Abstract
Using a combination of dye injections, clonal labeling, and molecular markers we have reconstructed the axonal connections between brain and ventral nerve cord of the first instar Drosophila larva. Out of the approximately 1400 neurons that form the early larval brain hemisphere, less than 50 cells have axons descending into the ventral nerve cord. Descending neurons fall into four topologically defined clusters located in the antero-medial, antero-lateral, dorsal, and baso-posterior brain, respectively. The antero-lateral cluster represents a lineage derived from a single neuroblast. Terminations of descending neurons are almost exclusively found in the anterior part of the ventral nerve cord, represented by the gnathal and thoracic neuromeres. This region also contains small numbers of neurons with axons ascending into the brain. Terminals of the ascending axons are found in the same basal brain regions that also contain descending neurons. We have mapped ascending and descending axons to the previously described scaffold of longitudinal fiber tracts that interconnect different neuromeres of the ventral nerve cord and the brain. This work provides a structural framework for functional and genetic studies addressing the control of Drosophila larval behavior by brain circuits.
Keywords: Drosophila, brain, ventral nerve cord, connectivity, ascending axons, descending axons
Introduction
Many aspects of insect behavior entail stereotyped sequences of movement which are controlled by neuronal circuits, called central pattern generators (CPGs; Marder et al., 2005). Central pattern generators are located in the ventral nerve cord (CPGs controlling behaviors involving movement of the wings, legs, and abdomen) and suboesophageal ganglion (CPGs controlling behaviors involving the mouth parts; Burrows, 1996; Heinrich, 2002). Given that many behaviors make use of the same muscles/motor neurons (e.g., wing beating in flies is part of flight and mating behavior), one must view the pattern generators as groups of interneurons that interconnect motor neurons in such a way that different motor neuron activity patterns result. Brain centers, which are mostly sensory in nature, exert their influence on central pattern generators of the nerve cord via descending neurons (DNs), and are in turn informed about the state of activation of these circuits through ascending neurons (ANs).
Neurons connecting brain and ventral nerve cord have been characterized in a number of insect species both anatomically and physiologically. Functionally, most DNs constitute command neurons (Kupfermann and Weiss, 1978), which can be subdivided into into trigger neurons, driver neurons, and modulator neurons (Gronenberg and Strausfeld, 1990). Trigger neurons are typically tuned to specific, often multimodal inputs and upon activation turn on/off the activity of central pattern generators. Trigger neurons are often large, fast conducting cells with low levels of spontaneous activity. A characteristic example is the giant fiber neuron in flies that is stimulated by visual interneurons in the lobula and activates a motor circuit controlling the initiation of flight (Koto et al., 1981). Driver neurons and modulator neurons act on individual elements of a motor circuit (rather than the circuit as a whole) and are able to modulate specific aspects of the behavior, such as wing beat frequency, in adaptation to specific stimuli (e.g., elevation of the horizon, wind direction; Rowell, 1989; Burrows, 1996).
Systematic anatomical studies, cutting the cervical connective and backfilling DNs and ANs, exist for adult cricket (Staudacher, 1998) and cockroach (Okada et al., 2003). The authors of both papers identified approximately 200 descending neurons per brain hemisphere and further subdivided the DNs on the basis of neuron location into discrete clusters. The pattern of DNs appears to be reasonably conserved: out of 17 groups of DNs defined in the cricket brain, at least 14 had homologs in the cockroach (Okada et al., 2003). These global studies gave little information regarding dendritic arborization in specific brain compartments, or axonal projections to distinct neuropile domains of the ventral nerve cord. More detailed information exists for a few selected DNs which were visualized (and often concomitantly physiologically characterized) by intracellular injections. Among these are DNs that control stridulation and walking in orthopterans (Heinrich, 2002). In dipterans, more than 50 pairs of DNs were studied with respect to function and dendritic/axonal topologies (Gronenberg and Strausfeld, 1990; Strausfeld and Gronenberg, 1990; Gronenberg et al., 1995). These neurons fall into two categories, the “ventral DNs” and “dorsal DNs”. Dendrites of the former innervate ventral neuropile domains, located around the ventral body (=lateral accessory lobe), and project to ventral areas of the thoraco-abdominal ganglion that are innervated by the dendrites of local interneurons and leg motoneurons. By contrast, dorsal DNs have dendritic arbors primarily in central visual neuropiles (“optic foci”) that are innervated by motion-detecting neurons of the lobula and lobula plate. These DNs project dorsally in the thoracic neuromeres, reaching neuropiles that contain dendrites of motoneurons innervating neck and flight muscles. Physiological recordings suggest that the dorsal DNs control flight velocity, stabilization and steering maneuvers (Gronenberg and Strausfeld, 1990).
The fruitfly Drosophila has proven increasingly useful to approach neuronal circuitry from a developmental and genetic perspective. The availability of specific molecular markers, used in wild-type and mutant backgrounds, make it possible to follow the formation of neuronal connections throughout development, that is, from a simple to complex condition. Studies in Drosophila have significantly advanced our knowledge of numerous subsystems of the brain, such as the olfactory system (Couto et al., 2005), the mushroom body (involved in learning and memory; Heisenberg, 2003; Fahrbach, 2006), or central complex (involved in walking and other behaviors; Strauss, 2002). Aside from the giant interneuron that, as in other dipterans, triggers the flight response (Sun and Wyman, 1997; Allen et al., 1998), virtually no information exists in regard to axonal pathways interconnecting the brain and ventral nerve cord of Drosophila. In the present work we have employed a combination of dye injections, clonal labeling, and molecular markers to study these connections in the larva. Larvae (shortly after hatching) have a brain consisting (on each side) of approximately 1400 neurons, and a ventral nerve cord of approximately 3000 neurons, which is more than an order of magnitude smaller than the adult brain (V.H., unpublished). Larval behavior is correspondingly much simpler than adult behavior (Green, 1983; Cattaert and Birman, 2001; Gerber and Stocker, 2007); however, it is clear that input from the brain is required for almost all of larval behavioral activities, from directing locomotion (phototaxis, chemotaxis) to controlling feeding (the confluence of input from interoreceptors sensing the chemical composition of the hemolymph or the distension of the gut happens in the brain) and the initiation of metamorphosis.
We used DiI injections of different sizes, applied at different locations of the ventral nerve cord, to visualize the pattern of ANs and DNs that connect the brain with the ventral nerve cord. In addition, GFP-labeled clones of neurons whose axon tracts crossed from the brain into the ventral nerve cord and vice versa furnished additional data. Finally, we took advantage of several Gal4 driver lines targeting subsets of ANs and DNs to visualize these fiber connections. Our data define groups of DNs located near distinct neuropile compartments of the basal brain. We have mapped the pathway taken by DNs, as well as ascending axons, with respect to the scaffold of fiber tracts described for the larval brain (Nassif et al., 1998; Nassif et al., 2003). These data will inform genetic and functional studies addressing the role of the brain in controlling larval behavior.
Material and Methods
Markers
Antibody markers used in this study were:
anti-FasII (Grenningloh et al., 1991; mouse monoclonal; antigen seq.: fusion protein containing C-terminal 496aa, which include cytoplasmic domain; antigen expression is absent in embryos deficient for the faciclin II gene; Developmental Studies Hybridoma Bank #1D4);
anti-DN-cadherin (anti-DNcad; Iwai et al., 1997; mouse monoclonal; antigen seq.: fusion protein containing N-terminal 1349 aa containing cadherin domains Cr2-Cr8; antibody recognized 300kDa band in embryo lysate; antigen expression disappears or is greatly reduced in embryos deficient for the DN-cad gene; Developmental Studies Hybridoma Bank #Ex#8);
anti-Dilp2 antibody (Rulifson et al., 2002; rabbit polyclonal; antigen seq.: fusion protein containing 75aa peptide sequence “YNPVIP….QGIVER”; antibody recognizes 12 and 14kDa band on Western blot; expression disappears in larvae in which Dilp2-producing cells are ablated; provided by Dr E. Rulifson).
To visualize reporter constructs, we used the following two antibodies:
anti-Beta-gal. This rabbit antiserum (Cappel #55976) was prepared against entire E. coli Beta-galactosidase protein;
anti-GFP. This rabbit antiserum (Sigma #G1544) was raised a synthetic peptide representing amino acids 3-17 of jellyfish green fluorescent protein.
Fly stocks and egg collections
As wild-type stock we used Oregon R. Flies were grown under standard conditions at room temperature (25 °C). Egg collections were done on yeasted apple juice agar plates (Ashburner, 1989). The following fly lines were used:
3741-Gal4 (Bloomington Stock Center); So-Gal4 (Chang et al., 2003); MzVum-Gal4; UAS-CD8-GFP (Landgraf et al 2003); ChAT-Gal4+UAS-GFP (Salvaterra and Kitamoto, 2001); Nc1-Gal4 (Larsen et al., 2006).
Clonal analysis
We used the FLP/FRT technique to induce labeled clones in early larval brains (Ito et al., 1997; Ward and Skeath, 2000). Briefly, a UAS-cd8-GFP construct containing a FRT flanked flip-out cassette is driven by a global neuronal driver line such as elav-Gal4. Beside these two chromosomes, the stock contains a hs-FLP construct that allows one to induce the flip-out event, leading to a GFP-expressing cell, by applying short, 30-40 minute heat pulses to early (3-5h) embryos. Clones can be assigned to specific lineages based on location of cell bodies and trajectory of lineage tract. There are two or three lineages, belonging to the DAL group, whose axons form the highly characteristic CAPT tract that can be identified in both early and late larvae. Thus, cell bodies of these lineages flank the spur/dorsal lobe of the mushroom body laterally; axon tracts project medially across the peduncle, right posterior to the dorsal lobe, and then turn ventrally. All of the labeled clones with this morphology (about four, out of approximately 150 clones evaluated for the first instar larva; example shown in Fig.2E) had descending axons that reached the anterior part of the ventral nerve cord.
