Abstract
Background and Purpose
It is unknown whether supraventricular arrhythmias other than atrial fibrillation or flutter are associated with stroke.
Methods
To examine the association between paroxysmal supraventricular tachycardia (PSVT) and stroke, we performed a retrospective cohort study using administrative claims data from all emergency department encounters and hospitalizations at California’s nonfederal acute care hospitals in 2009. Our cohort comprised all adult patients with ≥1 emergency department visit or hospitalization from which they were discharged alive and without a diagnosis of stroke. Our primary exposure was a diagnosis of PSVT recorded at an encounter before stroke or documented as present-on-admission at the time of stroke. To reduce confounding, we excluded patients with diagnoses of atrial fibrillation. We defined PSVT, stroke, and atrial fibrillation using International Classification of Diseases, Ninth Revision, Clinical Modification codes previously validated by detailed chart review.
Results
Of 4 806 830 eligible patients, 14 121 (0.29%) were diagnosed with PSVT and 14 402 (0.30%) experienced a stroke. The cumulative rate of stroke after PSVT diagnosis (0.94%; 95% confidence interval, 0.76%–1.16%) significantly exceeded the rate among patients without a diagnosis of PSVT (0.21%; 95% confidence interval, 0.21%–0.22%). In Cox proportional hazards analysis controlling for demographic characteristics and potential confounders, PSVT was independently associated with a higher risk of subsequent stroke (hazard ratio, 2.10; 95% confidence interval, 1.69–2.62).
Conclusions
In a large and demographically diverse sample of patients, we found an independent association between PSVT and ischemic stroke. PSVT seems to be a novel risk factor that may account for some proportion of strokes that are currently classified as cryptogenic.
Keywords: arrhythmia, embolism, epidemiology, fibrillation, risk factors, stroke, tachycardia
One third of ischemic strokes remain cryptogenic because diagnostic evaluation fails to reveal an exact cause.1 This uncertainty prevents optimal treatment of risk factors and impedes efforts to prevent stroke. Accumulating data suggest that some strokes arise from forms of atrial electric dysfunction other than clinically apparent atrial fibrillation or flutter (AF)—currently the only arrhythmias that are established stroke risk factors.2 Specifically, subclinical AF episodes as brief as 6 minutes seem sufficient to increase stroke risk.3 Furthermore, patients with cardioembolic-appearing cryptogenic stroke manifest nonspecific supraventricular arrhythmias more often than patients with other types of stroke.4 In addition, several studies have found an association between frequent supraventricular ectopy and stroke even in the absence of AF.5–7
At the same time, epidemiological evidence suggests that paroxysmal supraventricular tachycardia (PSVT) is more closely linked to cardiovascular disease than traditionally appreciated. On the basis of reports from referral centers, PSVT was long viewed as an isolated conduction disorder manifesting mostly in young and otherwise healthy patients.8 However, more recent population-based data indicate that PSVT affects mostly older patients with a high burden of cardiovascular disease.9 Therefore, rather than simply reflecting an atrial or atrioventricular nodal anomaly present at birth, PSVT may also be a manifestation of acquired atrial myocardial disease in the setting of cardiovascular risk factors.
Together, these findings suggest that even before AF develops, atrial disease may manifest as other supraventricular arrhythmias, such as PSVT, and increase stroke risk through some combination of hypercoagulability,10 inflammation,11 endothelial injury,12 and structural changes.13 A confirmed association between PSVT and stroke might explain some proportion of currently cryptogenic strokes, many of which seem radiographically to have resulted from cardiac embolism.14 However, despite these suggestive data and their potential implications, it remains unknown whether PSVT is a risk factor for stroke. Therefore, we, examined the association between clinically documented diagnoses of PSVT and subsequent stroke.
Materials and Methods
Design
We performed a retrospective cohort study using the 2009 California State Inpatient Database and State Emergency Department Database. The California Office of Statewide Healthcare Planning and Development collects data about all emergency department (ED) visits and hospital stays at nonfederal acute care hospitals in California. After quality-checking, these data are provided in a deidentified format to the Agency for Healthcare Quality and Research for its Healthcare Cost and Utilization Project.15 A unique record linkage number for each patient allows longitudinal tracking of ED encounters and hospitalizations.16 Up to 25 discharge diagnoses are coded at each encounter using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and a separate code indicates whether each diagnosis was present before hospital admission or developed during the hospitalization.17 Because this publicly available database includes only deidentified data, our study was exempt from review by the Weill Cornell institutional review board.
