Abstract
Stem cells of the adult vertebrate intestine (ISCs) are responsible for the continuous replacement of intestinal cells, but also serve as site of origin of intestinal neoplasms. The interaction between multiple signaling pathways, including Wnt/Wg, Shh/Hh, BMP, and Notch, orchestrate mitosis, motility, and differentiation of ISCs. Many fundamental questions of how these pathways carry out their function remain unanswered. One approach to gain more insight is to look at the development of stem cells, to analyze the “programming” process which these cells undergo as they emerge from the large populations of embryonic progenitors. This review intends to summarize pertinent data on vertebrate intestinal stem cell biology, to then take a closer look at recent studies of intestinal stem cell development in Drosophila. Here, stem cell pools and their niche environment consist of relatively small numbers of cells, and questions concerning the pattern of cell division, niche-stem cell contacts, or differentiation can be addressed at the single cell level. Likewise, it is possible to analyze the emergence of stem cells during development more easily than in vertebrate systems: where in the embryo do stem cells arise, what structures in their environment do they interact with, and what signaling pathways are active sequentially as a result of these interactions. Given the high degree of conservation among genetic mechanisms controlling stem cell behavior in all animals, findings in Drosophila will provide answers that inform research in the vertebrate stem cell field.
Stem Cells and their Niches
Adult stem cells are typically slowly cycling, undifferentiated and often multipotent cells. Through their mitotic activity, stem cells generate two types of offspring. First, they renew themselves, and thereby maintain a pool of proliferating stem cells. Secondly, they produce offspring that then become postmitotic and differentiate, or (more typically), that first undergo a phase of rapid cell division before differentiating. Since these dividing cells have a limited proliferative potential and eventually turn into differentiated progeny, they are referred to as transient amplifying (TA) cells.
The part of the stem cells’ environment that provides signals promoting stem cell self renewal is operationally defined as the stem cell niche. Niches can be quite different in the way in which they relate to stem cells spatially and developmentally (Martinez-Agosto et al., 2007). The Drosophila gonads provide examples where the niche is represented by a small group of cells (the hub cells of the testis, cap cells of the ovary) that are in direct contact with the germ line stem cells (GSCs). Asymmetric mitosis of the GSCs causes one daughter cell to remain in contact with the niche, whereas the other daughter is pushed away from it and thereby loses its stemness (Xie et al., 2005; Fuller and Spradling, 2007). In the vertebrate hematopoietic stem cells (HSCs), the osteoblast layer lining the bone marrow cavity may act as a niche, and it seems more likely that a stochastic mechanism causes the transition from HSC to amplifying progenitor (Arai and Suda, 2007; Levesque et al., 2010). In the Drosophila hematopoietic organ (lymph gland), diffusible signals and/or cellular extensions emanating from a specialized group of cells that form integral part of the lymph gland, called posterior signaling center (PSC) promote renewal of blood stem cells (Martinez-Agosto et al., 2007). For most adult stem cells, the niche and niche associated signaling mechanisms have not yet been elucidated.
Stem Cells of the Mammalian Intestine
The mammalian intestinal epithelium is composed of terminally differentiated enterocytes and several types of secretory and endocrine cells (Fig.1A). In many parts of the intestinal tract, such as the mammalian small intestine, the epithelium is folded into finger-like processes termed villi. Sub-mucosal epithelial invaginations, called crypts, are found at inter-villar spaces or scattered over the surface epithelium. Enterocytes are specialized for the uptake and processing of nutrients. Secretory cells located in the villi, called goblet cells, produce mucus; secretory cells found in the crypts, called Paneth cells, produce antimicrobial peptides. Specialized secretory cells of the stomach, called parietal cells, produce hydrochloric acid. Endocrine cells, which are scattered all over the intestinal epithelium, release peptide hormones with specific regional distributions and functions (Montuenga et al., 2003); examples are secretin or CKK, produced in the duodenum (stimulates pancreatic bicarbonate secretion), or gastrin, produced in the stomach (increases acid secretion from parietal cells).
Fig.1.
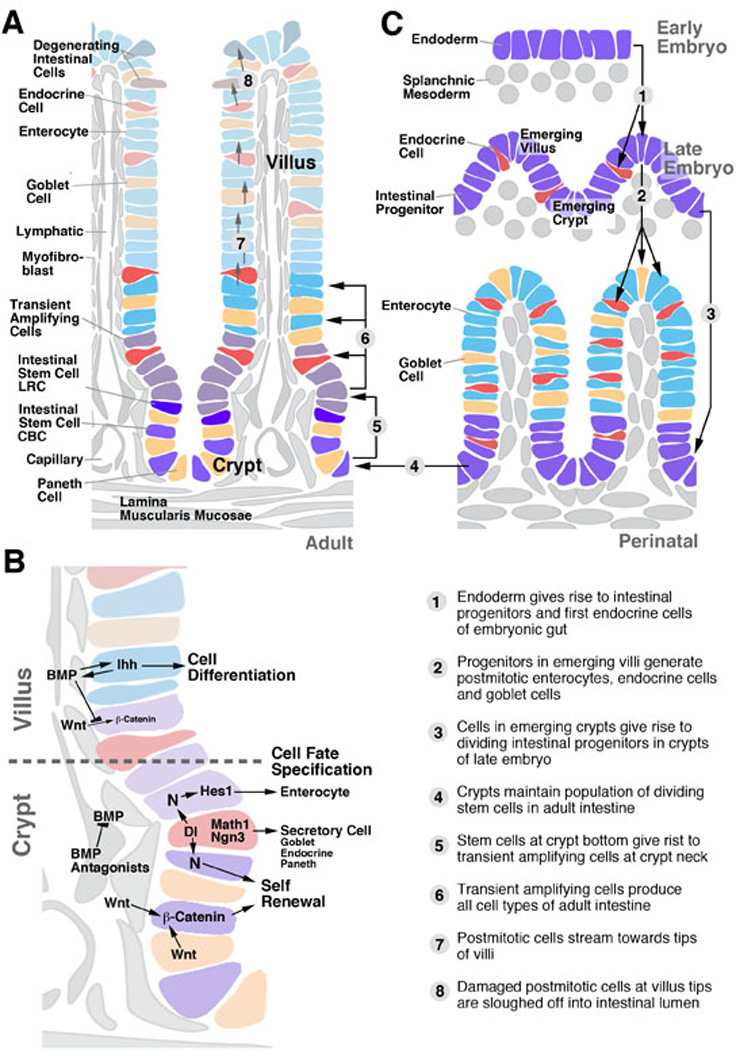
Intestinal stem cells in the vertebrate gut. A: Distribution of stem cells and differentiating cell types in the adult small intestine. B: Signaling pathways controlling intestinal stem cell proliferation and differentiation. C: Steps in intestinal development and stem cell specification. Numbered arrows on schematic representations (A) and (C) are explained in bottom part of (C). In this and other schematics of this review, dividing embryonic intestinal progenitors and stem cells are rendered purple; enterocytes are shown in blue (or green, for the Drosophila hindgut shown in Fig.5), and endocrine cells in red.
