Abstract
The Drosophila larval and adult midgut are derived from two populations of endodermal progenitors that separate from each other in the early embryo. As larval midgut cells differentiate into an epithelial layer, adult midgut progenitors (AMPs) remain as small clusters of proliferating, undifferentiated cells attached to the basal surface of the larval gut epithelium. During the first few hours of metamorphosis, AMPs merge into a continuous epithelial tube that overgrows the larval layer and differentiates into the adult midgut; at the same time, the larval midgut degenerates. As shown in this paper, there is a second, transient pupal midgut that develops from the AMPs at the beginning of metamorphosis, and that intercalates between the adult and larval midgut epithelia. Cells of the transient pupal midgut form a multilayered tube that exhibits signs of differentiation, in the form of septate junctions and rudimentary apical microvilli. Some cells of the pupal midgut develop as endocrine cells. The pupal midgut remains closely attached to the degenerating larval midgut cells. Along with these cells, pupal midgut cells are sequestered into the lumen where they form the compact “yellow body”. The formation of a pupal midgut has been reported from several other species, and may represent a general feature of intestinal metamorphosis in insects.
Keywords: Drosophila, midgut, metamorphosis, pupa, ultrastructure
Introduction
The midgut of Drosophila is formed by a monolayer of epithelial cells, surrounded by circular and longitudinal visceral muscle cells. Midgut epithelial cells are comprised of two main types, digestive enterocytes, and endocrine cells. The midgut epithelium of the larva arises from the embryonic endoderm, which starts out as an anterior and posterior cluster of proliferating mesenchymal cells. During mid-embryonic stages, anterior and posterior clusters elongate, merge, and undergo a mesenchymal-epithelial transition that results in the formation of the larval midgut epithelium (Campos-Ortega and Hartenstein, 1985; Tepass and Hartenstein, 1994a; Fig. 1A). Midgut epithelial cells are post-mitotic; growth of the larval midgut takes place by endoreplication, followed by an increase in cell volume of individual cells. A separate population of cells that remains undifferentiated and mitotically active serves as a pool of progenitors, for the adult gut (adult midgut progenitors, AMPs). AMPs also originate from the endoderm. They split from the cells that give rise to the differentiated larval midgut in early embryogenesis and form a separate layer in the lumen of the midgut primordium (Tepass and Hartenstein, 1994a; 1995; Fig. 1A). Subsequently, in late embryogenesis, AMPs migrate through the midgut wall and begin to lie as individual cells at the basal surface of the midgut epithelium. AMPs proliferate throughout the larval period. In the late larva, they form groups (“nests” or “nidi”) of small cells, scattered more or less evenly among the large, differentiated enterocytes (Robertson, 1936; Bodenstein, 1950; Hartenstein and Jan, 1992; Jiang and Edgar, 2009; Fig. 1B). Nests of such proliferating midgut progenitors, located at their characteristic position at the basal surface of the midgut epithelium, have been observed in developing stages of all insect taxa (Martoja and Ballan-Dufrancais, 1984; Lehane, 1998).
Fig. 1.
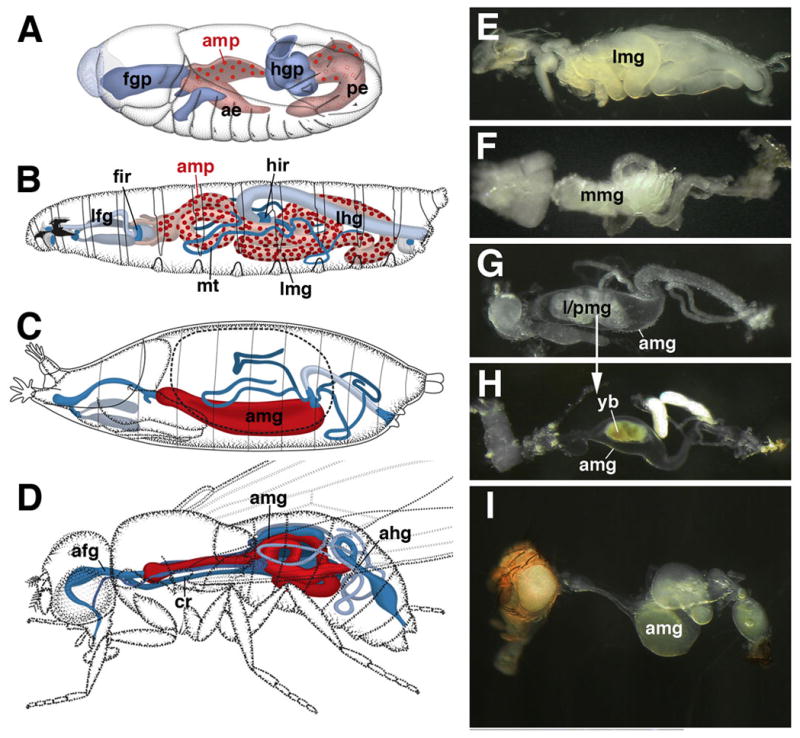
Development of the Drosophila midgut. A–D: Schematic drawings of embryo (A; stage 12), larva (B), pupa (C; 12h after puparium formation), and adult (D). The embryonic midgut primordium is comprised of the anterior endoderm (ae) and posterior endoderm (pe). At the stage shown, the endoderm is in the process of forming the larval midgut epithelium (light red). Progenitors of the adult midgut (amp; dark red) form a scattered population of cells attached to the larval midgut. After hatching (B), adult midgut progenitors proliferate, forming clusters (nests or nidi) distributed evenly over the basal surface of the larval midgut (lmg). With the onset of metamorphosis, adult midgut progenitors fuse into a continuous layer, the adult midgut (amg), that takes up the larval midgut into its lumen (C, D). The developing foregut and hindgut are shown in shades of blue. E–I: Photographs of dissected gut of third instar larva (E), 6h pupa (F), 16h pupa (G), 40h pupa (H), adult (I). ae anterior endoderm, afg adult foregut, ahg adult hindgut, amp adult midgut progenitor, amg adult midgut, cr crop, fgp foregut primordium, fir foregut imaginal ring, hgp hindgut primordium, hir hindgut imaginal ring, lfg larval foregut, lhg larval hindgut, lmg larval midgut, mmg metamorphosing midgut, mt: Malpighian tubule, pe posterior endoderm, pmg pupal midgut. yb yellow body.
