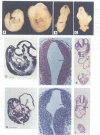
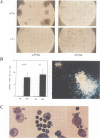
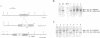Abstract
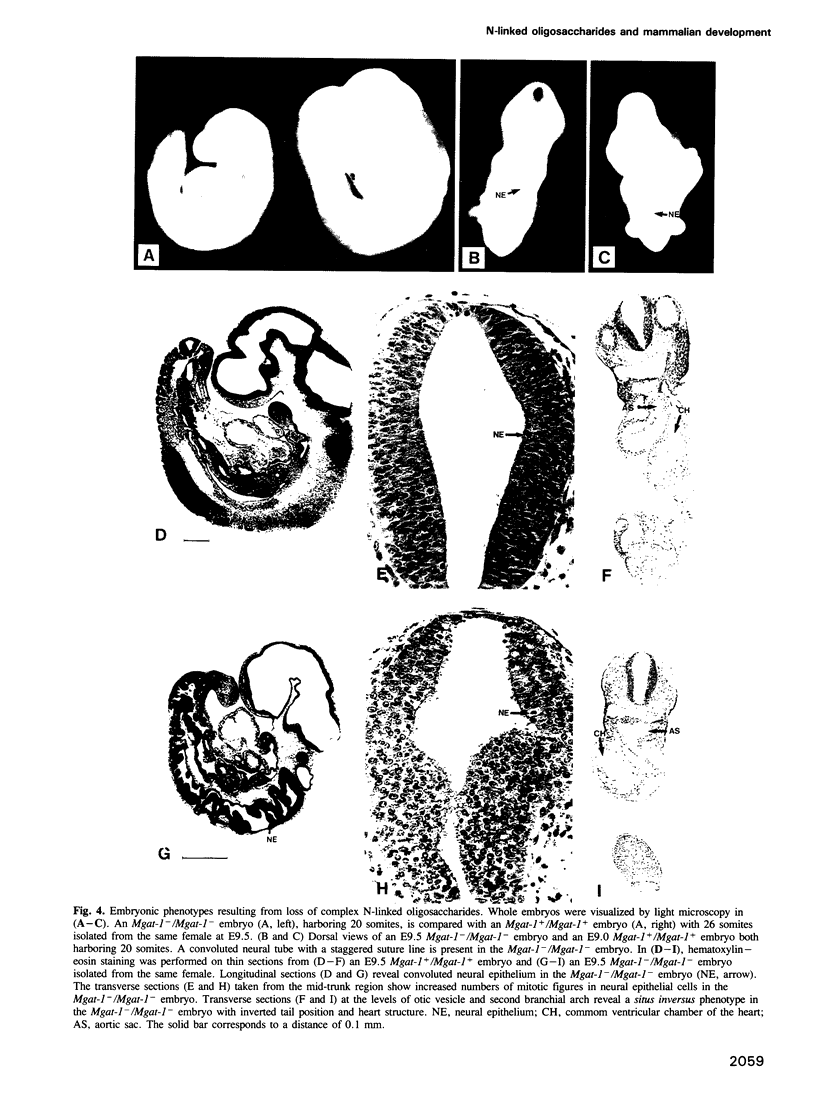
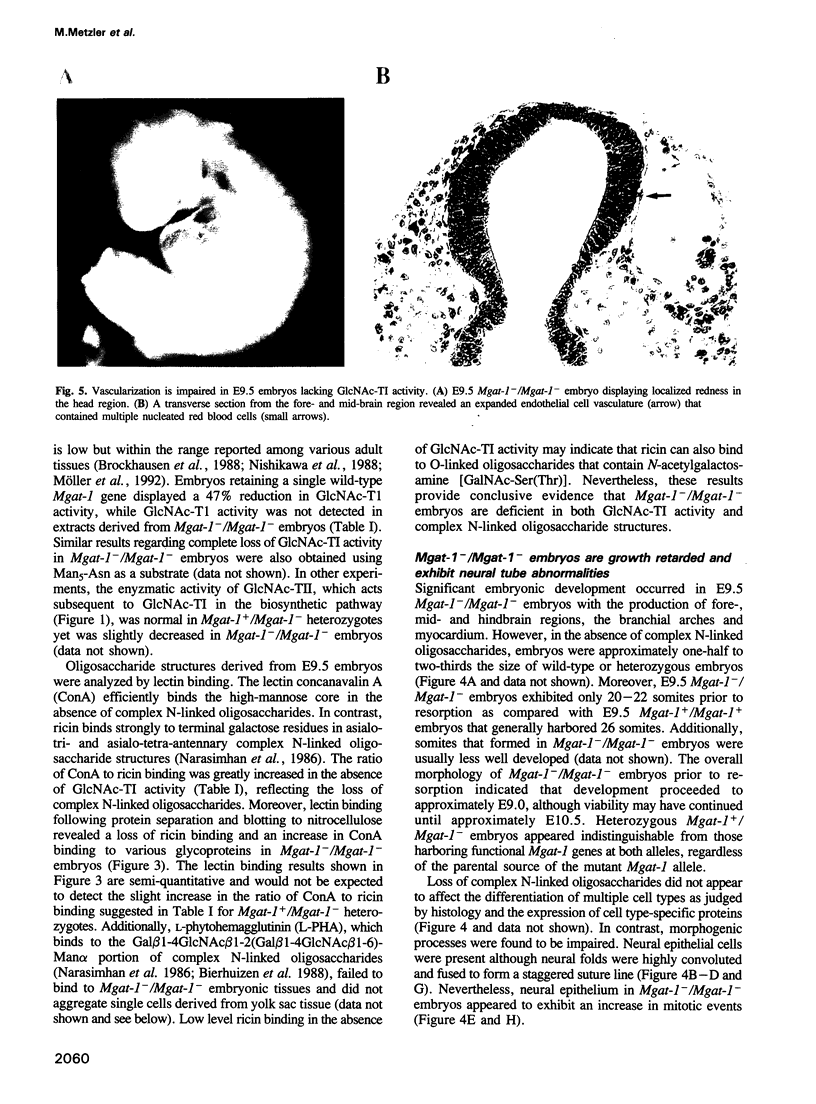
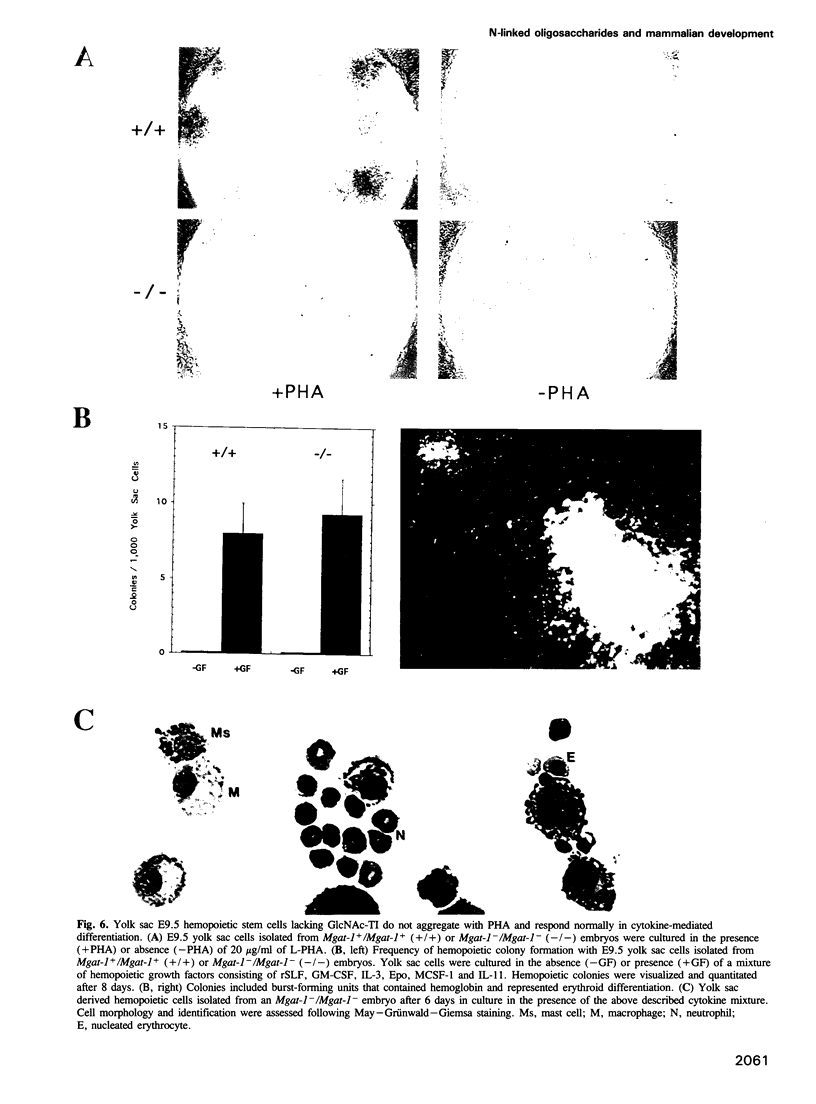
Complex asparagine (N)-linked oligosaccharides appear late in phylogeny and are highly regulated in vertebrates. Variations in these structures are found on the majority of cell-surface and secreted proteins. Complex N-linked oligosaccharide biosynthesis is initiated in the Golgi apparatus by the action of Mgat-1-encoded UDP-N-acetylglucosamine:alpha-3-D- mannoside beta-1,2-N-acetylglucosaminyltransferase I (GlcNAc-TI). To determine if these structures govern ontogenic processes in mammals, mouse embryos were generated that lacked a functional Mgat-1 gene. Inactivation of both Mgat-1 alleles produced deficiencies in GlcNAc-TI activity and complex N-linked oligosaccharides. Embryonic lethality occurred by day 10.5, thus establishing that complex N-linked oligosaccharides are required during post-implantation development. Remarkably, embryonic development proceeded into day 9 with the differentiation of multiple cell types. Complex N-linked oligosaccharides are important for morphogenic processes as neural tube formation, vascularization and the determination of left-right body plan asymmetry were impaired in the absence of a functional Mgat-1 gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby M. W., Gross J. A., Cooke M. P., Levin S. D., Qian X., Perlmutter R. M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992 Sep 4;70(5):751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- Bayna E. M., Shaper J. H., Shur B. D. Temporally specific involvement of cell surface beta-1,4 galactosyltransferase during mouse embryo morula compaction. Cell. 1988 Apr 8;53(1):145–157. doi: 10.1016/0092-8674(88)90496-5. [DOI] [PubMed] [Google Scholar]
