Abstract
Efficient, inexpensive and sensitive assays for the measurement of drugs are of interest for pharmacokinetic and pharmacodynamics (PK-PD) analysis. Dried blood spots (DBS) are a unique bioanaltyical matrix with the potential to fulfill this interest for the measurement of numerous analytes. Here we describe the development and validation of a reversed-phase high performance liquid chromatographic (LC), tandem mass spectrometry (MS/MS) assay for the determination of ribavirin (RBV) in DBS. A 3mm punch from spotted and dried whole blood was extracted in methanol utilizing isotopically labeled internal standard for LC-MS/MS analysis. Validation was performed over a range of 0.05 μg/mL to 10.0 μg/mL and the method was shown to be precise (coefficient of variation ≤ 15%) and accurate (within ±15% of control). These acceptance criteria were met for hematocrit ranges of 20-54%, for center versus edge punches and for spot volumes from 10-60 μL. RBV was stable for up to 140 days at room temperature and −20°C as well as for three freeze/thaw cycles. Correlation of RBV in DBS versus in plasma yielded r2 ≥ 0.98 demonstrating that DBS can be used as an alternative to plasma for PK-PD studies in human subjects.
Keywords: Ribavirin, dried blood spot, analytical method, nucleoside analog, hepatitis C, LC-MS/MS
1. Introduction
Ribavirin (RBV) is a nucleoside analog used for the past 15 years in the treatment of hepatitis C. Although the mechanism of action is still debated, RBV has been essential for increased cure rate of hepatitis C in combination with other drugs. Therapeutic drug monitoring of RBV is important because this drug causes hemolytic anemia leading to dose reduction and even the cessation of treatment [1]. However, existing assays to gain PK-PD information require venous blood draw, separation of plasma and a lengthy extraction process
Recently, dried blood spots (DBS) have become of interest for the quantification of various drugs for pharmacokinetic (PK) studies [2, 3]. Analysis of DBS by LC-MS/MS has been used for over 20 years to quantify a wide variety of analytes for different purposes, including genetic disease screening and therapeutic drug monitoring [4]. DBS is especially attractive for these studies because samples can be easily and less invasively obtained via finger or heel stick, processed cheaply and quickly and stored more efficiently compared to plasma samples. Additionally, the low volume requirement (~25 μL) is useful when dealing with special patient populations such as pediatrics or those suffering from anemia. It also allows for measurement of RBV in samples obtained from patients at sites without laboratory capabilities and there are no special shipping costs or requirements associated with DBS samples [5]. We present here the development and validation of an assay to quantify RBV in DBS using a sensitive LC-MS/MS technique.
2. Methods
2.1 Chemicals and Materials
RBV was purchased from Sigma Life Sciences (St. Louis, MO) and RBV isotopic internal standard (RBV-IS) was purchased from Toronto Research Chemicals (TRC Canada). HPLC grade methanol, formic acid and acetonitrile were purchased from Fisher Scientific (Fairlawn, NJ) as well as Whatman 903 protein saver cards, desiccants and humidity indicators. Human blank whole blood with K3 EDTA was acquired from Biological Specialty Corporation and K2 EDTA whole blood was obtained from volunteers who consented for IRB-approved protocols.
2.2 Preparation of stocks, standard calibrators, quality controls (QCs) and internal standard (IS)
Prep stocks at a concentration of 1 mg/mL were prepared for RBV standard calibrators and QCs separately by dissolving approximately 5 mg of RBV into an equal volume of ultrapure water (UPH2O). Each solution was used to make the standard and QC working stocks to be diluted for the appropriate DBS concentration. To prepare the calibration standards, 25 μL of working stock was added to 475 μL of whole blood followed by immediate spotting onto Whatman 903 protein saver cards at 30 μL per spot, except for the spot volume experiment in section 3.5. The pipet tip was not allowed to touch the paper when spotting and cards were set to dry for 2 hours and up to overnight at room temperature. Standards H-A were made at concentrations of 0.05, 0.125, 0.25, 0.5, 1.25, 2.5, 5.0 and 10.0 μg/mL. Quality controls were prepared in an identical fashion using their specific 1 mg/mL prep stock at levels of lower limit of quantitation (LLOQ): 0.05 μg/mL, QL (low): 0.15 μg/mL (3× LLOQ), QM (medium): 3.0 μg/mL and QH (high): 8.0 μg/mL (80% of highest standard).
Internal standard solution (RBV-IS) was prepared in two steps. First, a 40 pmol/μL stock solution was diluted to 0.5 pmol/μL. Secondly, 5 mL of this solution was transferred to a new 15 mL conical tube and diluted to a final concentration of 0.25 pmol/μL. All solutions were stored at 4°C.
2.3 DBS extraction
Once cards were dried, a single 3 mm diameter circle was punched from the edge (except for section 3.7 of this manuscript) for each standard and QC and placed in 200 μL of methanol followed by the addition of 20 μL RBV-IS. The samples were then subjected to 10 minutes of sonication. Samples were dried under nitrogen at 40°C, reconstituted in 100 μL of UPH2O and placed in auto-sampler vials for injection onto the LC-MS/MS system.
DBS samples were collected from patients consented to participate in IRB approved protocols. Each whole blood sample was collected in an EDTA Vacutainer tube followed by immediate spotting of 30 μL onto Whatman 903 protein saver paper and extracted as described above. Samples from human subjects were used to compliment stability testing in QCs. A total of 28 DBS samples collected from human subjects were paired to plasma results obtained from a previously validated method [6]to explore the correlation between the two methods.