Fig.2.
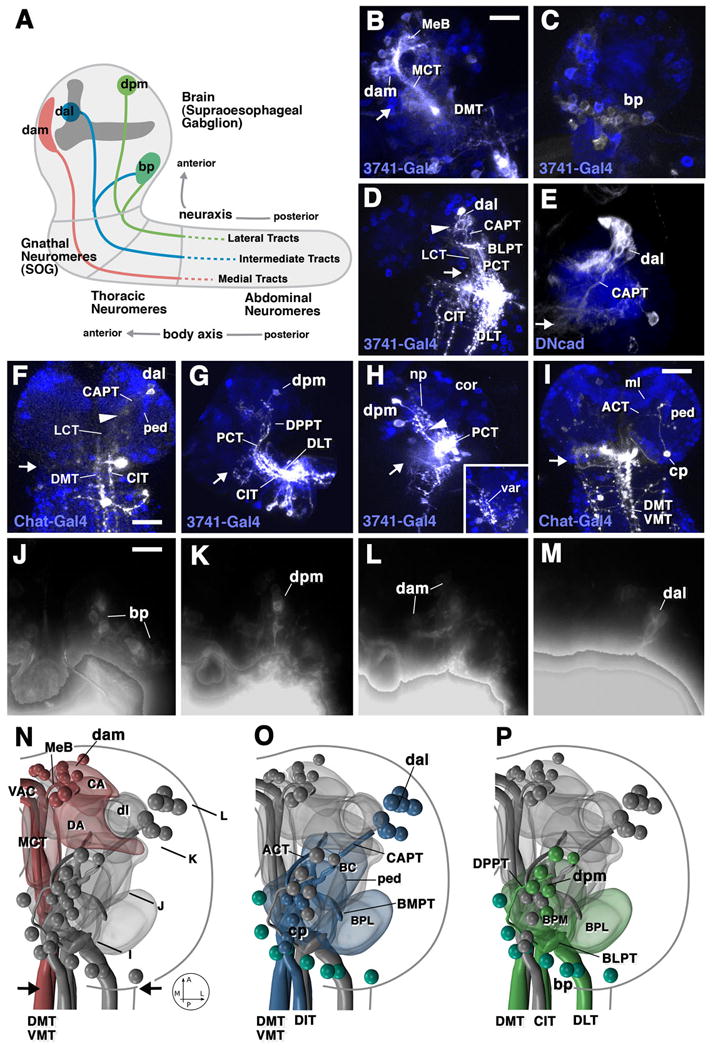
Descending brain neurons (DNs) of the first instar larval brain revealed by DiI injection. A: schematic presentation of first instar brain, illustrating neuraxis and body axis, and main groups of descending neurons. Only injections in the anterior cord (thoracic neuromeres upward) resulted in labeled cells in the brain. As suggested in the diagram, we conclude that descending axons are concentrated in this region, but cannot exclude the possibility that a few descending neurons project further posteriorly (dotted lines). B-I show Z-projections of horizontal or parasagittal confocal sections of preparations which had received small injections of DiI at discrete locations of the anterior ventral nerve cord. Backfilled fibers and cells are white. Blue color shows cells of the brain cortex (cor) and/or neuropile (np) labeled by expression of GFP reporter gene driven by Chat-Gal4 or 3741-Gal4 (indicated for each panel). J-M are Z-projections of frontal confocal sections of a preparation which had been injected with large bolus of DiI. Antero-posterior levels of the Z projections are indicated in panel N. N-P show digital 3D models of right brain hemisphere in dorsal view (anterior to the top). Different groups of descending neurons and the long axon tracts through which their axons descend, as well as neuropile compartments that are close to the neuronal cell bodies and contain the bulk of their (dendritic) arborizations are shown in matching colors. DAM-DNs (dam) are shown in N (red), DAL-DNs (dal) and CP-DNs (cp) in O (blue), BP-DNs (bp) and DPM-DNs (dpm) in P (green and turquoise, respectively). Centro-anterior and dorso-anterior neuropile compartments (CA, DA) are in red; baso-central (BC) compartment in blue; baso-posterior medial compartment (BPM) in green; baso-posterior lateral compartment (BPL) in blue (panel O) and green (panel P). Black arrows in N point at brain-ventral nerve cord boundary; this boundary is shown by small white arrows in panels B-I.
B, C: Injection at dorso-medial/dorso-intermediate position, labeling DAM-DNs descending through median bundle (MeB) and medial cervical tract (MCT), and BP-DNs (C). Both panels present lateral view (anterior to the left); B is a Z-projection of medial half of brain hemisphere, C shows lateral half. Compare schematic representation of DAM-DNs and CP-DNs in panels N and O. D: Injection at dorso-intermediate/dorso-lateral position. Dorsal view of both brain hemispheres and anterior ventral nerve cord. DAL-DNs descending via CAPT and ascending projections through PCT and BLPT are labeled; compare with panel P in which termination of BLPT at posterior surface of the BPL and BPM compartments is schematically shown. White arrowhead in D points at arborizations of DAL-DNs in BC compartment. E: GFP-labeled DAL lineage (flipout clone), containing the DAL-DNs that project through CAPT towards ventral nerve cord. Dorsal view of right hemisphere, lateral to the right. F: dorso-medial/dorso-intermediate injection labeling DAL-DNs descending through CAPT, then LCT towards CIT and DMT of ventral nerve cord. Note characteristic position of DAL-DN cell bodies and CAPT relative to peduncle (ped; compare with schematic representation shown in O). White arrowhead points at arborizations of DAL-DNs in BC compartment. Dorsal view of both brain hemispheres and anterior ventral nerve cord. G: Injection into dorso-intermediate region, labeling several DPM-DNs whose axons descend through DPPT, then PCT, towards CIT and DLT tracts of ventral nerve cord (compare schematic view in panel P). Lateral view. H: Injection similar to that in B, labeling DPM-DNs. Inset shows varicose endings (var) of ascending fibers terminating in dorsal neuropile near DPM cell bodies. Dorsal view, lateral to the left, anterior to the top. I: Medial injection. Labeling of CP-DN whose axon projects anteriorly near antenno-cerebral tract (ACT), then turns 180 degrees posterior around medial lobe of mushroom body (ml) and joins MCT that connects to DMT/VMT tracts of ventral nerve cord (compare schematic view of CP-DNs and their descending tract in panel N). J: Posterior Z-projection showing BP-DNs. K: Section through center of brain with DPM-DNs. L: Anterior section with DAM-DNs. M: Anterior section with DAL-DNs. White-grey “halo” at bottom of each of these panels is caused by bright fluorescence of DiI bolus placed at the cut surface of ventral nerve cord.
Bars: 10μm (for B-E, G, H; J-M); 20μm (F, I)
Immunohistochemistry and histology
For antibody labeling standard procedures were followed (e.g., Ashburner, 1989). The anti-FasII and anti-DN-cadherin antibodies were diluted 1:10 and 1:20, respectively. Anti-Dilp2 was diluted 1:500. Anti-beta-gal and anti-GFP were used at 1:5000 and 1:2000, respectively. For fluorescent staining, the following secondary antibodies were used:
Alexa Fluor 546 goat anti-Mouse IgG (H+L) at 1:500. This goat antiserum (Molecular Probes #A11039) was prepared against mouse immunoglobulin G (heavy and light chain).
Alexa Fluor 546 goat anti-Rat IgG (H+L) at 1:500. This goat antiserum (Jackson ImmunoResearchLaboratories # A11081) was prepared against rat immunoglobulin G (heavy and light chain).
Fluorescein (FITC) AffiniPure Goat Anti-rabbit IgG (H+L) at 1:200. This goat antiserum (Jackson ImmunoResearch Laboratories # 111-095-144) was prepared against rabbit immunoglobulin G (heavy and light chain).
After washing in PBT (1XPBS with 0.1% Tween-20), embryos or brains were mounted in Vectashield mounting medium (Cat# H1000, Vector Laboratories).
For permanent preparations and sections of anti-FasII labeled brains, the preparations were incubated with Biotin-SP-conjugated AffiniPure Goat Anti-Mouse IgG(H+L) at a 1:100 dilution. This goat antiserum (Jackson ImmunoResearch Laboratories #115-065-166) was prepared against mouse immunoglobulin G (heavy and light chain). For the histochemical color reaction, the VectaStain Elite ABC kit (Vector Laboratories) was used according to the manufacturer's specifications.
DiI Injections
First instar larvae were immersed in PBS, opened dorsally using a syringe needle (Beckton Dickinson; # BD30G1/2), and transferred to a slide coated with poly-lysine. Brains and attached nerve cords were removed with a sharp glass needle, transferred to a second, poly-lysine–coated slide, attached with the brain hemispheres facing up, and fixed with 4% formaldehyde in PBS. DiI injections (1mg/ml of pure ethanol) were made into the brain and ventral nerve cord under visual control (100x water lens, Zeiss fixed stage Axioscope microscope) iontophoretically, using a iontophoretic dye marker (Digitimer Ltd., Model D380).