At the onset of metamorphosis, the AMP nests expand, merge with each other, and transform into a continuous epithelial layer that overgrows the former larval midgut (Fig. 1C). The larval midgut cells contract into a dense mass, the yellow body, which is recognizable in the lumen of the developing adult gut throughout the pupal period (Fig. 1F–H). Recent findings indicate that the AMPs, in addition to generating the adult midgut, give rise to yet another, transient layer of cells that is interposed in between the outer adult and inner larval midgut epithelium. Thus, in the late larva, cells of the AMP nests segregate into two different types. Cells at the periphery of each nest (“peripheral cells”; Mathur et al., 2010; Takashima et al., 2011) become flattened and elongated, forming a cup-shaped envelope that encloses a cluster of “central cells”. The central cells go on and produce the adult gut: they are the adult midgut progenitors in the proper sense. By contrast, peripheral cells, which act as an important signaling center promoting proliferation during the larva, do not become part of the adult gut (Mathur et al., 2010). Instead, as described in more detail in this paper, they spread out underneath the adult gut and form a transient pupal epithelium surrounding the degenerating larval midgut (Takashima et al., 2011). A similar transient midgut has been described for several other insect species, among them the dragon fly, Aeshna cyanea (Andries, 1979; Martoja and Ballan-Dufrancais, 1984), but has thus far largely escaped notice in the literature on Drosophila development or physiology. In this paper, we use specific markers in conjunction with electron microscopy to characterize the morphological attributes of the pupal midgut. Our data demonstrate that its cells form a multilayered tube that shows several features of differentiation, including septate junctions and rudimentary apical microvilli. On the other hand, other aspects of gut epithelial differentiation, in particular the polarized expression of cell adhesion molecule complexes, fails to occur. As metamorphosis proceeds, the pupal midgut remains closely associated with the degenerating larval gut cells. Along with these, pupal midgut cells are sequestered into the lumen where they form the compact yellow body. Our findings shed light on a previously unknown aspect of Drosophila intestinal development; an area that has received considerable attention in recent years due to the presence of stem cells which remain active throughout the adult period.
Material and Methods
Fly strains
Flies used in this study were: OregonRw1118, UAS-myr-mRFP, UAS-flp, Act5c promoter>stop>lacZ, all obtained from the Bloomington Stock Center; esg-GAL4 was from the National Institute of Genetics, Mishima, Japan; 10XStat92E-GFP (Bach et al., 2007). The flies were maintained with normal fly food and kept at 25°C or at room temperature, except for the lineage trace experiment (see below). When staging pupae, they were reared at 25°C.
Immunostaining
Antibodies used in this study included: rabbit anti-Tachykinin (1:2000; kindly provided by Dr. Dick Nassel); anti-FMRF-amide (Thermo Fisher Scientific, Waltham, MA); mouse anti-Armadillo (1:5), anti-FasIII (1:25) (Developmental Studies Hybridoma Bank, University of Iowa); mouse anti-Beta-galactosidase (1:100; Promega, Madison, WI); rabbit anti-Beta-galactosidase (1:1000; Cappel/MP Biomedicals, Solon, OH); mouse anti-green fluorescent protein (GFP; 1:100; Sigma, St. Louis, IL); rabbit anti-GFP (1:100; Invitrogen, Carlsbad, CA), mouse anti-Fibrillarin (1:25; abcam, Cambridge, MA), and rabbit anti-Pdm-1 (1:1000; Yeo et al., 1995).
Antibody staining was performed according to Ashburner, (1989). Guts were fixed with 4% formaldehyde (electron microscopy grade; Polysciences, Warrington, PA), diluted with phosphate-buffered saline containing 0.1% TritonX-100 (PBT). The samples were incubated with primary antibodies, and then with secondary antibodies conjugated with fluorescent tags, at 4°C overnight, respectively. Samples were mounted in Vectashield (Vector laboratories, Burlingame, CA), which contains TOTO-3 (1:2000; Invitrogen) in several experiments.
Preparations were imaged using a LSM 700 confocal laser scanning microscope system with an Axio Imager.M2 microscope (Carl Zeiss, Germany).
Lineage tracing experiment
Animals were kept at 18°C before the temperature shift. Second instar larvae with the genotype, tub-GAL80ts/+; esg-GAL4 UAS-mRFP/UAS-flp; Act5c promoter>stop>lacZ (Struhl and Basler, 1993; Jung et al., 2005) were transferred to 29°C to inactivate GAL80ts for 6 hours, and then dissected at late 3rd instar stage or at 24 hour pupal stage, and then processed for immunohistochemistry.
Transmission electron microscopy
Guts were fixed with 2.5% glutaraldehyde and 1% osmium tetroxyde, and embedded in Epon resin following standard procedures, optimized for pupal tissue (Grigorian et al., 2011). Ultra-thin sections were stained with uranyl acetate and lead citrate. JEOL 100CX transmission electron microscope was used for observation.
Results
Formation of the transient pupal midgut during early metamorphosis
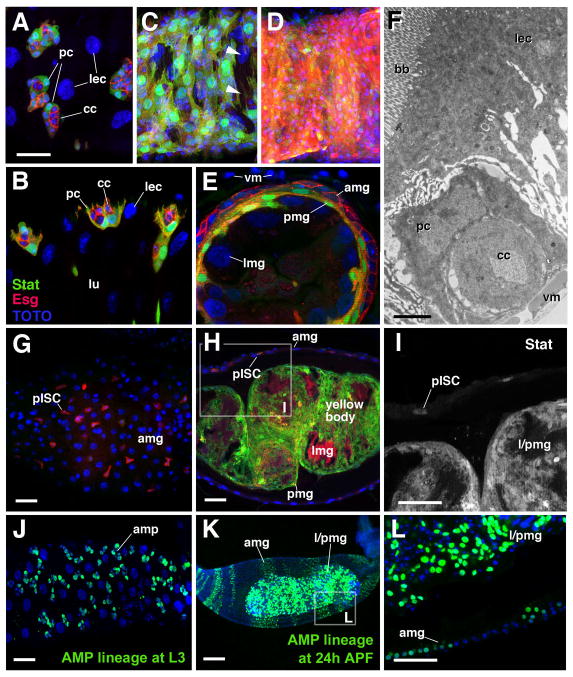
The clusters of adult midgut progenitors found in the late larval gut contain two structurally and molecularly different cell types, the central cells and peripheral cells. Peripheral cells, of which there are typically two to three per AMP cluster, can be recognized by a high level of expression of 10XStat92E-GFP (Mathur et al., 2010; Takashima et al., 2011), a reporter of Jak/Stat signaling activity (Bach et al., 2007). Peripheral cells are flattened, sheath-like cells that surround an inner mass of central cells, small, rounded cells with low 10XStat92E-GFP level (Fig. 2A, B). In most cases, peripheral cells already extend in between the central cells and the larval enterocytes (Fig. 2B, F).