Patients
Our cohort comprised adult patients with ≥1 ED visit or hospitalization from which they were discharged alive. To reduce the proportion of patients with pre-existing cerebrovascular disease and enrich our cohort for incident cases of stroke, we excluded patients with a stroke diagnosis at their index encounter. To maximize follow-up, we excluded non-California residents and patients with missing tracking numbers.
Measurements
Our primary outcome was ischemic stroke, defined as ICD-9-CM codes 433.x1, 434.x1, or 436 in any hospital discharge diagnosis position without an accompanying code for rehabilitation (V57), trauma (800–804 or 850–854), intracerebral hemorrhage (ICH; 431), or sub-arachnoid hemorrhage (430). This algorithm has been shown to have 86% sensitivity and 95% specificity for ischemic stroke when validated by detailed chart review.18 Patients were censored at the time of their first stroke hospitalization; this has been shown to produce samples in which 88% of strokes are incident cases.18
Our primary exposure variable was any ED or hospital discharge diagnosis of PSVT, defined by an ICD-9-CM code (427.0) previously validated by detailed chart review to have a 92% positive predictive value.19 Diagnoses of PSVT were considered exposures only if recorded at encounters before stroke or documented as present-on-admission (POA) at the time of the first stroke. POA codes in the California State Inpatient Database are rarely falsely positive (<14% of cases),20 and POA codes for arrhythmias have been shown to have 90% agreement with blinded chart review,21 indicating that PSVT diagnoses coded as POA are highly likely to represent pre-existing disease. Nevertheless, to address the possibility that some cases of PSVT labeled as POA were neurocardiogenic arrhythmias caused by stroke, we examined the association between PSVT and ICH, which tends to cause at least as much neurocardiogenic injury.22 ICH was defined using a validated algorithm similar to that for ischemic stroke.18 To further explore the temporal relationship between PSVT and stroke, and to address the possibility that PSVT may be more readily documented in patients hospitalized for stroke, we performed a sensitivity analysis limited to PSVT diagnoses recorded at ED visits or hospitalizations preceding the first stroke hospitalization.
When examining the association between PSVT and stroke, we excluded patients with AF documented before or anytime during the initial stroke hospitalization because AF is associated with PSVT23 and causes stroke.24 We defined AF based on ICD-9-CM codes (427.31 and 427.32) that have been validated as 95% sensitive and 99% specific.25
To control for other potential confounders in the relationships between our exposures and outcomes, we adjusted for age, sex, race, insurance status, and cardiovascular comorbidities previously reported as risk factors for stroke and PSVT: hypertension, diabetes mellitus, coronary heart disease, congestive heart failure, peripheral vascular disease, chronic kidney disease, and chronic obstructive pulmonary disease.9,19,26 All comorbidities were defined using the Healthcare Cost and Utilization Project’s Clinical Classification Software categorization scheme.15
Statistical Analyses
Patients were assumed to enter observation on January 1, 2009, and were censored at the time of death or first stroke or on December 31, 2009. Cumulative outcome rates were calculated using Kaplan–Meier survival statistics and compared using the log-rank test. Cox proportional hazards analyses were used to examine the associations between our exposures and outcomes, while controlling for the confounders listed above. The goal of our study was to isolate the relationship between PSVT and stroke, rather than to create a parsimonious prediction model, so all model covariates were left in place regardless of their statistical significance. We assessed for confounders that may have remained after these adjustments by using the same model to examine the association between PSVT and ICH, which shares many of the same risk factors27 but has no apparent reason to be independently associated with PSVT.
To gauge the face validity of associations between arrhythmias and stroke in this cohort, we also used the same model to examine the stroke risk associated with AF, which has been established to increase stroke risk 3-fold.24 To further explore the plausibility of stroke risk from PSVT, which would be expected to cause cardioembolic stroke and not be associated with other types of stroke, we performed 2 sensitivity analyses focused on stroke mechanisms. First, we limited our outcome to ICD-9-CM code 434.11, which has 73% specificity for embolic stroke subtypes.28 Second, we performed a multiple logistic regression analysis to test the hypothesis that among stroke patients, prior diagnoses of PSVT would be less common in those with documented carotid endarterectomy procedures (ICD-9-CM code 38.12) because these patients would predominantly have had large-artery atherosclerotic stroke mechanisms.