We injected approximately 400 ventral nerve cords. Of these, 71 contained interpretable labeling of ascending and/ or descending fibers. The remainder showed only labeling of local ventral cord tissue and were not further considered. Injections were done predominantly from dorsal, into a variety of medial and lateral positions, both superficial and deep. Out of the 71, 5 preparations had large DiI injections (hundreds of cells/processes); 27 had medium injections (approx. 4 to 20 cells labeled); 39 had small injections (1 to 3 cells). The data presented in the Results and in Figure 2 are mostly based on small injections. Counts of descending neurons were done on seven brain hemispheres of preparations with large ventral cord fills.
Generation of 3D digital models
Staged Drosophila larval brains labeled with anti-FasII and other suitable markers were viewed as wholemounts by confocal microscopy [Biorad MRC 1024ES microscope using Biorad Lasersharp version 3.2 software; lenses: 40x oil (numerical aperture 1.0; WD 0.17); 60x oil (numerical aperture 1.4; WD 0.21). Complete series of optical sections were taken at 2 mm intervals for at least five individuals per stage and compiled into a stack. The confocal stack was imported into TrakEM2 (http://www.ini.uzh.ch/∼acardona/trakem2.html ), and each neuropile compartment was manually segmented following flourescent labelings on the slices. From the lists of segmentations for each compartment, a 3D-mesh was generated using the VIB package (B. Schmid and J.Schindelin, unpublished) by marching cubes algorithm. A second, similarly oriented stack was imported into TrakEM2, and the ventral nerve cord tracts and cervical connectives were sketched with three-dimensional, variable radius tubes. The generated meshes were imported into Blender (Blender Foundation) for merging, volume-preserving smoothening, coloring, animation and rendering by ray-tracing.
Results
Long axon tracts connecting brain and ventral nerve cord
The ascending and descending axons that interconnect brain and ventral nerve cord and that represent the focus of this paper form part of larger fiber systems (“long axon tracts”) described in the previous literature (Power, 1948; Tyrer and Gregory, 1982; Pflueger et al., 1988; Nassif et al., 2003; Landgraf et al., 2003) and summarized in Fig.1. In the ventral nerve cord, one can distinguish a system of medial, intermediate, and lateral long axon tracts (Fig. 1A, D, J-L). Since all long axon tracts contain (subsets of) axons that express the Fasciclin II (FasII) antigen, an antibody against this protein has been widely used to visualize these structures (Nassif et al., 1998; 2003; Landgraf et al., 2003). Medially and laterally, one finds a dorsal tract (DMT and DLT, respectively) and a ventral tract (VMT and VLT, respectively; Fig.1I, L). At the intermediate level, a thick dorsal tract (DIT) is separated from a ventral system that is further split into several closely packed smaller bundles (CIT1-3; Fig.1I, L). According to the literature, dense terminal arborizations of afferent sensory axons, motor neurons and interneurons flank the long axon tracts. Sensory afferents terminate mainly ventrally (Pflüger et al., 1988; Schrader and Merritt, 2000; Zlatic et al., 2003): Tactile hairs (trichoid sensilla) and multidendritic neurons project to a ventromedial domain surrounding the VMT bundle. Terminal arbors of chordotonal organs extend dorsal of tactile projections, around the CIT bundles. Some stretch receptors, including the dbd neuron, terminate around the DMT bundle. Dendritic arborizations of motor neurons also surround the dorsal tracts (DIT, DLT; Landgraf and Thor, 2006).
Fig.1.

Pattern of long axon tracts connecting the brain and ventral nerve cord of the early first instar larva. A-D: Z-projections of confocal sections of brains labeled with anti-FasII in horizontal orientation; anterior is to the top. A shows a low magnification of the entire brain and ventral nerve cord. Note the continuity of the evenly spaced long fiber fascicles of the nerve cord (vcfs) through the cervical connective (ct) into the brain neuropile (brnnp). B-D are high magnification images of the right brain hemisphere. Each Z projection in B-D corresponds to a horizontal brain slice of approximately 20 microns; the mid-levels of the slices are indicated at left margin of panel J. B represents the level of the central brain, containing the mushroom body [dorsal lobe (dl); medial lobe (ml); peduncle (ped), spur (sp), calyx (CX)] and antenno-cerebral tract (ACT). C shows basal brain level, D the level of the cervical connective. E-I: Histological frontal sections of brain labeled with anti-Fasciclin II. Sections contain right brain hemisphere (lateral to the left, dorsal up) and are 3 microns thick. Levels of sections are indicated at lower margin of panel J. E shows section of antenno-cerebral tract in front of calyx; F and G are taken at level of calyx and show the medial and lateral cervical tracts (MCT, LCT), respectively. H shows level of optic lobe (OL) and posterior cervical tract (PCT). I shows level posterior to optic lobe and illustrates spatial arrangement of the longitudinal axon tracts in the ventral nerve cord. J-L: Digital 3D models of brain hemisphere in medial view (J), dorsal view (K) and dorso-medial-posterior view (L). In these models and the models of the following figures, systems of long axon tracts are shown in different colors: the medial cervical tract (MCT) is red, the lateral cervical tract (LCT) with its branches [central anterior protocerebral tract (CAPT), ACT, basomedial protocerebral tract (BMPT)] is blue, the posterior cervical tract (PCT) and its branches [dorsoposterior protocerebral tract (DPPT), basolateral protocerebral tract (BLPT)] green. The color of ventral nerve connectives hints at the cervical tracts they give rise to: the dorso-medial tract (DMT) contributes axons to all three cervical tracts (see also panels D, H) and is colored brown; the ventro-medial tract (VMT) is colored purple because it contributes to the medial and lateral cervical tract (see also panels D and G). The dorso-lateral and central-intermediate tracts (DLT, CIT) give rise to the posterior cervical tract (green; see panels D and H); the dorso-intermediate (DIT) and part of the centro-intermediate system contributes to the lateral cervical tract (blue). In all three panels, the mushroom body and optic lobe are shown in gray as landmarks. In J and K, neuropile compartments are shown in semi-transparent gray. M-Q: Z-projections of horizontal confocal sections of brain hemispheres labeled with anti-FasII (red) and a marker for neuropile (UAS-GFP driven by Chat-Gal4; Salvaterra and Kitamoto, 2001) in green; anterior is to the top. The levels of sections are indicated at left margin of panel J. M represents the level of the dorsal brain [dorsal lobe of mushroom body (dl), calyx (CX), dorso-anterior and dorso-posterior compartments (DA, DP)]. N is taken at central brain level, with peduncle (ped), medial lobe (ml), centro-anterior compartment (CA), centro-intermediate compartment (CPI), and centro-lateral compartment (CPL). O corresponds to basal brain level (baso-anterior compartment = antennal lobe, BA; baso-central compartment BC; baso-posterior medial compartment BPM; baso-posterior lateral compartment BPL). Note position of main long axon tracts (MCT, ACT, BMPT, BLPT) relative to compartment boundaries. P and Q show sections at level of cervical connective at the transition between brain and ventral nerve cord (vc) Other abbreviations: es esophagus; OL optic lobe; MeB median bundle; VAC ventral anterior commissures
Bars: 10μm (for A; B-D; E-I; M-Q)
Anteriorly, the long axon tracts of the ventral nerve cord continue into the brain, forming the cervical connectives. Here, the individual tracts converge and anastomose with each other. Out of this convergence zone emerge the three cervical tracts (Fig.1A, D, H, K; nomenclature used in the following was introduced by Nassif et al., 1998; 2003; Younossi-Hartenstein et al., 2006). Note that at the transition between ventral cord and brain, the neuraxis turns dorsally, thereby deviating from the body axis (Fig.2A). More importantly, the position of structures in the “dorsal” (according to body axis) brain relative to the neuraxis is not clear at all. We will therefore in the following always refer to the body axis. The cervical tracts show the following pattern:
Contingents of axons of all three dorsal tracts of the ventral nerve cord form the posterior cervical tract (PCT) that curves dorsally and laterally. It bifurcates into two branches. The main branch, the baso-lateral protocerebral tract (BLPT), reaches the posterior surface of the basal brain (the baso-posterior lateral and baso-posterior medial compartments; Fig.1D, H, L, O). A thin branch of the PCT, called dorso-posterior protocerebral tract (DPPT), continues straight vertically to reach the dorso-posterior compartment of the brain (Fig.1J, L).
The ventro-medial and ventro-intermediate tracts of the ventral nerve cord mainly give rise to the lateral cervical tract (LCT) which continues forward into the center of the baso-posterior medial compartment of the brain (Fig.1C, D, F, L). Here it splits into several components. A fiber bundle that curves backward and reaches into the baso-posterior lateral neuropile compartment forms the baso-medial protocerebral tract (BMPT; Fig.1C, J, L, O). A second branch, extending further dorsally, represents the forerunner of the antenno-cerebral tract (ACT; Fig.1C, E, J, L), clearly identifiable as the larval counterpart of the adult structure with the same name by its characteristic relationship to the antennal lobe and mushroom body (Nassif et al., 1998; 2003; Fig. 1). A third branch (centro-anterior protocerebral tract, CAPT), projects dorsally and laterally into the lateral neuropile of the protocerebrum (Fig.1B, J, K, L).
Axons of the ventro-medial and the dorso-medial tract of the ventral nerve cord form the medial cervical tract (MCT; Fig.1C, G, J-L). The MCT travels dorsally at the inner surface of the cervical connective, flanking the foregut. Most MCT axons turn medially and form the ventro-anterior part of the supraesophageal commissure (VAC; Fig.1E, J, L, O); other MCT fibers continue dorsally and then posteriorly into the anterior and dorso-medial protocerebrum as the median bundle (Fig.1K).