Fig. 2.
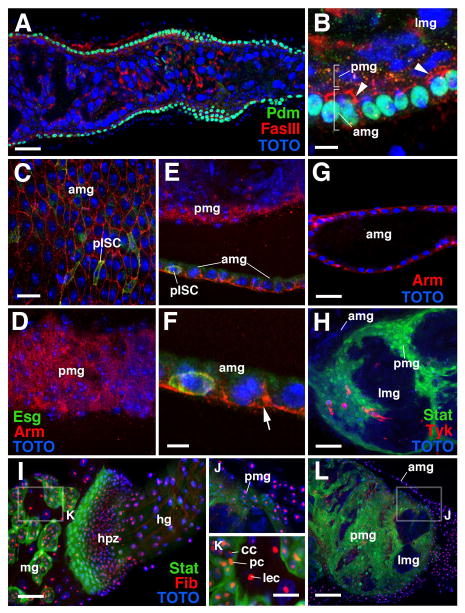
Formation of the transient pupal midgut. A–E: Z-projections of confocal sections of midgut of late third instar larva (L3; A, B), white prepupa (C), 6h prepupa (D, E), and 72h pupa (G–I). A, C, E and G present tangential confocal section of the outer gut epithelium; B, E and H show confocal cross sections. Adult midgut progenitors are labeled by esg-Gal4-driven mRFP (red) and by 10XStat92E-GFP (green; white in panel I). Cell nuclei are labeled by TOTO-3 (blue). A, B: clusters of small adult midgut progenitors form nests (nidi) scattered among large larval enterocytes (lec). Outer cells of each nest have differentiated into peripheral cells (pc) which express higher levels of 10XStat92E-GFP; central cells (cc) are small, round and Stat-negative. C: In white pre-pupa, the midgut contracts. Clusters of adult midgut progenitors approach each other and form a lose cell layer, still interrupted by gaps (arrowheads), that encloses the larval midgut epithelium. D, E: In the 6h pupa, peripheral cells and central cells of the former nests of adult midgut progenitors have fused into two complete layers. Central cells (Stat-negative, red) form the presumptive adult midgut (amg) at the outer surface; peripheral cells (Stat-positive, green) form the transient pupal midgut (pmg) underneath. In the center are the large larval midgut cells (lmg). Which undergo programmed cell death. Note that these cells express various levels of esg-Gal4 driven GFP throughout the pupal period. F: Electron micrograph of section of late larval midgut. At the top is a large larval enterocyte (lec) with a brush border (bb) of long microvilli at its apical surface. At the bottom is a nest of adult midgut progenitors, differentiated into central cells (cc) and peripheral cells (pc), and visceral muscle (vm).
G–I: In the 72h pupa, the degenerating larval midgut (lmg), surrounded and septated by the transient pupal midgut (pmg), has become the compact yellow body that rests in the lumen of the presumptive adult midgut (amg). 10xStat92-GFP is still expressed in the pupal midgut. In addition, it is expressed in the presumptive midgut stem cells (pISCs) which at this stage are scattered throughout the adult midgut layer. J–L: Lineage tracing experiments, activating a stably expressed lacZ reporter in adult midgut progenitors in the first instar larva by esg-Gal4 (see Material and Methods for detail). By late larval stage (J), adult midgut progenitors have proliferated and form clusters of 8–10 cells. In 24h pupa (K, L) labeled cells (descended from adult midgut progenitors) are found in both adult midgut (amg) and transient pupa midgut (pmg). amp adult midgut progenitors, bb brush border, cc central cells, lec larval enterocyte, lmg larval midgut, lu: lumen, pc peripheral cell, pmg pupal midgut, vm visceral muscle. Bars: 10μm (A–E; G–I; J; L); 2μm (F); 50μm (K)
During the first hours after puparium formation, the larval midgut constricts in the longitudinal axis, which brings the previously scattered AMP clusters in close proximity (Fig. 2C). As neighboring clusters meet, the first cells to establish contact are the peripheral cells. By 6h after puparium formation (APF), peripheral cells have formed a complete cellular layer around the central mass of contracted larval midgut cells (Fig. 2D, E). At the same time, central cells of the AMP clusters flatten apico-basally and expand laterally, to form the outer, adult midgut layer (Fig. 2E). During a phase that lasts approximately until 12h APF, the midgut exhibits a clear, three layered appearance, with an inner, differentiated larval midgut epithelium, an outer, undifferentiated adult midgut, and a transient pupal midgut sandwiched in between. During later stages of metamorphosis, the larval and transient pupal midgut separate from the outer layer, further contract, and become the compact yellow body resting in the lumen of the developing adult midgut. The pupal midgut remains detectable using 10xStat92-GFP as a marker until approximately 72 APF (Fig. 2G–I).
The adult midgut and transient pupal midgut both derive from AMPs
To confirm that the adult and pupal midgut cells share a common origin we used a lineage tracing construct (Act5c promoter>Stop>lacZ; see Materials and Methods), where the flip-out of the stop-cassette was driven by esg-Gal4, to trace the lineages derived from the AMPs. The construct was activated by heat treatment in the early first instar larva, resulting in the stable expression of a lacZ reporter in cells expressing esg-Gal4 at that stage. In midguts fixed at the late third instar we observed many lacZ-positive AMP clusters, whereas larval midgut cells were lacZ-negative (Fig. 2J). In midguts fixed at 24h APF, lacZ was found in both cells of the adult gut, as well as the transient pupal midgut (Fig. 2K, L).