2.4 LC-MS/MS instrumentation
RBV and RBV-IS were quantified using a Thermo Scientific TSQ Vantage® triple quadrupole mass spectrometer coupled with a Thermo Scientific Accela® UHP pump and CTC analytics HTC PAL® autosampler based on previous methods [7-9]. The system utilized an electrospray ionization (ESI) probe (Ion Max HESI II®) operated in positive mode. Selective and highly selective (SRM and hSRM) reaction monitoring [precursor/product] m/z transitions were: RBV (245.056/133.110) and RBV-IS (250.100/113.110). The instrument injection volume was 10 μL and separation was achieved using an isocratic mobile phase (0.1% formic acid in 2% acetonitrile: 98% UPH2O) and a flow rate of 200 μL/min. A Develosil 3μ Reverse Phase-Aqueous, C30, 140A, 150×2.0mm column was purchased from Phenomenex for chromatographic separation. Total run time was 4.0 minutes with an approximate retention time of 3.0 minutes for RBV. Data were analyzed using Xcalibur™2.0.7 software from Thermo Scientific.
2.5 Validation procedure
Validation was carried out according to the FDA guidelines for typical bioanalytical assays [10]. Important items to show are accuracy and precision of the assay, matrix effect (ME), recovery (RE) and process efficiency (PE) as well as general stability of QCs and clinical samples. DBS also has its own set of unique properties that should be assessed including spot volume, punch location, hematocrit and dilution/punch stacking effects [11]. The acceptance criteria for all of these tests are within ±15% deviation from control/nominal and ≤15% coefficient of variation (CV).
Stability is of particular interest for RBV DBS samples because RBV is a nucleoside analog which is transported, phosphorylated and accumulated within red blood cells [12]. Since this accumulation increases with continued dosing, we found it vital to assess the effect of increased intracellular concentrations on parent RBV levels at various time points of dosing (i.e. day 1 up to week 48). This is especially important since the matrix for DBS consists mainly of red blood cells.
3. Validation results
3.1.1 Accuracy, precision and performance
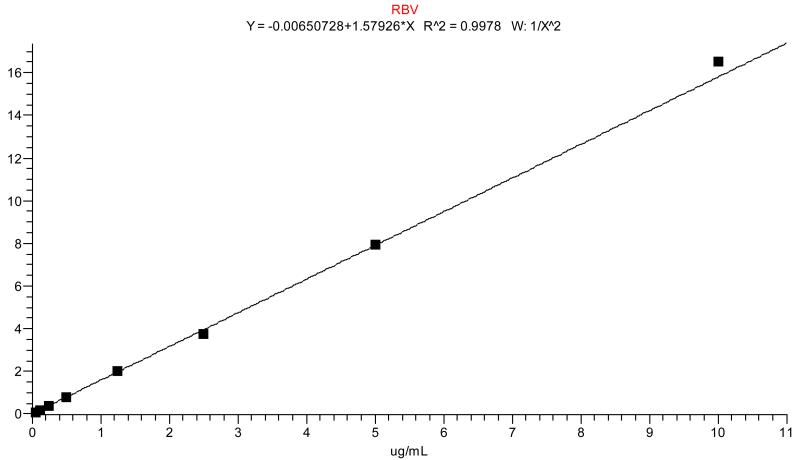
Performance of the calibration standards was shown by averaging the r2 values from each of the three analytical runs. Each calibration sample contained internal standard and peak area ratios versus concentration were fitted with 1/x2 weighed linear regression. Acceptance criteria were similar to QCs with the lowest standard (STD H at 0.05 μg/mL) treated the same as the LLOQ (within ±20%, all other levels within ±15%). Any standard outside of this range was dropped from the curve as long as 6/8 (75%) calibration standards were within this range. An r2 of 0.980 or higher was considered acceptable. A representative standard curve is shown in Figure 1 and curve performance is summarized in Table 1a.
Figure 1.
A typical standard curve of RBV in DBS. Regression was performed with 1/x2 weighting.
Table 1.
Assay accuracy and precision validation results. 1a: summary of standard performance from 3 validation runs. Linear regression curves were fit with 1/x2 weighting. 1b: Quality control performance of 4 quality control levels.