The scaffold of long axon tracts connecting brain and ventral nerve cord was used to map groups of descending and ascending fibers that were labeled by injection of DiI or clonally restricted expression of GFP. These populations of fibers will be described in the following two sections.
Descending Neurons
Large DiI backfills that affect the entire ventral cord neuropile provide a map of the brain neurons whose axons descend into the ventral nerve cord. Small injections of individual tracts, as well as labeled clones, give further insight into the exact trajectories of these descending connections. In many instances, the pattern of proximal neurite branches, presumably dendrites, could be discerned. Significantly, only the anterior part of the larval ventral nerve cord (suboesophageal ganglion, anterior thoracic segments) appear to be reached by descending axons originating in the brain; backfills at any level further posterior does not result in labeled brain neurons. We performed more than 20 large backfills at anterior levels. In the majority of these attempts, the high fluorescence of the bolus of dye applied to the cut surface prevented the visualization of individually backfilled cells. We were able to image six preparations, all of which showed a very similar pattern of backfilled neurons. The number of small injections into discrete regions of the anterior ventral nerve cord was approximately 200; of these, 50 resulted in successful backfills that were recorded. Backfills allowed us to distinguish the following populations of descending neurons:
Baso-posterior group (BP-DN; Fig.2C, J, P): Large ventral cord back fills yield an average of 11 BP-DNs (range: 9-14) that are arranged in a crescent around the posterior surface of the BPM and BPL compartments. Proximal branches of baso-posterior descending neurons are found in the BPL and posterior BPM compartments. Axons of these neurons descend towards the dorsal neuropile of the ventral nerve cord via the PCT, the same fiber system that carries ascending ventral cord axons towards the BPL and BPM (see below).
Dorso-anterior medial group (DAM-DN; Fig.2B, L, N): Backfills reveal an average of 13 DAM-DNs (range: 9-18) that flank the DA and CA compartments, with both ipsilateral and crossed descending axons projecting through the MCT towards the medial ventral nerve cord (Fig.2B, N). Some antero-medial descending neurons are located quite dorsally, above the medial lobe of the mushroom body; axons of these neurons initially travel anteriorly, curve downward around the anterior surface of the medial lobe and supraesophageal commissure, then join the MCT towards the ventral nerve cord. Neurons of the pars intercerebralis form part of the antero-medial group of brain output neurons (see below). Proximal neurite branches of antero-medial descending neurons fill the DA, CA, and BCv compartments, the same neuropile domains that receive input from the ventral nerve cord through the MCT (see below).
Dorso-anterior lateral group (DAL-DN; Fig.2D, F, M, O): This group contains an average of 5 neurons (range: 4-7) located laterally adjacent to the spur and dorsal lobe of the mushroom body. Axons of the DAL-DNs travel with the central-anterior protocerebral tract (CAPT), crossing over the peduncle and then turning ventrally to join the lateral cervical tract that guides the DAL-DN axons towards the ventral nerve cord (Fig.2F, O). DAL-DNs are regularly backfilled with injections into the dorsal and ventral intermediate tracts of the ventral nerve cord. Proximal branches of DAL neurons extend around the spur and medial lobe of the mushroom body (the CA compartment), as well as throughout the BC compartment located ventral of the medial lobe (Fig.2O). The DAL group of descending neurons was repeatedly visualized in clones (Fig.2E), indicating that these neurons form a lineage derived from a single neuroblast.
Dorso-posterior medial group (DPM-DN; Fig.2G, K, P): Has an average of 4 neurons (range: 3-6), located dorsal of the DP compartment, medial of the calyx of the mushroom body. Axons of the descending DPM neurons form part of the dorso-posterior protocerebral tract (DPPT) which projects straight ventrally, then laterally to join the PCT. Proximal arborizations of DPM neurons extend through the DP compartment; terminal arborizations, made visible by molecular markers expressed in the DPM neurons (see below), surround the PCT and LCT as these tracts enter the anterior end of the ventral nerve cord.
Centro-posterior group (CP-DN): Some local injections into medial tracts reveal a small set (1-3) of neurons located at the posterior pole of the brain, ventral of the mushroom body, whose axons project anteriorly, then join the LCT and descend into the ventral nerve cord (Fig.2I, O). In large ventral cord backfills, the CP-DNs would be part of the BP-DN population.
Ascending projections
Ascending axons were visualized with the help of the Gal4 driver line Nc1-Gal4, which came out of a screen for larval brain specific Gal4 driver lines (Larsen et al., 2006), and which is almost exclusively expressed in neurons of the ventral nerve cord. Based on number and density of labeled neurons, it appears as if most, if not all neurons of the cord express the driver line. Outside the cord, only a single small cluster of brain neurons that probably corresponded to the DPM-DN, expressed Nc1-Gal4. In addition to the Nc1-Gal4 marker, labeled clones of neurons located in the ventral nerve cord, as well as DiI injections that filled preferentially ascending fibers, helped to define ascending fiber tracts.
In general, regions of the brain receiving ascending projections from the ventral nerve cord closely coincide with those housing descending neurons. Antero-medial regions (the BCv, CA and DA compartments) and postero-basal regions (the BPL and posterior BPM compartment) receive the densest input. All dorsal tracts of the cord (DMT, DIT, DLT) carry ascending axons that converge in the BLPT and terminate in the superficial layer of the BPL and BPM compartments (Fig.3A, D, H, J). There appears to be a topographic order to this BLPT projection, in that axons from the medial cord reach more medial positions of the posterior brain, and vice versa (Fig.3I, J). A topographic order probably also exists in the antero-posterior axis, since small DiI injections at thoracic levels labeled terminals only in the most basal parts of the brain, whereas injections further anteriorly, in the suboesophageal ganglion, resulted in labeling of more dorsal terminals (not shown). However, the exact topography of connections needs to be substantiated by single cell labelings, either through clones or small injections.
Fig.3.
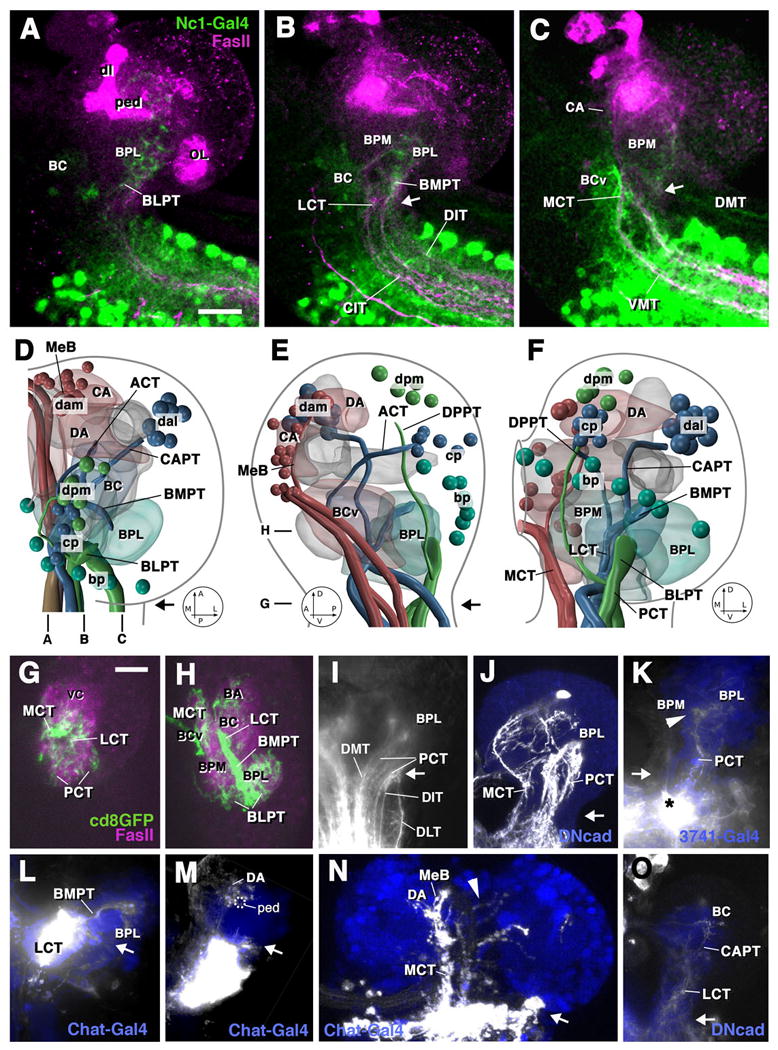
Ascending projections into the first instar brain. A-C: Z projections of parasagittal confocal sections of brain and anterior ventral nerve cord of preparation in which GFP is driven by the Nc1-Gal4 driver line which is expressed in most, if not all, neurons of the ventral nerve cord from thoracic levels posteriorly (green label). An antibody against FasII labels long axon tracts (red). Levels of sections are indicated at bottom of panel D. Arrows in these and all other panels indicate boundary between brain and ventral nerve cord. Ascending projections reach mainly the basal compartments of the brain neuropile. Laterally (A), ascending fibers enter the BLP compartment via BLPT. At intermediate level (B), fibers ascending via CIT/DIT tracts, continuing through BMPT, carry input to BC and lateral BPM. At a medial level (C), fibers ascending via the VMT and DMT tracts terminate in the BCv, CA, and BPM compartments. D-F show digital 3D models of right brain hemisphere in dorsal view (D; anterior to the top), medial view (E; anterior to the left), and posterior view (F; medial to the left). Groups of descending neurons, long axon tracts carrying ascending and descending fibers, and neuropile compartments receiving ascending input are color coded and annotated as in the models shown in Fig.2M-O. G, H: horizontal confocal sections of right brain hemisphere of preparation where GFP (green) is expressed in large clones of ventral nerve cord neurons. Neuropile is labeled red by anti-DNcad. The levels of sections are indicated to the left of panel E. G shows horizontal confocal section at level of brain-ventral nerve cord boundary. Ascending fibers are highly concentrated in MCT and LCT; the PCT appears more spread out, consisting of several thin fascicles arranged along the posterior surface of the neuropile. At the slightly more dorsal level shown in H, the LCT gives rise to the BMPT which carries ascending fibers into the BPL compartment from anteriorly, whereas PCT afferents (forming the BLPT tract at this level) reach the BPL from posteriorly. Note scattering of terminal fibers also in BCv, BC and BA compartments.