Ultrastructure of the midgut during metamorphosis
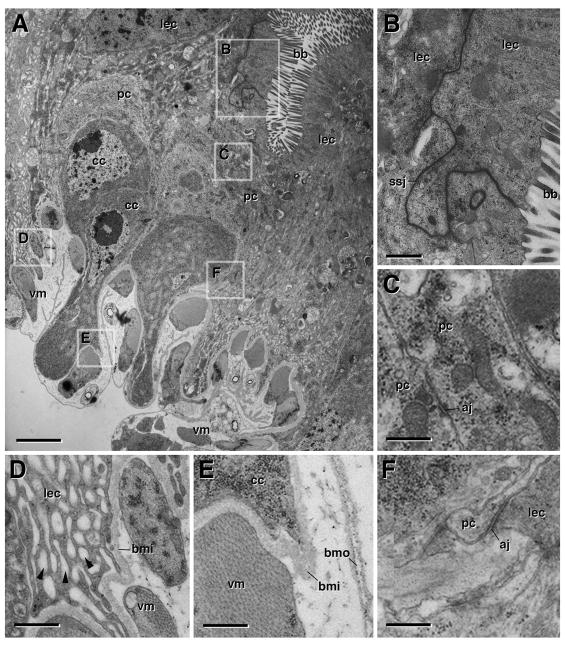
During the first two hours APF (white pre-pupa), the larval midgut epithelium is still recognizable as an intact tissue with an apical brush border (Fig. 3A, B) and basal labyrinth (the profuse membrane infoldings characteristic of insect enterocytes; Martoja and Ballan-Dufrancais, 1984; Fig. 3A, D). Smooth septate junctions connect larval enterocytes apico-laterally (Fig. 3B). Outside the larval midgut, AMP clusters have not yet fully extended, but still form distinct “nests”, separated by regions where larval enterocytes contact the basal surface (Fig. 3A). Visceral muscle (which, as in arthropods generally forms an inner layer of densely spaced circular fibers and more widely spaced longitudinal strands; Crossley, 1985; Jirikowski et al., 2010) contracts with the onset of metamorphosis. The basal surface of the pre-pupal gut epithelium is covered by contracted muscle fibers and two basal laminae, one in between the gut epithelium and the muscle, the other outside of the muscle layer (Fig. 3A, E). Adult midgut progenitors (the former “central cells”), as well as the transient pupal midgut cells, (the former “peripheral cells”) undergo a mesenchymal-epithelial transition. Both cell types become flattened cells connected by spot adherens junctions (Fig. 3C). During the first hours APF, the outer surface of the contracted midgut is deformed into ruffles and folds; as a result, many of the adult and pupal midgut cells appear to lengthen apico-basally (Fig. 3A). However, by 6h APF, the outer midgut surface has smoothened, revealing the definitive configuration of adult and pupal midgut cells as squamous epithelial cells (Fig. 4A).
Fig. 3.
Ultrastructure of the metamorphosing midgut. White Prepupa. A: Cross section of wall of white pre-pupal midgut at low magnification. Boxed areas indicate regions shown at higher magnifications in panels B–F. B: Enterocytes of larval midgut (lec) with apical brush border (bb) and smooth septate junctions (ssj). C: peripheral cells (pc), stretching out to become transient pupal midgut, form leaflets interconnected by spot adherens junctions (aj). D: Basal part of larval enterocyte, containing numerous membrane invaginations (“labyrinth”; arrowheads). Inner layer of basement membrane (bmi) separates larval midgut cells from visceral muscle (vm). E: Basal process of central cell (cc) separated from visceral muscle by inner layer of basement membrane. Outer layer of basement membrane (bmo) covers visceral muscle. F: Spot adherens junctions (aj) link larval enterocytes and peripheral cells. aj adherens junction, bb brush border, cc central cells, bmi inner layer of basement membrane, bmo outer layer of basement membrane, lec larval enterocyte, pc peripheral cell, ssj smooth septate junction, vm visceral muscle. Bars: 2μm (A); 0.5μm (B); 0.4μm (C–F)
Fig. 4.
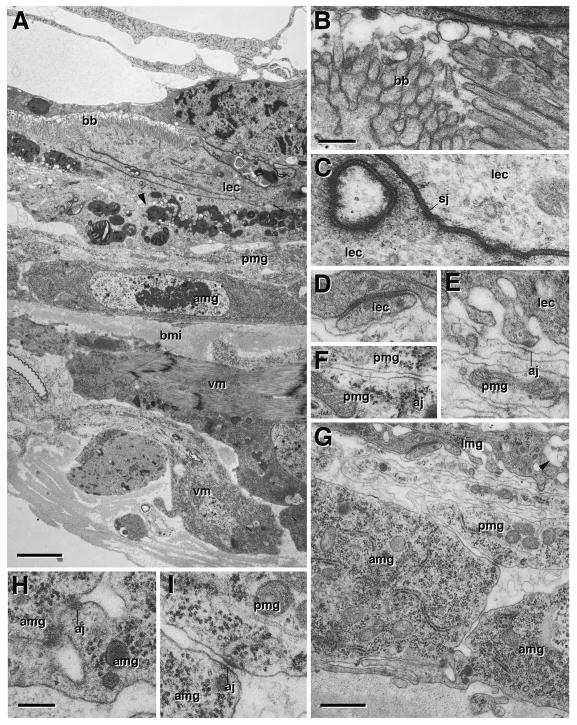
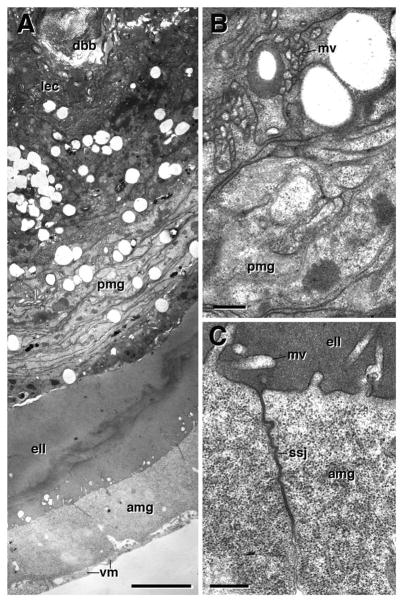
Ultrastructure of metamorphosing midgut, 6h pupa. A: Cross section showing degenerating larval gut (lmg), pupal midgut (pmg), adult midgut (amg), and visceral muscle (vm). Microvillar brush border (bb) is still visible in larval midgut. Mitochondria are fragmenting (arrowheads). B: Brush border of larval midgut at higher magnification. C, D: Septate junction (sj) between enterocytes of larval midgut (lec). E: Spot adherens junction (aj) between larval enterocyte and pupal midgut cell (pmg). F: Spot adherens junction between leaflets of pupal midgut. G–I: Cross section of basal part of larval midgut, pupal midgut, and adult midgut. Note that the labyrinth of basal membrane invaginations in larval enterocytes (see Fig. 3D) is all but gone; arrowhead in (G) points at one of the remnants of these invaginations. Adult midgut cells are connected basally by spot adherens junctions (aj; see higher magnification in H). Spot adherens junctions also connect adult to pupal midgut cells (I). Microvilli or septate junctions have not formed yet in adult midgut or pupal midgut. aj adherens junction, amg adult midgut, bb brush border, bmi inner layer of basement membrane, lec larval enterocyte, pmg pupal midgut, sj septate junction, vm visceral muscle. Bars: 2μm (A); 0.2μm (B–F; H, I); 1μm (G)
The transient pupal midgut (tPMG) appears multilayered in confocal cross-sections (Fig. 2E), an impression that is confirmed electron microscopically. Thus, in the 6h pupa, the tPMG consists of 2–3 loosely packed leaflets (Fig. 4E); by 20h APF, this number has increased to 4–6, and leaflets are more tightly packed (Fig. 5A, D). Initially, scattered spot adherens junctions interconnect the leaflets to each other, as well as to the apically adjacent larval enterocytes and basally adjacent adult midgut cells (Fig. 4E, F, G, I). In the 20h and 48h pupa, pleated septate junctions have been added to the array of junctional contacts of the tPMG (Fig. 5D, E). By that stage, the tPMG separates from the surrounding adult midgut, and a liquid filled, electron dense cleft appears between the two tissues (Figs. 5G, 6A). The adult midgut epithelium shows structural signs of differentiation, including the first irregularly scattered apical microvilli, and the smooth septate junctions at the subapical membrane (Figs. 5G, K; 6A, C). At the same time, the larval midgut cells exhibit hallmarks of degeneration, including fragmentation of mitochondria and nuclei (Fig. 6A). By contrast, cells of the tPMG remain intact at least as long as 48h (Fig. 6A); eventually, these cells also undergo cell death (Mathur et al., 2010).