Intestinal stem cells are localized in the crypts. Similar to other organs, such as the skin or bone marrow, the intestinal crypts house two populations of stem cells, one that cycles very slowly (called “label-retaining cell”; LRC), and another one (“active stem cell”) that cycles faster and is responsible for the rapid turnover of intestinal cells (Li and Clevers, 2010; Fig.1A). Active stem cells are intermingled with the Paneth cells at the crypt bottom, and are therefore also referred to as ‘crypt-base columnar cells (CBCs). Both types of stem cells can be labeled by a number of specific markers, among them Lgr5 (CBCs), and Bmi1 (label retaining cells). Recent findings (Tian et al., 2011) indicate a hierarchical relationship between these two stem cell types; removal of the CBC population resulted in an increased proliferation of Bmi1 cells, followed by the eventual replacement of the CBCs. Progeny of the intestinal stem cells (transient amplifying cells) populate the upper region of the crypts and, after a limited number of cell divisions, become postmitotic. Moving apically into the villus/surface epithelium they differentiate into the different types of intestinal cells (Crosnier et al., 2006; Scoville et al., 2008; Fig.1A). Clonal analyses indicate that stem cells are pluripotent; labeled clones, originating from individual CBCs, contained enterocytes, as well as secretory and/or endocrine cells (Bjerknes and Cheng, 2006). This indicates that at the level of the stem cells in which the clones were induced, a decision between endocrine, secretory, and enterocyte fate had not yet been made. Multiple signaling pathways act among TAs and early postmitotic cells to specify intestinal cell fate.
Stem Cell Niche and Niche Signaling
Signals acting on ISCs are derived from mesenchymal cells, as well as differentiated intestinal epithelial cells. Villi of the small intestine contain several classes of mesenchymal cells, notably the smooth muscle cells surrounding the lymphatic vessel in the core of the villus, and the myofibroblasts that form a network around capillaries subjacent to the intestinal epithelium (Powell et al., 1999; Yen and Wright, 2006; Scoville et al., 2008; McLin et al., 2009; Shaker and Rubin, 2010; Powell et al., 2011; Fig.1A, B). Smooth muscle cells of the lamina muscularis mucosae extend basally of the crypts. Given their proximity to the epithelium, myofibroblasts are generally considered as the most likely source of signals acting on ISCs. Recent studies have shown that differentiated Paneth cells, which are intermingled with the stem cells at the crypt bottom, also provide signals that promote stem cell renewal (Sato et al., 2011). Thus, crypt bottom stem cells and Paneth cells, grown in culture in the absence of mesenchyme, are able to produce complete crypt-villus organoids.
Members of the Wnt/Wingless family appear to reside at the center of the signaling network that promotes ISC renewal (Haegebarth and Clevers, 2009; Yeung et al., 2011; Fig.1B). A crucial role of Wnt proteins as niche signals appears to be conserved in most stem cell systems. Wnt proteins are secreted by myofibroblasts and Paneth cells in the vertebrate intestine, and by visceral muscle cells and epithelial stem cells of the Drosophila intestine. The activity of Wnt signaling is restricted to the crypt by the graded expression of several other signals, notably Wnt antagonists, Bone Morphogenetic Protein (BMP), BMP antagonists, and Hedgehog (Scoville et al., 2008; Yeung et al., 2011; Fig.1B). BMP signals are secreted by myofibroblasts of the villi. They antagonize the Wnt pathway by stabilizing PTEN and thereby reducing nuclear b-catenin (He et al., 2004). Removal of BMP signals from the villi causes the stem cell population to invade the villi. BMP signaling is maintained by Hedgehog signals (Indian hedgehog, Ihh), secreted from the villus epithelium. Besides antagonizing Wnt, BMP and Ihh promote the differentiation of epithelial cells and mesenchymal cells in the villus. In the crypt, myofibroblasts produce the BMP antagonists Noggin and Gremlin, inhibiting the BMP pathway, and allowing for the Wnt signal (derived from crypt myofibroblasts and Paneth cells) to be active (Scoville et al., 2008; Yeung et al., 2011).
Notch signaling plays a complex role, (1) controlling the balance between self renewing stem cells and their differentiating progeny, and (2) determining the type of progeny. These same two functions of Notch were also observed in the Drosophila intestine, as detailed below. In neither vertebrate nor Drosophila is it clear whether these two functions are truly separable, or whether they represent aspects of one single signaling interaction. It was observed in numerous vertebrate systems that by decreasing Notch signaling, the ratio of enterocytes to secretory/endocrine cells is shifted towards the latter (Jensen et al., 2000; Crosnier et al., 2005; van Es et al., 2005; Fre et al., 2011). At the same time, the number of proliferating cells decreases. These findings indicate that proliferating cells, as well as the absorptive enterocytes derived from them, require the activity of Notch. Enterocytes express the bHLH transcription factor, Hes-1, which is activated by the Notch pathway (Fig.1B). By contrast, secretory cells and endocrine cells exhibit a low level of Notch activity, and express the bHLH proteins Math1 and Neurogenin 3 (Schonhoff et al., 2004; Lee and Kaestner, 2004; Fig.1B). The fact that the same bHLH family members act as fate determinants in neurons and endocrine cells may attest to a common evolutionary origin of these cell types (Hartenstein, 2006).