- Bird J. M., Kimber S. J. Oligosaccharides containing fucose linked alpha(1-3) and alpha(1-4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev Biol. 1984 Aug;104(2):449–460. doi: 10.1016/0012-1606(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Bolscher J. G., van der Bijl M. M., Neefjes J. J., Hall A., Smets L. A., Ploegh H. L. Ras (proto)oncogene induces N-linked carbohydrate modification: temporal relationship with induction of invasive potential. EMBO J. 1988 Nov;7(11):3361–3368. doi: 10.1002/j.1460-2075.1988.tb03208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. E., Behringer R. R., Peschon J. J., Brinster R. L., Palmiter R. D. Genetically haploid spermatids are phenotypically diploid. Nature. 1989 Jan 26;337(6205):373–376. doi: 10.1038/337373a0. [DOI] [PubMed] [Google Scholar]
- Brockhausen I., Carver J. P., Schachter H. Control of glycoprotein synthesis. The use of oligosaccharide substrates and HPLC to study the sequential pathway for N-acetylglucosaminyltransferases I, II, III, IV, V, and VI in the biosynthesis of highly branched N-glycans by hen oviduct membranes. Biochem Cell Biol. 1988 Oct;66(10):1134–1151. doi: 10.1139/o88-131. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Eggens I., Fenderson B. A., Toyokuni T., Hakomori S. A role of carbohydrate-carbohydrate interaction in the process of specific cell recognition during embryogenesis and organogenesis: a preliminary note. Biochem Biophys Res Commun. 1989 Feb 15;158(3):913–920. doi: 10.1016/0006-291x(89)92809-x. [DOI] [PubMed] [Google Scholar]
- Fenderson B. A., Zehavi U., Hakomori S. A multivalent lacto-N-fucopentaose III-lysyllysine conjugate decompacts preimplantation mouse embryos, while the free oligosaccharide is ineffective. J Exp Med. 1984 Nov 1;160(5):1591–1596. doi: 10.1084/jem.160.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes B., Sagman U., Auger M., Demetrio M., Dennis J. W. Beta 1-6 branched oligosaccharides as a marker of tumor progression in human breast and colon neoplasia. Cancer Res. 1991 Jan 15;51(2):718–723. [PubMed] [Google Scholar]