Fig. 5.
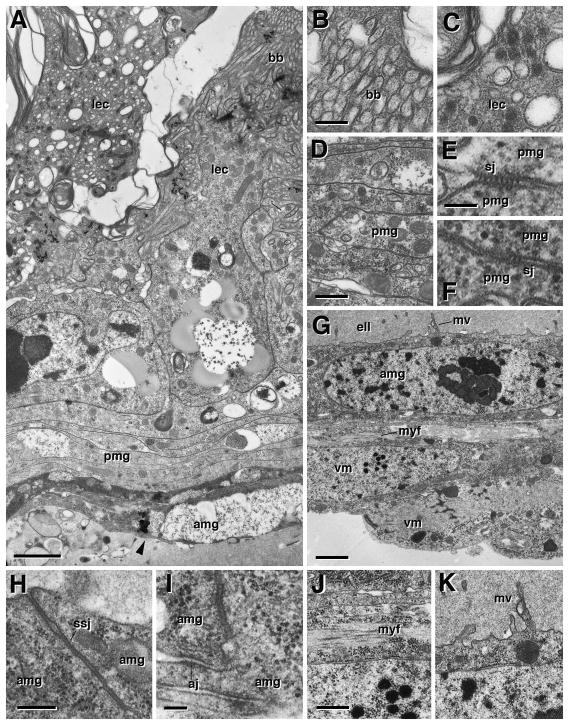
Ultrastructure of metamorphosing midgut, 20h pupa. A: Cross section of pupal midgut at 20h, showing degenerating larval enterocytes (lec), transient pupal midgut (pmg) and adult midgut (amg). B: Microvillar brush border (bb) is still present in larval enterocytes. C: Fragmented mitochondria in degenerating larval enterocytes. D–F: Transient pupal midgut cells (pmg) have expanded and form multi-layered leaflets connected by many scattered pleated septate junctions (sj). G–K: cross section of adult midgut layer and visceral muscle. At the position where photo (G) was taken, a cleft filled with electron-dense liquid (ell) had developed between the adult midgut and the pupal/larval midgut, which form the yellow body (not on photo). Cell bodies of visceral muscle (vm) persist throughout metamorphosis, but have withdrawn most myofibril filled processes. Small patches of myofibrils (myf) are left (G, J). Adult midgut cells have started to differentiate, forming scattered microvilli (mv in G, K) and smooth septate junctions (ssj; H) at their apical pole. Basally, adult midgut cells are connected by spot adherens junctions (aj in I). aj adherens junction, amg adult midgut, bb brush border, ell electron dense liquid, lec larval enterocyte, mv microvilli, myf miofibrils, pmg pupal midgut, ssj smooth septate junction, vm visceral muscle. Bars: 1μm (A); 0.25μm (B, C); 0.5μm (D); 0.1μm (E, F; I); 1μm (G); 0.2μm (H); 0.4μm (J, K)
Fig. 6.
Ultrastructure of metamorphosing midgut, 48h pupa. A: cross section of midgut wall, with degenerating larval cells (lec) in center, surrounded by multilayered transient pupal midgut (pmg). By this stage, larval and pupal midgut have shrunken into a compact mass, the yellow body, separated from the adult midgut wall (amg) by a wide cleft filled with electron-dense liquid (ell). A thin muscle layer (vm) has been reconstituted. B: Apical domain of pupal midgut, showing densely packed, irregular membrane protrusions and infoldings which may be interpreted as rudimentary microvilli (mv). C: Adult midgut cells have extended their microvilli and form large smooth septate junctions (ssj). amg adult midgut, dbb degenerating brush border, ell electron dense liquid, lec larval enterocyte, mv microvilli, pmg pupal midgut, ssj smooth septate junction, vm visceral muscle. Bars: 2μm (A); 0.25μm (B, C)
The above changes in midgut epithelial structure take place within an outer envelope formed by the visceral muscle cells. As described in more detail elsewhere (Aghajanian et al., 2011), visceral muscle also undergoes a phase of profound reorganization during metamorphosis. Thus, myofibrils break down around 12–24h APF, and muscle cells loose their regular shape and spatial arrangement. This process has reached its apogee around 24h APF, when muscle cells have lost most myofibrils and form irregular clusters of cells around the developing adult midgut epithelium (Fig. 5A, G, J). Basement membranes are also lost. By 48h APF a thin muscle layer with myofibrils has reappeared, and a thin basement membrane surrounds muscle and adult midgut epithelial cells (Fig. 6A).
Partial differentiation of the pupal midgut
Even though the pupal midgut, along with the larval midgut, forms no part of the adult body, it initiates several aspects of epithelial differentiation during the first half of the pupal period. As already mentioned above, tPMG cells form a junctional complex of septate junctions and adherens junctions. They also start to form microvilli at their apical membrane. Thus, in the 4h pupal gut shown in Fig. 6, dense arrays of short, apically oriented membrane processes can be observed in the innermost leaflets of the tPMG (Fig. 6B). However, other aspects of membrane polarization, one of the hallmarks of epithelia, do not materialize. Two markers, Drosophila β-catenin/Armadillo (Arm) and Fasciclin III (FasIII), document the polarization of the adult midgut epithelium as early as 6h APF (Fig. 7A–F). Interestingly, the expression of both markers is the reverse of that found in ectodermal epithelia, where Arm marks the apically located zonula adherens (Woods and Bryant, 1993), and FasIII is concentrated latero-basally (Patel et al., 1987; Tepass et al., 1996; Uemura et al., 1996). In the developing adult midgut, Arm is concentrated basally, and FasIII apically (Fig. 7A–G). This unusual distribution is most likely related to the endoderm-specific junctional complex, which lacks an apical zonula adherens. The tPMG expresses low levels of Arm and FasIII, but shows no signs of a polarized distribution of these markers. Another sign for the incomplete differentiation of the tPMG is its failure to express the molecular differentiation marker Pdm-1 (Affolter et al., 1993) which comes on transiently in both larval and adult midgut cells, but never in the transient pupal midgut (Fig. 7A, B).