I-O: Z-projections of confocal sections of brain preparations in which ascending fibers are labeled by injection of DiI or clonal expression of GFP. Backfilled fibers and cells appear white. Blue color shows cells of the cortex or neuropile labeled by expression of GFP reporter gene driven by Chat-Gal4 or 3741-Gal4. Note varicose endings (“boutons”) of labeled axons in K, M, and N, which is typical of axonal terminations. I: Large injection into dorsal part of ventral nerve cord, labeling ascending fibers in all three dorsal tracts (DMT, DIT, DLT). These fibers continue on their parallel course, forming the spread-out PCT that carries the fibers towards the BPL compartment. J: Clones of ventral nerve cord neurons projecting ascending fibers into the basal brain via PCT and MCT. K: Ascending fibers terminating in BPL and BPM compartments, labeled via small DiI injection into dorsal tier of ventral nerve cord. L: Labeling of fibers ascending through BMPT into BPL compartment. M, N: Ascending fibers reaching the DA compartment via MCT and median bundle. A small number of fibers crosses to the contralateral hemisphere in the brain commissure (arrowhead in N). O: Small contingents of fibers ascending through LCT and CAPT towards BC compartment. Other abbreviations: dl dorsal lobe of mushroom body; MeB median bundle; OL optic lobe; ped peduncle of mushroom body; vc ventral nerve cord Bars: 10μm (for A-C; G-O)
Ventral tracts of the cord that converge and form the LCT contain a second major population of ascending axons that reach the BPL compartment from antero-medially, through the BMPT tract (Fig.3B, D, H, L). Ascending BMPT axons form a dense projection extending throughout most of the volume of the BPL; smaller numbers of branches are given off to the BPM, as well as to the BC compartment which flanks the BMPT anteriorly (Fig.3H). Note then that input from the ventral cord enters the BPL compartment on two sides: ascending axons from the dorsal cord (traversing the “motor neuropile” of the cord) reach the BPL compartment at its posterior surface, axons from the ventral part of the cord (“sensory neuropiles”) enter the BPL from anteriorly.
The third major ascending projection travels with the MCT and branches throughout the BCv, CA and DA compartments (Fig.3C, E, F, J, N). Some of these fibers send a commissural branch through the supraesophageal commissure towards the opposite hemisphere (Fig.3N), a behavior not encountered in the other ascending projections. Smaller groups of ascending axons seem to also be associated with the DPPT and the CAPT tract, since some of the small injections labeling these tracts resulted in labeling of the surrounding neuropile, i.e., the DP and the anterior CPL, respectively (Fig.3M, O). Since no cell bodies were visibly labeled in these experiments, we assume that the label represents terminal branches of ascending axons; however, we cannot exclude that the label corresponds to proximal branches of descending neurons (DAL and DPM, respectively) whose somata did not receive enough DiI to become visible.
One of the main insights gained from this study is that descending neurons, and ascending terminal axons, are largely confined to the ventral compartments of the brain. By contrast, dorsal regions are largely populated by neurons whose dendrites and axons remain confined to the brain. Among these dorsal protocerebral neurons, one major class (DPM and DPL neurons; Younossi-Hartenstein et al., 2006) have neurites directed from laterally to medially, many of them reaching the opposite hemisphere; the other class is represented by the mushroom body, which (in the first instar larva) constitutes 20-25% of the brain in terms of cell number and volume. The separation of a dorsal and ventral brain is also impressively shown by many of the clones (data not shown). Only a relatively small subset of clones has projections that connect brain and ventral nerve cord, or even dorsal and ventral brain, for that matter. Exceptions are lineages such as the DAL lineage (Fig.2D) that produces the descending CAPT axons, or the BA lineages that have dendrites in the (basal) antennal compartment and projects dorsally through the ACT into the calyx of the dorsal protocerebrum (not shown).
Molecular markers of individual populations of descending brain neurons
Among the Gal4 driver lines expressed in the central nervous system we could identify several that (among other neurons) target subsets of descending neurons (Fig.4). 3741-Gal4 (Bloomington #3741; P{w[+mW.hs]=GawB}167Y, w[1118]) is expressed strongly in the antero-medial descending neurons, in particular in a small group of 3-4 cells with dense proximal arborizations in the CA and DA compartments and thick axons that descend through the MCT into the dorso-medial tract of the cord (Fig.4A, B). While passing through the BCv compartment, the axons form additional short branches. At least one of the neurons, that forms a collateral leaving the brain and projecting to the ring gland, represents a neurosecretory cell (Fig.4A, arrowhead).
Fig.4.
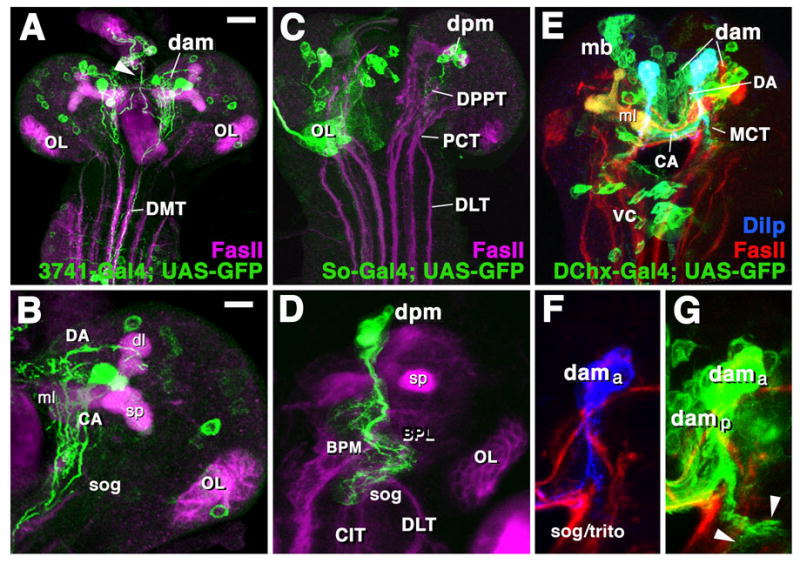
Expression of GFP reporter gene (green) in subset of descending axons targeted by three different Gal4 driver lines. All panels show Z projections of confocal images of first instar brains. Anti-FasII labels long axon tracts (red). Upper panels (A, C, E) show low magnification views, lower panels (B, D, F/G) high magnifications of the same preparations.
A, B: 3741-Gal4 drives in a subset of DAM-DNs (dam) with proximal arborizations in the DA and CA compartments and axons descending through the MCT into the DMT of the ventral cord. C, D: So-Gal4 is expressed in subset of DPM-DNs (dpm) which descend via DPPT tract. Left hemisphere in C shows complete So-Gal4 pattern; in right hemisphere, labeling in all neurons except for DPM has been removed. Note dense arborizations of DPM neurons in basal brain (BPM, BPL) and anteriormost part of ventral nerve cord (primordium of suboesophageal ganglion (sog in D)]. E-G: Expression of DChx-Gal4 and Drosophila insulin-related peptide (Dilp) in subset of DAM-DNs. In left hemisphere of E, complete DChx-Gal4 is shown; aside from dam neurons, it is turned on in the mushroom body (mb) and some neurons of the ventral nerve cord (vc). As reported by De Velasco et al. (2006), DChx is expressed by a large number of DAM neurons (dama anterior DAM neurons; damp posterior DAM neurons), most of which have arborizations confined to the dorso-anterior brain compartments (DA, CA). A subset of these neurons, co-labeled here with an antibody against Dilp (blue), are the neurosecretory cells projecting to the ring gland (not shown); in addition, the same cells have descending branches that terminate in the tritocerebrum/anterior suboesophageal ganglion (sog/trito in F, G).
Other abbreviations: dl dorsal lobe; ml medial lobe; mb mushroom body; OL optic lobe; sp spur
Bars: 10μm (for A, C, E); 5μm (for B, D, F, G)
Sine oculis-(So)-Gal4 (Chang et al., 2003) is expressed in the optic lobe and a few lineages derived from the optic lobe placode; the driver is expressed also in several other clusters of central brain neurons, among them the DPM descending neurons described above. Axons of these cells descend through the DPPT and give off widespread branches in the CPI and BPM compartment (Fig.4C, D). Terminal arborizations are seen in the anterior ventral cord around the zone where the DLT and DIT tracts converge (Fig.4D). We note that the descending projections visualized with the So-Gal4 and MzVUM-Gal4 (see below) drivers conform with the general pattern reconstructed from injections, in terminating in the most anterior parts of the ventral cord, rather than continuing to more posterior levels.
