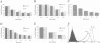Abstract
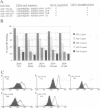
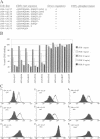
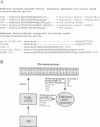
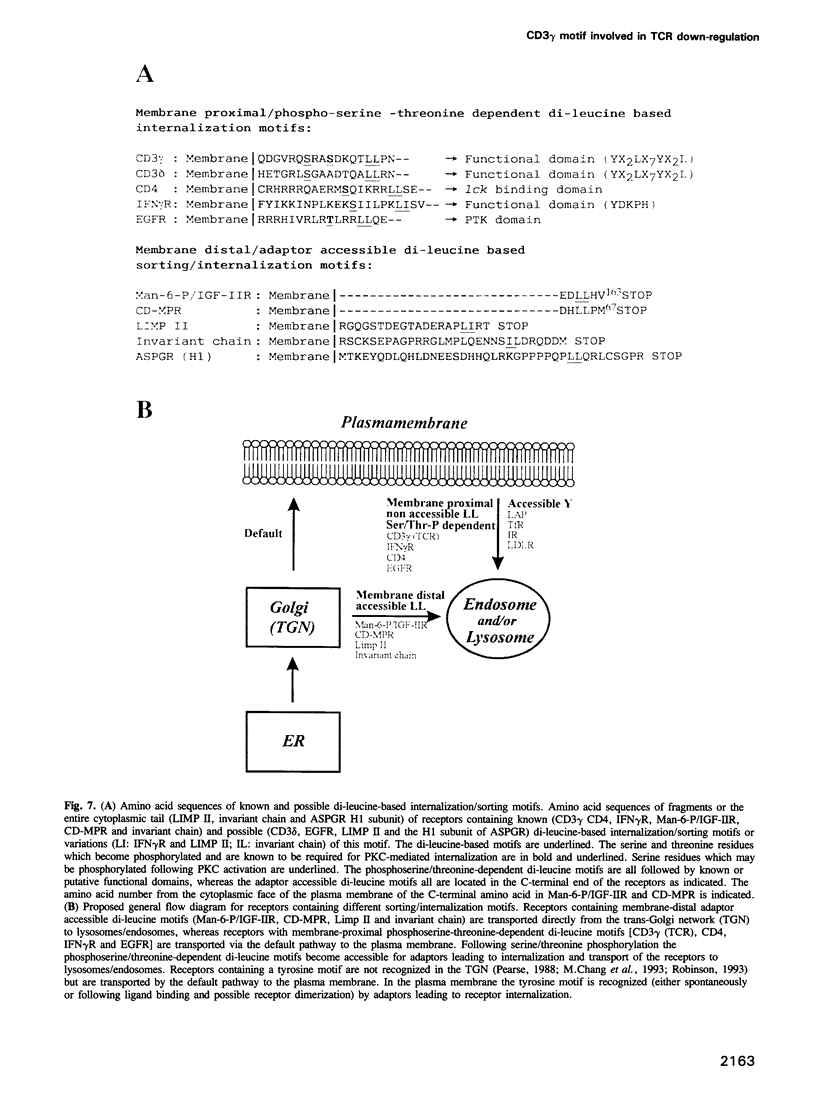
Several cell surface receptors including the T cell receptor (TCR) are phosphorylated and down-regulated following activation of protein kinase C (PKC). Among other substrates the activated PKC in T cells phosphorylates the CD3 gamma subunit of the TCR. To investigate the role of CD3 gamma phosphorylation in PKC-mediated TCR down-regulation, point mutated CD3 gamma cDNA was transfected into the CD3 gamma-negative T cell line JGN and CD3 gamma transfectants were analysed. Phosphorylation at S126 but not S123 in the cytoplasmic tail of CD3 gamma was required for PKC-mediated down-regulation of the TCR. Furthermore, analysis of a series of CD3 gamma truncation mutants indicated that in addition to S126 phosphorylation a motif C-terminal of S126 was required for TCR down-regulation. Point mutation analyses confirmed this observation and demonstrated that a membrane-proximal di-leucine motif (L131 and L132) in the cytoplasmic tail of CD3 gamma was required for PKC-mediated TCR down-regulation in addition to phosphorylation at S126. Incubation of T cells in hypertonic medium known to disrupt normal clathrin lattices severely inhibited PKC-mediated TCR down-regulation in non-mutated T cells, indicating that the TCR was down-regulated by endocytosis via clathrin coated pits. Based on the present results and previously published observations on intracellular receptor sorting, a general model for intracellular sorting of receptors containing di-leucine- or tyrosine-based motifs is proposed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R. T., Karnitz L. M., Secrist J. P., Leibson P. J. Signal transduction through the T-cell antigen receptor. Trends Biochem Sci. 1992 Oct;17(10):434–438. doi: 10.1016/0968-0004(92)90015-2. [DOI] [PubMed] [Google Scholar]
- Alexander D. R., Brown M. H., Tutt A. L., Crumpton M. J., Shivnan E. CD3 and CD2 antigen-mediated CD3 gamma-chain phosphorylation in permeabilized human T cells. Regulation by cytosolic phosphatases. Biochem J. 1992 Nov 15;288(Pt 1):69–77. doi: 10.1042/bj2880069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando I., Hariri G., Wallace D., Beverley P. Tumor promoter phorbol esters induce unresponsiveness to antigen and expression of interleukin 2 receptor on T cells. Eur J Immunol. 1985 Feb;15(2):196–199. doi: 10.1002/eji.1830150217. [DOI] [PubMed] [Google Scholar]
- Baniyash M., Garcia-Morales P., Luong E., Samelson L. E., Klausner R. D. The T cell antigen receptor zeta chain is tyrosine phosphorylated upon activation. J Biol Chem. 1988 Dec 5;263(34):18225–18230. [PubMed] [Google Scholar]
- Bansal A., Gierasch L. M. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991 Dec 20;67(6):1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- Beguinot L., Hanover J. A., Ito S., Richert N. D., Willingham M. C., Pastan I. Phorbol esters induce transient internalization without degradation of unoccupied epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1985 May;82(9):2774–2778. doi: 10.1073/pnas.82.9.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Boyer C., Auphan N., Luton F., Malburet J. M., Barad M., Bizozzero J. P., Reggio H., Schmitt-Verhulst A. M. T cell receptor/CD3 complex internalization following activation of a cytolytic T cell clone: evidence for a protein kinase C-independent staurosporine-sensitive step. Eur J Immunol. 1991 Jul;21(7):1623–1634. doi: 10.1002/eji.1830210707. [DOI] [PubMed] [Google Scholar]