On the basis of prior reports of age- and sex-dependent heterogeneity in PSVT mechanisms and stroke risk factors,9,29,30 we performed prespecified secondary analyses stratified by sex and age (dichotomized as <65 or ≥65 years, approximately the median age in a population-based cohort of patients with PSVT)9 and checked interaction terms between PSVT and age and sex in multivariable models.
We analyzed PSVT as a time-varying covariate and verified the proportional hazards assumption by including an interaction term between PSVT and time. To account for facility-level characteristics that may differentially affect the diagnosis of PSVT, all models used robust standard errors accounting for clustering by facility. In addition to those described above, we performed numerous other sensitivity analyses to explore the possibility of residual confounding and bias further (Appendix in the online-only Data Supplement).
Statistical analysis was performed using Stata MP (version 12, StataCorp, TX). The threshold of statistical significance was set at α=0.05 for all analyses.
Results
Among 4 806 830 adults without a diagnosis of stroke at their index visit (Table I in the online-only Data Supplement), 14 121 (0.29%; 95% confidence interval [CI], 0.29%–0.30%) had or eventually received a diagnosis of PSVT, and 14 402 (0.30%; 95% CI, 0.29%–0.30%) eventually received a diagnosis of ischemic stroke (Table II in the online-only Data Supplement). Patients with PSVT were older and had significantly more cardiovascular risk factors than those without PSVT (Table 1).
Table 1.
Baseline Characteristics of Cohort Stratified by Diagnosis of Paroxysmal Supraventricular Tachycardia*
| Characteristic | Overall (n=4 806 830) | PSVT (n=14 121) | No PSVT (n=4 792 709) |
|---|---|---|---|
| Age, median (IQR), y | 46 (30–62) | 69 (55–81) | 46 (30–62) |
| Women | 2 833 725 (59.0) | 8522 (60.3) | 2 825 203 (58.9) |
| Race/ethnicity† | |||
| White | 2 451 146 (53.8) | 9005 (66.3) | 2 442 141 (53.8) |
| Black | 441 114 (9.7) | 1238 (9.1) | 439 876 (9.7) |
| Hispanic | 1 205 595 (26.5) | 1952 (14.4) | 1 203 643 (26.5) |
| Asian or Pacific Islander | 314 120 (6.9) | 1081 (8.0) | 313 039 (6.9) |
| Native American | 6074 (0.1) | 7 (0.1) | 6067 (0.1) |
| Other | 135 763 (3.0) | 295 (2.2) | 135 468 (3.0) |
| Payment source | |||
| Medicare | 1 144 318 (23.8) | 8007 (56.7) | 1 136 311 (23.7) |
| Medicaid | 708 395 (14.7) | 1039 (7.4) | 707 356 (14.8) |
| Private insurance | 1 992 935 (41.5) | 4202 (29.8) | 1 988 733 (41.5) |
| Self-pay | 662 013 (13.8) | 539 (3.8) | 661 474 (13.8) |
| Other | 298 476 (6.2) | 331 (2.3) | 298 145 (6.2) |
| Vascular risk factors | |||
| Hypertension | 1 101 552 (22.9) | 7269 (51.5) | 1 094 283 (22.8) |
| Diabetes mellitus | 535 186 (11.1) | 3193 (22.6) | 531 993 (11.1) |
| Coronary heart disease | 300 321 (6.2) | 3155 (22.3) | 297 166 (6.2) |
| COPD | 200 162 (4.2) | 1911 (13.5) | 198 251 (4.1) |
| Congestive heart failure | 152 373 (3.2) | 2188 (15.5) | 150 185 (3.1) |
| Renal insufficiency | 146 031 (3.0) | 1589 (11.3) | 144 442 (3.0) |
| PVD | 67 154 (1.4) | 754 (5.3) | 66 400 (1.4) |
COPD indicates chronic obstructive pulmonary disease; IQR, interquartile range; PSVT, paroxysmal supraventricular tachycardia; and PVD, peripheral vascular disease.
Data are presented as number (%) of participants unless otherwise specified.
The apparent racial/ethnic imbalances among patients with and without PSVT are attenuated after accounting for age; for example, among those ≥65 y of age, 70.6% of patients with PSVT and 67.8% of patients without PSVT were white.