Fig. 7.
Differentiation of transient pupal midgut. A, B: Z-projection of confocal sections of 6h pupal misgut labeled with anti-Pdm, a differentiation marker for midgut (green), anti-Fasciclin III (FasIII; red), and Sytox (blue). Note lack of Pdm expression in transient pupal midgut (pmg). FasIII is expressed apically and laterally in primordium of adult midgut (amg; arrowheads), but lacks polarized expression in pupal midgut. C-F: Z-projections of 24h pupal midgut labeled with anti-β-catenin/Armadillo (Arm; red) and Sytox (blue). GFP expression is driven by the escargot-Gal4 line (Esg, green). C shows plane of adult midgut (amg), where membrane-bound Arm forms a crisp network outlining the lateral membranes of cells. D is at the plane of the transient pupal midgut; Arm is expressed at moderate levels throughout the cells. E and F show cross section of adult and transient pupal midgut. Note strong membrane bound Arm signal basally and laterally around adult cells, and diffuse staining of pupal cells. Esg is upregulated in presumptive adult midgut stem cells (pISC), which at this stage are integrated in the adult midgut epithelium. G: Confocal section of 72h pupal midgut, showing polarized expression of Arm (red). H: 6h pupal midgut labeled by Stat92E-GFP (Stat; green) and anti-Tachykinin (Tyk; red). Tyk is expressed in differentiated midgut endocrine cells; note population of these cells integrated in the transient pupal midgut. I-L: Expression of Fibrillarin (Fib; red) in midgut and hindgut of late larva (I, K) and 20h pupa (J, L). amg adult midgut, cc central cell, hg hindgut, hpz hindgut proliferation zone/imaginal ring, mg midgut, lec larval enterocyte, lmg larval midgut, lu: lumen, pc peripheral cell, pISC presumptive adult midgut stem cell, pmg pupal midgut, vm visceral muscle. Bars: 10μm (A, G, H, I, L); 3μm (B); 5μm (C–E); 2μm (F); 5μm (J, K)
Differentiation programmed death of the tPMGs could also be followed with an antibody against the nuclear protein Fibrillarin. Fibrillarin localizes in the nucleolus as well as in the Cajal body (Liu et al., 2006), and functions in the ribosomal RNA processing machinery. Thus we used anti-Fibrillarin antibody to monitor the physiology of the tPMG. Fibrillarin expression occurs at a high level in the nuclei of differentiated cells, as shown for the larval enterocytes of the midgut and hindgut in Fig. 7 I, K. Weak expression is seen in the undifferentiated progenitors of midgut and hindgut (Fig. 7I, K). Peripheral cells of the larval AMP clusters also express high levels of Fibrillarin (Fig. 7K). In the pupa, Fibrillarin disappears from the degenerating larval enterocytes (Fig. 7J, L), but is maintained at high levels in cells of the adult midgut and tPMG (Fig. 7J, L). Expression disappears from the tPMG as it degenerates around 50h APF (not shown).
A subset of pupal midgut cells develops as enteroendocrine cells; these can be labeled by antibodies against specific peptide hormones, such as anti-Tachykinin (Fig. 7H), and anti-FMRF-amide (data not shown). Both are peptides found widely in neurons and endocrine cells in many taxa (Krajniak and Greenberg, 1992; Satake and Kawada, 2006; Kwok et al., 2005; Haselton et al., 2008; Gallus et al., 2008; Veenstra et al., 2008; Nakanishi et al., 2009). Expression of these markers already begins among the peripheral cells of the AMP nests found in the late larva (Takashima et al., 2011). The endocrine cells in tPMG do not flatten, like the remainder of the pupal midgut cells; they remain detectable until at least 24h APF as somewhat irregular, spindle shaped cells scattered throughout the pupal midgut.
Discussion
The structure and development of the insect midgut is the object of a large number of studies. Findings are all in agreement in regard to the general mechanism by which clusters (“nests” or “nidi”) of mitotically active progenitor cells (also called “regeneration cells” by many authors) adhere to the basal surface of the midgut epithelium and generate midgut cells (Andries 1970; 1979; Kathuria, 1971; Martoja and Ballan-Dufrancais, 1984; Uwo et al., 2002; Jiang and Edgar, 2009; for recent review, see Hakim et al., 2010). In a number of species, newly produced daughter cells of these progenitors are continuously incorporated into the differentiated midgut epithelium, both in the larva and the adult. By this mechanism, progenitor cell proliferation contributes to the growth of the midgut in the larva. In Drosophila and some other holometabolous insects, the larval midgut grows by endoreplication and its differentiated cells increase in size; progenitor cells divide, but remain undifferentiated until the onset of metamorphosis. Only in the adult, does the progeny formed by the scattered stem cell population, differentiate (Ohlstein and Spradling, 2006; Michelli and Perrimon, 2006).
Metamorphosis appears to bring about a sudden replacement of the midgut epithelium in its entirety in all insect species studied, including holometabolans and at least some hemimetabolans (e.g., Odonata; Andries, 1970; 1979). This may come as no surprise, in view of the fact that the transition from larva to adult typically brings with it a radical change in diet, which would necessitate different types of secretions, food uptake mechanisms, and hormonal control of the midgut. The way in which the larval midgut is replaced within the first hours after onset of the pupal period, seems also quite similar across the insect taxa: the progenitor cell clusters expand, merge, and form an outer replacement layer that covers the larval midgut at its basal surface. Subsequently, the larval midgut undergoes programmed cell death (Denton et al., 2009; 2010), and the replacement differentiates into the adult and/or the transient pupal midgut.
