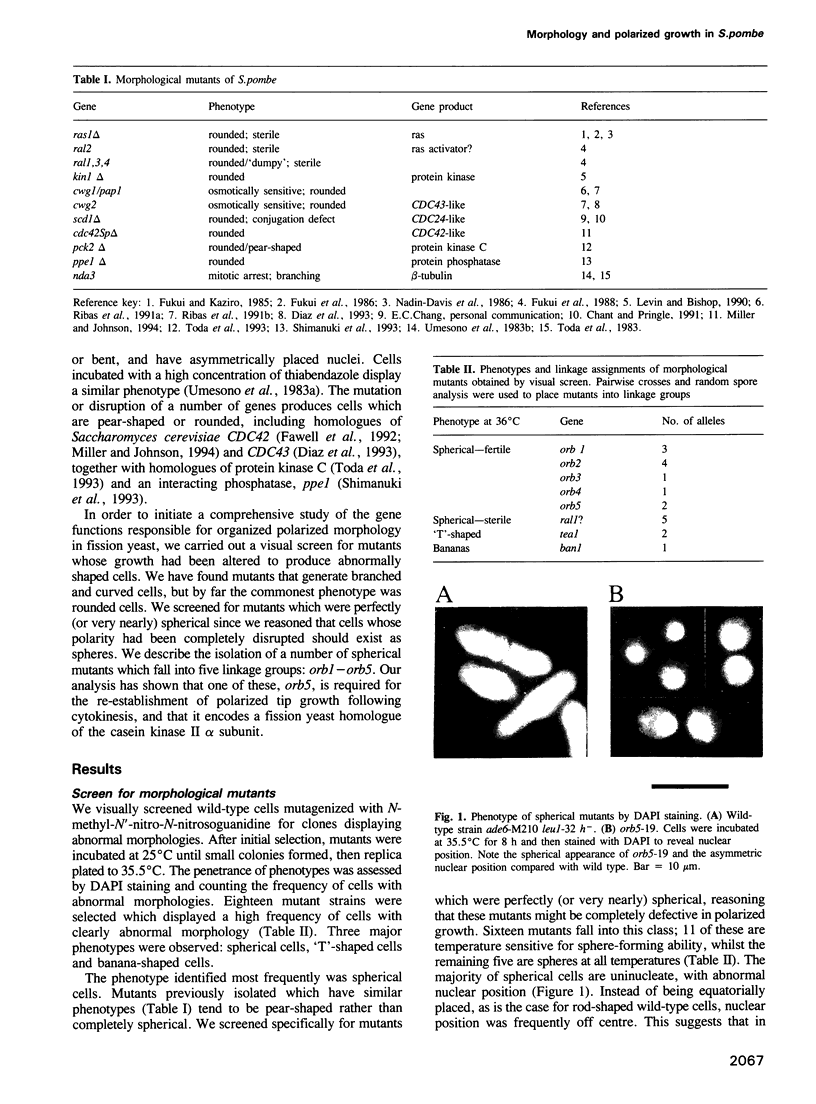
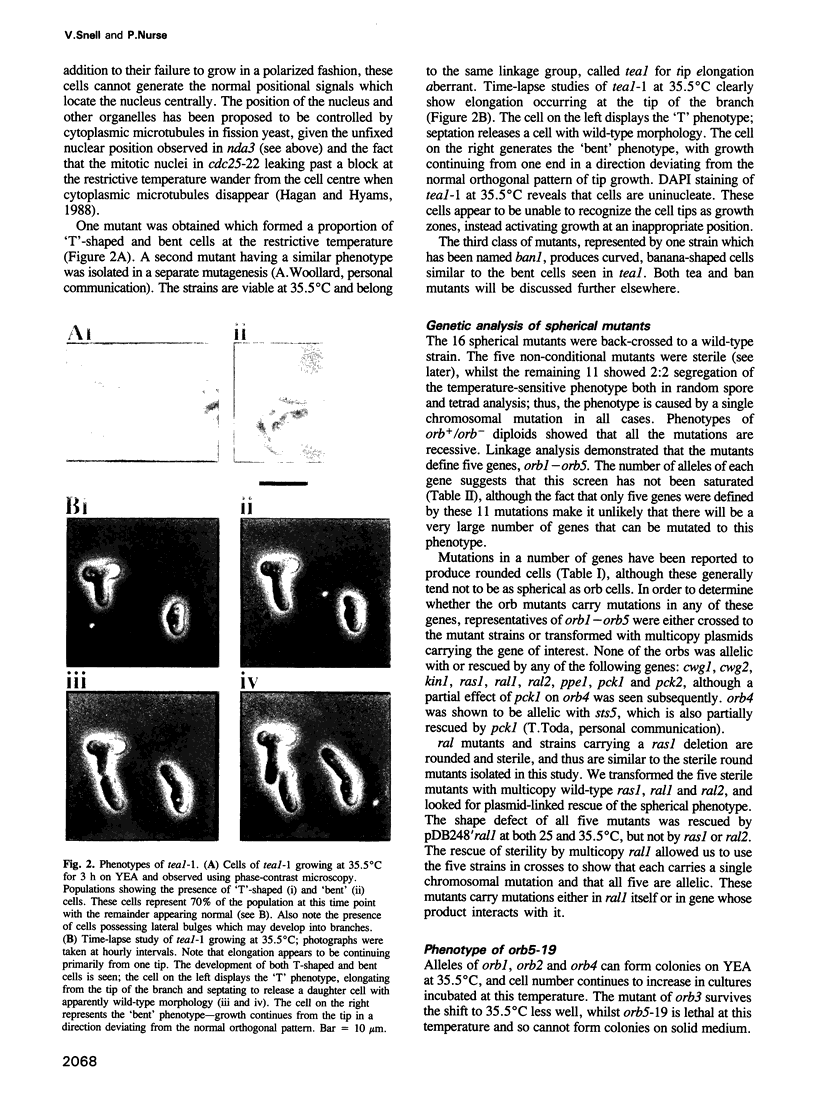
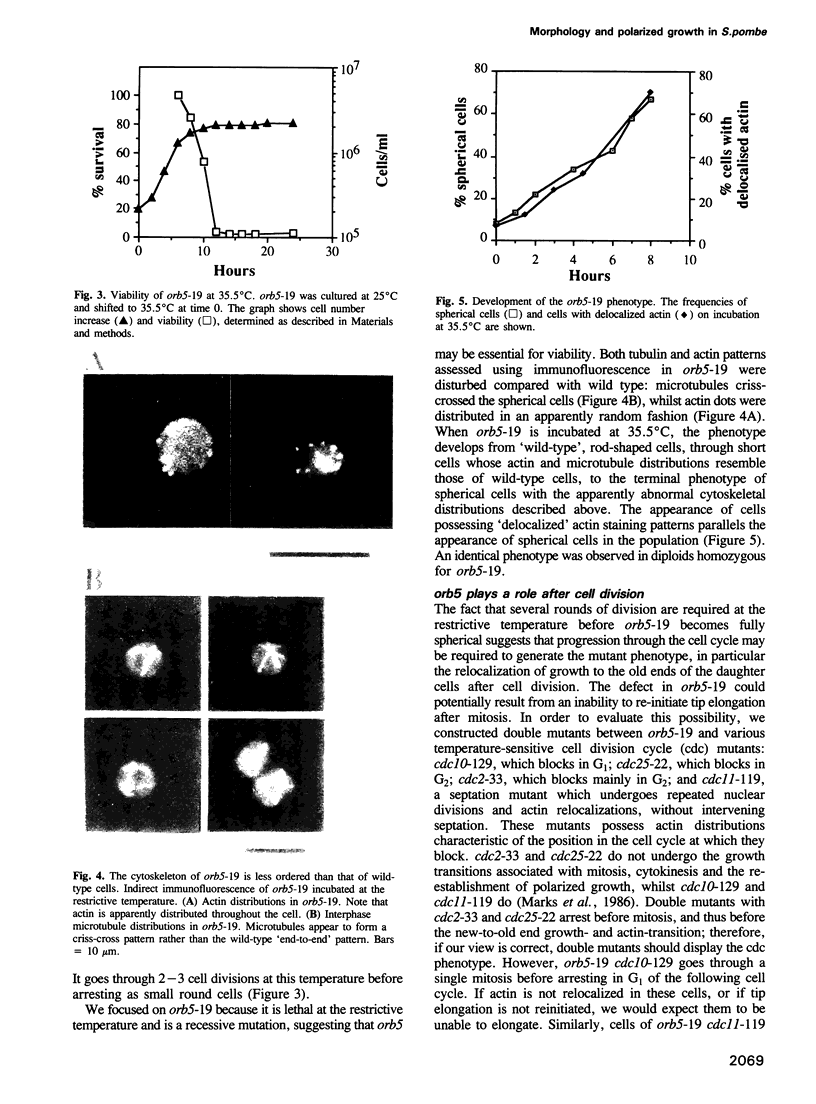
Abstract
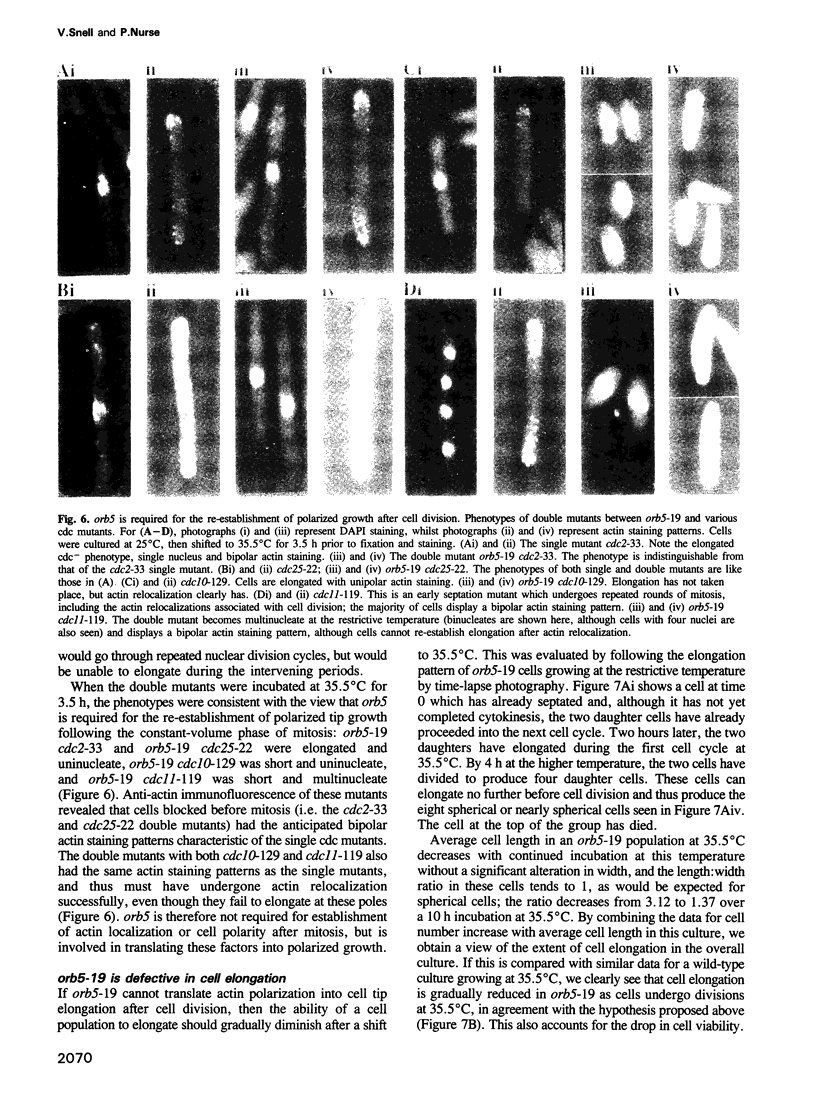
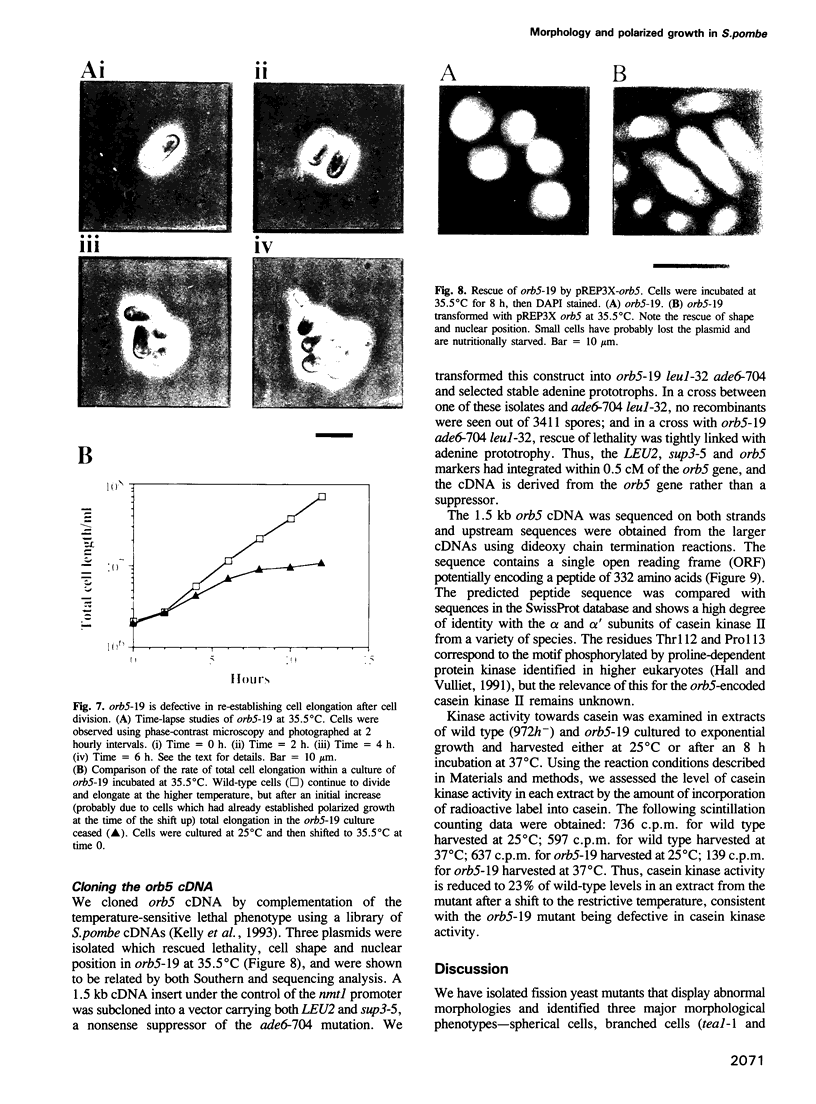
We have initiated a study to identify genes regulating cell morphogenesis in the fission yeast Schizosaccharomyces pombe. Five genes have been identified, orb1-orb5, whose mutation gives rise to spherical cells, indicative of an inability to polarize growth. Two further genes have been identified, tea1 and ban1, whose mutant alleles have disturbed patterns of tip growth, leading to T-shaped and curved cells. In fission yeast, sites of cell wall deposition are defined by actin localization, with actin distributions and therefore growth patterns undergoing cell cycle stage-specific reorganization. Studies of double mutants constructed between orb5-19 and various cdc mutants blocked before and after cell division show that orb5 is required for the re-establishment of polar growth following cytokinesis. This indicates that the mutant allele orb5-19 is defective in the reinitiation of polarized growth, even though actin reorganization to the cell tips occurs normally. orb5 encodes a fission yeast homologue of casein kinase II alpha. We propose that this kinase plays a role in the translation of cell polarity into polarized growth, but not in the establishment of polarity itself.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chant J., Corrado K., Pringle J. R., Herskowitz I. Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. Cell. 1991 Jun 28;65(7):1213–1224. doi: 10.1016/0092-8674(91)90016-r. [DOI] [PubMed] [Google Scholar]
- Chant J., Herskowitz I. Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell. 1991 Jun 28;65(7):1203–1212. doi: 10.1016/0092-8674(91)90015-q. [DOI] [PubMed] [Google Scholar]
- Chant J., Pringle J. R. Budding and cell polarity in Saccharomyces cerevisiae. Curr Opin Genet Dev. 1991 Oct;1(3):342–350. doi: 10.1016/s0959-437x(05)80298-9. [DOI] [PubMed] [Google Scholar]
