Abstract
The midgut epithelium is formed by absorptive enterocytes, secretory cells and endocrine cells. Each of these lineages is derived from the pluripotent progenitors that constitute the embryonic endoderm; the mature midgut retains pools of self-renewing stem cells that continue to produce all lineages. Recent findings in vertebrates and Drosophila shed light on the genetic mechanism that specifies the fate of the different lineages. A pivotal role is played by the Notch signaling pathway that, in a manner that appears to be very similar to the way in which Notch signaling selects neural progenitors within the neurectoderm, distinguishes the fate of secretory/endocrine cells and enterocytes. Proneural genes encoding bHLH transcription factors are expressed and required in prospective endocrine cells; activation of the Notch pathways restricts the number of these cells and promotes enterocyte development. In this review we compare the development of the intestinal endocrine cells in vertebrates and insects and summarize recent findings dealing with genetic pathways controlling this cell type.
Keywords: Drosophila, vertebrate, endocrine, gut, development, stem cell, Notch
The diffuse endocrine system (DES): A brief overview
Digestive function, including motility of the gut, secretion of enzymes, resorption of nutrients, ions and water, is regulated by two systems, the autonomic nervous system, and the endocrine system. In vertebrates, the latter is formed by specialized endocrine glands, in particular the pancreas, as well as scattered endocrine cells integrated in the intestinal wall. These cells, which outnumber all other endocrine organs by a wide margin, form the diffuse endocrine system (DES). Within the DES, at least 14 different cell types have been identified which produce many different peptide hormones with a specific regional distribution (for review, see Rehfeld, 1998; Montuenga et al., 2003; Rindi et al., 2004). For example, secretin, produced in the duodenum, was one of the first hormones discovered and characterized around the turn of the 20th century (reviewed in Modlin et al., 2006); released by gastric acid, secretin stimulates secretion of bicarbonate-rich pancreatic juice. Other well characterized DES hormones are gastrin (produced in the stomach) and cholecystokinin (CKK; produced in the small intestine). Release of gastrin is triggered by protein rich food and in turn increases acid secretion from parietal cells; likewise, CKK, triggered by fats and proteins, stimulates the secretion of pancreatic enzymes and gall bladder contraction.
Enteroendocrine cells are elongated, epithelial cells with a cell body located basally, and a neck that reaches the the luminal surface of the epithelium (“open endocrine cells”; Fig.1A, B). In other cases, the apical contact to the lumen is lost (“closed endocrine cells”). Both types of endocrine cells are characterized by two regulated pathways of secretion which are morphologically defined by large dense core vesicles (LDCV) and synaptic-like microvesicles (SLMV; Rindi et al., 2004). Vesicles are targeted to the basal cell membrane and the hormones are released into the interstitial space or into capillaries. With regard to the cellular mechanisms controlling vesicle trafficking and docking, as well as the hormones themselves, enteroendocrine cells share many characteristics with neurons, a theme that will reoccur when looking at development (see below). For example, typical neuronal markers like N-CAM, synaptophysin, or vesicular monoamine transporter, are also found in enteroendocrine cells.
Fig.1.
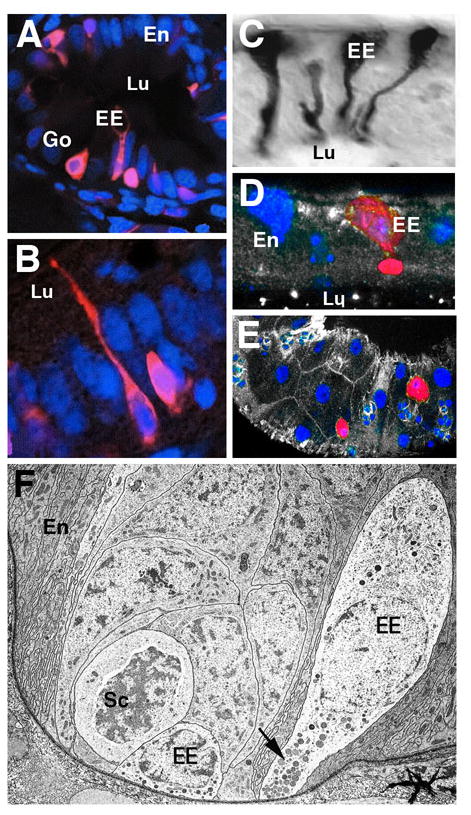
Enteroendocrine cells in the vertebrate and Drosophila intestine. A, B: Five day zebrafish posterior intestine (from Crosnier et al., 2005). Enteroendocrine cells (EE) are labeled by monoclonal antibody 2F11 (red; nuclei of all cells labeled by TOPRO-3 in blue) exhibit an elongated, neuron-like shape, with a basal cell body and a slender apical process integrated into the enterocyte layer (En) and contacting the gut lumen (Lu). Exocrine goblet cells (Go) are also labeled. C: Endocrine cells in locust midgut, labeled by antibody against locust tachkinin-related peptide (from Winther and Nässel, 2001). Note characteristic shape and position of cells, resembling vertebrate enteroendocrine cells. D: Cross section of Drosophila adult midgut epithelium. Enteroendocrine cell labeled by anti-Tachykinin antibody (red). Cell nuclei labeled with Sytox (blue). As in locusts, endocrine cell body is located basally and possesses a club-shaped apical protrusion. E: Tangential section of Drosophila adult midgut epithelium, showing scattered distribution of tachykinin-positive endocrine cells (red). F: Electron micrograph of basal portion of midgut epithelium, showing enteroendocrine cells (EE) in close spatial association with proliferating stem cell “nests” (Sc; from Lehane, 1998). Note dense-core vesicles near basal membrane of endocrine cells (arrow).