Development of the vertebrate intestine and its stem cell system
At an early stage of embryonic development, the vertebrate endoderm forms an epithelial tube in which all cells are mitotically active (Henning et al., 1994; Crosnier et al., 2005; Fig.1C). In mammals, the embryonic gut wall is multilayered (Mathan et al., 1976; Madara et al., 1981; Kim et al., 2007). As the gut tube increases in surface area, the epithelium becomes folded into longitudinal folds (fish, amphibians) or villi (mammals). Villus formation is initiated with the condensation of mesodermal cells at locations that foreshadow the incipient villi, which happens around E14 in mouse development. Endoderm contacting the mesodermal condensations convert into a single layered epithelium that folds towards the lumen. Epithelial cells of the villi exit the mitotic cycle and differentiate as enterocytes, secretory cells and endocrine cells (Fig.1C). Endoderm in between villi remains multilayered, undifferentiated and mitotically active throughout the remainder of embryogenesis (Fig.1C).
In amphibians, which undergo metamorphosis, the larval gut degenerates by programmed cell death and is replaced by a new, adult gut. The larval gut does not have villi or folds, with the exception of one ventral, longitudinal fold called typhlosole (Ishizuya-Oka and Shi, 2007). Mitotic cells are scattered evenly throughout the larval gut epithelium. At the onset of metamorphosis, all larval gut cells initiate apoptosis. Progenitors of adult gut form clusters of mitotically active cells that reside underneath the basal surface of the larval epithelium, reminiscent of the adult gut progenitors in Drosophila (Fig.2A, B). Adult gut progenitor clusters grow, merge with each other and form a complete layer enclosing the degenerating larval gut in its lumen. The adult gut epithelium increases in surface area and forms longitudinal folds. At that point, as mentioned above for mouse intestinal development, cells within the folds exit the cell cycle and differentiate; only the troughs in between the folds retain populations of cycling, self renewing stem cells.
Fig.2.

A-C: Dynamic expression of Wnt activity reporter (blue) in developing mouse small intestine (from Kim and Shivdasani, 2007; with permission). A: Embryonic day 16.25; B: Postnatal day 3; C: Postnatal day 20. D, E: Intestinal metamorphosis in Xenopus. (from Ishizuya-Oka and Shi, 2007; with permission). D: Histological section, showing clusters of adult gut progenitors (arrows) attached to the basal surface of degenerating larval gut (arrowhead). E: Adult gut progenitors express Shh (brown; arow).
The same signaling pathways that control proliferation and differentiation along the adult crypt-villus axis are active during development. Endoderm cells secrete Hedgehog signals that act on the underlying mesoderm, promoting the proliferation, patterning and differentiation of smooth muscle cells and myofibroblasts (Ramalho-Santos et al., 2000; Madison et al., 2005; Mao et al., 2009). Hh signals are also required to initiate reciprocal signaling of BMP from mesoderm to endoderm. Along with Hh, BMP signals are necessary for the proliferation and differentiation of mesodermal lineages (Batts et al., 2006; Torihashi et al., 2009). Wnt signals are expressed throughout the mesoderm; unlike in the adult, where Wnt activity is restricted to the crypt compartment (Fig.2E), Wnt signaling activity in the embryo is found in a widespread manner in the gut epithelium (Kim et al., 2007; Fig.2C). This may reflect the fact that during the early stages of gut development, proliferation is still found in all cells.
How does the transition in Wnt activation and function from embryonic to adult occur? How are the graded patterns of expression of BMPs and their antagonists built from an initially uniform pattern? These questions need to be addressed in order to understand the birth of the adult stem cell population and its niche. We speculate that the adults ISCs are a “left over” of the population of embryonic gut progenitors. As the signaling environment changes at late embryonic/early postnatal stages, most gut progenitors are driven towards differentiation. However, small niches that maintain an “embryonic milieu” remain, and protect gut progenitors in contact with the niches from differentiation. One prediction of this hypothesis is that the “specialized” adult ISCs share molecular and cellular characteristics with the “generic” embryonic gut progenitors. The extent to which that is the case remains to be established for vertebrate ISCs; in Drosophila, it clearly is true: as discussed in more detail below, all of the molecular markers of adults ISCs are expressed already by embryonic/larval gut progenitors.
ISCs of the Drosophila intestine
The Drosophila intestine shows four main divisions, the foregut, midgut, Malpighian tubules, and hindgut (Skaer, 1993; Fig.3A). The foregut and hindgut are descended from the embryonic ectoderm. These parts of the gut, which each is further subdivided into several smaller segments (pharynx, esophagus, proventriculus and crop for foregut; small intestine, large intestine and rectum for the hindgut), consist of epithelial enterocytes which secrete a cuticular layer at their apical surface. The foregut is required for the transport, storage, and mechanical processing of ingested food; the main role of the hindgut is the reabsorption of water. The midgut, derived from embryonic endoderm, is formed by enterocytes that lack cuticle and have a pronounced microvillar brushborder instead. Midgut enterocytes absorb and metabolize nutrients. The fly midgut also contains numerous types of secretory and endocrine cells. For example, the so called “copper cells” secrete hydrochloric acid, thereby corresponding to the vertebrate stomach parietal cells (Dubreuil, 2004). Endocrine cells produce different peptide hormones that act on gut and heart motility, as well as water reabsorption (Veenstra, 2008; 2009; Hartenstein et al., 2010; Takashima et al., 2011a) The Malpighian tubules, a set of four epithelial tubes that filter and excrete hemolymph (the “blood” filling the body cavity), open into the digestive tract at the junction between midgut and hindgut.
Fig.3.