| Table 1a. Back Calculated Calibration Standards | RBV |
| Calibration Standard Range | 0.05-10.0 μg/mL |
| Interassay Accuracy (% Dev ) (n=3) | −7.0 to 2.2 |
| Interassay Precision (%CV ) (n=3) | 1.0 to 5.4 |
| Slope Mean (n=3) | 1.579 |
| Slope Precision (%CV) (n=3) | 4.02 |
| Coefficient of Determination (r^2) Mean (n=3) | 0.9965 |
|
| |
|
Table 1b. Quality Control Accuracy and
Precision |
RBV |
| Quality Control Level 1 (LLOQ) | 0.05 μg/mL |
| Interassay Accuracy (%Dev) (n=18) | 8.3 |
| Interassay Precision (%CV) (n=18) | 5.2 |
| Intraassay Accuracy (%Dev)(n=6) | 8.0 to 9.3 |
| Intraassay Precision (%CV) (n=6) | 3.3 to 6.3 |
| Quality Control (Low) | 0.15 μg/mL |
| Interassay Accuracy (%Dev) (n=18) | 7.3 |
| Interassay Precision (%CV) (n=18) | 7.9 |
| Intraassay Accuracy (%Dev)(n=6) | 5.4 to 8.9 |
| Intraassay Precision (%CV) (n=6) | 2.6 to 6.6 |
| Quality Control (Medium) | 3.0 μg/mL |
| Interassay Accuracy (%Dev) (n=18) | 5.9 |
| Interassay Precision (%CV) (n=18) | 4.8 |
| Intraassay Accuracy (%Dev)(n=6) | 3.8 to 8.2 |
| Intraassay Precision (%CV) (n=6) | 2.8 to 5.9 |
| Quality Control (High) | 8.0 μg/mL |
| Interassay Accuracy (%Dev) (n=18) | 8.9 |
| Interassay Precision (%CV) (n=18) | 4.2 |
| Intraassay Accuracy (%Dev)(n=6) | 7.3 to 11.4 |
| Intraassay Precision (%CV) (n=6) | 2.2 to 5.2 |
Accuracy and precision were assessed according to FDA guidelines for general bioanalytical matrices [10]. Six replicates of each of the four prepared QC levels (LLOQ: 0.05, QL: 0.15, QM: 3.0 and QH: 8.0 μg/mL) were extracted and analyzed against a standard calibration curve consisting of eight levels from 0.05-10.0 μg/mL as described above. This was repeated in three separate analytical runs to determine both inter- and intra-accuracy (% deviation from nominal) and precision (% coefficient of variation (CV)). Assay was considered valid if both of these parameters were within ±15% at all levels except for the LLOQ which was considered valid within ±20%. Table 1b gives a summary of inter- and intra-assay accuracy and precision. For the LLOQ, accuracy was within ±9.3% and precision was ≤6.3% while all other levels were within ±11.4% deviation from nominal and ≤7.9% CV.
3.1.2 Precision in clinical samples
Intra-assay precision using four human subject samples at both room temperature and −20°C was also assessed. Each of the four patient samples was run in triplicate per storage condition (room temperature (RT) and −20°C) with mean RBV concentration ranges of 1.91-7.57 μg/mL. Precision was determined to be within acceptance criteria at 12.9%. This was consistent with QC precision assessment signifying a precise method of measurement for RBV in DBS.
3.2 Specificity and selectivity
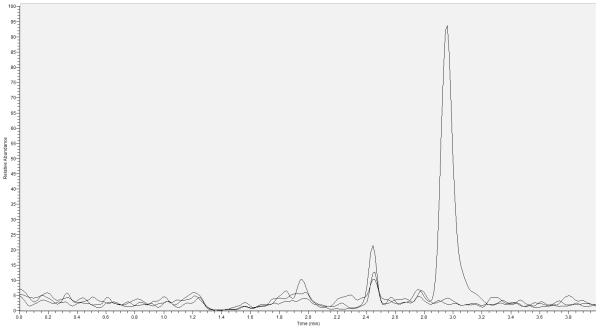
Specificity of DBS was determined by preparing DBS from 5 separate lots of blank whole blood. Cross talk was determined by injecting STD A (10.0 μg/mL) without internal standard followed by injection of an extracted blank with IS. Carryover was assessed by injecting STD A followed by injection of an extracted blank sample. No significant cross talk or carryover was observed from these tests. Chromatographs representing blank, blank IS and the LLOQ are provided in Figure 2. When assessing unit resolution (SRM) and hSRM (first quadrant resolution at 0.2 full width half mass) peak area ratio responses, no significant differences in peak area were detected between the two instrument settings.
Figure 2.
Lower limit of quantitation overlay with blank and blank plus internal standard chromatographs. All peaks are scaled to the LLOQ response. Retention time for RBV is approximately 3 minutes. X –axis represents time to peak elution and Y-axis is relative abundance.
3.3 Matrix effect (ME), recovery (RE) and process efficiency (PE)
Three RBV concentrations (QC-low 0.15μg/mL, QC-Medium 3.0μg/mL and QC-high 8.0μg/mL) were used to complete this experiment. Three sets of samples were prepared to assess ME, RE and PE: set 1 was neat samples in UPH2O, set 2 was spiked into blank extracted DBS and set 3 was regular extracted DBS samples. Five different lots of blood were used to determine the matrix effect at these three concentrations as described above. ME was determined by comparing set 2 to set 1, RE by comparing set 3 to set 2 and overall PE was calculated by comparing set 3 to set 1 [13]. All three tests were consistent over the tested concentration ranges. The mean ME, RE and PE for RBV were calculated to be 85.7%, 74.0% and 63.7%, respectively. Calculated slopes for each of the five linear regressions were within 5% CV indicating insignificant matrix effect.