- Chen-Wu J. L., Padmanabha R., Glover C. V. Isolation, sequencing, and disruption of the CKA1 gene encoding the alpha subunit of yeast casein kinase II. Mol Cell Biol. 1988 Nov;8(11):4981–4990. doi: 10.1128/mcb.8.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolska G., Boldyreff B., Issinger O. G. Cloning and sequencing of the casein kinase 2 alpha subunit from Zea mays. Biochim Biophys Acta. 1991 Dec 2;1129(1):139–140. doi: 10.1016/0167-4781(91)90230-j. [DOI] [PubMed] [Google Scholar]
- Díaz-Nido J., Serrano L., Méndez E., Avila J. A casein kinase II-related activity is involved in phosphorylation of microtubule-associated protein MAP-1B during neuroblastoma cell differentiation. J Cell Biol. 1988 Jun;106(6):2057–2065. doi: 10.1083/jcb.106.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz M., Sanchez Y., Bennett T., Sun C. R., Godoy C., Tamanoi F., Duran A., Perez P. The Schizosaccharomyces pombe cwg2+ gene codes for the beta subunit of a geranylgeranyltransferase type I required for beta-glucan synthesis. EMBO J. 1993 Dec 15;12(13):5245–5254. doi: 10.1002/j.1460-2075.1993.tb06220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell E., Bowden S., Armstrong J. A homologue of the ras-related CDC42 gene from Schizosaccharomyces pombe. Gene. 1992 May 1;114(1):153–154. doi: 10.1016/0378-1119(92)90724-4. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Kaziro Y. Molecular cloning and sequence analysis of a ras gene from Schizosaccharomyces pombe. EMBO J. 1985 Mar;4(3):687–691. doi: 10.1002/j.1460-2075.1985.tb03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kozasa T., Kaziro Y., Takeda T., Yamamoto M. Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell. 1986 Jan 31;44(2):329–336. doi: 10.1016/0092-8674(86)90767-1. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Yamamoto M. Isolation and characterization of Schizosaccharomyces pombe mutants phenotypically similar to ras1-. Mol Gen Genet. 1988 Dec;215(1):26–31. doi: 10.1007/BF00331298. [DOI] [PubMed] [Google Scholar]