Open enteroendocrine cells exist in all animals, from cnidarians to vertebrates. The DES of insects has been studied in considerable detail, and its complexity, in terms of number of different hormones produced and the control of hormone release, is comparable to that of vertebrates (for review, see Zitnan et al., 1993; Veenstra et al., 2008, 2009; Winther and Nässel, 2001). As in vertebrates, the peptide hormones found in insect enteroendocrine cells also occur as neurotransmitters in neurons of the central nervous system and stomatogastric nervous system (comparable to the vertebrate autonomic nervous system) and are therefore frequently referred to as “brain-gut peptides” (Fujita et al., 1981). For example, the peptides of the tachykinin family are found both in DES cells of the midgut (Fig.1C-E), as well as in neurons. Local tachykinin release from neurons has spatially restricted effects on muscle contractility; systemic release into the hemolymph (insects have an open circulatory system, with a blood-like hemolymph filling the body cavity) acts on many effector organs, including the excretory Malpighian tubules, the heart, and the somatic musculature (Winther and Nässel, 2001). The storage and systemic release of peptide hormones involves dense core vesicles located at the basal membrane of the cell (Fig.1F). Peptides of the FMRFamide, myosupressin and leucomyosuppressin family act on the visceral musculature (inhibition of midgut muscle tone; Lange and Orchard, 1998) and secretory cells of the midgut (stimulation of digestive enzyme release; Fuse et al., 1999). Many other brain-gut peptides have been identified (for review see Veenstra et al., 1995; 2008; 2009); as for vertebrate DES-derived hormones, the parameters of release and physiology of most of these peptides have not yet been elucidated.
Development of enteroendocrine cells in vertebrates
It was known for a long time that the autonomic neurons populating the intestinal wall and ganglia associated with it arise in the neural crest and migrate to their final destination during embryogenesis (Anderson et al., 2006). Several decades ago, the hypothesis was put forward that enteroendocrine cells, given their strong similarities with neurons, were also derived from migrating cell populations originating in the neural crest (reviewed in Modlin et al., 2006). Subsequent investigations (Pictet et al., 1976) showed convincingly that that is not the case, and that, instead, enteroendocrine cells segregate from within the same endodermal primordium that gives rise to the enterocytes of the gut epithelium. More recently, using appropriate markers it was possible to study how the different cell lineages (endocrine cells, exocrine secretory cells, enterocytes) relate to each other, and what molecular mechanisms control their fate.
At an early stage of development, the endoderm forms an epithelial tube in which all cells are mitotically active (Henning et al., 1994; Crosnier et al., 2005; Fig.2A, B, E). As the gut tube increases in surface area, the epithelium is gradually folded into the villi and crypts that are characteristic of the gut. At that point, proliferation becomes restricted to the crypts, and cells in the villi undergo differentiation (Fig.2C, F). Eventually, proliferation settles into its adult pattern, in which a small number of slowly dividing stem cells populate the crypts; next to the crypts, at the base of the villi, cells that were produced by the stem cells enter a phase of fast proliferation (“transient amplifying progenitors”); moving further apically, towards the tip of the villi, cells become postmitotic and differentiate (reviewed in Hauck et al., 2005; Fig.2D, G, H). At the villus tip is a region where old and/or damaged cells, including enterocytes and endocrine cells, are sequestered and undergo apoptosis (Potten and Allen, 1977). In this way, there is a constant streaming of cells from the crypts where they are born upward into the villi where they differentiate and eventually die.
Fig.2.
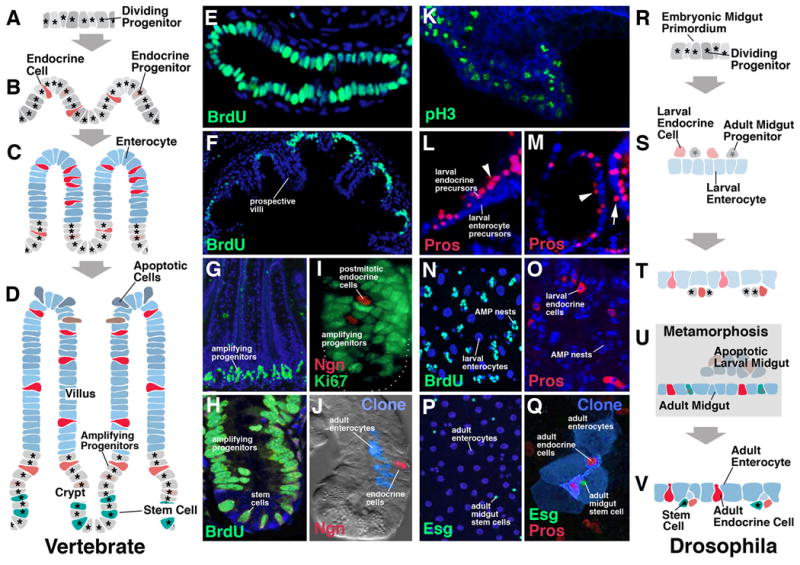
Development of enterocytes and enteroendocrine cells. A-J: Vertebrate. K-V: Drosophila. Panels of left column (A-D) schematically illustrate steps in development of vertebrate intestinal epithelium and enteroendocrine cells. Panels of right column (R-V) show steps in Drosophila development. Panels of middle columns show confocal sections of developing vertebrate gut (E-J) and Drosophila gut (K-Q), respectively. A: Vertebrate embryonic endoderm, consisting of proliferating progenitors of the intestinal epithelium. As an example, panel E (from Crosnier et al., 2005) shows the three day zebrafish intestine that is labeled entirely by the marker for proliferation, BrdU. B: Enteroendocrine cell appear scattered throughout the proliferating primordium of the intestinal epithelium. These cells can either be already postmitotic, or represent dividing progenitors that give rise to multiple endocrine cells. C: The growing epithelium is folded into prospective villi and crypts (called intervillar pockets in zebrafish). Proliferation becomes restricted to the intervillar pockets (shown in panel F; one month zebrafish gut; from Crosnier et al., 2005). Villar cells represent postmitotic, differentiating enterocytes, secretory cells, and enteroendocrine cells. D: mature intestinal epithelium. Dividing cells in the crypts have sorted out into two major populations. One is the slowly cycling intestinal stem cells; the other comprise fast dividing (“amplifying”) progenitors. As these cell divide, progeny are pushed apically into the villus where they differentiate. The choice