- Canfield W. M., Johnson K. F., Ye R. D., Gregory W., Kornfeld S. Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24-29 of the cytoplasmic tail. J Biol Chem. 1991 Mar 25;266(9):5682–5688. [PubMed] [Google Scholar]
- Cantrell D. A., Davies A. A., Crumpton M. J. Activators of protein kinase C down-regulate and phosphorylate the T3/T-cell antigen receptor complex of human T lymphocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8158–8162. doi: 10.1073/pnas.82.23.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. A., Friedrich B., Davies A. A., Gullberg M., Crumpton M. J. Evidence that a kinase distinct from protein kinase C induces CD3 gamma-subunit phosphorylation without a concomitant down-regulation in CD3 antigen expression. J Immunol. 1989 Mar 1;142(5):1626–1630. [PubMed] [Google Scholar]
- Cantrell D., Davies A. A., Londei M., Feldman M., Crumpton M. J. Association of phosphorylation of the T3 antigen with immune activation of T lymphocytes. Nature. 1987 Feb 5;325(6104):540–542. doi: 10.1038/325540a0. [DOI] [PubMed] [Google Scholar]
- Carpentier J. L., Paccaud J. P., Backer J., Gilbert A., Orci L., Kahn C. R., Baecker J [corrected to Backer J. ]. Two steps of insulin receptor internalization depend on different domains of the beta-subunit. J Cell Biol. 1993 Sep;122(6):1243–1252. doi: 10.1083/jcb.122.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Irving B. A., Fraser J. D., Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. P., Lazar C. S., Walsh B. J., Komuro M., Collawn J. F., Kuhn L. A., Tainer J. A., Trowbridge I. S., Farquhar M. G., Rosenfeld M. G. Ligand-induced internalization of the epidermal growth factor receptor is mediated by multiple endocytic codes analogous to the tyrosine motif found in constitutively internalized receptors. J Biol Chem. 1993 Sep 15;268(26):19312–19320. [PubMed] [Google Scholar]
- Chang M. P., Mallet W. G., Mostov K. E., Brodsky F. M. Adaptor self-aggregation, adaptor-receptor recognition and binding of alpha-adaptin subunits to the plasma membrane contribute to recruitment of adaptor (AP2) components of clathrin-coated pits. EMBO J. 1993 May;12(5):2169–2180. doi: 10.1002/j.1460-2075.1993.tb05865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. J., Remmler J., Delaney J. C., Messner D. J., Lobel P. Mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor. A consensus casein kinase II site followed by 2 leucines near the carboxyl terminus is important for intracellular targeting of lysosomal enzymes. J Biol Chem. 1993 Oct 25;268(30):22338–22346. [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Collawn J. F., Stangel M., Kuhn L. A., Esekogwu V., Jing S. Q., Trowbridge I. S., Tainer J. A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990 Nov 30;63(5):1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Countaway J. L., Nairn A. C., Davis R. J. Mechanism of desensitization of the epidermal growth factor receptor protein-tyrosine kinase. J Biol Chem. 1992 Jan 15;267(2):1129–1140. [PubMed] [Google Scholar]