- Hagan I. M., Hyams J. S. The use of cell division cycle mutants to investigate the control of microtubule distribution in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1988 Mar;89(Pt 3):343–357. doi: 10.1242/jcs.89.3.343. [DOI] [PubMed] [Google Scholar]
- Hall F. L., Vulliet P. R. Proline-directed protein phosphorylation and cell cycle regulation. Curr Opin Cell Biol. 1991 Apr;3(2):176–184. doi: 10.1016/0955-0674(91)90136-m. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y., Toda T., Yanagida M. The NDA3 gene of fission yeast encodes beta-tubulin: a cold-sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984 Dec;39(2 Pt 1):349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Horio T., Uzawa S., Jung M. K., Oakley B. R., Tanaka K., Yanagida M. The fission yeast gamma-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci. 1991 Aug;99(Pt 4):693–700. doi: 10.1242/jcs.99.4.693. [DOI] [PubMed] [Google Scholar]
- Hu E., Rubin C. S. Casein kinase II from Caenorhabditis elegans. Properties and developmental regulation of the enzyme; cloning and sequence analyses of cDNA and the gene for the catalytic subunit. J Biol Chem. 1990 Mar 25;265(9):5072–5080. [PubMed] [Google Scholar]
- Huffaker T. C., Thomas J. H., Botstein D. Diverse effects of beta-tubulin mutations on microtubule formation and function. J Cell Biol. 1988 Jun;106(6):1997–2010. doi: 10.1083/jcb.106.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C. W., Adams A. E., Szaniszlo P. J., Pringle J. R. Functions of microtubules in the Saccharomyces cerevisiae cell cycle. J Cell Biol. 1988 Oct;107(4):1409–1426. doi: 10.1083/jcb.107.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Prendergast J. A., Singer R. A. The Saccharomyces cerevisiae MYO2 gene encodes an essential myosin for vectorial transport of vesicles. J Cell Biol. 1991 May;113(3):539–551. doi: 10.1083/jcb.113.