- Fukuda M. N. HEMPAS disease: genetic defect of glycosylation. Glycobiology. 1990 Sep;1(1):9–15. doi: 10.1093/glycob/1.1.9. [DOI] [PubMed] [Google Scholar]
- Grey A. A., Narasimhan S., Brisson J. R., Schachter H., Carver J. P. Structure of the glycopeptides of a human gamma 1-immunoglobulin G (Tem) myeloma protein as determined by 360-megahertz nuclear magnetic resonance spectroscopy. Can J Biochem. 1982 Dec;60(12):1123–1131. doi: 10.1139/o82-144. [DOI] [PubMed] [Google Scholar]
- Gu H., Zou Y. R., Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993 Jun 18;73(6):1155–1164. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- Imamoto A., Soriano P. Disruption of the csk gene, encoding a negative regulator of Src family tyrosine kinases, leads to neural tube defects and embryonic lethality in mice. Cell. 1993 Jun 18;73(6):1117–1124. doi: 10.1016/0092-8674(93)90641-3. [DOI] [PubMed] [Google Scholar]
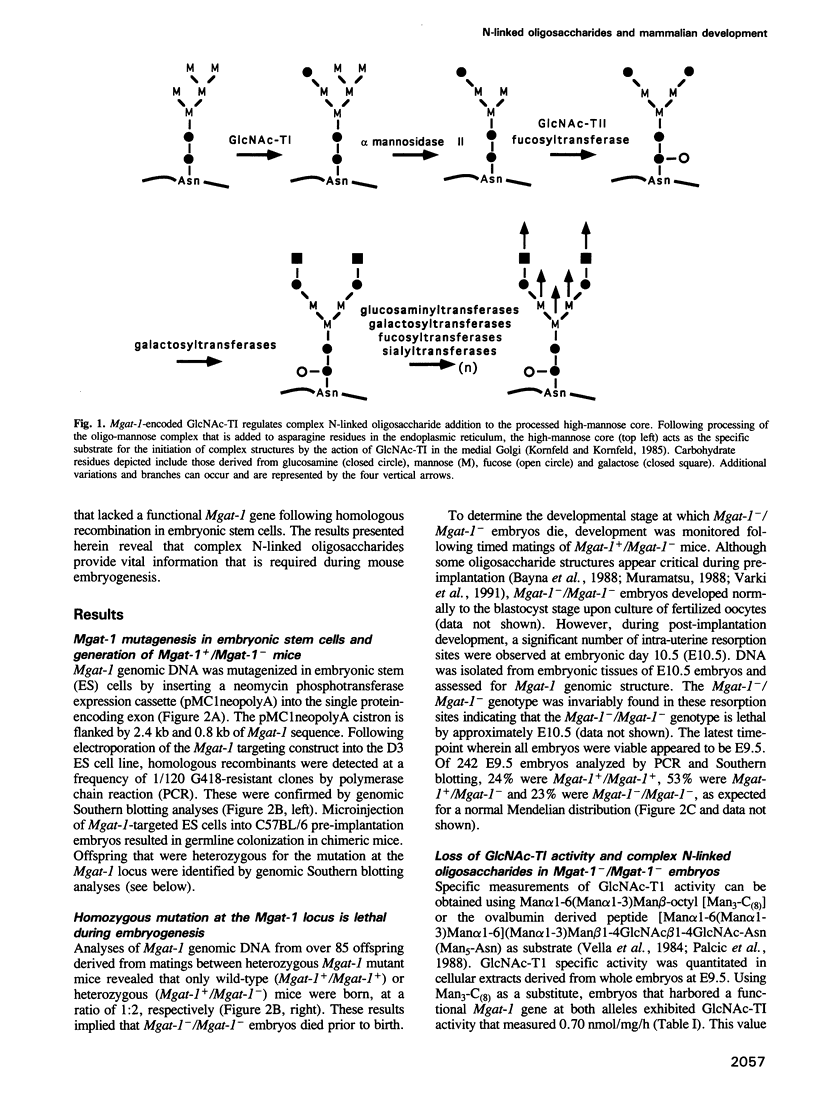
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Bergh M. L., Jackson B. J. Protein glycosylation in yeast. Annu Rev Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- Kumar R., Stanley P. Transfection of a human gene that corrects the Lec1 glycosylation defect: evidence for transfer of the structural gene for N-acetylglucosaminyltransferase I. Mol Cell Biol. 1989 Dec;9(12):5713–5717. doi: 10.1128/mcb.9.12.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Yang J., Eddy R. L., Byers M. G., Shows T. B., Stanley P. Cloning and expression of the murine gene and chromosomal location of the human gene encoding N-acetylglucosaminyltransferase I. Glycobiology. 1992 Aug;2(4):383–393. doi: 10.1093/glycob/2.4.383. [DOI] [PubMed] [Google Scholar]