between enteroendocrine cells and enterocytes is continuously being made in the compartment of amplifying progenitors. G and H show proliferating cells in crypt of mouse intestinal epithelium (from Aiken and Roth, 1992). In I, some postmitotic (Ki67-negative) endocrine cells have separated from dividing progenitors (Ki67-positive; from Bjerknes and Cheng, 2006). J shows clone derived from labeled progenitor, containing enterocytes and one endocrine cell (from Bjerknes and Cheng, 2006). In Drosophila, the midgut develops from the proliferating embryonic midgut primordium (K, R). At an early stage, a separation of prospective larval enterocytes, larval enteroendocrine cells, and adult midgut progenitors (AMPs) takes place (L, M, S). Endocrine cells and AMPs initially lie at the apical (luminal) surface of the emerging enterocyte layer (arrow head in L and M); subsequently they migrate through the epithelium to adopt a basal position (arrow in M, which shows later embryonic stage than L). During the larval stage, enterocytes and endocrine cells constitute the larval midgut (N, O, T); adult midgut progenitors form proliferating cluster (“nests”) of cells attached to the basal surface of the midgut epithelium. During metamorphosis (U), these nests expand and fuse, forming the adult midgut that engulfs the larval midgut. At the same time, a cell fate choice is made within the AMP nests: the majority of cells differentiate as adult enterocytes; some cells are selected as (adult) enteroendocrine cells; a small population retains its proliferatory capacity and will give rise to the midgut stem cells of the adult. In the adult midgut (P, Q, V), these stem cells undergo self-renewing cell divisions; with each division, one daughter cells forms the next stem cell, the other one differentiates as either enterocyte or endocrine cell. Q shows a clone (green) including four enterocytes, two endocrine cells and the one stem cell (from Ohlstein and Spradling, 2006).
The first enteroendocrine cells first appear at an early stage of development before the gut epithelium has formed villi and crypts. Expressing markers for endocrine fate (Math-1; Ngn-3; for review, see Lee and Kaestner, 2004; Schonhoff et al., 2004a) these cells seem to be the first ones to become postmitotic; surrounding enterocyte progenitors continue to divide. Also at later stages, when the characteristic spatio-temporal pattern of proliferation has been set up (stem cells in crypts, transient amplifying progenitors at crypt-villus boundary), enteroendocrine cells are continuously produced. As in the embryo, cells committed to the endocrine fate often withdraw from the mitotic cycle earlier than presumptive enterocytes (Bjerknes and Cheng, 2006; Fig.2I). Differentiating endocrine cells migrate apically into the microvilli, although their speed seems to be slower than that of enterocytes.
Much attention has been given to the question how enterocytes, endocrine cells and other secretory cell types are related. The most direct approach to address this question is to generate labeled clones. To this end, markers are activated in individual proliferating progenitor (or stem) cells. These markers are then inherited by all of the progeny of the labeled cell, thereby showing what different cell types are derived from the one individual progenitor. The analysis performed by Bjerknes and Cheng (2006) yielded clones that contained both enterocytes and (smaller numbers of) enteroendocrine cells. This results indicates that at the level of stem cells, a decision between endocrine and enterocyte fate has not yet been made; rather, cell-cell interactions among the progeny of the stem cells decide cell fate. It is unclear whether this decision happens at the level of progenitors or postmitotic cells. A number of studies showed that endocrine cells expressing different peptides may derive from one progenitor, which would favor the first possibility. Thus, inserting the gene herpes simplex virus 1 thymidine kinase in secretin-expressing cells and thereby rendering these cells susceptible to the antiviral drug ganciclovir did not only kill secretin-positive cells, but several other endocrine cell types as well (CKK, peptide Y, GLP-1; Rindi et al., 1999). This and other studies (e.g., Aiken and Roth, 1992; Schonhoff et al., 2004b) indicate that secretin-expressing cells are still mitotically active and produce progeny that switch to the expression of other peptides. On the other hand, it has been shown that cells that produce a peptide hormone and thereby exhibit their endocrine fate, are postmitotic (e.g.; Bjernes and Cheng, 2006; see Fig.2I). Such findings show that a fate choice is made between either becoming a postmitotic endocrine cell or a cell that continues to divide, to then produce more future enterocytes and endocrine cells. This scenario would also explain the phenotype resulting from disrupting the Notch signaling pathway during gut development (see below).
Development of the Drosophila enteroendocrine system
Drosophila belongs to the group of holometabolous insects which undergo complete metamorphosis. Most organs (including the intestinal tract) of the larval body which is formed during embryogenesis are destroyed during metamorphosis and are replaced by adult-specific organs. Enteroendocrine cells are found scattered throughout the larval and adult midgut (Siviter et al., 2000; Ohlstein and Spradling, 2007; Veenstra et al., 2008, 2009). Recent studies have started to elucidate some of the pertinent facts regarding the origin of these cells, as well as their lineage relationship to other cells of the intestinal epithelium.