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbe T., Kobayashi I., Tanaka K. Dynamics of cytoplasmic organelles in the cell cycle of the fission yeast Schizosaccharomyces pombe: three-dimensional reconstruction from serial sections. J Cell Sci. 1989 Dec;94(Pt 4):647–656. doi: 10.1242/jcs.94.4.647. [DOI] [PubMed] [Google Scholar]
- Kelly T. J., Martin G. S., Forsburg S. L., Stephen R. J., Russo A., Nurse P. The fission yeast cdc18+ gene product couples S phase to START and mitosis. Cell. 1993 Jul 30;74(2):371–382. doi: 10.1016/0092-8674(93)90427-r. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Mann S. K., Firtel R. A., Hunter T. Molecular cloning of casein kinase II alpha subunit from Dictyostelium discoideum and its expression in the life cycle. Mol Cell Biol. 1992 Dec;12(12):5711–5723. doi: 10.1128/mcb.12.12.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori H., Yamada N., Taki A., Osumi M. Actin is associated with the formation of the cell wall in reverting protoplasts of the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1989 Dec;94(Pt 4):635–646. doi: 10.1242/jcs.94.4.635. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Bishop J. M. A putative protein kinase gene (kin1+) is important for growth polarity in Schizosaccharomyces pombe. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8272–8276. doi: 10.1073/pnas.87.21.8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozeman F. J., Litchfield D. W., Piening C., Takio K., Walsh K. A., Krebs E. G. Isolation and characterization of human cDNA clones encoding the alpha and the alpha' subunits of casein kinase II. Biochemistry. 1990 Sep 11;29(36):8436–8447. doi: 10.1021/bi00488a034. [DOI] [PubMed] [Google Scholar]
- Madden K., Costigan C., Snyder M. Cell polarity and morphogenesis in Saccharomyces cerevisiae. Trends Cell Biol. 1992 Jan;2(1):22–29. doi: 10.1016/0962-8924(92)90140-i. [DOI] [PubMed] [Google Scholar]