MzVUM-Gal4 (Landgraf et al., 2003), an insertion in the DChx1 gene (DeVelasco et al., 2006; Erclik et al., 2008), is expressed in neurons of the pars intercerebralis (PI), as well as the mushroom body. Forming the medial rim of the larval protocerebrum (the PI as an unpaired structure truly located in between (“inter-”) the brain hemispheres does not develop prior to metamorphosis; DeVelasco et al., 2006), the pars intercerebralis contains many of the antero-medial (DAM) descending neurons (Fig.4E, G). Proximal arborizations of these neurons ramify throughout the DA and CA compartments (Fig.4G); axons cross the midline in the supraesophageal commissure and/or descend through the MCT into the anterior part of the ventral cord, i.e., the suboesophageal ganglion. Here axons terminate in a tuft of branches that span the entire width of the suboesophageal ganglion (arrowheads in Fig.4G). The DChx1-positive descending neurons include a subsets of cells with the above described morphology that also express Drosophila insulin-like peptide (Dilp) and can be visualized with an antibody against this peptide (Rulifson et al., 2002; deVelasco et al., 2006; Fig.4F).
Discussion
DN clusters: possible homologies among insects
Neurons connecting the insect brain and ventral nerve cord have been visualized by dye fillings in several different species, notably cricket (Staudacher, 1998), cockroach (Okada et al., 2003), and blow fly (Gronenberg and Strausfeld, 1990; Strausfeld and Gronenberg, 1990). The fact that these studies were done in adult specimens makes it difficult to compare the data of the literature with the present map of connections in the early Drosophila larva. Further complicating the comparisons are semantic issues. For example, in cockroach, the part of the central brain housing the antennal lobe and α-lobe of the mushroom is called “ventral” in Okada et al. (2003); the same region in the Drosophila brain is referred to as “anterior” by workers in the fly field (e.g., Strausfeld, 1976). Such complications notwithstanding, most of the clusters of descending neurons defined in cockroach and cricket can be tentatively homologized with clusters identified here in the Drosophila larva.
Our data reveal five groups of descending neurons which can be distinguished based on cell body location and fiber tract. In regard to location and trajectory of descending fibers it seems likely that our basal-posterior group (BP-DN) corresponds to clusters i/c1, i/c2, i/c3 defined for cricket and cockroach (Staudacher, 1998; Okada et al., 2003). The designation “i” and “c” indicates whether the given cluster projects its descending ipsi- or contralaterally, respectively; the numeral refers to the location of the cluster. Clusters i1 and c1, for example, share a similar location, but differ with respect to the side on which their axons descend. Clusters i/c1, i/c2, i/c3 occupy a position that is medial, basal and posterior relative to the calyx and peduncle of the mushroom body, which corresponds to the location of the Drosophila larval BP group. Furthermore, descending axons of this group travel directly posteriorly towards the suboesophageal ganglion, traversing and distributing neurite arbors to the dorsal (i.e., posterior) deuterocerebrum. This is exactly the trajectory of the BP neurons seen in the Drosophila larval brain.
On the opposite side of the brain, i.e., more anteriorly and dorsally, is the DAM-DN group in the fly larva. This location corresponds to that of cockroach/cricket groups Pi, i/c4, i/c6 and i/c7, whose neurons are situated anterior and medial to the dorsal lobe (α-lobe) of the mushroom body. Likewise, axons of this group descend anterior to the medial/β-lobe and form neurite arbors in the ventral deuterocerebrum, as is the case in the fly DAM-DN group. The Pi (pars intercerebralis) cluster in Drosophila includes the neurons that express neuropeptides (e.g., insulin-like peptide; Rulifson et al., 2002) and the molecular marker DChx1 (DeVelasco et al., 2006). These neurons have branched axons that project towards the ring gland and towards the suboesophageal ganglion, as shown in this paper.
Another group of descending neurons with a highly characteristic location is DAL-DN, whose cell bodies are located anterior-lateral of the spur of the mushroom body, that is the joint at which peduncle, medial lobe (β/g) and dorsal lobe (α) come together. The CAPT tract formed by the descending axons of this group, which (at least in Drosophila) most likely forms a clone derived from one neuroblast, passes postero-medially over the peduncle, before turning sharply ventrally and eventually joining the lateral cervical tract. In cockroach/cricket, clusters i5 and i5n are located at a position corresponding to that of DAL-DN. Significantly, there exists no contralaterally descending (“c”) component for this cell cluster, just as in the case of the Drosophila DAL-DNs. The fact that two neighboring, yet separate clusters are found in cockroach (Okada et al., 2003) may indicate that in this species, the DAL-like group of descending neurons represents two different lineages, rather then a single one as in flies.
It is unclear to which of the clusters of descending neurons the CP-DNs and DPM-DNs might correspond to. CP-DNs have a similar cell body location as the BP-DNs, and are distinguished from the latter merely by the characteristic axonal trajectory, which is directed forward before turning ventrally. Since details of axon pathways are not elucidated in the cockroach or cricket map of descending neurons, it is not possible to tell whether any neurons of the i/c-i/c3 clusters, which in position could include CP-like neurons, have axons with CP-axonal characteristics. The same reason precludes identifying a potential homolog of the Drosophila DPM-DN cluster. DPM -DNs are located far dorsally, on the “crown” of the protocerebrum, medially adjacent to the calyx of the mushroom body. No somata with such dorsal location are depicted in the cricket/cockroach map, suggesting that the DPM homologs, if they exist, have moved to a slightly different position.
Dye labeling of descending neurons of dipterans has been carried out in a number of studies by Strausfeld and collaborators on Calliphora (Strausfeld and Gronenberg, 1990; Gronenberg and Strausfeld, 1990). These authors estimate the total number of DNs to be 300 pairs, which would even exceed the number of DNs established for cricket and cockroach by Staudacher (1998) and Okada et al., (2003), respectively. Detailed studies exist for two subpopulations of DNs, those located in the basal anterior (ventral, relative to neuraxis) and basal-posterior brain. The former may correspond to the DAM-DN group defined for the Drosophila larva in this paper. Dendrites of the anterior DNs arborize in the neuropile between the lateral accessory lobe (called ventral body in the classical fly literature) and central complex, which corresponds to the location that harbors DAM-DN arborizations in the Drosophila larva (i.e., CA and DA compartment).
The second group of well characterized Calliphora DNs introduced by Strausfeld and coworkers are the “dorsal DNs”. This group, formed by several discrete clusters that add up to a total of approximately 50 cells, forms dendritic arborizations in the ventro-lateral protocerebrum that receives afferent input mostly from the optic lobe, but also from other sensory modalities (see below). Dendritic arbors and their presynaptic afferents form discrete modules, called “optic foci”, not unlike the olfactory glomeruli of the antennal lobe (or the vertebrate olfactory bulb, for that matter). The dorsal DNs project into the dorsal thoracic neuropile that contains circuits controlling flight. Based on location of their cell bodies, as well dendritic arborization, the dorsal DNs of the adult fly brain most likely correspond to the neurons of the larval BP group of this study. Thus, BP neuronal somata are located in the baso-lateral cortex, as their adult counterparts; BP neuronal axons project through the dorsal fascicles of the cervical connective and, most likely, terminate in the dorsal neuropile of the ventral nerve cord; and, dendritic arbors of the BP neurons are seen in the BPL compartment, that gives rise to the adult ventrolateral protocerebrum which harbors the optic foci (Younossi-Hartenstein et al., 2003).
Output compartments of the insect brain are prefigured in the early larva
Directed behaviors like flight or walking are controlled by central pattern generators in the thoracic ganglia. CPGs are modulated both by feed back input (e.g., proprioceptors in the wings and legs), as well as exteroreceptors that inform the CPG about the dynamically changing parameters of the animal's environment (e.g., wind speed, gravity, olfactory and visual cues). Several brain neuropile compartments with descending neurons receiving multimodal sensory input have been identified in both orthopterans and dipterans. Notable among these are the ventro-lateral protocerebrum (VLP), the antennal mechanosensory and motor center (AMMC), and the lateral accessory lobe (LAL). All of these brain centers evolve from larval brain compartments that, according to the present study, contain ascending and descending fibers connecting the brain with the ventral nerve cord. Thus, despite of the fact that fly larvae exhibit an extremely reduced behavioral repertoire that includes neither walking nor flight, the brain centers subserving the central modulation of these behaviors in the adult are prefigured already in the larval brain.
The VLP represents the domain within the central brain neuropile that receives afferent visual input from neurons located in the optic lobe. Note that because of uncertainties of the neuraxis in the brain, as well as neuromere identities of individual parts of the brain neuropile, the VLP has received numerous different names in the literature, including posterior protocerebrum (Homberg, 1994), and dorsal deuterocerebrum (Strausfeld and Gronenberg, 1990). However, the VLP can be unambigously defined by the presence of the so called optic foci, which form discrete, synapse-rich domains receiving afferents from the optic lobe (Strausfeld and Bacon, 1984; Strausfeld and Gronenberg, 1990). For example, in the fly Calliphora, input to the VLP arrives from the lobula via three axon bundles containing 400-500 fibers each; nine bundles with several hundred axons each arrive from the lobula plate. The pattern of optic foci and optic lobe afferents in Drosophila resembles closely the one described for larger flies (Otsuna and Ito, 2006). Among the intrinsic neurons of the VLP, a large number (at least 50 per hemisphere in Calliphora) with descending axons to the thoracic flight motor neuropile have been studied anatomically and physiologically. In flies (Strausfeld and Gronenberg, 1990), as well as locusts (Hensler, 1992; Rowell, 1989; 1993), these DNs receive input from large horizontal motion-sensitive neurons of the optic lobe, as well as from small-field retinotopic axons. These neurons convey information about deviation from straight flight and thereby enable the DNs of the VLP to modulate the activity of their targets in the thoracic flight neuropile. At least some of the DNs [best studied example: the giant descending neuron (GDN) in flies (Strausfeld and Bacon, 1984)] receive multimodal input (visual, olfactory, mechanosensory).