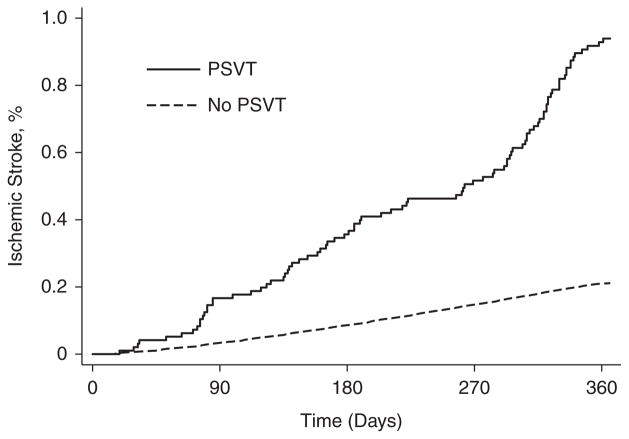
Stroke occurred a median 70 days (interquartile range, 18–157 days) after the index visit. After excluding patients with AF, the cumulative stroke rate after a diagnosis of PSVT (0.94%; 95% CI, 0.76%–1.16%) significantly exceeded the rate among patients without PSVT (0.21%; 95% CI, 0.21%–0.22%; P<0.001, log-rank test; Figure). In a Cox proportional hazards model controlling for demographic characteristics and potential confounders, PSVT was independently associated with subsequent stroke (hazard ratio [HR], 2.10; 95% CI, 1.69–2.62). This association remained essentially unchanged in numerous sensitivity analyses (Table III in the online-only Data Supplement). No violation of the proportional hazards assumption was apparent.
Figure.
Cumulative rates of ischemic stroke are shown according to whether or not patients had a preexisting diagnosis of paroxysmal supraventricular tachycardia (PSVT).
Interaction terms revealed significant evidence of variation in the association between PSVT and stroke by age (P=0.03) but not by sex (P=0.66). PSVT was associated with a higher risk of stroke in patients ≥65 years of age (unadjusted cumulative rate, 1.72% versus 0.66%; adjusted HR, 2.48; 95% CI, 1.96–3.13) but not in patients <65 years of age (unadjusted cumulative rate, 0.17% versus 0.10%; adjusted HR, 0.53; 95% CI, 0.23–1.25). Post hoc exploratory analyses across increasing decades of age confirmed that the association becomes apparent at ≈65 years of age (Table IV in the online-only Data Supplement).
Supporting the face validity of these findings, we found a heightened cumulative rate of stroke among patients with AF (2.20%; 95% CI, 2.14%–2.27%) and an expectedly strong association between AF diagnoses and subsequent stroke (HR, 3.35; 95% CI, 3.19–3.52). In sensitivity analyses limited to arrhythmias documented before the first stroke hospitalization, these associations were attenuated but remained significant for both PSVT (HR, 1.33; 95% CI, 1.02–1.73) and AF (HR, 1.86; 95% CI, 1.76–1.96). Conversely, we found no association between PSVT and the 2700 incident cases of ICH in this cohort (HR, 0.92; 95% CI, 0.53–1.58).
Our main finding remained essentially unchanged when we limited our outcome to diagnoses of embolic stroke (HR, 1.80; 95% CI, 0.89–3.65), although the result was not statistically significant given the smaller sample size. Similarly, patients who underwent documented carotid endarterectomy procedures (and, therefore, presumably had large-artery atherosclerosis) seemed to have fewer PSVT diagnoses than other patients with stroke (odds ratio, 0.29; 95% CI, 0.04–2.18).
Discussion
In a large and demographically diverse sample of patients, we found an independent association between diagnoses of PSVT and subsequent ischemic stroke. This relationship remained significant after controlling for patient-level confounders and clustering by hospital facility and persisted in a sensitivity analysis limited to diagnoses of PSVT recorded at ED visits or hospitalizations before stroke. As expected, we also found a strong association between diagnoses of AF and subsequent stroke.24 However, we found no association between diagnoses of PSVT and ICH, which shares many of the same risk factors as ischemic stroke,27 but has no obviously apparent reason to be caused by PSVT. These secondary findings provide some assurance that the association we found between PSVT and stroke is valid and not because of bias or residual confounding.