- Maridor G., Park W., Krek W., Nigg E. A. Casein kinase II. cDNA sequences, developmental expression, and tissue distribution of mRNAs for alpha, alpha', and beta subunits of the chicken enzyme. J Biol Chem. 1991 Feb 5;266(4):2362–2368. [PubMed] [Google Scholar]
- Marks J., Hagan I. M., Hyams J. S. Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J Cell Sci Suppl. 1986;5:229–241. doi: 10.1242/jcs.1986.supplement_5.15. [DOI] [PubMed] [Google Scholar]
- Meisner H., Heller-Harrison R., Buxton J., Czech M. P. Molecular cloning of the human casein kinase II alpha subunit. Biochemistry. 1989 May 2;28(9):4072–4076. doi: 10.1021/bi00435a066. [DOI] [PubMed] [Google Scholar]
- Miller P. J., Johnson D. I. Cdc42p GTPase is involved in controlling polarized cell growth in Schizosaccharomyces pombe. Mol Cell Biol. 1994 Feb;14(2):1075–1083. doi: 10.1128/mcb.14.2.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison J. M., Nurse P. Growth in cell length in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1985 Apr;75:357–376. doi: 10.1242/jcs.75.1.357. [DOI] [PubMed] [Google Scholar]
- Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nadin-Davis S. A., Nasim A., Beach D. Involvement of ras in sexual differentiation but not in growth control in fission yeast. EMBO J. 1986 Nov;5(11):2963–2971. doi: 10.1002/j.1460-2075.1986.tb04593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., Thuriaux P., Nasmyth K. Genetic control of the cell division cycle in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet. 1976 Jul 23;146(2):167–178. doi: 10.1007/BF00268085. [DOI] [PubMed] [Google Scholar]
- Padmanabha R., Chen-Wu J. L., Hanna D. E., Glover C. V. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol. 1990 Aug;10(8):4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Ribas J. C., Diaz M., Duran A., Perez P. Isolation and characterization of Schizosaccharomyces pombe mutants defective in cell wall (1-3)beta-D-glucan. J Bacteriol. 