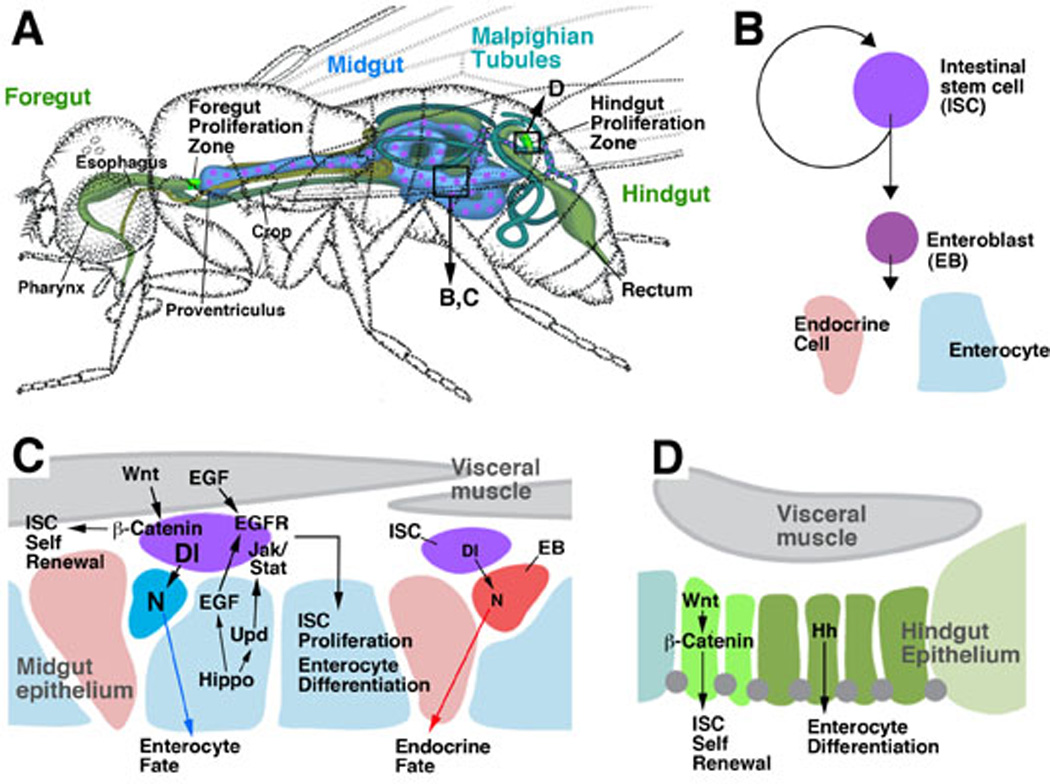
Intestinal stem cell populations of Drosophila. A: Schematic of the adult intestinal tract. Scattered mesenchymal proliferating stem cells are found in the midgut and Malpighian tubules (magenta); zones of epithelial stem cells are found at the boundary between foregut and midgut (foregut proliferation zone), and midgut and hindgut (hindgut proliferation zone), respectively (bright green). B: Lineage produced by stem cells in the adult midgut. C: Signaling pathways controlling proliferation and differentiation of adult midgut stem cells. D: Signaling pathways acting in the hindgut proliferation zone.
Adult intestinal stem cells (ISCs) have been found in all four subdivisions of the Drosophila intestine. In the midgut (Micchelli and Perrimon, 2006; Ohlstein and Spradling, 2006) and Malpighian tubules (where they were called renal stem cells or RNSCs; Singh et al., 2007), ISCs are evenly distributed, mesenchymal cells, scattered underneath the basal surface of the gut epithelium (Figs.3A; 4A, A’, C). In the hindgut, ISCs form integral part of the gut epithelium. They are restricted to a narrow segment at the midgut-hindgut boundary (hindgut proliferation zone, HPZ; Takashima et al., 2008; Figs.3A, 4B, B’). A similar zone of epithelial, proliferating cells has been recently described for the adult foregut (Singh et al., 2011). Midgut ISCs divide frequently; in average, midgut cells are renewed approximately four times during the eight week life span of the adult female fly (Jiang et al., 2009). Similar to the Drosophila germ line stem cells or neuroblasts, midgut ISCs undergo asymmetric divisions. One daughter cell maintains ISC characteristics and keeps dividing; the other daughter cell, called enteroblast, exits the cell cycle and differentiates into either an absorptive enterocyte, or an endocrine cell (Fig.3B). In either case, this daughter cell then undergoes a mesenchymal-epithelial transition as it inserts itself into the midgut epithelium. In the Malpighian tubules, stem cells behave similarly to the ISCs of the midgut. Restricted to the proximal part of the tubules, stem cells divide asymmetrically into one stem cell daughter that keeps self renewing, and one postmitotic daughter (“renalblast”, Singh et al., 2007). This postmitotic cell either differentiates locally, or migrates distally. Proximal tubules consist of a single type of cuboideal, epithelial cells, characterized by a large or intermediate size nucleus and a pronounced brush border (type I cell or “renalcyte”). Distally, one finds type I cells and a second type of cell, called type II or “stellate” cell, which is smaller and sends several lateral processes that intercalate in between neighboring type I cells (hence the descriptive name “stellate cell”). According to the clonal analysis ofSingh et al. (2007), RBs differentiate into both type I and type II cells. Unlike midgut, the Malpighian tubules do not contain endocrine cells.
Fig.4.

Intestinal stem cells of Drosophila. Z-projections of confocal sections of the adult midgut (A, A’), anterior hindgut (B, B’), and Malighian tubules (C). Schematic D illustrates postion along intestinal tracts where A-C’ were taken. A-C show surface views of gut epithelium; A’ and B’ represent segtions of gut wall. Intestinal stem cells (ISCs) are labeled in green by expression of Esg-GFP (midgut, Malpighian tubules) or Stat-GFP (hindgut proliferation zone; HPZ), respectively. Cell boundaries made visible by antibody against the b-catenin/Armadillo protein (Arm; red). Note high levels of Arm in stem cells. Cell nuclei in blue.
The stem cell zones at the boundaries of midgut-hindgut boundary, and the midgut-foregut boundary, consist entirely of small, undifferentiated, epithelial cells (Fig.4B, B’). Under physiological conditions, they cycle slowly, in a stochastic pattern (Takashima et al., 2008; Fox and Spradling, 2009; Singh et al., 2011). In the hindgut proliferation zone, two different subdivisions were defined on the basis of molecular marker expression, cell shape, and cell cycle characteristics. The anterior, narrow segment of the HPZ, called spindle cell zone, is marked by high levels of expression of the JAK/STAT activity reporter Stat82E-GFP and the Wnt/Wg-target gene b-catenin. It contains cells that cycle very slowly. The posteriorly adjacent zone, marked by expression of Hh, cycles slightly faster (Takashima et al., 2008).