3.4 Stability Testing
3.4.1 Stability in whole blood prior to spotting for standard and QC prep
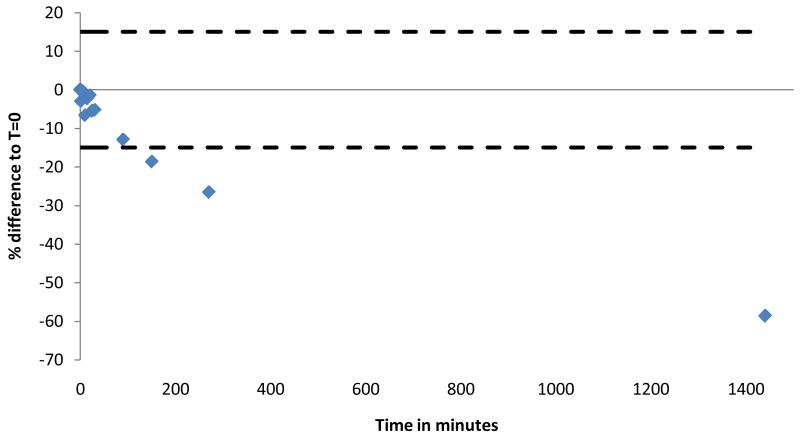
The maximum allowable time for a spiked whole blood sample to remain at room temperature was determined using STD A (10.0 μg/mL). The sample was prepared by spiking 25 μL of STD A working stock into 475 μL of whole blood. 30 μL of this sample was spotted immediately as a control. The remaining whole blood sample was left at room temperature and 30 μL of blood was spotted at various time points up to 24 hours (T=2, 5, 10, 15, 20, 25, 30, 90, 150, 270 minutes and 24 hours). Spots were allowed to dry and triplicate 3 mm discs were punched and extracted for analysis of RBV concentration. Average responses were compared to mean response from control of T=0 minutes. Deviation from control was within ±12.3% (T=90) up until 150 minutes where deviation varied ≤−18.6% for the remaining samples. These data indicate that samples should be spotted prior to T=30-90 minutes after preparation in whole blood in order to obtain accurate results for RBV quantification. These results are summarized in Figure 3.
Figure 3.
Ribavirin is rapidly transported into red blood cells. The highest standard (10.0 μg/mL) was spiked into whole blood and spotted at various time points while remaining at room temperature. The X-axis represents the time in whole blood (minutes) and the Y axis shows the percent difference to T=0. The time points shown are from 30 to 1440 minutes. Dotted lines represent the ±15% cut-off for acceptance.
3.4.1.1 Stability in patient samples prior to spotting
Stability of RBV in whole blood that had been drawn into EDTA Vacutainer tubes was assessed to establish the length of time patient samples could remain at room temperature prior to spotting. Five subjects who had been taking RBV for ≥12 weeks were consented to donate three separate tubes of blood to determine differences in analysis at T=24 and 48 hr compared to T=0 hr (control). All subject samples were outside the acceptable range of ±15% compared to control. Tubes left at room temperature for 24 hours prior to spotting were between 57.7 and 803% greater than control while the 48 hour samples ranged from 78.9 to 1065% greater than control. These results indicate that phosphorylated RBV anabolites were being released from red blood cells (hemolysis of samples) and then hydrolyzed to parent RBV in the plasma. Because of this result, it is best practice to spot DBS cards from patient samples as soon as possible after obtaining the whole blood.
3.4.2 Long term storage stability of QCs at −20°C and room temperature
QCs at the high (QH=8.0 μg/mL) and low (QL=0.15 μg/mL) concentrations were prepared in DBS and stored at both room temperature and −20°C. To test for stability, five replicates of each level were run at both storage conditions and compared. After storage for 415 days at −20°C, QCs tested at −12.4% for QL and −14.9% for QH compared to nominal concentrations. Room temperature storage for 415 days gave results for QL of −7.7% and −12.2% for QH compared to nominal. Storage at room temperature compared to storage at −20°C was within 5.3% for QL and 3.2% for QH. QC performance was also tracked over time and compared to initial analysis. QCs stored at −20°C and RT conditions for 415 days resulted in values within 5.0% of initial analysis for both QL and QH. Precision for the five replicates was within the acceptable range with values ≤3.7% for QL and ≤4.4% for QH indicating stability for >1 year.
3.4.2.1 Long term storage stability in clinical samples at multiple storage conditions
Spiked ribavirin samples are not a completely accurate portrayal of what may actually be occurring with this drug in-vivo, as shown in section 3.4.1.1. Because RBV is anabolized in red blood cells, a large component of DBS, it is essential to use clinical samples to determine whether this affects stability at various conditions these samples may be exposed to. Specimens were collected at multiple time points of treatment varying from first dose to week 48. Because the steady state (week 9-12 of treatment) maximum concentrations of RBV in plasma are 3-6 fold higher [14] than first dose, it was important to analyze clinical samples from these two time points as well as from the middle and end of treatment (week 1-4 and week >12). To accomplish this we analyzed two samples at first dose, two samples at steady state, seven samples between week 1 and week 4 and three samples collected at week >12. Nine subjects and fifteen total samples were used to analyze the effects of long term storage at four different conditions: RT, 4°C, −20°C and −80°C.