The AMMC represents a neuropile compartment located laterally and posteriorly (i.e., dorsally, relative to neuraxis) of the antennal lobe (Rospars, 1988; Homberg et al., 1989; Horseman et al., 1997). The AMMC receives sensory input from external mechanoreceptors and stretch receptors (chordotonal organs) located in the antenna. In addition, ascending fibers carrying mechanosensory input from the thoracic ganglia, as well as visual input, reach the AMMC. Functional studies indicate that the AMMC controls antennal movement during flight and walking, as well as during tracking of visual objects; furthermore, descending neurons with dendritic arborizations in the AMMC convey information from antennal mechanoreceptors towards the thoracic flight centers (Horseman et al., 1997).
The VLP evolves from the larval BPL compartment (Younossi-Hartenstein et al., 2003). The AMMC does not form a distinctive compartment in the larval brain (Pereanu et al., 2008), but arises in tight relationship to the BPL. Thus, the BMPT tract, that carries ascending fibers entering the BPL from antero-medially, can be followed continuously through larval development and metamorphosis; in the adult, this tract forms a conspicuous bundle connecting the baso-medial midbrain with the AMMC (VH, ubpublished). Sensory afferents to the larval BPL also include elements that may be comparable to the input of the adult VLP and AMMC: the larval antennal organ includes mechanoreceptors, among them a chordotonal organ (Campos-Ortega and Hartenstein, 1997), which project alongside the (mostly olfactory) antennal nerve into the basal brain, and may well pioneer the mechanosensory input to the presumptive AMMC. The larval eye (Bolwig's organ) projects into the minute larval optic neuropile (LON); from here, a group of postsynaptic neurons, among them the “optic lobe pioneers”, relay visual input to the central brain (Campos et al., 1995; Helfrich-Foerster, 1997; Chang et al., 2003). For neither the rudimentary central antenno-mechanosensory nor the visual pathway of the larva has there been a detailed anatomical study. Given that many of the descending neurons described here (e.g., the BP-DNs), as well as afferent input to the larval brain, are concentrated in the BPL, developmental-anatomical studies on the growth and metamorphosis of these connections will most likely provide important insights into the wiring and working of brain output centers.
The lateral accessory lobe (LAL; called ventral body in much of the classical fly literature) forms a distinct neuropile compartment located ventrally of the horizontal lobes of the mushroom body (Strausfeld, 1976). The adult LAL evolves from the BC compartment of the larva (Younossi-Hartenstein et al., 2003). In locusts, the LAL receives ascending afferents from the thoracic ganglia that carry sensory information from external mechanoreceptors and proprioceptors of the wing and notum (Homberg, 1994). Descending neurons with dendritic terminals in the LAL show an enhanced tonic spiking activity during, before or after flight; some neurons are stimulated by mechanoreceptive hairs of the head, others by proprioceptors and visual stimuli. A similar, flight-related activity increase was recorded from columnar and tangential neurons of the central complex that had terminal arbors in the LAL. Recent studies in locusts show impressively the central role of the LAL in polarized light-guided flight (Homberg et al., 2004). Previous studies in Drosophila had shown de-oxyglucose incorporation (an assay for increased neuronal activity) into the LAL during flight, but not walking (Mueller et al., 1993).
The larval forerunner of the ventral body is the BC compartment which, like its adult counterpart, is one of the compartments associated with descending and ascending neurons. The former are represented by the DAL-DNs whose cell bodies are located dorsal and lateral of the BC, and which have neurite arborizations in the BC. As has been reported for adult insect brains, the larval LAL/BC is closely connected to the compartment that will metamorphose into the central complex (Pereanu et al., 2008).
Aside from the BPL and BC, the CA and DA compartments and the DAM-DNs associated with these compartments appear as a major output center of the larval brain. One subpopulation of DAM-DNs are the neurosecretory cells (e.g., insulinergic cells; Rulifson et al., 2002; DeVelasco et al., 2006) which project to the ring gland, but which also have proximal neurite arborization in the DA/CA compartments and project axonal collaterals to the tritocerebrum and the SOG, both of which are the brain centers controlling feeding behavior (Stocker and Schorderet, 1981; Rajashekhar and Singh, 1994; Thorne et al., 2004). The DA/CA compartments and their descending neurons might therefore be best compared to the hypothalamus of the vertebrate brain, where multimodal sensory information about the external and internal milieu of the organism converge, and from where motor centers controlling homeostatic behaviors are controlled.
Descending neurons: overall number and clonal organization
It is understandable that descending neurons of the early larval fly brain are considerably lower in number than their counterparts in adult insects. The number of primary neurons that form the larval brain is less than 10% of the number of adult neurons. Secondary lineages with descending projections leaving the late larval brain have been identified (Pereanu and Hartenstein, 2006); for example, the DALd and DALcl lineages produce secondary axon tracts that follow the descending CAPT axon tract, which is the fiber bundle also used by the primary DAL-DNs described here. We have evidence that the primary neurons of one of the DALcl lineages forms the DAL-DNs described for the first instar larva in this paper (WP and VH, unpublished). It is not yet known how many of the secondary neurons produced by the DALd and DALcl lineages actually produce descending axons, and how far posteriorly these axons project. Based on tract diameter (in confocal sections) the late larval CAPT contain between 50 and 100 axons that reach at least as far as the suboesophageal ganglion. This implies that the number of secondary neurons added to the DAL group is at least five times of that present in the early larva. One might expect a similar increase in cell number in the other groups of DNs.
An important future goal is to establish the lineage identity of all of the descending neurons, both primary and secondary. So far, we can make the argument that the groups of descending neurons form several discrete lineages only for the DAL group, for which we have isolated MARCM clones. And, as discussed in the previous paragraph, it is highly likely that the same neuroblast that had produced the primary DAL-DNs of the larva will continue to produce secondary DNs with the same trajectory as their older siblings. We would like to propose that the other groups of DNs also belong to a small number of discrete lineages, although a more extensive clonal analysis of the larval brain is needed to confirm this idea. The identification of lineage-specific Gal4 driver lines will play an important part in this quest. Each of the lines introduced in this study (e.g., So-Gal4; Chang et al., 2003) targets several lineages, some of which include DNs. We anticipate that in screens that are currently under way several more suitable lineage-specific drivers will be identified.
Acknowledgments
This work was supported by National Science Foundation Grant IOB-0445365 to V.H.