Signaling pathways controlling ISC proliferation and differentiation in the Drosophila intestine
Recent genetic studies have elucidated several of the signaling pathways that control the proliferation and differentiation of gut progenitors in the larva, and ISCs in the adult. Best understood are the stem cells of the midgut. At least five signaling pathways, Notch, Wnt, JAK/STAT, EGFR, and Hippo, impinge upon the stem cell and control its cell cycle length (rate of proliferation), type of division (asymmetric vs symmetric), and differentiation (enterocyte vs endocrine cell). Wnt/Wg signaling, as described for vertebrate ISCs, promotes the rate of proliferation of midgut ISCs (Lin et al., 2008; Lee et al., 2009; Xu et al., 2011; Fig.3C), and also leads to divisions that are (in part) symmetric. Thus, clones derived from ISCs lacking Wnt pathway activity are smaller than wild-type clones because they lose self-renewing ISCs (Lin et al., 2008). The ligand, Wg, is expressed in a restricted population of visceral muscle cells (Lin et al., 2008); another member of the Wnt family, DWnt-4, is expressed in a more widespread manner in the visceral musculature (ST and VH, unpublished). These findings suggest that, similar to the vertebrate myofibroblasts, visceral muscle fibers provide a signal that promotes ISC self renewal.
Notch signaling plays a key role in midgut ISC self renewal, by promoting asymmetric as opposed to symmetric division of the ISC (Ohlstein and Spradling, 2006; 2007; Micchelli and Perrimon, 2006; Fig.3C). ISCs express the ligand Delta (Dl), which activates the Notch pathway in the neighboring enteroblasts. Enteroblasts require Notch activity to differentiate; in the absence of Dl, or Notch, ISCs divide symmetrically, forming “tumors” of undifferentiated, mitotically active cells. By contrast, Notch activity is absent in ISCs; ectopic expression of Notch in ISCs drives these cells to exit the cell cycle and differentiate as enterocytes. Note that the role Notch in self renewal described here is exactly the opposite of that seen in vertebrate ISCs, where activation of Notch promotes ISC proliferation (Fre et al., 2005; Wang and Hou, 2010). This does not reflect a general, species-related difference: in numerous instances in Drosophila, such as larval neuroblasts (Bowman et al., 2008), as well as ISCs of the hindgut (ST and VH, unpublished), Notch activity drives proliferation and inhibits differentiation. What complicates the interpretation of Notch function in midgut ISCs is that Notch also controls the fate of the enteroblasts. High levels of Notch activity in an enteroblast promote this cell to differentiate as enterocyte; low levels result in endocrine cell formation (Ohlstein and Spradling, 2007). Tight adhesion between the ISC and its daughters are required for the Dl signal to become effective; the reduction in adhesion caused by loss of Drosophila E-cadherin results in a decreased frequency of enterocyte formation (Maeda et al., 2008). Enteroblasts, under wild-type conditions, will generate both cell types because the level of Dl, presented by the ISC, oscillates; it is not yet clear what mechanism causes this oscillation.
Two other signaling pathways, JAK/STAT and EGFR, are activated in the ISCs to promote the rate of proliferation (Fig.3C). Ligands of EGFR are produced by visceral muscle cells, as well as differentiated enterocytes (Jiang et al., 2011; Xu et al., 2011); ligands activating the JAK/STAT pathway (Upd, Upd1, Upd2) are expressed in enterocytes, as well as ISCs themselves (Jiang et al., 2009; Liu et al., 2010). This indicates that the Drosophila midgut does not harbor a spatially restricted niche; all cell midgut types seem to contribute signals that promote self renewal of ISCs. EGFR and JAK/STAT signaling are required to maintain gut homeostasis under normal conditions; they are upregulated when enterocytes are killed or stressed by bacterial infection. Under these conditions, Upd expression is greatly enhanced (Jiang et al., 2009). Recent data show that the Hippo pathway acts upstream of JAK/STAT and EGFR (Shaw et al., 2010; Ren et al., 2010). Cellular stress in differentiated enterocytes blocks Hippo, which in turn leads to an increase in the production of Upd and EGFR ligands. In addition, the Hippo target, Yorkie, can act cell autonomously in the ISC itself as an activator of proliferation (Karpovicz et al., 2010).
Gene expression and genetic data suggest that all of the signaling pathways discussed above for the midgut ISCs may play a role in the remainder of the intestine as well. However, fewer details have been worked out to date. Wingless is expressed in a narrow ring of cells that form part of the HPZ stem cell region. Wingless activates proliferation; loss of this signal results in a loss of dividing cells, and also causes apoptotic cell death (Takashima et al., 2008). Hh, which is expressed further posteriorly in the HPZ and the adjoining differentiated hindgut, is required for hindgut differentiation (Takashima et al., 2008). JAK/STAT activity is found in the anterior region (the spindle cell zone) of the HPZ. Loss of JAK/STAT activity does not cause any proliferation defect in the HPZ (ST and VH, unpublished); however, enhanced activation of this pathway causes enhanced proliferation of this cell population (ST and VH, unpublished). The strong upregulation of HPZ proliferation that results from experimental ablation of hindgut (Fox and Spradling, 2009) is most likely the result of enhanced Upd signaling from the damaged tissue. Activation of the JAK/STAT pathway also drives proliferation of the stem cell population of the Malpighian tubules (Singh et al., 2007).
Development of Drosophila intestinal stem cells
Drosophila belongs to the group of holometabolous insects which undergo complete metamorphosis. Most organs (including the intestinal tract) of the larval body, which develops in the embryo, are destroyed during metamorphosis and are replaced by adult-specific organs. Progenitors of the adult intestine first appear in the embryo; they proliferate throughout the larval period, and undergo differentiation during the pupal stage (metamorphosis). We will summarize the major events of intestinal development, paying special attention to the mechanisms by which adult stem cell populations sort out from the larger pool of adult gut progenitors that differentiate.
The midgut arises from the embryonic endoderm, which forms an anterior and posterior cluster of proliferating, mesenchymal cells (Campos-Ortega and Hartenstein, 1985; Skaer, 1993; Tepass and Hartenstein, 1994). Initially, all dividing endoderm cells express esg, the marker for undifferentiated midgut stem cells (Takashima et al., 2011a). The endoderm splits into an outer layer that reorganizes into the larval midgut epithelium, and an inner layer, that includes the progenitors of the adult gut (AMPs), as well as precursors of larval endocrine cells and secretory cells. As these two layers appear, expression of esg disappears first from the outer layer, indicating that cells of this layer lose their state as undifferentiated progenitors first. The outer layer is also the first to become postmitotic. The inner layer continues to divide for several hours and maintains expression of esg, but then downregulates this gene in all cells except for the prospective AMPs (Fig.5, step 1); the remainder of the inner cells differentiate as endocrine cells. In the larva, AMPs undergo 7–8 rounds of division in a parasynchronous manner (Jiang and Edgar, 2009). Daughter cells resulting from the first three mitoses spread out over the basal surface of the early larval midgut epithelium (Fig. 1D). During late divisions, AMPs stay in contact to form “islands” or “nests” of small, diploid cells distributed evenly among the large, polyploid larval enterocytes (Fig.5, step 4). Within these nests, cells sort out into two structurally and molecularly different types, the central cells and peripheral cells. Peripheral cells, marked by high expression levels of 10XStat92E-GFP (Mathur et al., 2010; Takashima et al., 2011a), are flattened, sheath-like cells that surround an inner mass of central cells, small, rounded cells with low 10XStat92E-GFP level, and continued proliferation. With the onset of metamorphosis in the early pupa, these two cell populations give rise to two separate epithelial layers: the central cells become the adult midgut epithelium (Fig.5, step 5), the peripheral cells form a transient pupal midgut epithelium, which is sandwiched in between the adult epithelium, and the inner mass of degenerating larval midgut cells (Takashima et al., 2011a, b; Fig.5, steps 6, 8).