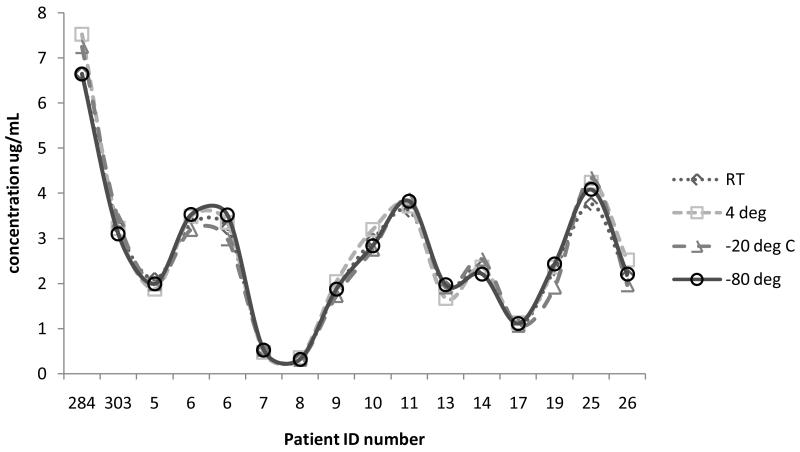
Storage condition data are shown in Figure 4. The data show no impact on RBV analysis in DBS regardless of the storage condition. The largest variation from the −80°C control was 14.2%. All samples tested above LLOQ with concentrations ranging from 0.3 to 7.5 μg/mL. Duration of treatment (i.e. first dose versus steady state) did not impact the analysis for any storage condition tested. Further testing was done on four subjects at weeks 2, 4, 13 and 18 of treatment to determine stability of clinical samples over long periods of time at RT and/or −20°C. Figure 5a shows the data for these four samples at room temperature and −20°C at the initial analysis and analysis 120-140 days later. As shown, there was no effect of storing clinical samples at room temperature or at −20°C for this amount of time (all within ±9.0% of initial run).
Figure 4.
Results for varying storage conditions of clinical samples. Patient identification numbers are on the X axis with each point representing one patient’s result at the varying temperatures. Along the Y axis are the concentrations measured for each storage condition. −80°C (open circles, solid line), −20°C (open triangles, dashed line), 4°C (open squares, round dashed line), room temperature (open diamonds, dotted line) are represented on the graph.
Figure 5.
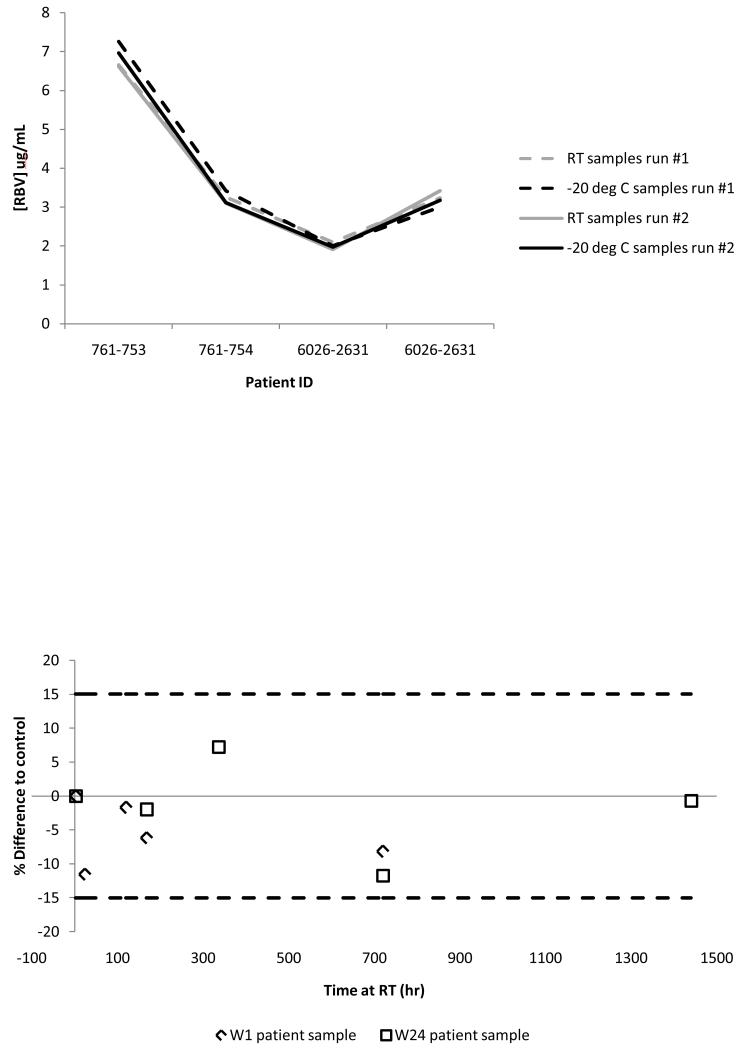
(a) Long term storage stability of patient samples. Dotted lines represent the initial run and solid lines represent analysis at 120-140 days later. Black is for −20°C and grey is for room temperature storage. (b) Storage stability of two patient samples exposed at room temperature. The Y axis is the % difference from the control (immediate analysis after 3 hours of drying) and the X axis is the storage time in hours. The week 1 patient sample is represented by diamond shape and the week 24 patient sample is represented by the square shape.
Room temperature stability of a clinical sample at pre- and post- steady state without the card being stored in a plastic bag with desiccant was also performed to assess potential humidity effects. A clinical sample at week 1 of treatment (average concentration 1.8 μg/mL) was spotted and assayed immediately after drying followed by assessment at several time points post initial analysis (T= 2, 5, 7 days, 2 weeks and one month). A different subject at week 24 of treatment (average concentration 3.3 μg/mL) was assessed in an identical manner except an additional two months post-dry analysis was performed. There was no significant impact on analysis of either of these clinical samples over the tested time period (all within ±11.6% of the immediately analyzed control, Figure 5b).