The Drosophila midgut originates from the endoderm. In the early embryo, the endoderm forms an anterior and posterior cluster of proliferating mesenchymal cells which eventually migrate towards each other and merge, and at the same time re-organize into an epithelial layer (Tepass and Hartenstein, 1994; Campos-Ortega and Hartenstein, 1997; Fig.2K, R). At an early stage, before the mesenchymal-epithelial transition takes place, a cell fate choice between at least three cell types is made. The majority of cells, those which form the epithelial layer, will give rise to the enterocytes of the larval gut. Two smaller populations of cells remain outside the epithelium. They are the progenitors of the enteroendocrine cells of the larva, and the cells that eventually will give rise to the adult midgut (adult midgut progenitors; AMPs; Tepass and Hartenstein, 1995; Takashima et al., 2009). Both populations initially occupy a position in the lumen of the midgut (Fig.2L, S); during late embryonic stages, they migrate through the gut epithelium and adopt a position at its basal surface (Fig.2M). Enteroendocrine cells maintain a slender process in the epithelium that connects to its apical (luminal) surface; AMPs are no longer in contact with lumen.
During the larval period, AMPs proliferate and produce clusters of cells scattered more or less evenly over the entire midgut surface (Hartenstein and Jan, 1992; Jiang and Edgar, 2009; Takashima et al., 2009; Fig.2N, O, T). When metamorphosis begins during the first hours of the pupal stage, these clusters stretch and rapidly form a continuously layer which surrounds the former larval midgut (Fig.2U). The larval midgut (including enterocytes and endocrine cells) undergoes apoptosis, whereas the layer of AMPs differentiates as the adult midgut. As in the embryo, a cell fate choice takes place among the AMPs of the late larva and early pupa: the majority of cells differentiates as enterocytes, and smaller populations of cells scattered throughout the metamorphosing epithelium become adult endocrine cells on the one hand side, and adult midgut stem cells on the other hand. Thus, after eclosion of adult flies, the midgut epithelium houses scattered undifferentiated cells. Located between the basal surface of the epithelium and the surrounding muscle layer (like their predecessors, the AMPs of the larval midgut), the stem cells undergo periodic asymmetric divisions that give rise to renewed stem cells, as well as postmitotic daughter cells (“enteroblasts”) that differentiate as enterocyte or endocrine cells (Ohlstein and Spradling, 2006; 2007; Michelli and Perrimon, 2006; Fig.2P, Q, V).
It appears then that there exist a number of common elements between the developmental histories of endocrine cells in vertebrate and Drosophila. Notably, in both, common progenitors produce both enterocytes and endocrine cells. In the Drosophila adult gut, the choice between the two fates is made at a level where, after the division of a stem cell, one daughter becomes postmitotic and differentiates as endocrine cell or enterocyte, whereas the other daughter continues to divide as a renewed stem cell. Possibly, presumptive larval endocrine cells are selected in a similar scenario in the embryonic endoderm, where cells that become postmitotic endocrine cells are segregated from a majority of cells that continue to divide for one or two more rounds before differentiating as larval enterocytes (Takashima et al., 2009). In vertebrates, the fate choice is probably often made in the same manner (postmitotic endocrine versus proliferating progenitor), although in other cases, two dividing progenitor populations split, one giving rise to enterocytes, the other to different types of endocrine cells. It should be noted that also in Drosophila, there may exist a much greater complexity that is not yet revealed, given the fact that Drosophila possesses many different subpopulations of endocrine cells expressing different peptides (Veenstra et al., 2008, 2009); it is entirely unknown how these different subpopulations are developmentally related to each other.
Role of the Notch signaling pathway in enteroendocrine cell fate choice
A wealth of recent studies in vertebrates (for review, see Schonhoff et al., 2004a) and Drosophila (Ohlstein and Spradling, 2006; Michelli and Perrimon, 2006) suggest that there is an evolutionarily conserved role of Notch in intestinal lineage specification, where Notch activation favors enterocytes at the expense of endocrine/secretory cells. Signaling through the Notch receptor has many essential roles in modulating cell fate decisions and patterning events, and is conserved from Drosophila to humans (Kopan and Llagan, 2009). In most developmental scenarios investigated to date, the Notch signaling pathway forms part of a larger “gene cassette” which always becomes active when, from an initially homogenous cell population, different fates are selected. This cassette was first studied in Drosophila neurulation (review of classical studies in Campos-Ortega, 1995), but is deployed in a very similar fashion in many other developmental events. Briefly, the Drosophila neurectoderm forms an epithelial layer which, in a matter of a few hours, splits into two major cell populations (Fig.3A). One population is that of neural progenitor cells, called neuroblasts; these cells delaminate from the neurectoderm into the interior of the embryo and produce the central nervous system. The second population comprises epidermoblasts, which are the epithelial cells that remain at the surface and later give rise to the epidermis of the animal. The mechanism controlling the specification of these two fates involves two steps. During the first step, discrete clusters of neurectodermal cells (“proneural clusters”) express a combination of regulatory genes, the proneural genes, which makes the cells “comptetent” to form neuroblasts. Proneural clusters are equivalence groups, which means that all cells within a given proneural cluster initiate a neural fate. In the fly embryonic neurectoderm, proneural clusters are relatively small groups of cells, each of which gives rise to a single neuroblast; in other systems, proneural clusters can be much larger and deliver many neuroblasts (or other cells selected from a larger pool of uncommitted cells). Proneural genes encode DNA binding proteins that belong to the large family of basic helix-loop-helix (bHLH) transcription factors, including the Achaete-Scute proteins and their vertebrate homologs (e.g., Mash-1 in mouse), and Atonal and its vertebrate homologs (Math and neurogenins in mouse; reviewed in Campuzano and Modolell, 1992; Kageyama et al., 1995; Guillemot, 1999; Lo et al., 2002). Proneural genes switch on a set of as yet mostly unknown genes essential for an ectoderm cell to express a neural fate. If one removes proneural genes from a Drosophila embryo, most neuroblasts do not form.
Fig.3.