Fig.5.

Development of intestinal stem cells in Drosophila. Column in the center shows schematic length sections of the intestine at different stages (embryo, early larva, late larva, pupa, adult). Depicted is the border region of midgut (blue, to the left), Malpighian tubules (turquois, center and hindgut (green, to the right). Column on the left shows details of intestinal stem cell formation at higher magnification (boxed areas). Steps in the formation of intestinal stem cells are indicated by numbered arrows. Explanation of these developmental steps are given in right column.
Precursors of the adult midgut stem cells (pISCs) can be first detected within the adult midgut layer shortly after onset of metamorphosis (Fig.5, step 5). These cells maintain high levels of Esg, whereas all other descendants of the AMP islands (forming the pupal and adult midgut epithelium) lose this marker (Jiang and Edgar, 2009; Takashima et al., 2011a). pISCs perform several rounds of divisions during the pupal stage. Interestingly, these early divisions are different from the canonical asymmetric divisions described for the adult (Takashima et al., 2011a). Thus, the first 1–2 rounds of mitosis are symmetric, increasing the overall number of pISCs (Fig.6A). During an additional 1–2 rounds performed in the late pupa, divisions become asymmetric; they produce the endocrine cells that populate the midgut of the eclosing adult fly (Fig.6B). Only after eclosion does the mode of proliferation of ISCs switch to a mode that produces both endocrine cells and enterocytes.
Fig.6.

Migration and proliferatory behavior of Drosophila intestinal stem cells during metamorphosis. A, B: Boundary region of midgut (left) and hindgut (right) of late third instar larva (L3). Adult midgut progenitors (AMPs) are labeled by Esg reporter construct (red; A); adult hindgut progenitors, labeled by Byn reporter construct (red; B) are concentrated in hindgut proliferation zone (hpz). They can be distinguished from Byn-positive differentiated larval hindgut (hg) by their small size and high packing density. C, D: Lineage tracing of adult midgut progenitors (C) and adult hindgut progenitors (D). Progenitors were labeled at early larval stage by stably expressing a lacZ reporter by Esg-Gal4 (C) or Byn-Gal4 (D), respectively. Labeled progeny of progenitors at in adult gut are depicted. Note that progeny of AMPs (C) occupy proximal portion of Malpighian tubules (ureter, ure), but not posterior segment of midgut (mgps). This part of the midgut is populated by descendants of adult hindgut progenitors (mgps in D). E, F: Lineages produced by division of presumptive intestinal stem cells during the pupal period. The lacZ tracing construct was expressed by esg-Gal4 in 24h pupa. At 48h (E), lacZ expression (blue) is confined to presumptive stem cells (pISCs; GFP-positive, green), indicating that division of these cells from 24–48h results merely in the production of additional pISCs. At 72h, lacZ-positive cells include pISCs (green), but also endocrine cells (aen; labeled by anti-Prospero; red). This result demonstrates that the Pros-positive cells are born during pISC divisions taking place between 48 and72h after onset of metamorphosis.
The picture that emerges from the findings summarized so far is that (1) a pool of adult gut progenitors is specified in the embryo; (2) these cells proliferate in the larva; (3) adult gut stem cells arise as a subpopulation from within the progenitor pool during metamorphosis. The same principle holds true when looking at the stem cells of the foregut and hindgut. From mid-embryonic stages onward, coinciding with the end of proliferation and onset of differentiation of larval hindgut cells, one can recognize near the anterior hindgut boundary a narrow domain, called “imaginal ring of the hindgut” or larval HPZ, whose cells remain small and undifferentiated (Robertson, 1936; Skaer, 1993; Fig.5, step 3). Cells of the larval HPZ are the adult hindgut progenitors; like midgut progenitors, they express high levels of JAK/STAT activity, but are negative for Esg (Takashima et al., 2008). Hindgut progenitors proliferate rapidly during the larval period until shortly after onset of metamorphosis. At that time, between 20 and 40h after puparium formation, the larval HPZ rapidly expands, in a “telescope-like” motion, posteriorly (Fig.5, step 7), thereby displacing the larval hindgut whose cells undergo apoptosis. While expanding, adult hindgut cells start to differentiate. Surprisingly, cells at the anterior margin of the HPZ expand anteriorly, and differentiate as midgut cells (Takashima et al., 2011c; Fig.6C, D). This demonstrates that, even though the larval HPZ shows structural characteristics of ectoderm and expresses molecular markers of hindgut, cells are able to differentiate into “endodermal” midgut cells. A small subset of cells of the larval HPZ remain undifferentiated. After upregulating Stat92E-GFP these cells resume proliferatory activity and give rise to the adult HPZ.
Adult stem cells of the Malpighian tubules derive from a subset of midgut stem cells that migrate posteriorly (Takashima et al., 2011c). Given that larval MTs cells survive metamorphosis and form the adult tubules, no proliferating progenitors are present in the larval MTs (Singh et al., 2007). However, recent findings show that the proximal segments of the larval ureters degenerate, and are replaced by cells derived from the adult midgut progenitors. Thus, as AMPs merge into the adult midgut epithelium (see above), this layer also spreads posteriorly and gives rise to the new proximal segments of the Malpighian tubules (Takashima et al., 2011c; Figs.5, step 9; 6E, F). The spreading epithelium carries with it presumptive stem cells which proliferate and subsequently spread out into the tubules.