Our findings are supported by prior studies suggesting a relationship between stroke and other forms of atrial electric dysfunction besides AF. Engström et al7 prospectively performed ambulatory cardiac monitoring in a cohort of 402 men and found that AF-free patients with the highest quintile of supraventricular ectopy faced a 2-fold elevated risk of stroke. In a similar study, Binici et al5 also found that the highest decile of supraventricular ectopy conferred an elevated risk of subsequent stroke (HR, 2.79), even after censoring patients who developed AF. Furthermore, patients with stroke seem to manifest more supraventricular ectopy than nonstroke controls.31 Finally, patients with cryptogenic stroke demonstrate more nonspecific supraventricular arrhythmias than those with established noncardioembolic stroke mechanisms.4,32 Although these studies have relatively small sample sizes and homogenous populations, they complement and support our findings that stroke risk from supraventricular arrhythmias is not limited to AF alone.
Our results also resonate with epidemiological findings that PSVT is more heterogeneous and closely linked to cardiovascular disease than originally appreciated.9 These data are buttressed by evidence that the mechanism of PSVT varies with age. Although atrioventricular nodal reentrant tachycardia is most common across all ages, younger patients often have atrioventricular reentrant tachycardia—reflecting the presence of a bypass tract, which is a remnant of abnormal embryogenesis—whereas older patients often have focal atrial tachycardia, which reflects possible age-related changes to the atrioventricular node and atrial myocardium29 and additional injury from acquired factors associated with cardiovascular disease.9 Consistent with this age-dependent heterogeneity in the mechanisms of PSVT, our results indicate that age may be an effect modifier in the relationship between PSVT and stroke. Although PSVT in younger patients seems benign, PSVT in older patients may reflect underlying atrial disease that predisposes to stroke, possibly through some of the same acquired mechanisms by which AF increases stroke risk.10–13,33 Given the limitations of administrative claims data, further exploring this mechanistic hypothesis will require prospective studies incorporating detailed investigations, such as transesophageal echocardiography, biomarkers, and cardiac imaging.
Our study has 3 key limitations. First, we lacked important clinical information, such as medication use and detailed characterization of cardiac disease, which may have resulted in residual confounding. Second, although our cohort was geographically and demographically diverse, it was not strictly population-based because we included only patients evaluated in an ED or hospitalized at least once in 2009. These patients are likely to have been more severely ill than PSVT patients without an ED visit or hospitalization, which may have resulted in significant case ascertainment bias and increased the apparent association between PSVT and stroke. However, the same limitation could also have led to underascertainment of PSVT in patients hospitalized with stroke because we used a PSVT diagnosis code with high specificity19 but low sensitivity.9 Overall, the baseline characteristics of patients with PSVT in our cohort closely resemble those of patients in a strictly population-based study of PSVT9 (Table 2); this supports the generalizability of our results because it argues that our cohort was representative of the overall population of patients with PSVT. Third, some patients with PSVT diagnoses may have had undiagnosed or undocumented AF, thereby confounding the relationship between PSVT and stroke. We attempted to guard against this by using highly sensitive ICD-9-CM codes25 to exclude AF patients from our analyses of PSVT and stroke. Furthermore, a prospective study using periodic cardiac monitoring documented AF in 12% of PSVT patients,23 and undiagnosed or undocumented AF, which confers a 3-fold higher risk of stroke,24 in 10% to 20% of patients with PSVT would not completely explain the 2-fold higher risk of stroke seen with PSVT in our cohort. Our results instead suggest that underlying atrial disease may sometimes manifest as PSVT and increase stroke risk before AF develops. This would be consistent with recent findings that a substantial proportion of patients with cryptogenic stroke manifest nonspecific supraventricular arrhythmias but no AF during several weeks of continuous poststroke cardiac monitoring,34,35 only to manifest AF several months later.36
Table 2.
Baseline Characteristics of Patients With Paroxysmal Supraventricular Tachycardia Compared With Population-Based Marshfield Epidemiological Study Area Cohort*
| Characteristic | California Cohort | Marshfield Cohort9 |
|---|---|---|
| Age, median, y | 69 | 63 |
| Women | 60 | 70 |
| Hypertension | 52 | 46 |
| Coronary heart disease | 22 | 21 |
| Congestive heart failure | 16 | 18 |
Data are presented as percentage of participants unless otherwise specified.