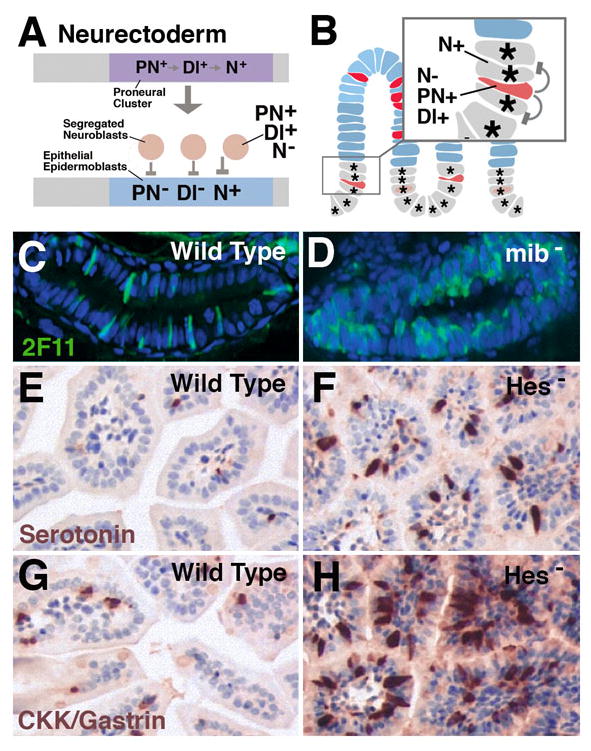
Notch signaling controls the ratio of enteroendocrine cells and enterocytes in vertebrate intestinal epithelium. A: Canonical role of Notch signaling in proneural clusters of Drosophila neurectoderm. From an initially homogenous cell population forming the proneural cluster, two subpopulations are selected. Cells that upregulate Delta (Dl+) and proneural genes (PN+) and downregulate Notch activity (N-) become neuroblasts; the remainder upregulate Notch (N+) which suppresses proneural genes and Delta (PN-; Dl-, respectively. B: Intestinal progenitors can be compared to proneural cluster(s). Cells that upregulate Delta and proneural genes and downregulate Notch give rise to endocrine cells (postmitotic or progenitors); these cells activate Notch activity in the adjacent cells, which thereby are inhibited (‘lateral inhibition’) to adopt an endocrine fate, and become enterocytes and/or continue to proliferate. C: Wild type zebrafish larval gut in which secretory cells are labeled with antibody 2F11. D: In mindbomb (mib) mutant (elimination of Delta function), the majority of gut cells develops as secretory cells (endocrine and exocrine; from Crosnier et al., 2005). E, G: Wild type mouse intestinal epithelium in which endocrine cells expressing serotonin (E) or CKK/gastrin (G) are labeled. Both types of cells are increased in number in mutants where the Hes-1 gene (loss of Notch signaling activity) is impaired (F, H; from Jensen et al., 2000).
In a second step of neuroblast/epidermoblast specification, called lateral inhibition, cells of each proneural cluster “compete” with each other to become a neuroblast. On the molecular level, this competition is initiated by the signaling molecule Delta (or other Notch ligands like Serrate/Jagged), whose expression is upregulated within proneural clusters by the proneural bHLH genes. Delta encodes a membrane-bound signal molecule that interacts with its receptor, Notch. Notch receptor activation is mediated by a sequence of proteolytic events that release the Notch intracellular domain, which is subsequently translocated to the nucleus and interacts with the DNA-binding protein Suppressor of Hairless (Su(H); CSL in vertebrates) to regulate transcription of its target genes, among them another class of bHLH transcription factors, Hairy and the Enhancer of split genes (Hes in vertebrates; Kopan and Llagan, 2009). These genes act as repressors; among other targets, they repress the transcription of proneural genes. Now, if all cells in the proneural cluster were to behave in exactly the same manner, no neuroblasts would be formed since following a short burst of expression of proneural genes and Delta, these same genes would be turned off again as a result of activating Notch and E(spl)! A (stochastic?) mechanism must exist that gives some cells a “competitive advantage”, so that these cells express proneural genes/Notch ligands at a slightly higher level (“high Delta” cells). This initially slight advantage is rapidly amplified because the cells contacting the “high Delta” cells activate Notch and E(spl) stronger, and thereby turn down proneural genes and Delta more (“low Delta”); in turn, they cannot as efficiently signal to the “high Delta” cells, so that these cells become even “higher Delta”. As the end result, two populations of cells emerge: one that expresses high levels of proneural genes and Delta, and is itself low in Notch activity; and another one that has high Notch activity levels and is low in proneural genes and Delta. The former will become neuroblasts; the latter epidermoblasts.
The proneural/neurogenic gene cassette may act in a manner very similar to that described above in the developing midgut epithelium to separate enterocytes from endocrine cells (or secretory cells more general). Many details about the molecular events that lead to the specification of these fates are not yet known. However, what is clear in the vertebrate system, is that (1) emerging endocrine cells express and require proneural genes, in particular Math-1 and Neurogenin; (2) the same cells express higher levels of Notch ligands (e.g., Delta); (3) loss of Delta or Notch pathway function results in higher number of endocrine cells, often at the expense of enterocytes.
The mouse proneural gene Math-1 is expressed in the zone of transient amplifying progenitors and then becomes restricted to postmitotic exocrine and endocrine cells. Loss of this gene results in the absence of both cell populations (Yang et al., 2001). Another proneural gene, neurogenin 3, may act downstream of Math-1 in a more restricted progenitor populations that include only endocrine cell types (Jenny et al., 2002; Lee et al., 2002; reviewed in Schonhoff et al., 2004; Lee and Kaestner, 2004). Thus, loss of neurogenin 3 in mouse results in the absence or strong reduction of several endocrine cell populations, in particular glucagon, somatostatin, and gastrin expressing cells.