Signaling pathways acting during Drosophila ISC development
The signaling pathways controlling proliferation and differentiation in adult stem cells appear to act in a similar manner during development, where they control the behavior of adult progenitors. This comes as no surprise, because the task at hand is the same: to maintain a subset of cells in an undifferentiated state, and regulate their proliferation. In the developing midgut, Notch signaling is instrumental in restricting progenitor proliferation, and promoting the specification of enterocytes over endocrine cells. In embryos lacking Notch, or the ligand Dl, the endoderm remains mesenchymal and overproliferates; enterocytes do not form, and most cells express markers of endocrine cells (Takashima et al., 2011a). During larval stages, expression of Delta becomes restricted to the central cells; concomitantly, Notch activity decreases in central cells, and increases in the peripheral cells, which as a result differentiate as the epithelial cells forming the transient pupal midgut (Mathur et al., 2010; Takashima et al., 2011a). A reciprocal Dpp signal from the peripheral cells maintains low Notch activity and thereby promotes continued proliferation; abrogation of Dpp in peripheral cells causes central AMPs to differentiate into epithelial enterocytes (Mathur et al., 2010). EGF signals promote proliferatory activity of the AMPs (Jiang and Edgar, 2009). An effect of JAK/STAT signaling and Wg signaling (from the visceral muscle layer) is likely, given the high level of activity of reporters for these pathways in the AMPs, but has not been tested experimentally yet. This also applies for the restructuring of the signaling environment that occurs during metamorphosis. During the first 24h of metamorphosis, the larval visceral musculature de-differentiates; myofibrils and extracellular matrix is lost, even though the muscle cells themselves survive (Klapper et al., 2000; Aghajanian et al., 2011). Around 40 hour APF these cells re-differentiate into adult visceral muscle. This stage coincides with the time when adult stem cell precursors change their mode of division from symmetric self renewal to an asymmetric division that gives rise to endocrine cells. It is possible that the re-occurrence of visceral muscle and signals, like Wg, associated with these cells, may differentially activate Delta protein in pISC to change the fate of their daughter cells.
In the HPZ of the early larva, signaling activity of Wg, Hh and JAK/STAT are found in an overlapping manner in all cells. As the HPZ grows in length, these signals sort out such that Wg becomes restricted to the anterior border of the HPZ where it maintains cells in a proliferating mode (Takashima et al., 2008). Ectopic activity of Wg keeps the cells of the larval HPZ in a proliferating mode, and prevents differentiation. Hh has the opposite effect, priming cells of the larval HPZ to differentiate. A role of other pathways, including Notch and EGFR, appear likely, but have not been explored to date.
Conclusion
The findings reviewed above suggest that in Drosophila, ISCs originate from larger populations of adult gut progenitor cells which exist during the larval stage (Fig.7). A network of signaling pathways, presented by a “larval niche”, are responsible for the ordered proliferation of the adult progenitors. It appears that the signaling pathways associated with the larval niche are largely the same that also act on adult ISCs. During metamorphosis, the “larval niche” largely breaks down, and adult gut progenitors differentiate. However, a small subset of progenitors survive the cataclysm, and become the founders of adult ISCs, which we call the “pISCs” (Fig.7). pISCs initially show several unique characteristics that gradually evolve into the distinctive phenotype of the adult ISCs. This “maturation” of pISCs is paralleled by the step-by-step reconstitution of niche elements. It therefore stands to reason that the signaling pathways that control ISC behavior, and that are associated with different niche elements (and dependent on a certain stage of differentiation of these elements) also “come back on line” in a step-wise fashion. In this lies the general significance of studying the development of stem cells: it helps one to understand the complex network of signaling mechanisms controlling stem cell biology, because development “dissects” this network in time. We postulate that the same will apply for the analysis of stem cell specification in the developing vertebrate intestine. As for the adult ISCs, one may anticipate that studies in Drosophila will be a driving force in formulating new hypotheses and approaches.
Fig.7.

Origin of Drosophila intestinal stem cells originate from within larger pool of adult gut progenitors.
References
- Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- Batts LE, Polk DB, Dubois RN, Kulessa H. Bmp signaling is required for intestinal growth and morphogenesis. Dev Dyn. 2006;235:1563–1570. doi: 10.1002/dvdy.20741. [DOI] [PubMed] [Google Scholar]
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, Ramos-Mejia V, Rouleau A, Yang J, Bossé M, Lajoie G, Bhatia M. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722–735. doi: 10.1016/j.ydbio.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell. 2008;14(4):535–546. doi: 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. Berlin: Springer; 1985. The Embryonic Development of Drosophila melanogaster. [Google Scholar]
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132:1093–1104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- Dubreuil RR. Copper cells and stomach acid secretion in the Drosophila midgut. Int J Biochem Cell Biol. 2004;36(5):745–752. doi: 10.1016/j.biocel.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Fox DT, Spradling AC. The Drosophila hindgut lacks constitutively active adult stem cells but proliferates in response to tissue damage. Cell Stem Cell. 2009;5(3):290–297. doi: 10.1016/j.stem.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435(7044):964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Fre S, Bardin A, Robine S, Louvard D. Notch signaling in intestinal homeostasis across species: the cases of Drosophila, Zebrafish and the mouse. Exp Cell Res. 2011 doi: 10.1016/j.yexcr.2011.06.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316(5823):402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174(3):715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V. The neuroendocrine system of invertebrates: a developmental and evolutionary perspective. J Endocrinol. 2006;190(3):555–570. doi: 10.1677/joe.1.06964. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Takashima S, Adams KL. Conserved genetic pathways controlling the development of the diffuse endocrine system in vertebrates and Drosophila. Gen Comp Endocrinol. 2010;166(3):462–469. doi: 10.1016/j.ygcen.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Adams K, Ortiz PA, Ying C, Moridzadeh R, Younossi-Hartenstein A, Hartenstein V. Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev Biol. 2011a;353:161–172. doi: 10.1016/j.ydbio.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Younossi-Hartenstein A, Ortiz PA, Hartenstein V. A novel tissue in an established model system: the Drosophila pupal midgut. Dev Genes Evol. 2011b;221:69–81. doi: 10.1007/s00427-011-0360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Henning SJ, Rubin DC, Shulman RJ. Ontogeny of the intestinal mucosa. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York NY: Raven press; 1994. pp. 571–610. [Google Scholar]