- Davies A. A., Cantrell D. A., Hexham J. M., Parker P. J., Rothbard J., Crumpton M. J. The human T3 gamma chain is phosphorylated at serine 126 in response to T lymphocyte activation. J Biol Chem. 1987 Aug 15;262(23):10918–10921. [PubMed] [Google Scholar]
- Davis C. G., van Driel I. R., Russell D. W., Brown M. S., Goldstein J. L. The low density lipoprotein receptor. Identification of amino acids in cytoplasmic domain required for rapid endocytosis. J Biol Chem. 1987 Mar 25;262(9):4075–4082. [PubMed] [Google Scholar]
- Dembić Z., Haas W., Weiss S., McCubrey J., Kiefer H., von Boehmer H., Steinmetz M. Transfer of specificity by murine alpha and beta T-cell receptor genes. Nature. 1986 Mar 20;320(6059):232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- Duprez V., Ferrer M., Dautry-Varsat A. High-affinity interleukin 2 receptor alpha and beta chains are internalized and remain associated inside the cells after interleukin 2 endocytosis. J Biol Chem. 1992 Sep 15;267(26):18639–18643. [PubMed] [Google Scholar]
- Eberle W., Sander C., Klaus W., Schmidt B., von Figura K., Peters C. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a beta turn. Cell. 1991 Dec 20;67(6):1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- Fallon R. J., Schwartz A. L. Asialoglycoprotein receptor phosphorylation and receptor-mediated endocytosis in hepatoma cells. Effect of phorbol esters. J Biol Chem. 1988 Sep 15;263(26):13159–13166. [PubMed] [Google Scholar]
- Farrar M. A., Schreiber R. D. The molecular cell biology of interferon-gamma and its receptor. Annu Rev Immunol. 1993;11:571–611. doi: 10.1146/annurev.iy.11.040193.003035. [DOI] [PubMed] [Google Scholar]
- Fuhrer C., Geffen I., Spiess M. Endocytosis of the ASGP receptor H1 is reduced by mutation of tyrosine-5 but still occurs via coated pits. J Cell Biol. 1991 Aug;114(3):423–431. doi: 10.1083/jcb.114.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler C. Failure to synthesize the CD3-gamma chain. Consequences for T cell antigen receptor assembly, processing, and expression. J Immunol. 1992 Apr 15;148(8):2437–2445. [PubMed] [Google Scholar]
- Geisler C., Plesner T., Pallesen G., Skjødt K., Odum N., Larsen J. K. Characterization and expression of the human T cell receptor-T3 complex by monoclonal antibody F101.01. Scand J Immunol. 1988 Jun;27(6):685–696. doi: 10.1111/j.1365-3083.1988.tb02402.x. [DOI] [PubMed] [Google Scholar]
- Geisler C., Rubin B., Caspar-Bauguil S., Champagne E., Vangsted A., Hou X., Gajhede M. Structural mutations of C-domains in members of the Ig superfamily. Consequences for the interactions between the T cell antigen receptor and the zeta 2 homodimer. J Immunol. 1992 Jun 1;148(11):3469–3477. [PubMed] [Google Scholar]
- Hansen S. H., Sandvig K., van Deurs B. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J Cell Biol. 1993 Apr;121(1):61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hausdorff W. P., Campbell P. T., Ostrowski J., Yu S. S., Caron M. G., Lefkowitz R. J. A small region of the beta-adrenergic receptor is selectively involved in its rapid regulation. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):2979–2983. doi: 10.1073/pnas.88.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans M. H., Malissen B. The cytoplasmic tail of the T cell receptor zeta chain is dispensable for antigen-mediated T cell activation. Eur J Immunol. 1993 Sep;23(9):2257–2262. doi: 10.1002/eji.1830230931. [DOI] [PubMed] [Google Scholar]