Similar to proneural genes, Notch ligands such as DeltaD (Delta1 in mouse) are upregulated in enteroendocrine and secretory cells of zebrafish and mouse gut (Crosnier et al., 2005). By contrast, expression of Hes-1, which signifies elevated Notch signaling activity, occurs in enterocytes surrounding Hes-1 negative secretory cells (Jensen et al., 2000). Loss of the mib gene in zebrafish, resulting in complete disruption of Delta function, causes an almost complete conversion of all gut enterocytes into secretory (exocrine/endocrine) cells (Crosnier et al., 2005; Fig.3C, D). Similarly, loss of the Notch activated Hes-1 or CSL gene in developing mouse embryos results in a decrease in number of many intestinal epithelial cells, including pancreas; at the same time, endocrine and/or secretory lineages are enhanced (Jensen et al., 2000; van Es et al., 2005; Fig.3E-H).
A requirement of Notch signaling has also been shown for the fate decision beteen enterocendocrine cells and enterocytes in Drosophila. As in vertebrates, proneural genes (e.g., the bHLH transcription factor Lethal of scute) are expressed in the embryo midgut primordium, where the enteroendocrine cells split from the enterocytes of the presumptive larval midgut (Tepass and Hartenstein, 2005; Takashima et al., 2009). Loss of Delta results in a strong increase of the former, at the expense of the latter (Fig.4A-D). The activation of Notch causes the opposite phenotype: enteroendocrine cells are reduced in number. It is not yet clear how (different levels of?) Notch signaling contributes to the ratio of enteroendocrine cells versus AMPs, the other cell population that segregates from the larval enterocytes.
Fig.4.
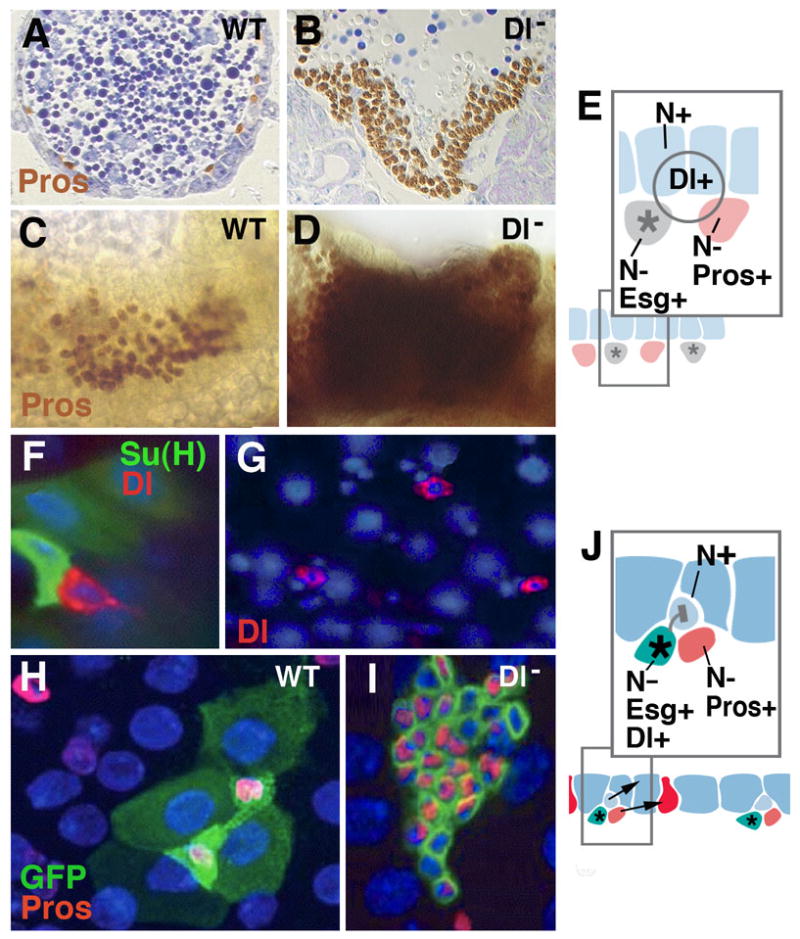
Notch signaling controls enteroendocrine cell formation at all stages of Drosophila development. A-E: Specification of larval endocrine and enterocyte precursors during embryogenesis. A, C: Section (A) and wholemount (C) of wild-type embryo, showing normal pattern of prospective endocrine cells (prospero-positive). B, D: Section (B) and wholemount (D) of Delta mutant embryo. Endocrine precursors are strongly increased at the expense of enterocytes. E: Model of Notch signaling function in embryonic midgut primordium. Fluctuations of the signaling Delta, widely expressed in the midgut primordium, result in cells that upregulate Notch and become epithelial larval enterocytes (blue); cells with low levels of Notch activity develop as prospective endocrine cells (red) and adult midgut progenitors (gray). The former upregulate the gene prospero (pros); the latter express the marker escargot (esg). F-J: adult midgut. Scattered stem cells express fluctuating levels of the signal Delta (red in F, G; from Ohlstein and Spradling, 2007). High levels of Delta activate Notch activity in neighboring, postmitotic enteroblast (monitored by Su(H), green in F). This will become enterocyte (shown in light blue in schematic of panel K). Lower levels of Delta in stem cell will fail to activate Notch in enteroblast, which thereby develops as endocrine cell (red in schematic J). H: Wild-type clone (labeled green), containing four enterocytes and two endocrine cells. Clones derived from stem cells lacking Delta (I) have increased number cells which all express endocrine fate (from Ohlstein and Spradling, 2007).
In the adult midgut, several aspects of Notch signaling have been investigated in great detail, although also here, many open questions remain. Delta is expressed in the midgut stem cell (Ohlstein and Spradling, 2007; Fig.4F, G). After each division of this cell, Delta activates Notch signaling in the adjacent daughter postmitotic cell (enteroblast), while no Notch signaling activity is detectable in the stem cell itself (Fig.4F). Due to periodic oscillations in the level of Dl, Notch signal reception in daughter cells varies, and the corresponding level of Notch activity in the adjacent enteroblast determines the fate of this cell. If the Notch level is high, the enteroblast differentiated as enterocyte; if it is low, it becomes an endocrine cell. Loss of Delta results in clones that are increased in cell number and express the endocrine marker prospero (Fig.4H, I).