Given that ≈750 000 patients in the United States have PSVT,9 our results point to a novel stroke risk factor that may account for some proportion of currently cryptogenic strokes. In light of the limitations of the administrative claims data on which our study is based, our findings are hypothesis-generating and require confirmation in prospective studies of the epidemiological and mechanistic link between PSVT and stroke. If confirmed in other studies, knowledge of this association may lead to the evaluation and adoption of new antithrombotic strategies that could ultimately reduce the substantial public health burden of stroke.
Supplementary Material
Acknowledgments
Sources of Funding
This study was funded by grant 13SDG14560037 from the American Heart Association (Dr Kamel).
Footnotes
Disclosures
Dr Elkind serves as a consultant for BMS-Pfizer. Dr Okin serves on an advisory board for GE Medical Systems. The other authors have no conflict to report.
References
- 1.Marnane M, Duggan CA, Sheehan OC, Merwick A, Hannon N, Curtin D, et al. Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, A-S-C-O, and Causative Classification system. Stroke. 2010;41:1579–1586. doi: 10.1161/STROKEAHA.109.575373. [DOI] [PubMed] [Google Scholar]
- 2.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 3.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 4.Alhadramy O, Jeerakathil TJ, Majumdar SR, Najjar E, Choy J, Saqqur M. Prevalence and predictors of paroxysmal atrial fibrillation on Holter monitor in patients with stroke or transient ischemic attack. Stroke. 2010;41:2596–2600. doi: 10.1161/STROKEAHA.109.570382. [DOI] [PubMed] [Google Scholar]
- 5.Binici Z, Intzilakis T, Nielsen OW, Køber L, Sajadieh A. Excessive supraventricular ectopic activity and increased risk of atrial fibrillation and stroke. Circulation. 2010;121:1904–1911. doi: 10.1161/CIRCULATIONAHA.109.874982. [DOI] [PubMed] [Google Scholar]
- 6.Chong BH, Pong V, Lam KF, Liu S, Zuo ML, Lau YF, et al. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2012;14:942–947. doi: 10.1093/europace/eur389. [DOI] [PubMed] [Google Scholar]
- 7.Engström G, Hedblad B, Juul-Möller S, Tydén P, Janzon L. Cardiac arrhythmias and stroke: increased risk in men with high frequency of atrial ectopic beats. Stroke. 2000;31:2925–2929. doi: 10.1161/01.str.31.12.2925. [DOI] [PubMed] [Google Scholar]
- 8.Ganz LI, Friedman PL. Supraventricular tachycardia. N Engl J Med. 1995;332:162–173. doi: 10.1056/NEJM199501193320307. [DOI] [PubMed] [Google Scholar]
- 9.Orejarena LA, Vidaillet H, Jr, DeStefano F, Nordstrom DL, Vierkant RA, Smith PN, et al. Paroxysmal supraventricular tachycardia in the general population. J Am Coll Cardiol. 1998;31:150–157. doi: 10.1016/s0735-1097(97)00422-1. [DOI] [PubMed] [Google Scholar]
- 10.Inoue H, Nozawa T, Okumura K, Jong-Dae L, Shimizu A, Yano K. Prothrombotic activity is increased in patients with nonvalvular atrial fibrillation and risk factors for embolism. Chest. 2004;126:687–692. doi: 10.1378/chest.126.3.687. [DOI] [PubMed] [Google Scholar]
- 11.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 12.Conway DS, Pearce LA, Chin BS, Hart RG, Lip GY. Plasma von Willebrand factor and soluble p-selectin as indices of endothelial damage and platelet activation in 1321 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors. Circulation. 2002;106:1962–1967. doi: 10.1161/01.cir.0000033220.97592.9a. [DOI] [PubMed] [Google Scholar]
- 13.Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012;60:531–538. doi: 10.1016/j.jacc.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, et al. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382–390. doi: 10.1002/ana.410250410. [DOI] [PubMed] [Google Scholar]
- 15.Agency for Healthcare Research and Quality. [Accessed February 7, 2013];Healthcare Cost and Utilization Project. Available at http://hcupnet.ahrq.gov.
- 16.Agency for Healthcare Research and Quality. [Accessed February 7, 2013];HCUP methods series: methodological issues when studying readmissions and revisits using hospital administrative data. Available at http://www.hcup-us.ahrq.gov/reports/methods/2011_01.pdf.
- 17.Agency for Healthcare Research and Quality. [Accessed February 7, 2013];HCUP methods series: the case for the POA indicator. Available at http://www.hcup-us.ahrq.gov/reports/methods/2011_05.pdf.