1991 Jun;173(11):3456–3462. doi: 10.1128/jb.173.11.3456-3462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas J. C., Roncero C., Rico H., Durán A. Characterization of a Schizosaccharomyces pombe morphological mutant altered in the galactomannan content. FEMS Microbiol Lett. 1991 Apr 15;63(2-3):263–267. doi: 10.1016/0378-1097(91)90096-s. [DOI] [PubMed] [Google Scholar]
- Roussou I., Draetta G. The Schizosaccharomyces pombe casein kinase II alpha and beta subunits: evolutionary conservation and positive role of the beta subunit. Mol Cell Biol. 1994 Jan;14(1):576–586. doi: 10.1128/mcb.14.1.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A., Padmanabha R., Glover C. V. Isolation and sequencing of cDNA clones encoding alpha and beta subunits of Drosophila melanogaster casein kinase II. Mol Cell Biol. 1987 Oct;7(10):3409–3417. doi: 10.1128/mcb.7.10.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Díaz-Nido J., Wandosell F., Avila J. Tubulin phosphorylation by casein kinase II is similar to that found in vivo. J Cell Biol. 1987 Oct;105(4):1731–1739. doi: 10.1083/jcb.105.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Hernández M. A., Díaz-Nido J., Avila J. Association of casein kinase II with microtubules. Exp Cell Res. 1989 Mar;181(1):263–272. doi: 10.1016/0014-4827(89)90200-0. [DOI] [PubMed] [Google Scholar]
- Shimanuki M., Kinoshita N., Ohkura H., Yoshida T., Toda T., Yanagida M. Isolation and characterization of the fission yeast protein phosphatase gene ppe1+ involved in cell shape control and mitosis. Mol Biol Cell. 1993 Mar;4(3):303–313. doi: 10.1091/mbc.4.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiblová E., Wolf A. Cell wall growth during the cell cycle of Schizosaccharomyces pombe. Z Allg Mikrobiol. 1972;12(8):673–684. [PubMed] [Google Scholar]
- Strome S. Determination of cleavage planes. Cell. 1993 Jan 15;72(1):3–6. doi: 10.1016/0092-8674(93)90041-n. [DOI] [PubMed] [Google Scholar]