Conclusion
The data reviewed here allow two major conclusions. One: the selection of enteroendocrine cells (and the same also implies for many “glandular” endocrine cells, like those of the pancreas) from epithelial cells follows a similar mechanism, and is controlled by the same gene cassette, as the selection of neural precursors from the neurectoderm. Two: this process is highly conserved between vertebrates and Drosophila, and, therefore, probably all bilaterian animals.
Both of these conclusions have some important implications. One is that, to understand the specification, differentiation and function of endocrine cells, the study of neurons and their development will be highly relevant. This has been realized since quite some time, given the shared expression of numerous molecular and ultrastructural features (Falkmer, 1993). However, the extent of “genetic overlap” between endocrine and neural cells maybe even greater than previously expected. Moreover, the extent to which the developmental pathways specifying endocrine lineages in vertebrates and Drosophila resemble each other is truly astounding. It gives reason to hope that insights gained in the “simple, genetically tractable model” Drosophila will continue to pave the way for studies in vertebrates.
It is tempting to interpret the similarities concerning the molecular mechanisms controlling the development of neurons and endocrine cells in terms of the phylogenetic origin of these cell types. Cell communication through secreted, diffusible signals is phylogenitcally older than neural transmission. Animals without nervous system (e.g., sponges; Robitzki et al., 1989) and even protests (Csaba and Pallinger, 2008) produce a wide array of hormones which are in some cases identical to the corresponding compounds found in highly derived taxa. One may speculate that in primitive multicellular animals, specialized epithelial cells integrated into the epidermis and the intestinal lining reacted to certain stimuli, chemical ar physical, by secreting metabolites that diffused throughout the body and evoked adaptive responses in other tissues. As a nervous system was “invented” (cells sending out processes and forming specialized synaptic contacts), many of the endocrine cells became incorporated into the emerging nervous system.
Acknowledgments
This work was funded by NIH Grant RO1 GM 087373-01 to VH.
References
- Aiken KD, Roth KA. Temporal differentiation and migration of substance P, serotonin, and secretin immunoreactive enteroendocrine cells in the mouse proximal small intestine. Dev Dyn. 1992;194:303–310. doi: 10.1002/aja.1001940406. [DOI] [PubMed] [Google Scholar]
- Anderson RB, Newgreen DF, Young HM. Neural crest and the development of the enteric nervous system. Adv Exp Med Biol. 2006;589:181–196. doi: 10.1007/978-0-387-46954-6_11. [DOI] [PubMed] [Google Scholar]
- Bjerknes M, Cheng H. Neurogenin 3 and the enteroendocrine cell lineage in the adult mouse small intestinal epithelium. Dev Biol. 2006;300:722–735. doi: 10.1016/j.ydbio.2006.07.040. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA. Genetic mechanisms of early neurogenesis in Drosophila melanogaster. Mol Neurobiol. 1995 Apr-Jun;10(2-3):75–89. doi: 10.1007/BF02740668. 1995. Review. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The Embryonic Development of Drosophila melanogaster. 2nd. Springer; 1997. [Google Scholar]
- Campuzano S, Modolell J. Patterning of the Drosophila nervous system: the achaete-scute gene complex. Trends Genet. 1992;8:202–208. doi: 10.1016/0168-9525(92)90234-u. [DOI] [PubMed] [Google Scholar]
- Csaba G, Pállinger E. Is there a hormonal network in Tetrahymena? A systematic investigation of hormonal effects on the hormone content. Cell Biochem Funct. 2008;26:303–308. doi: 10.1002/cbf.1435. [DOI] [PubMed] [Google Scholar]
- Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132:1093–1104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- Falkmer S. Phylogeny and ontogeny of the neuroendocrine cells of the gastrointestinal tract. Endocrinol Metab Clin North Am. 2003;22:731–752. [PubMed] [Google Scholar]
- Fujita T, Yui R, Iwanaga T, Nishiitsutsuji-Uwo J, Endo Y, Yanaihara N. Evolutionary aspects of “brain-gut peptides”: an immunohistochemical study. Peptides. 1981;2(Suppl 2):123–131. doi: 10.1016/0196-9781(81)90023-1. [DOI] [PubMed] [Google Scholar]
- Fusé M, Zhang JR, Partridge E, Nachman RJ, Orchard I, Bendena WG, Tobe SS. Effects of an allatostatin and a myosuppressin on midgut carbohydrate enzyme activity in the cockroach Diploptera punctata. Peptides. 1999;20:1285–1293. doi: 10.1016/s0196-9781(99)00133-3. [DOI] [PubMed] [Google Scholar]
- Guillemot F. Vertebrate bHLH genes and the determination of neuronal fates. Exp Cell Res. 1999;253:357–364. doi: 10.1006/excr.1999.4717. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Jan YN. Studying Drosophila embryogenesis with P-lacZ enhancer trap lines. Wilhelm Roux's Arch Dev Biol. 1992;201:194–220. doi: 10.1007/BF00188752. [DOI] [PubMed] [Google Scholar]
- Hauck AL, Swanson KS, Kenis PJ, Leckband DE, Gaskins HR, Schook LB. Twists and turns in the development and maintenance of the mammalian small intestine epithelium. Birth Defects Res C Embryo Today. 2005;75:58–71. doi: 10.1002/bdrc.20032. [DOI] [PubMed] [Google Scholar]
- Henning SJ, Rubin DC, Shulman RJ. Ontogeny of the intestinal mucosa. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York NY: Raven press; 1994. pp. 571–610. [Google Scholar]
- Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, Jensen J, Kedinger M, Gradwohl G. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 2002;21:6338–6347. doi: 10.1093/emboj/cdf649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Jiang H, Edgar BA. EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development. 2009;136:483–493. doi: 10.1242/dev.026955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama R, Sasai Y, Akazawa C, Ishibashi M, Takebayashi K, Shimizu C, Tomita K, Nakanishi S. Regulation of mammalian neural development by helix-loop-helix transcription factors. Crit Rev Neurobiol. 1995;9:177–188. [PubMed] [Google Scholar]
- Kopan R, Ilagan MXG. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AB, Orchard I. The effects of SchistoFLRFamide on contractions of locust midgut. Peptides. 1998;19:459–467. doi: 10.1016/s0196-9781(97)00465-8. [DOI] [PubMed] [Google Scholar]
- Lee CS, Perreault N, Brestelli JE, Kaestner KH. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and the maintenance of gastric epithelial cell identity. Genes Dev. 2002;16:1488–1497. doi: 10.1101/gad.985002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Kaestner KH. Development of gut endocrine cells. Best Pract Res Clin Endocrinol Metab. 2004;18:453–462. doi: 10.1016/j.beem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Lehane MJ. The Midgut. In: Harrison FW, Locke M, editors. Microscopic Anatomy of Invertebrates. Wiley Liss; New York: 1998. pp. 725–746. [Google Scholar]
- Lo L, Dormand E, Greenwood A, Anderson DJ. Comparison of the generic neuronal differentiation and neuron subtype specification functions of mammalian achaete-scute and atonal homologs in cultured neural progenitor cells. Development. 2002;129:1553–1567. doi: 10.1242/dev.129.7.1553. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- Modlin IM, Champaneria MC, Bornschein J, Kidd M. Evolution of the diffuse neuroendocrine system--clear cells and cloudy origins. Neuroendocrinology. 2006;84:69–82. doi: 10.1159/000096997. [DOI] [PubMed] [Google Scholar]
- Montuenga LM, Guembe L, Burrell MA, Bodegas ME, Calvo A, Sola JJ, Sesma P, Villaro AC. The diffuse endocrine system: from embryogenesis to carcinogenesis. Prog Histochem Cytochem. 2003;38:155–272. doi: 10.1016/s0079-6336(03)80004-9. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential Notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- Pictet RL, Rall LB, Phelps P, Rutter WJ. The neural crest and the origin of the insulin-producing and other gastrointestinal hormone-producing cells. Science. 1976;191:191–192. doi: 10.1126/science.1108195. [DOI] [PubMed] [Google Scholar]
- Potten CS, Allen TD. Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res. 1977;60:272–277. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. The new biology of gastrointestinal hormones. Physiol Rev. 1998;78:1087–1108. doi: 10.1152/physrev.1998.78.4.1087. [DOI] [PubMed] [Google Scholar]
- Rindi G, Ratineau C, Ronco A, Candusso ME, Tsai M, Leiter AB. Targeted ablation of secretin-producing cells in transgenic mice reveals a common differentiation pathway with multiple enteroendocrine cell lineages in the small intestine. Development. 1999;126:4149–4156. doi: 10.1242/dev.126.18.4149. [DOI] [PubMed] [Google Scholar]
- Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- Robitzki A, Schröder HC, Ugarkovic D, Pfeifer K, Uhlenbruck G, Müller WE. Demonstration of an endocrine signaling circuit for insulin in the sponge Geodia cydonium. EMBO J. 1989;8:2905–2909. doi: 10.1002/j.1460-2075.1989.tb08439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Development and differentiation of gut endocrine cells. Endocrinology. 2004a;145:2639–2644. doi: 10.1210/en.2004-0051. [DOI] [PubMed] [Google Scholar]
- Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004b;270:443–454. doi: 10.1016/j.ydbio.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Siviter RJ, Coast GM, Winther AM, Nachman RJ, Taylor CA, Shirras AD, Coates D, Isaac RE, Nässel DR. Expression and functional characterization of a Drosophila neuropeptide precursor with homology to mammalian preprotachykinin A. J Biol Chem. 2000;275:23273–23280. doi: 10.1074/jbc.M002875200. [DOI] [PubMed] [Google Scholar]
- Takashima S, Adams K, Moridzadeh R, Aghajanian P, Younossi-Hartenstein A, Hartenstein V. Genetic specification of Drosophila enteroendocrine cells during embryonic and larval development (submitted) 2009 [Google Scholar]
- Tepass U, Hartenstein V. The formation of the midgut epithelium in Drosophila depends on the interaction of endoderm and mesoderm. Development. 1994;120:579–590. doi: 10.1242/dev.120.3.579. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. Neurogenic and proneural genes control the specification of non-neuronal cell types in the Drosophila endoderm. Development. 1995;121:393–405. doi: 10.1242/dev.121.2.393. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Veenstra JA, Lau GW, Agricola HJ, Petzel DH. Immunohistological localization of regulatory peptides in the midgut of the female mosquito Aedes aegypti. Histochem Cell Biol. 1995;104:337–347. doi: 10.1007/BF01458127. [DOI] [PubMed] [Google Scholar]
- Veenstra JA, Agricola HJ, Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- Veenstra JA. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 2009;336:309–323. doi: 10.1007/s00441-009-0769-y. [DOI] [PubMed] [Google Scholar]
- Winther AM, Nässel DR. Intestinal peptides as circulating hormones: release of tachykinin-related peptide from the locust and cockroach midgut. J Exp Biol. 2001;204:1269–1280. doi: 10.1242/jeb.204.7.1269. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Zitnan D, Sauman I, Sehnal F. Peptidergic innervation and endocrine cells of insect midgut. Arch Insect Biochem Physiol. 1993;22:113–132. [Google Scholar]