References
- Allen MJ, Drummond JA, Moffat KG. Development of the giant fiber neuron of Drosophila melanogaster. J Comp Neurol. 1998;397:519–31. doi: 10.1002/(sici)1096-9861(19980810)397:4<519::aid-cne5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Ashburner M. A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. Drosophila. [Google Scholar]
- Burrows M. The neurobiology of an insect brain. Oxford University Press; OxfordNew York: 1996. [Google Scholar]
- Campos AR, Lee KJ, Steller H. Establishment of neuronal connectivity during development of the Drosophila larval visual system. J Neurobiol. 1995;28:313–329. doi: 10.1002/neu.480280305. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hartenstein V. The Embryonic Development of Drosophila melanogaster. 2nd. Springer; 1997. [Google Scholar]
- Cattaert D, Birman S. Blockade of the central generator of locomotor rhythm by noncompetitive NMDA receptor antagonists in Drosophila larvae. J Neurobiol. 2001;48:58–73. doi: 10.1002/neu.1042. [DOI] [PubMed] [Google Scholar]
- Chang T, Younossi-Hartenstein A, Hartenstein V. Development of neural lineages derived from the sine oculis positive eye field of Drosophila. Arthropod Struct Dev. 2003;32:303–317. doi: 10.1016/j.asd.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–47. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- De Velasco B, Erclik T, Shy D, Sclafani J, Lipshitz H, McInnes R, Hartenstein V. Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev Biol. 2007;302:309–23. doi: 10.1016/j.ydbio.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE. Structure of the mushroom bodies of the insect brain. Annu Rev Entomol. 2006;51:209–32. doi: 10.1146/annurev.ento.51.110104.150954. [DOI] [PubMed] [Google Scholar]
- Gerber B, Stocker RF. The Drosophila larva as a model for studying chemosensation and chemosensory learning: a review. Chem Senses. 2007;32:65–89. doi: 10.1093/chemse/bjl030. [DOI] [PubMed] [Google Scholar]
- Green CH, Burnet B, Connolly KJ. Organization and patterns of inter and intraspecific variation in the behaviour of Drosophila melanogaster larvae. Anim Behav. 1983;31:262–291. [Google Scholar]
- Grenningloh G, Rehm EJ, Goodman CS. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell. 1991;67(1):45–57. doi: 10.1016/0092-8674(91)90571-f. [DOI] [PubMed] [Google Scholar]
- Gronenberg W, Strausfeld NJ. Descending neurons supplying the neck and flight motor of Diptera: physiological and anatomical characteristics. J Comp Neurol. 1990;302:973–91. doi: 10.1002/cne.903020420. [DOI] [PubMed] [Google Scholar]
- Gronenberg W, Milde JJ, Strausfeld NJ. Oculomotor control in calliphorid flies: organization of descending neurons to neck motor neurons responding to visual stimuli. J Comp Neurol. 1995;361:267–84. doi: 10.1002/cne.903610206. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Pereanu W, Jennett A, Simpson J, Rubin GM. A developmental guide to the compartmentalization of the Drosophila brain. 2008 in preparation. [Google Scholar]
- Heinrich R. Impact of descending brain neurons on the control of stridulation, walking, and flight in orthoptera. Microsc Res Tech. 2002;56:292–301. doi: 10.1002/jemt.10033. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–75. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Helfrich-Foerster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol. 1997;380:335–354. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Hensler K. Neuronal co-processing of course deviation and head movement in locusts. I. Descending deviation detectors. J Comp Physiol A. 1992;171:257–271. [Google Scholar]
- Homberg U. Flight-correlated activity changes in neurons of the lateral accessory lobes in the brain of the locust Schistocerca gregaria. J Comp Physiol A. 1994;175:597–610. [Google Scholar]
- Homberg U, Christensen TA, Hildebrand JG. Structure and function of the deuterocerebrum in insects. Annu Rev Entomol. 1989;34:477–501. doi: 10.1146/annurev.en.34.010189.002401. [DOI] [PubMed] [Google Scholar]
- Homberg U, Hofer S, Mappes M, Vitzthum H, Pfeiffer K, Gebhardt S, Müller M, Paech A. Neurobiology of polarization vision in the locust Schistocerca gregaria. Acta Biol Hung. 2004;55:81–9. doi: 10.1556/ABiol.55.2004.1-4.10. [DOI] [PubMed] [Google Scholar]
- Horseman BG, Gebhardt MJ, Honegger HW. Involvement of the suboesophageal and thoracic ganglia in the control of antennal movements in crickets. J Comp Physiol A. 1997;181:195–204. [Google Scholar]
- Ito K, Sass H, Urban J, Hofbauer A, Schneuwly S. GAL4-responsive UAS-tau as a tool for studying the anatomy and development of the Drosophila central nervous system. Cell Tissue Res. 1997;290:1–10. doi: 10.1007/s004410050901. [DOI] [PubMed] [Google Scholar]
- Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T. Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron. 1997;19:77–89. doi: 10.1016/s0896-6273(00)80349-9. [DOI] [PubMed] [Google Scholar]
- Koto M, Tanouye MA, Ferrus A, Thomas JB, Wyman RJ. The morphology of the cervical giant fiber neuron of Drosophila. Brain Res. 1981;221:213–7. doi: 10.1016/0006-8993(81)90772-1. [DOI] [PubMed] [Google Scholar]
- Kupfermann I, Weiss KR. The command neuron concept. Behav Brain Sci. 1978;1:3–39. [Google Scholar]
- Landgraf M, Sanchez-Soriano N, Technau GM, Urban J, Prokop A. Charting the Drosophila neuropile: a strategy for the standardised characterisation of genetically amenable neurites. Dev Biol. 2003;260:207–25. doi: 10.1016/s0012-1606(03)00215-x. [DOI] [PubMed] [Google Scholar]
- Landgraf M, Thor S. Development of Drosophila motoneurons: specification and morphology. Semin Cell Dev Biol. 2006;17:3–11. doi: 10.1016/j.semcdb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Larsen C, Franch-Marro X, Hartenstein V, Alexandre C, Vincent JP. An efficient promoter trap for detection of patterned gene expression and subsequent functional analysis in Drosophila. Proc Natl Acad Sci USA. 2006;103:17813–7. doi: 10.1073/pnas.0607652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–R699. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Mueller NR, Buchner E, Heisenberg M. 3H-deoxyglucose activity labeling in the central complex of Drosophila melanogaster monitors different behavioral situations and different visual stimuli. In: Elsner N, Heisenberg M, editors. Gene-brain-behavior. Stuttgart New York: 1993. 2005. [Google Scholar]
- Nassif C, Noveen A, Hartenstein V. Embryonic development of the Drosophila brain I. The pattern of pioneer tracts. J Comp Neur. 1998;402:10–31. [PubMed] [Google Scholar]
- Nassif C, Noveen A, Hartenstein V. Early development of the Drosophila brain III. The pattern of neuropile founder tracts during the larval period. J Comp Neurol. 2003;455:417–434. doi: 10.1002/cne.10482. [DOI] [PubMed] [Google Scholar]
- Okada R, Sakura M, Mizunami M. Distribution of dendrites of descending neurons and its implications for the basic organization of the cockroach brain. J Comp Neurol. 2003;459:158–74. [PubMed] [Google Scholar]
- Otsuna H, Ito K. Systematic analysis of the visual projection neurons of Drosophila melanogaster. I. Lobula-specific pathways. J Comp Neurol. 2006;497:928–58. doi: 10.1002/cne.21015. [DOI] [PubMed] [Google Scholar]
- Pereanu W, Hartenstein V. Neural lineages of the Drosophila brain: A 3D digital atlas of the pattern of lineage location and projection at the late larval stage. J Neurosci. 2006;26:5534–5553. doi: 10.1523/JNEUROSCI.4708-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereanu W, Jennett A, Kumar A, Reichert H, Hartenstein V. A development-based compartmentalization of the Drosophila central brain. 2008 doi: 10.1002/cne.22376. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflueger HJ, Braeunig P, Hustert R. The organization of mechanosensory neuropiles in locust thoracic ganglia. Phil Trans R Soc Lond B. 1988;321:1–26. [Google Scholar]
- Power ME. The thoraco-abdominal nervous system of an adult insect Drosophila melanogaster. J Comp Neur. 1948;88:347–409. doi: 10.1002/cne.900880303. [DOI] [PubMed] [Google Scholar]
- Rajashekhar KP, Singh RN. Neuroarchitecture of the tritocerebrum of Drosophila melanogaster. J Comp Neurol. 1994;349:633–645. doi: 10.1002/cne.903490410. [DOI] [PubMed] [Google Scholar]
- Rowell CHF. Descending interneurones of the locust reporting deviation from flight course: what is their role in steering? J Exp Biol. 1989;146:177–194. [Google Scholar]
- Rowell CHF. Intersegmental coordination of flight steering in locusts. Sem Neurosci. 1993;5:59–66. [Google Scholar]
- Rospars JP. Structure and development of the insect antennodeuterocerebral system. Int J Insect Morphol Embryol. 1988;17:243–294. [Google Scholar]
- Rulifson EJ, Kim SK, Nusse R. Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science. 2002;296:1118–20. doi: 10.1126/science.1070058. [DOI] [PubMed] [Google Scholar]
- Salvaterra PM, Kitamoto T. Drosophila cholinergic neurons and processes visualized with Gal4/UAS-GFP. Gene Express Patt. 2001;1:73–82. doi: 10.1016/s1567-133x(01)00011-4. [DOI] [PubMed] [Google Scholar]
- Schrader S, Merritt DJ. Central projections of Drosophila sensory neurons in the transition from embryo to larva. J Comp Neurol. 2000;425:34–44. [PubMed] [Google Scholar]
- Staudacher E. Distribution and morphology of descending brain neurons in the cricket Gryllus bimaculatus. Cell Tissue Res. 1998;294:187–202. doi: 10.1007/s004410051169. [DOI] [PubMed] [Google Scholar]
- Stocker RF, Schorderet M. Cobalt filling of sensory projections from internal and external mouthparts in Drosophila. Cell Tissue Res. 1981;216:513–523. doi: 10.1007/BF00238648. [DOI] [PubMed] [Google Scholar]
- Strausfeld N. Atlas of an insect Brain. Berlin: Springer; 1976. [Google Scholar]
- Strausfeld NJ, Bacon JP. Multimodal convergence in the central nervous system of insects. In: Horn E, editor. Multimodal convergence in sensory systems. Stuttgart: Gustav Fischer; 1984. pp. 47–76. [Google Scholar]
- Strausfeld NJ, Gronenberg W. Descending neurons supplying the neck and flight motor of Diptera: organization and neuroanatomical relationships with visual pathways. J Comp Neurol. 1990;302:954–72. doi: 10.1002/cne.903020419. [DOI] [PubMed] [Google Scholar]
- Strauss R. The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol. 2002;12:633–8. doi: 10.1016/s0959-4388(02)00385-9. [DOI] [PubMed] [Google Scholar]
- Sun YA, Wyman RJ. Neurons of the Drosophila giant fiber system: I. Dorsal longitudinal motor neurons. J Comp Neurol. 1997;387:157–66. [PubMed] [Google Scholar]
- Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–79. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Tyrer NM, Gregory GE. A guide to the neuroanatomy of locust suoesophageal and thoracic ganglia. Phil Trans R Soc Lond B. 1982;297:91–123. [Google Scholar]
- Ward EJ, Skeath JB. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development. 2000;127(22):4959–69. doi: 10.1242/dev.127.22.4959. [DOI] [PubMed] [Google Scholar]
- Younossi-Hartenstein A, Salvaterra P, Hartenstein V. Early development of the Drosophila brain IV. Larval neuropile compartments defined by glial septa. J Comp Neurol. 2003;455:435–450. doi: 10.1002/cne.10483. [DOI] [PubMed] [Google Scholar]
- Zlatic M, Landgraf M, Bate M. Genetic specification of axonal arbors: atonal regulates robo3 to position terminal branches in the Drosophila nervous system. Neuron. 2003;37:41–51. doi: 10.1016/s0896-6273(02)01131-5. [DOI] [PubMed] [Google Scholar]