For a number of insects, the transient pupal midgut has been described in the classical literature, but received little attention in the more recent literature. On the other hand, for Drosophila, the transient pupal midgut, was overlooked in both classical studies (Robertson, 1936; Bodenstein, 1950) and modern papers (e.g., Lee et al., 2002) that utilize specific markers or electron microscopy. One reason for this is that cells of the transient pupal midgut do not acquire the large size and characteristic shape of differentiated larval or adult midgut cells. Instead, they form flattened, leaflet like cells lacking a prominent microvillar brush border, or the abundance of secretory vesicles, by which enterocytes of the larval and adult midgut stand out. A type of transient pupal midgut structurally similar to the one described here in Drosophila has been observed also for other insects, such as the dragon fly Aeshna cyanea (Andries, 1979), where cells form a multilayer of flattened cells (“reticulated tissue”) wrapping around the compact mass formed by the degenerating larval midgut cells. By contrast, in other species such as the beetle Chilomenes sexmaculata (Kathuria, 1971), the transient pupal midgut differentiates more fully, forming secretory vesicles and a brush border.
Using electron microscopy and a variety of molecular markers we investigated the junctional complex and the appearance of apico-basal polarity in the transient pupal midgut. The emerging picture is that certain aspects of polarity, in particular rudimentary microvilli at the apical side of the epithelium, do appear. On the other hand, proteins with clear polarized distribution in larval or adult midgut, such as FasIII or β-catenin/Armadillo, are not expressed in a polarized manner in the transient pupal midgut. Junctions comprise spot adherens junctions (which are essentially ubiquitous in developing tissues; Tepass and Hartenstein, 1994b), and scattered pleated septate junctions. The presence of adherens junctions (called ‘desmosomes’ in much of the classical literature) and absence of smooth septate junctions (‘zonula continua’) was also reported in the transient pupal midgut described for Aeshna (Andries, 1979). The appearance of pleated septate junctions is unusual for an endodermally derived tissue: both larval and adult midgut epithelial cells possess only smooth septate junctions (Noirot-Timothee and Noirot, 1980). However, it has been shown that pleated septate junctions appear transiently in the midgut, as well as the adjacent Malpighian tubules and proventriculus (Baldwin and Hakim, 1987; Tepass and Hartenstein, 1994). The pleated junctions are later replaced by smooth, septate junctions. These findings support the idea that the transient pupal midgut in Drosophila and other species differentiates up to a certain stage, which includes the formation of pleated septate junctions. After that, development is aborted before reaching a mature stage, where smooth septate junctions would appear.
What could be the function of the transient pupal midgut? There seems to be good evidence that material of the degenerating larval gut, whose cells undergo autophagy (Denton et al., 2009; 2010), serves as a source of nutrients for the pupa (Hakim et al., 2010). This would explain the formation of a regular layer of enterocytes, active in absorption and secretion, as has been observed in some insects like the beetle Chilomenes (Kathuria, 1971). On the other hand, it is unlikely that the flattened cells observed in Aeshna or Drosophila, carry out any digestive action, due to the lack of pronounced microvilli or a secretory apparatus. More importantly, it has been shown experimentally that by blocking autophagy in Drosophila larval midgut cells, pupal development is not impaired (Lee et al., 2002). One therefore has to interpret the transient pupal midgut differently. One possibility is to consider this tissue as an evolutionary vestige: given that at some point in phylogeny, the pupal midgut did have a bona fide role in digestion. The developmental program creating it remains to the present day, just that it stops short of terminal differentiation of most enterocyte-specific features. Alternatively, one could think about different roles of the transient pupal midgut, for example in protecting the delicate pupal tissues from digestive enzymes released prematurely from the degenerating larval enterocytes, or in providing a more suitable environment for the differentiating adult midgut cells, as has been proposed by Andries (1979). It will be informative to ablate the transient pupal midgut genetically, and analyze ensuing changes in pupal development.
As described in previous works (Mathur et al., 2010; Takashima et al., 2011), it is clear that the specification of the transient pupal midgut employs the same genetic cassette as the larval or adult midgut. Centrally involved is the Notch signaling pathway. When activated in the endoderm of the embryo, or the midgut stem cells of the adult, Notch activity drives cells towards the differentiated enterocyte fate. In the case of the adult midgut progenitors clusters that populate the larval gut, Notch activity promotes the fate of transient pupal midgut cells. In turn, cells with high Notch activity signal back to the progenitors. This has been most clearly shown for the nascent pupal midgut cells (“peripheral cells”) of the larva, which via Dpp maintain the central cells in a proliferatory, undifferentiated state (Mathur et al., 2010). It is also likely that after the onset of metamorphosis, the pupal midgut cells communicate with the neighboring adult or larval enterocytes and thereby control aspects of differentiation/programmed cell death. Drosophila offers the opportunity to genetically eliminate cell populations that can be addressed by specific promoters. These studies will be informative to gain more insight into the developmental role of the pupal midgut.
Acknowledgments
We thank Dr Dick Nässel for providing anti-tachykinin antibody, the Bloomington stock center (Indiana Univ.) and National Institute of Genetics (Japan) for fly stocks; and the Developmental Studies Hybridoma Bank (Univ. Iowa) for antibodies. We also thank Marianne Ciluffo of the BRI electron microscopy facility for help with tissue sectioning, and members of the Hartenstein lab for helpful discussion. This work was supported by NIH/1 R01 GM087373 to V.H.