- 18.Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 19.Sidney S, Sorel M, Quesenberry CP, Jr, DeLuise C, Lanes S, Eisner MD. COPD and incident cardiovascular disease hospitalizations and mortality: Kaiser Permanente Medical Care Program. Chest. 2005;128:2068–2075. doi: 10.1378/chest.128.4.2068. [DOI] [PubMed] [Google Scholar]
- 20.Goldman LE, Chu PW, Osmond D, Bindman A. The accuracy of present-on-admission reporting in administrative data. Health Serv Res. 2011;46:1946–1962. doi: 10.1111/j.1475-6773.2011.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248–2254. doi: 10.1001/jama.2011.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hays A, Diringer MN. Elevated troponin levels are associated with higher mortality following intracerebral hemorrhage. Neurology. 2006;66:1330–1334. doi: 10.1212/01.wnl.0000210523.22944.9b. [DOI] [PubMed] [Google Scholar]
- 23.Hamer ME, Wilkinson WE, Clair WK, Page RL, McCarthy EA, Pritchett EL. Incidence of symptomatic atrial fibrillation in patients with paroxysmal supraventricular tachycardia. J Am Coll Cardiol. 1995;25:984–988. doi: 10.1016/0735-1097(94)00512-o. [DOI] [PubMed] [Google Scholar]
- 24.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 25.Glazer NL, Dublin S, Smith NL, French B, Jackson LA, Hrachovec JB, et al. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167:246–252. doi: 10.1001/archinte.167.3.246. [DOI] [PubMed] [Google Scholar]
- 26.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics, 2013 update. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGrath ER, Kapral MK, Fang J, Eikelboom JW, óConghaile A, Canavan M, et al. Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke. 2012;43:2048–2054. doi: 10.1161/STROKEAHA.112.654145. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29:1602–1604. doi: 10.1161/01.str.29.8.1602. [DOI] [PubMed] [Google Scholar]
- 29.Porter MJ, Morton JB, Denman R, Lin AC, Tierney S, Santucci PA, et al. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm. 2004;1:393–396. doi: 10.1016/j.hrthm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Kristensen B, Malm J, Carlberg B, Stegmayr B, Backman C, Fagerlund M, et al. Epidemiology and etiology of ischemic stroke in young adults aged 18 to 44 years in northern Sweden. Stroke. 1997;28:1702–1709. doi: 10.1161/01.str.28.9.1702. [DOI] [PubMed] [Google Scholar]
- 31.Abboud H, Berroir S, Labreuche J, Orjuela K, Amarenco P GENIC Investigators. Insular involvement in brain infarction increases risk for cardiac arrhythmia and death. Ann Neurol. 2006;59:691–699. doi: 10.1002/ana.20806. [DOI] [PubMed] [Google Scholar]
- 32.Todo K, Moriwaki H, Saito K, Naritomi H. Frequent premature atrial contractions in stroke of undetermined etiology. Eur Neurol. 2009;61:285–288. doi: 10.1159/000206853. [DOI] [PubMed] [Google Scholar]
- 33.Stöllberger C, Chnupa P, Kronik G, Brainin M, Finsterer J, Schneider B, et al. Transesophageal echocardiography to assess embolic risk in patients with atrial fibrillation. ELAT Study Group. Embolism in Left Atrial Thrombi. Ann Intern Med. 1998;128:630–638. doi: 10.7326/0003-4819-128-8-199804150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Tayal AH, Tian M, Kelly KM, Jones SC, Wright DG, Singh D, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology. 2008;71:1696–1701. doi: 10.1212/01.wnl.0000325059.86313.31. [DOI] [PubMed] [Google Scholar]
- 35.Kamel H, Navi BB, Elijovich L, Josephson SA, Yee AH, Fung G, et al. Pilot randomized trial of outpatient cardiac monitoring after cryptogenic stroke. Stroke. 2013;44:528–530. doi: 10.1161/STROKEAHA.112.679100. [DOI] [PubMed] [Google Scholar]
- 36.Wallmann D, Tüller D, Wustmann K, Meier P, Isenegger J, Arnold M, et al. Frequent atrial premature beats predict paroxysmal atrial fibrillation in stroke patients: an opportunity for a new diagnostic strategy. Stroke. 2007;38:2292–2294. doi: 10.1161/STROKEAHA.107.485110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.