- Hershey G. K., McCourt D. W., Schreiber R. D. Ligand-induced phosphorylation of the human interferon-gamma receptor. Dependence on the presence of a functionally active receptor. J Biol Chem. 1990 Oct 15;265(29):17868–17875. [PubMed] [Google Scholar]
- Heuser J. E., Anderson R. G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989 Feb;108(2):389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. Effects of cytoplasmic acidification on clathrin lattice morphology. J Cell Biol. 1989 Feb;108(2):401–411. doi: 10.1083/jcb.108.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991 Mar 8;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Johnson K. F., Kornfeld S. A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J Biol Chem. 1992 Aug 25;267(24):17110–17115. [PubMed] [Google Scholar]
- Johnson K. F., Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol. 1992 Oct;119(2):249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Harford J., van Renswoude J. Rapid internalization of the transferrin receptor in K562 cells is triggered by ligand binding or treatment with a phorbol ester. Proc Natl Acad Sci U S A. 1984 May;81(10):3005–3009. doi: 10.1073/pnas.81.10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning F., Maloy W. L., Coligan J. E. The implications of subunit interactions for the structure of the T cell receptor-CD3 complex. Eur J Immunol. 1990 Feb;20(2):299–305. doi: 10.1002/eji.1830200211. [DOI] [PubMed] [Google Scholar]
- Krangel M. S. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J Exp Med. 1987 Apr 1;165(4):1141–1159. doi: 10.1084/jem.165.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissansen G. W., Owen M. J., Verbi W., Crumpton M. J. Primary structure of the T3 gamma subunit of the T3/T cell antigen receptor complex deduced from cDNA sequences: evolution of the T3 gamma and delta subunits. EMBO J. 1986 Aug;5(8):1799–1808. doi: 10.1002/j.1460-2075.1986.tb04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktistakis N. T., Thomas D., Roth M. G. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990 Oct;111(4):1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann J., Geisler C. Structure of the T-cell receptor in a Ti alpha beta, Ti gamma delta double positive T-cell line. Scand J Immunol. 1993 Feb;37(2):271–275. doi: 10.1111/j.1365-3083.1993.tb01766.x. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992 Jun 26;69(7):1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Letourneur F., Klausner R. D. Activation of T cells by a tyrosine kinase activation domain in the cytoplasmic tail of CD3 epsilon. Science. 1992 Jan 3;255(5040):79–82. doi: 10.1126/science.1532456. [DOI] [PubMed] [Google Scholar]
- Lin C. R., Chen W. S., Lazar C. S., Carpenter C. D., Gill G. N., Evans R. M., Rosenfeld M. G. Protein kinase C phosphorylation at Thr 654 of the unoccupied EGF receptor and EGF binding regulate functional receptor loss by independent mechanisms. Cell. 1986 Mar 28;44(6):839–848. doi: 10.1016/0092-8674(86)90006-1. [DOI] [PubMed] [Google Scholar]
- Malissen B., Schmitt-Verhulst A. M. Transmembrane signalling through the T-cell-receptor-CD3 complex. Curr Opin Immunol. 1993 Jun;5(3):324–333. doi: 10.1016/0952-7915(93)90049-x. [DOI] [PubMed] [Google Scholar]