- Kumar R., Yang J., Larsen R. D., Stanley P. Cloning and expression of N-acetylglucosaminyltransferase I, the medial Golgi transferase that initiates complex N-linked carbohydrate formation. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9948–9952. doi: 10.1073/pnas.87.24.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasky L. A. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992 Nov 6;258(5084):964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- Layton W. M., Jr Random determination of a developmental process: reversal of normal visceral asymmetry in the mouse. J Hered. 1976 Nov-Dec;67(6):336–338. doi: 10.1093/oxfordjournals.jhered.a108749. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Muramatsu T. Alterations of cell-surface carbohydrates during differentiation and development. Biochimie. 1988 Nov;70(11):1587–1596. doi: 10.1016/0300-9084(88)90294-5. [DOI] [PubMed] [Google Scholar]
- Möller G., Reck F., Paulsen H., Kaur K. J., Sarkar M., Schachter H., Brockhausen I. Control of glycoprotein synthesis: substrate specificity of rat liver UDP-GlcNAc:Man alpha 3R beta 2-N-acetylglucosaminyltransferase I using synthetic substrate analogues. Glycoconj J. 1992 Aug;9(4):180–190. doi: 10.1007/BF00731163. [DOI] [PubMed] [Google Scholar]
- Nada S., Yagi T., Takeda H., Tokunaga T., Nakagawa H., Ikawa Y., Okada M., Aizawa S. Constitutive activation of Src family kinases in mouse embryos that lack Csk. Cell. 1993 Jun 18;73(6):1125–1135. doi: 10.1016/0092-8674(93)90642-4. [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Freed J. C., Schachter H. The effect of a "bisecting" N-acetylglucosaminyl group on the binding of biantennary, complex oligosaccharides to concanavalin A, Phaseolus vulgaris erythroagglutinin (E-PHA), and Ricinus communis agglutinin (RCA-120) immobilized on agarose. Carbohydr Res. 1986 Jun 1;149(1):65–83. doi: 10.1016/s0008-6215(00)90370-7. [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Wilson J. R., Martin E., Schachter H. A structural basis for four distinct elution profiles on concanavalin A--Sepharose affinity chromatography of glycopeptides. Can J Biochem. 