3.4.3 Freeze/thaw stability of clinical sample
Stability of a clinical sample after multiple freeze/thaw cycles (n=3) was assessed. Two clinical samples (week 1 and week 16 of treatment) were analyzed prior to storing at −20°C as a control. Cards were allowed to thaw to room temperature for 1-3 hours prior to extraction. Each sample was analyzed after 1, 2 and 3 freeze/thaw cycles and compared to control. Neither sample showed significant differences compared to control (within ± 9.3% for week 1 and ±7.0% for week 16). These data suggest that clinical samples are stable for up to three freeze/thaw cycles from storage at −20°C and therefore at least three separate analyses of the same sample can be determined.
3.4.4 Extracted sample stability
Stability of samples in the injected matrix was assessed using six replicates of each of the three QC levels retained in the autosampler (20°C) for 7 days after initial injection. These samples were re-injected against a fresh calibration curve and quality controls. Mean responses were compared to the initial injection control as well as to nominal QC concentrations. The largest deviation to control was −7.6% and all were within ±4.1% of nominal concentrations. Precision was within acceptance criteria at ≤8.6% indicating that extracted samples are stable for up to 7 days in the tested conditions.
3.5 Spot volume assessment (QC samples)
Spot volume effect was assessed at two QC levels (QL=0.15 μg/mL and QH=8.0 μg/mL) in triplicate at various spot volumes (from 5-60 μL) spotted on DBS cards. Concentrations assessed from each spot were compared to the standard spot volume of 30 μL. Acceptance criteria were a mean difference to this control of ±15% for each level. Because lower volume spots could only be punched from the center, all spots (including calibration curve and quality controls) were punched from center to eliminate possible effects of punch location. Compared to control, the 5 μL spot volume was outside of the acceptance criteria and all others (10-60 μL) were within 13.6% except for the 40 μL sample at 30% deviation in the QL concentration due to 1/3 replicates failing though it was not a significant outlier.
3.6 Hematocrit (HCT) effects
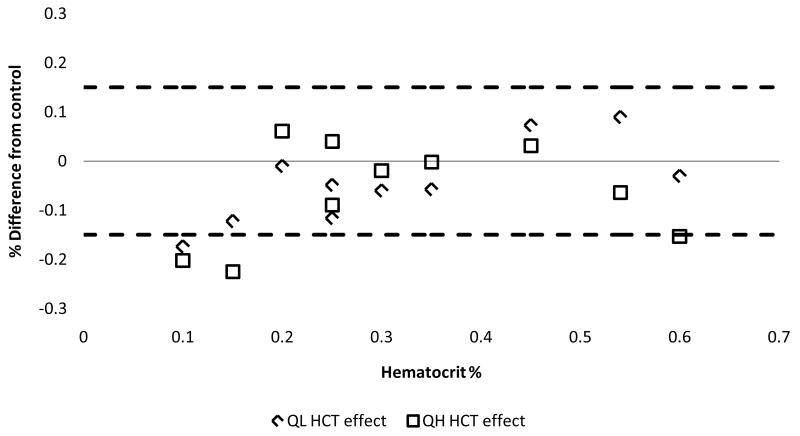
The major dose-limiting toxicity of RBV is hemolytic anemia. Thus, it is necessary to determine the potential impact of low hematocrit on DBS results. Hematocrit testing was performed by first preparing varying hematocrit whole blood samples using blood collected in EDTA Vacutainer tubes from healthy volunteers. These were prepared by either diluting whole blood with plasma or concentrating whole blood by removal of plasma to create a range of HCT (10%, 15%, 20%, 25%, 30%, 35%, 45%, 54% and 60%). Each of these HCT samples were then spiked with either QH (8.0 μg/mL) or QL (0.15 μg/mL) and extracted in triplicate. Mean RBV values from these HCT levels had to be within ±15% of the original whole blood (HCT=50%) in order to pass acceptance criteria. Figure 6 depicts the results of this experiment. When comparing tested HCT to control, it was shown that a range of 20-54% was within acceptance criteria. The QH level was out of acceptance criteria for the 60% HCT at −15.3% so this was not included in the acceptable range for analysis of RBV.
Figure 6.
Test of hematocrit effects on a low (diamond shape) and high (square shape) concentration quality control. Y axis shows the % difference to the 50% HCT control and X axis shows the various HCT% tested. Dotted lines depict the ±15% acceptance criteria window.
3.7 Effect of punch location
Punches were taken from the edge or the center to test the effect of punch location. Similarly to the above experiments, two QC levels (QL=0.15 μg/mL and QH=8.0 μg/mL) were utilized to make this assessment in triplicate for each location. Edge punch was used as the control and mean center punch value had to be within ±15% of mean edge value for acceptance. Difference of center to edge for QL was −3.3% and QH was −9.1%. These results show that punch location has no significant effect on the quantification of RBV from DBS.
3.8 Overcoming the limits of quantitation
“Punch stacking” (extracting multiple DBS punches from the same spot) was investigated as a method for accurately analyzing clinical samples that are below the limit of quantitation (BLQ). Three, 3 mm punches from DBS at two QC levels (LLOQ=0.05 μg/mL and QL=0.15 μg/mL) were added to a 1.5 mL centrifuge tube for “stacked” extraction in triplicate (n=9 punches total per level). Mean responses were then divided by three and compared to nominal concentration. Precision and accuracy were within ±15% for acceptance criteria. For LLOQ the %CV was 5.3% and accuracy was within 0.74% of nominal. For QL the %CV was 3.4% and accuracy was within 8.1% of nominal. These data show that “punch stacking” is a valid method for correcting BLQ levels of RBV in DBS.