- Manolios N., Letourneur F., Bonifacino J. S., Klausner R. D. Pairwise, cooperative and inhibitory interactions describe the assembly and probable structure of the T-cell antigen receptor. EMBO J. 1991 Jul;10(7):1643–1651. doi: 10.1002/j.1460-2075.1991.tb07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C., Merlin G., Ballotti R., Metzler M., Aguet M. Rapid increase of the human IFN-gamma receptor phosphorylation in response to human IFN-gamma and phorbol myristate acetate. Involvement of different serine/threonine kinases. J Immunol. 1990 Dec 15;145(12):4257–4264. [PubMed] [Google Scholar]
- Margolis B., Hu P., Katzav S., Li W., Oliver J. M., Ullrich A., Weiss A., Schlessinger J. Tyrosine phosphorylation of vav proto-oncogene product containing SH2 domain and transcription factor motifs. Nature. 1992 Mar 5;356(6364):71–74. doi: 10.1038/356071a0. [DOI] [PubMed] [Google Scholar]
- McCaffrey P. G., Friedman B., Rosner M. R. Diacylglycerol modulates binding and phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1984 Oct 25;259(20):12502–12507. [PubMed] [Google Scholar]
- Minami Y., Samelson L. E., Klausner R. D. Internalization and cycling of the T cell antigen receptor. Role of protein kinase C. J Biol Chem. 1987 Sep 25;262(27):13342–13347. [PubMed] [Google Scholar]
- Nishibe S., Wahl M. I., Hernández-Sotomayor S. M., Tonks N. K., Rhee S. G., Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990 Nov 30;250(4985):1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Mak T. W., Van den Elsen P., Yanagi Y., Yoshikai Y., Calman A. F., Terhorst C., Stobo J. D., Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985 Aug 15;316(6029):606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Oka J. A., Christensen M. D., Weigel P. H. Hyperosmolarity inhibits galactosyl receptor-mediated but not fluid phase endocytosis in isolated rat hepatocytes. J Biol Chem. 1989 Jul 15;264(20):12016–12024. [PubMed] [Google Scholar]
- Park D. J., Rho H. W., Rhee S. G. CD3 stimulation causes phosphorylation of phospholipase C-gamma 1 on serine and tyrosine residues in a human T-cell line. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5453–5456. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Receptors compete for adaptors found in plasma membrane coated pits. EMBO J. 1988 Nov;7(11):3331–3336. doi: 10.1002/j.1460-2075.1988.tb03204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchen-Matthews A., Parsons I. J., Marsh M. Phorbol ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J Exp Med. 1993 Oct 1;178(4):1209–1222. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C., Braun M., Weber B., Wendland M., Schmidt B., Pohlmann R., Waheed A., von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990 Nov;9(11):3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieters J., Bakke O., Dobberstein B. The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J Cell Sci. 1993 Nov;106(Pt 3):831–846. doi: 10.1242/jcs.106.3.831. [DOI] [PubMed] [Google Scholar]