- Ishizuya-Oka A, Shi YB. Regulation of adult intestinal epithelial stem cell development by thyroid hormone during Xenopus laevis metamorphosis. Dev Dyn. 2007;236(12):3358–3368. doi: 10.1002/dvdy.21291. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136(3):483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Patel PH, Kohlmaier A, Grenley MO, McEwen DG, Edgar BA. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8(1):84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137(24):4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BM, Mao J, Taketo MM, Shivdasani RA. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133(2):529–538. doi: 10.1053/j.gastro.2007.04.072. [DOI] [PubMed] [Google Scholar]
- Klapper R. The longitudinal visceral musculature of Drosophila melanogaster persists through metamorphosis. Mech Dev. 2000 Jul;95(1–2):47–54. doi: 10.1016/s0925-4773(00)00328-2. [DOI] [PubMed] [Google Scholar]
- Lee CS, Kaestner KH. Development of gut endocrine cells. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:453–462. doi: 10.1016/j.beem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136(13):2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- Lévesque JP, Helwani FM, Winkler IG. The endosteal 'osteoblastic' niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24(12):1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327(5965):542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455(7216):1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010;109(5):992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Takemura M, Umemori M, Adachi-Yamada T. E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes Cells. 2008;13(12):1219–1227. doi: 10.1111/j.1365-2443.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- Mao J, Kim BM, Rajurkar M, Shivdasani RA, McMahon AP. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development. 2010;137(10):1721–1729. doi: 10.1242/dev.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara JL, Neutra MR, Trier JS. Junctional complexes in fetal rat small intestine during morphogenesis. Dev Biol. 1981;86(1):170–178. doi: 10.1016/0012-1606(81)90327-4. [DOI] [PubMed] [Google Scholar]
- Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132(2):279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21(23):3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- Mathan M, Moxey PC, Trier JS. Morphogenesis of fetal rat duodenal villi. Am J Anat. 1976;146(1):73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009 Jun;136(7):2074–2091. doi: 10.1053/j.gastro.2009.03.001. Epub 2009 Mar 17. Review. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Montuenga LM, Guembe L, Burrell MA, Bodegas ME, Calvo A, Sola JJ, Sesma P, Villaro AC. The diffuse endocrine system: from embryogenesis to carcinogenesis. Prog. Histochem. Cytochem. 2003;38:155–272. doi: 10.1016/s0079-6336(03)80004-9. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277(2 Pt 1):C183–C201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213–237. doi: 10.1146/annurev.physiol.70.113006.100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127(12):2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U.S.A. 2010;107(49):21064–21069. doi: 10.1073/pnas.1012759107. Epub 2010 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CW. Metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J Morphol. 1936;59:351–399. [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Development and differentiation of gut endocrine cells. Endocrinology. 2004a;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134(3):849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- Shaker A, Rubin DC. Intestinal stem cells and epithelial-mesenchymal interactions in the crypt and stem cell niche. Transl Res. 2010;156(3):180–187. doi: 10.1016/j.trsl.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137(24):4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Liu W, Hou SX. The adult Drosophila malpighian tubules are maintained by multipotent stem cells. Cell Stem Cell. 2007;1(2):191–203. doi: 10.1016/j.stem.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SR, Zeng X, Zheng Z, Hou SX. The adult Drosophila gastric and stomach organs are maintained by a multipotent stem cell pool at the foregut/midgut junction in the cardia (proventriculus) Cell Cycle. 2011;10(7):1109–1120. doi: 10.4161/cc.10.7.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaer H leB. The alimentary canal. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Cold Spring Habor Laboratory Press; 1993. pp. 941–1012. [Google Scholar]
- Takashima S, Mkrtchyan M, Younossi-Hartenstein A, Merriam JR, Hartenstein V. The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature. 2008;454(7204):651–655. doi: 10.1038/nature07156. [DOI] [PubMed] [Google Scholar]
- Takashima S, Adams KL, Ortiz PA, Ying CT, Moridzadeh R, Younossi-Hartenstein A, Hartenstein V. Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev Biol. 2011a;353(2):161–172. doi: 10.1016/j.ydbio.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Younossi-Hartenstein A, Ortiz PA, Hartenstein V. A novel tissue in an established model system: the Drosophila pupal midgut. Dev Genes Evol. 2011b;221(2):69–81. doi: 10.1007/s00427-011-0360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Aghajanian P, Paul M, Younossi-Hartenstein A, Hartenstein V. Trans-germ layer migration of Drosophila intestinal stem cells at the developing midgut-hindgut boundary. 2011c doi: 10.1242/dev.082933. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The formation of the midgut epithelium in Drosophila depends on the interaction of endoderm and mesoderm. Development. 1994b;120:579–590. doi: 10.1242/dev.120.3.579. [DOI] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torihashi S, Hattori T, Hasegawa H, Kurahashi M, Ogaeri T, Fujimoto T. The expression and crucial roles of BMP signaling in development of smooth muscle progenitor cells in the mouse embryonic gut. Differentiation. 2009;77(3):277–289. doi: 10.1016/j.diff.2008.10.003. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 2009;336(2):309–323. doi: 10.1007/s00441-009-0769-y. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 2009;336(2):309–223. doi: 10.1007/s00441-009-0769-y. [DOI] [PubMed] [Google Scholar]
- Wang P, Hou SX. Regulation of intestinal stem cells in mammals and Drosophila. J Cell Physiol. 2010;222(1):33–37. doi: 10.1002/jcp.21928. [DOI] [PubMed] [Google Scholar]
- Xie T, Kawase E, Kirilly D, Wong MD. Intimate relationships with their neighbors: tales of stem cells in Drosophila reproductive systems. Dev Dyn. 2005;232:775–790. doi: 10.1002/dvdy.20317. [DOI] [PubMed] [Google Scholar]
- Xu N, Wang SQ, Tan D, Gao Y, Lin G, Xi R. EGFR, Wingless and JAK/STAT signaling cooperatively maintain Drosophila intestinal stem cells. Dev Biol. 2011;354(1):31–43. doi: 10.1016/j.ydbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2(3):203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- Yeung TM, Chia LA, Kosinski CM, Kuo CJ. Regulation of self-renewal and differentiation by the intestinal stem cell niche. Cell Mol Life Sci. 2011:2513–2525. doi: 10.1007/s00018-011-0687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