References
- Affolter M, Walldorf U, Kloter U, Schier AF, Gehring WJ. Regional repression of a Drosophila POU box gene in the endoderm involves inductive interactions between germ layers. Development. 1993;117:1199–1210. doi: 10.1242/dev.117.4.1199. [DOI] [PubMed] [Google Scholar]
- Aghajanian P, Takashima S, Ortiz P, Hartenstein V. Metamorphosis of the visceral musculature of Drosophila. 2011 doi: 10.1016/j.ydbio.2016.10.011. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries JC. Activité des nids de régénération de l’intestin moyen de la larve d’Aeschna cyanea au cours d’un cycle digestif. J Ins Phys. 1970;16:1961–1973. [Google Scholar]
- Andries JC. Junctional structures in the metamorphosing midgut of Aeshna cyanea (Insecta, Odonata) Cell Tiss Res. 1979;202:9–15. doi: 10.1007/BF00239216. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH. GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns. 2007;7:323–331. doi: 10.1016/j.modgep.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Hakim RS. Change of form of septate and gap junctions during development of the insect midgut. Tiss Cell. 1987;19:549–558. doi: 10.1016/0040-8166(87)90047-4. [DOI] [PubMed] [Google Scholar]
- Bodenstein D. The postembryonic development of Drosophila. In: Demerec M, editor. Biology of Drosophila. Wiley; New York: 1950. pp. 275–367. [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Springer-Verlag; Berlin: 1985. [Google Scholar]
- Crossley AC. Nephrocytes and pericardial cells. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry, and Pharmacology. Vol. 3. Pergamon; Oxford: 1985. [Google Scholar]
- Denton D, Shravage B, Simin R, Mills K, Berry DL, Baehrecke EH, Kumar S. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr Biol. 2009;19:1741–1746. doi: 10.1016/j.cub.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Shravage B, Simin R, Baehrecke EH, Kumar S. Larval midgut destruction in Drosophila: not dependent on caspases but suppressed by the loss of autophagy. Autophagy. 2010;6:163–165. doi: 10.4161/auto.6.1.10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallus L, Ferrando S, Bottaro M, Diaspro A, Girosi L, Faimali M, Ramoino P, Tagliafierro G. Presence and distribution of FMRFamide-like immunoreactivity in the cyprid of the barnacle Balanus amphitrite (Cirripedia, Crustacea) Microsc Res Tech. 2009;72:101–109. doi: 10.1002/jemt.20649. [DOI] [PubMed] [Google Scholar]
- Grigorian M, Mandal L, Hartenstein V. Hematopoiesis at the onset of metamorphosis: Terminal differentiation and dissociation of the Drosophila lymph gland. Dev Genes Evol. 2011 doi: 10.1007/s00427-011-0364-6. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakim RS, Baldwin K, Smagghe G. Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol. 2010;55:593–608. doi: 10.1146/annurev-ento-112408-085450. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Jan YN. Studying Drosophila embryogenesis with P-lacZ enhancer trap lines. Wilhelm Roux’s Arch Dev Biol. 1992;201:194–220. doi: 10.1007/BF00188752. [DOI] [PubMed] [Google Scholar]
- Haselton AT, Yin CM, Stoffolano JG. FMRFamide-like immunoreactivity in the central nervous system and alimentary tract of the non-hematophagous blow fly, Phormia regina, and the hematophagous horse fly, Tabanus nigrovittatus. J Insect Sci. 2008;8:1–17. doi: 10.1673/031.008.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirikowski G, Kreissl S, Richter S, Wolff C. Muscle development in the marbled crayfish--insights from an emerging model organism (Crustacea, Malacostraca, Decapoda) Dev Genes Evol. 2010;220:89–105. doi: 10.1007/s00427-010-0331-7. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kathuria OP. Metamorphosis of the midgut of a six-spotted ladybird beetle, Chilomenes sexmaculata (Coleoptera: Coccinellidae) Int J Ins Morph Embryol. 1971;1:87–93. [Google Scholar]
- Krajniak KG, Greenberg MJ. The localization of FMRFamide in the nervous and somatic tissues of Nereis virens and its effects upon the isolated esophagus. Comp Biochem Physiol C. 1992;101:93–100. doi: 10.1016/0742-8413(92)90205-l. [DOI] [PubMed] [Google Scholar]
- Kwok R, Chung D, Brugge VT, Orchard I. The distribution and activity of tachykinin-related peptides in the blood-feeding bug, Rhodnius prolixus. Peptides. 2005;26:43–51. doi: 10.1016/j.peptides.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Lee CY, Cooksey BA, Baehrecke EH. Steroid regulation of midgut cell death during Drosophila development. Dev Biol. 2002;250:101–111. doi: 10.1006/dbio.2002.0784. [DOI] [PubMed] [Google Scholar]
- Lehane MJ. The Midgut. In: Harrison FW, Locke M, editors. Microscopic Anatomy of Invertebrates. Wiley Liss; New York: 1998. pp. 725–746. [Google Scholar]
- Liu JL, Murphy C, Buszczak M, Clatterbuck S, Goodman R, Gall JG. The Drosophila melanogaster Cajal body. J Cell Biol. 2006;172:875–884. doi: 10.1083/jcb.200511038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martoja Rm, Ballan-Dufrancais C. The Ultrastructure of the Digestive and Excretory Organs. In: King RC, Akai H, editors. Insect Ultrastructure. Vol. 2. Plenum Press; New York London: 1984. pp. 199–268. [Google Scholar]
- Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Hartenstein V, Jacobs DK. Development of the rhopalial nervous system in Aurelia sp.1 (Cnidaria, Scyphozoa) Dev Genes Evol. 2009;219:301–317. doi: 10.1007/s00427-009-0291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noirot-Timothee C, Noirot C. Septate and scalariform junctions in arthropods. Int Rev Cytol. 1980;63:97–140. doi: 10.1016/s0074-7696(08)61758-1. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Patel NH, Snow PM, Goodman CS. Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell. 1987;48:975–988. doi: 10.1016/0092-8674(87)90706-9. [DOI] [PubMed] [Google Scholar]
- Robertson CW. Metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J Morphol. 1936;59:351–399. [Google Scholar]
- Satake H, Kawada T. Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets. 2006;7:963–974. doi: 10.2174/138945006778019273. [DOI] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–40. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Takashima S, Adams K, Ortiz P, Ying C, Moridzadeh R, Younossi-Hartenstein A, Hartenstein V. Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.01.039. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. Epithelium formation in the Drosophila midgut depends on the interaction of endoderm and mesoderm. Development. 1994a;120:579–590. doi: 10.1242/dev.120.3.579. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994b;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. Neurogenic and proneural genes control cell fate specification in the Drosophila endoderm. Development. 1995;121:393–405. doi: 10.1242/dev.121.2.393. [DOI] [PubMed] [Google Scholar]
- Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Török T, Hartenstein V. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev. 1996;10:672–685. doi: 10.1101/gad.10.6.672. [DOI] [PubMed] [Google Scholar]
- Uemura T, Oda H, Kraut R, Hayashi S, Kotaoka Y, Takeichi M. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 1996;10:659–671. doi: 10.1101/gad.10.6.659. [DOI] [PubMed] [Google Scholar]
- Uwo MF, Ui-Tei K, Park P, Takeda M. Replacement of midgut epithelium in the greater wax moth, Galleria mellonela, during larval-pupal moult. Cell Tiss Res. 2002;308:319–331. doi: 10.1007/s00441-002-0515-1. [DOI] [PubMed] [Google Scholar]
- Veenstra JA, Agricola HJ, Sellami A. Regulatory peptides in fruit fly midgut. Cell Tiss Res. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. Apical junctions and cell signalling in epithelia. J Cell Sci Suppl. 1993;17:171–181. doi: 10.1242/jcs.1993.supplement_17.25. [DOI] [PubMed] [Google Scholar]
- Yeo SL, Lloyd A, Kozak K, Dinh A, Dick T, Yang X, Sakonju S, Chia W. On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes Dev. 1995;9:1223–1236. doi: 10.1101/gad.9.10.1223. [DOI] [PubMed] [Google Scholar]