- Rajagopalan M., Neidigh J. L., McClain D. A. Amino acid sequences Gly-Pro-Leu-Tyr and Asn-Pro-Glu-Tyr in the submembranous domain of the insulin receptor are required for normal endocytosis. J Biol Chem. 1991 Dec 5;266(34):23068–23073. [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Internalization of interleukin 2 is mediated by the beta chain of the high-affinity interleukin 2 receptor. J Exp Med. 1987 Apr 1;165(4):1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. S. Adaptins. Trends Cell Biol. 1992 Oct;2(10):293–297. doi: 10.1016/0962-8924(92)90118-7. [DOI] [PubMed] [Google Scholar]
- Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991 Mar 8;64(5):1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- Saito H., Koyama T., Georgopoulos K., Clevers H., Haser W. G., LeBien T., Tonegawa S., Terhorst C. Close linkage of the mouse and human CD3 gamma- and delta-chain genes suggests that their transcription is controlled by common regulatory elements. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9131–9134. doi: 10.1073/pnas.84.24.9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Weiss A., Miller J., Norcross M. A., Germain R. N. Specific antigen-Ia activation of transfected human T cells expressing murine Ti alpha beta-human T3 receptor complexes. Nature. 1987 Jan 8;325(7000):125–130. doi: 10.1038/325125a0. [DOI] [PubMed] [Google Scholar]
- Secrist J. P., Karnitz L., Abraham R. T. T-cell antigen receptor ligation induces tyrosine phosphorylation of phospholipase C-gamma 1. J Biol Chem. 1991 Jul 5;266(19):12135–12139. [PubMed] [Google Scholar]
- Shin J., Dunbrack R. L., Jr, Lee S., Strominger J. L. Phosphorylation-dependent down-modulation of CD4 requires a specific structure within the cytoplasmic domain of CD4. J Biol Chem. 1991 Jun 5;266(16):10658–10665. [PubMed] [Google Scholar]
- Smith R. M., Jarett L. Receptor-mediated endocytosis and intracellular processing of insulin: ultrastructural and biochemical evidence for cell-specific heterogeneity and distinction from nonhormonal ligands. Lab Invest. 1988 Jun;58(6):613–629. [PubMed] [Google Scholar]
- Smith R. M., Seely B. L., Shah N., Olefsky J. M., Jarett L. Tyrosine kinase-defective insulin receptors undergo insulin-induced microaggregation but do not concentrate in coated pits. J Biol Chem. 1991 Sep 15;266(26):17522–17530. [PubMed] [Google Scholar]
- Telerman A., Amson R. B., Romasco F., Wybran J., Galand P., Mosselmans R. Internalization of human T lymphocyte receptors. Eur J Immunol. 1987 Jul;17(7):991–997. doi: 10.1002/eji.1830170715. [DOI] [PubMed] [Google Scholar]
- Tunnacliffe A., Buluwela L., Rabbitts T. H. Physical linkage of three CD3 genes on human chromosome 11. EMBO J. 1987 Oct;6(10):2953–2957. doi: 10.1002/j.1460-2075.1987.tb02600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. The structure of an endocytosis signal. Trends Cell Biol. 1992 Jul;2(7):189–192. doi: 10.1016/0962-8924(92)90232-c. [DOI] [PubMed] [Google Scholar]
- Vega M. A., Rodriguez F., Seguí B., Calés C., Alcalde J., Sandoval I. V. Targeting of lysosomal integral membrane protein LIMP II. The tyrosine-lacking carboxyl cytoplasmic tail of LIMP II is sufficient for direct targeting to lysosomes. J Biol Chem. 1991 Sep 5;266(25):16269–16272. [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Weiss A., Koretzky G., Schatzman R. C., Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanders E. D., Lamb J. R., Feldmann M., Green N., Beverley P. C. Tolerance of T-cell clones is associated with membrane antigen changes. Nature. 1983 Jun 16;303(5918):625–627. doi: 10.1038/303625a0. [DOI] [PubMed] [Google Scholar]