1979 Jan;57(1):83–96. doi: 10.1139/o79-011. [DOI] [PubMed] [Google Scholar]
- Nishikawa Y., Pegg W., Paulsen H., Schachter H. Control of glycoprotein synthesis. Purification and characterization of rabbit liver UDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I. J Biol Chem. 1988 Jun 15;263(17):8270–8281. [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Orban P. C., Chui D., Marth J. D. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passaniti A., Hart G. W. Metastasis-associated murine melanoma cell surface galactosyltransferase: characterization of enzyme activity and identification of the major surface substrates. Cancer Res. 1990 Nov 15;50(22):7261–7271. [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Pownall S., Kozak C. A., Schappert K., Sarkar M., Hull E., Schachter H., Marth J. D. Molecular cloning and characterization of the mouse UDP-N-acetylglucosamine:alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I gene. Genomics. 1992 Apr;12(4):699–704. doi: 10.1016/0888-7543(92)90297-6. [DOI] [PubMed] [Google Scholar]
- Sarkar M., Hull E., Nishikawa Y., Simpson R. J., Moritz R. L., Dunn R., Schachter H. Molecular cloning and expression of cDNA encoding the enzyme that controls conversion of high-mannose to hybrid and complex N-glycans: UDP-N-acetylglucosamine: alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):234–238. doi: 10.1073/pnas.88.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H., Brockhausen I., Hull E. High-performance liquid chromatography assays for N-acetylglucosaminyltransferases involved in N- and O-glycan synthesis. Methods Enzymol. 1989;179:351–397. doi: 10.1016/0076-6879(89)79138-2. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz L. D., Schweitzer P. A., Rajan T. V., Yi T., Ihle J. N., Matthews R. J., Thomas M. L., Beier D. R. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993 Jul 2;73(7):1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- Stanley P., Narasimhan S., Siminovitch L., Schachter H. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine--glycoprotein N-acetylglucosaminyltransferase activity. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3323–3327. doi: 10.1073/pnas.72.9.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner W., Lehle L. Protein glycosylation in yeast. Biochim Biophys Acta. 1987 Apr 27;906(1):81–99. doi: 10.1016/0304-4157(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993 Apr;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A., Hooshmand F., Diaz S., Varki N. M., Hedrick S. M. Developmental abnormalities in transgenic mice expressing a sialic acid-specific 9-O-acetylesterase. Cell. 1991 Apr 5;65(1):65–74. doi: 10.1016/0092-8674(91)90408-q. [DOI] [PubMed] [Google Scholar]
- Vella G. J., Paulsen H., Schachter H. Control of glycoprotein synthesis. IX. A terminal Man alpha l-3Man beta 1- sequence in the substrate is the minimum requirement for UDP-N-acetyl-D-glucosamine: alpha-D-mannoside (GlcNAc to Man alpha 1-3) beta 2-N-acetylglucosaminyltransferase I. Can J Biochem Cell Biol. 1984 Jun;62(6):409–417. doi: 10.1139/o84-056. [DOI] [PubMed] [Google Scholar]
- Yokoyama T., Copeland N. G., Jenkins N. A., Montgomery C. A., Elder F. F., Overbeek P. A. Reversal of left-right asymmetry: a situs inversus mutation. Science. 1993 Apr 30;260(5108):679–682. doi: 10.1126/science.8480178. [DOI] [PubMed] [Google Scholar]
- Yost H. J. Regulation of vertebrate left-right asymmetries by extracellular matrix. Nature. 1992 May 14;357(6374):158–161. doi: 10.1038/357158a0. [DOI] [PubMed] [Google Scholar]