In order to dilute samples that are above the limit of quantitation (ALQ), an approach called “internal standard-tracked dilution” was implemented [15]. If a clinical DBS sample required a dilution of “X” times to fall within the assay range, then internal standard that was “X” concentrated was added to the sample to be re-analyzed with “X” dilution. The acquired value of this diluted sample concentration to RBV/IS area ratio is then multiplied by the “X” dilution factor to obtain the true value of the diluted sample. To investigate this strategy, two-fold and five-fold concentrated internal standards were prepared in triplicate using the QH quality control (8.0 μg/mL). Mean values were compared to nominal after multiplying by the appropriate dilution factor. The %deviation and %CV were both within 2.8% of nominal for the dilution factors tested indicating that IS-tracked dilution may be used for RBV DBS sample analysis.
3.9 Relationship of RBV concentration in DBS vs. plasma
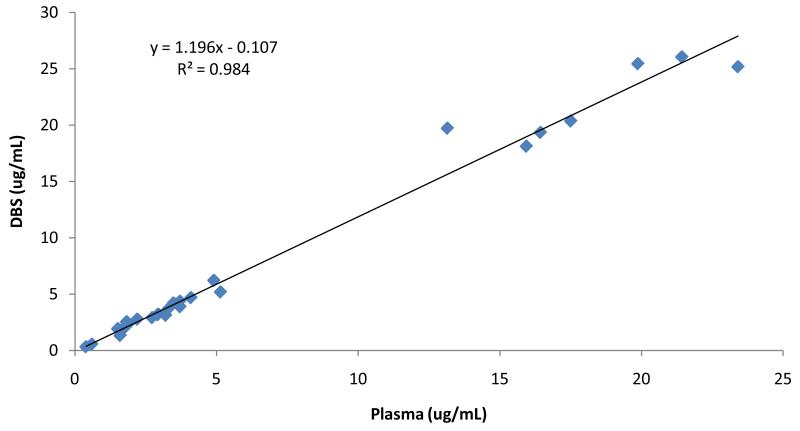
A total of 28 paired DBS and plasma samples from the same blood draw were evaluated to establish the relationship between concentrations of each of these matrices in a similar fashion to other analytes studied in our research group [9]. Linear regression analysis demonstrated coefficient of determination (r2)≥0.98 (Figure 7). The RBV plasma versus DBS relationship was defined as y=1.2x-0.1. The excellent correlation between these two methods suggests that DBS is a plausible alternative to plasma for the analysis of RBV.
Figure 7.
Linear regression comparing DBS (Y axis) to plasma (X axis) RBV concentrations in μg/mL. Samples are from 28 different patient samples at various points in treatment from day 1 to week 48. Linear relationship is shown by the equation y=1.2x-0.1 with r2 ≥ 0.98.
4. Discussion
DBS was developed and validated for the precise, accurate and sensitive measurement of ribavirin using liquid chromatography tandem-mass spectrometry. The extraction process and chromatography produced accuracy and precision data consistent with FDA guidelines [10]. The lower limit of quantification used for this assay was 0.05 μg/mL and is identical to that used for current plasma assays developed for RBV quantification [16].Correlation between RBV DBS and RBV plasma concentrations supports the use of DBS for pharmacokinetic studies in the future. Because this assay is highly sensitive with a low blood volume requirement, it is especially attractive for special patient populations such as pediatrics, infants and those who have conditions that limit the amount of allowable blood obtained for pharmacokinetic studies (e.g., those with anemia).
Qualities that are unique to DBS such as spot volume, punch location, hematocrit effects and overcoming limits of quantitation were examined in this study. Clinical samples were of importance for these experiments because of the unique metabolic profile of RBV that allows it to be phosphorylated and trapped within red blood cells [12]. Because this causes accumulation of the drug in a human subject with up to a 40 day half-life in the cell and a 12 day half-life in plasma [14] the stability of patient DBS samples have the potential for destabilization due to breakdown of phosphorylated ribavirin. However, we have shown that this method has sound stability in a variety of conditions for various clinical samples over the treatment period of 48 weeks. It is of interest to explore whether the intracellular RBV components are breaking down in DBS especially considering the observed release of intracellular RBV when patient whole blood was left at room temperature. The process may not be detectable by measuring parent concentrations because of the steady state profile of this drug [14]. The measurement of intracellular RBV from DBS would be of great value to understand how breakdown may affect storage conditions for this matrix.
Accuracy and precision were not affected by HCT ranges between 20% and 54% which is wider than the normal range of ~35%-50%. This finding is critical for implementation of this assay in clinical practice because it allows the use of this tool in patients who have HCT abnormalities due to toxicity from the drug, disease state or other circumstances [17]. This validation defined guidelines for DBS specific properties. Edge vs. center punching did not affect results and edge punches can be used as standard practice to maximize the number of analyses possible per spot. Spot volumes between 10 and 60 μL were shown to be accurate and precise allowing a large variety of possible spot volumes to be used in clinical practice. We have therefore shown that acceptable practice for RBV DBS extraction procedures are not dependent on punch location and can be accomplished with a wide margin of spot volumes.