- Toda T., Shimanuki M., Yanagida M. Fission yeast genes that confer resistance to staurosporine encode an AP-1-like transcription factor and a protein kinase related to the mammalian ERK1/MAP2 and budding yeast FUS3 and KSS1 kinases. Genes Dev. 1991 Jan;5(1):60–73. doi: 10.1101/gad.5.1.60. [DOI] [PubMed] [Google Scholar]
- Toda T., Shimanuki M., Yanagida M. Two novel protein kinase C-related genes of fission yeast are essential for cell viability and implicated in cell shape control. EMBO J. 1993 May;12(5):1987–1995. doi: 10.1002/j.1460-2075.1993.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Umesono K., Hirata A., Yanagida M. Cold-sensitive nuclear division arrest mutants of the fission yeast Schizosaccharomyces pombe. J Mol Biol. 1983 Aug 5;168(2):251–270. doi: 10.1016/s0022-2836(83)80017-5. [DOI] [PubMed] [Google Scholar]
- Ulloa L., Díaz-Nido J., Avila J. Depletion of casein kinase II by antisense oligonucleotide prevents neuritogenesis in neuroblastoma cells. EMBO J. 1993 Apr;12(4):1633–1640. doi: 10.1002/j.1460-2075.1993.tb05808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Toda T., Hayashi S., Yanagida M. Cell division cycle genes nda2 and nda3 of the fission yeast Schizosaccharomyces pombe control microtubular organization and sensitivity to anti-mitotic benzimidazole compounds. J Mol Biol. 1983 Aug 5;168(2):271–284. doi: 10.1016/s0022-2836(83)80018-7. [DOI] [PubMed] [Google Scholar]
- Walker G. M. Cell cycle specificity of certain antimicrotubular drugs in Schizosaccharomyces pombe. J Gen Microbiol. 1982 Jan;128(1):61–71. doi: 10.1099/00221287-128-1-61. [DOI] [PubMed] [Google Scholar]
- Woods A., Sherwin T., Sasse R., MacRae T. H., Baines A. J., Gull K. Definition of individual components within the cytoskeleton of Trypanosoma brucei by a library of monoclonal antibodies. J Cell Sci. 1989 Jul;93(Pt 3):491–500. doi: 10.1242/jcs.93.3.491. [DOI] [PubMed] [Google Scholar]