R.J. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]