Follow-up studies will be necessary to further this method and address some limitations. The first goal would be to compare the difference (if any) between capillary (finger-stick) blood and venous blood that was used for this validation. Additionally the question of exactly how long a patient sample can remain at room temperature before spotting is of interest to improve sample processing consistency. Long term storage data will continue to be accrued to monitor stability of this matrix for clinical and quality control samples. Hematocrit effects in clinical samples should also be explored and compared to quality controls.
Highlights.
Measurement of RBV in dried blood spot to 0.05 μg/mL
DBS storage was stable at room temperature for up to 140 days
Analysis was accurate and precise for hematocrit in the normal range
Punch location and spot volume (10-60 μL) did not affect measurement
DBS can be used for pharmacokinetic/dynamic analyses in place of plasma
Acknowledgments
We would like to thank the study personnel and nursing staff who aided with the clinical protocol and the study subjects who participated. This work is supported by grants from NIH NIDDK to J Kiser: K23 DK082621 and R03 DK096121.
Abbreviations
- RBV
ribavirin
- DBS
dried blood spots
- LC-MS/MS
liquid chromatography tandem-mass spectrometry
- PK-PD
pharmacokinetics/dynamics
- STD
standard
- QC
quality control
- LLOQ
lower limit of quantitation
- ALQ
above limit of quantitation
- HCT
hematocrit
- RT
room temperature
- UPH2O
ultrapure water
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
Authors report no conflicts of interest related to this manuscript.
References
- [1].Schering Plough Corporation Rebetol (ribavirin, USP) Capsules Product Information. 1998 [cited 2013 07/30/2013]; Available from: http://www.fda.gov/downloads/Drugs/DrugSafety/ucm089017.pdf.
- [2].Ji Qin C, G.L., D’Arienzo Celia J, Olah Timothy V, Arnold Mark E. What is next for dried blood spots? Bioanalysis. 2012;4:16, 2059–2065. doi: 10.4155/bio.12.168. [DOI] [PubMed] [Google Scholar]
- [3].Ronald JW, Meesters JZ, van Huizen Nick A, Hooff Gero P, Gruters Rob A, Luider Theo M. Dried Matrix on paper disks: the next generation DBS microsampling technique for managing the hematocrit effect in DBS analysis. Bioanalysis. 2012;4(16):2027–2035. doi: 10.4155/bio.12.175. [DOI] [PubMed] [Google Scholar]
- [4].Keevil BG. The analysis of dried blood spot samples using liquid chromatography tandem mass spectrometry. Clin Biochem. 2011;44(1):110–8. doi: 10.1016/j.clinbiochem.2010.06.014. [DOI] [PubMed] [Google Scholar]
- [5].Control C.f.D. Bloodspot Transportation Guidelines. [cited 2013 07/31/2013]; Available from: http://www.cdc.gov/labstandards/pdf/nsqap/Bloodspot_Transportation_Guidelines.pdf.
- [6].Kiser JJ, B.L., Anderson PL, Tise S, Klein B, Rower J, Zheng JH, Everson GT. Ribavirin mono-, di-, and triphosphate concentrations in the peripheral blood mononuclear and red blood cells of Hepatitis C virus infected persons; 5th International Workshop on Clinical Pharmacology of Hepatitis Therapy; Boston, MA. 2010. [Google Scholar]
- [7].Bushman LR, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal. 2011;56(2):390–401. doi: 10.1016/j.jpba.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Delahunty T, et al. The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(20-21):1907–14. doi: 10.1016/j.jchromb.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Castillo-Mancilla JR, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29(2):384–90. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Viswanathan CT, et al. Quantitative bioanalytical methods validation and implementation: best practices for chromatographic and ligand binding assays. Pharm Res. 2007;24(10):1962–73. doi: 10.1007/s11095-007-9291-7. [DOI] [PubMed] [Google Scholar]
- [11].Li W, Tse FL. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomed Chromatogr. 2010;24(1):49–65. doi: 10.1002/bmc.1367. [DOI] [PubMed] [Google Scholar]
- [12].Durand-Gasselin L, et al. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob Agents Chemother. 2007;51(6):2105–11. doi: 10.1128/AAC.00831-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75(13):3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- [14].Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis. 1999;19(Suppl 1):17–24. [PubMed] [Google Scholar]
- [15].Liu G, Snapp HM, Ji QC. Internal standard tracked dilution to overcome challenges in dried blood spots and robotic sample preparation for liquid chromatography/tandem mass spectrometry assays. Rapid Commun Mass Spectrom. 2011;25(9):1250–6. doi: 10.1002/rcm.4990. [DOI] [PubMed] [Google Scholar]
- [16].Loregian A, et al. Measurement of ribavirin and evaluation of its stability in human plasma by high-performance liquid chromatography with UV detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856(1-2):358–64. doi: 10.1016/j.jchromb.2007.05.039. [DOI] [PubMed] [Google Scholar]
- [17].Holub M, et al. Influence of hematocrit and localisation of punch in dried blood spots on levels of amino acids and acylcarnitines measured by tandem mass spectrometry. Clin Chim Acta. 2006;373(1-2):27–31. doi: 10.1016/j.cca.2006.04.013. [DOI] [PubMed] [Google Scholar]