Summary
The placenta provides the interface for gas and nutrient exchange between the mother and the fetus. Despite its critical function in sustaining pregnancy, the stem/progenitor cell hierarchy and molecular mechanisms responsible for the development of the placental exchange interface are poorly understood. We identified an Epcamhi labyrinth trophoblast progenitor (LaTP) in mouse placenta that at a clonal level generates all labyrinth trophoblast subtypes, syncytiotrophoblasts I and II and sinusoidal trophoblast giant cells. Moreover, we discovered that Hgf/c-Met signaling is required for sustaining proliferation of LaTP during midgestation. Loss of trophoblast c-Met also disrupted terminal differentiation and polarization of syncytiotrophoblasts, leading to intrauterine fetal growth restriction, fetal liver hypocellularity and demise. Identification of a this c-Met dependent multipotent labyrinth trophoblast progenitor provides a landmark in the poorly defined placental stem/progenitor cell hierarchy and may help understand pregnancy complications caused by a defective placental exchange.
Introduction
The mammalian placenta serves as the interface for gas and nutrient exchange during development. The placenta also provides an immunological barrier between the fetus and the mother and secretes hormones that regulate pregnancy. Recent studies identified the placenta also a hematopoietic organ that generates hematopoietic stem/progenitor cells (HS/PC) and macrophages and provides a niche that protects definitive HS/PC from premature erythroid differentiation (Gekas et al., 2005; Rhodes et al., 2008; Van Handel et al., 2010; Chhabra et al., 2012). Dysfunctional placental development has been associated with maternal hypertension and pre-eclampsia (Young et al., 2010), while disruption of placental circulation and fetal-maternal exchange can lead to intrauterine fetal growth restriction (IUGR) and demise (Scifres and Nelson, 2009). Therefore, proper placental function is critical for healthy pregnancy.
In the mouse placenta, substance exchange occurs in the labyrinth (La; analogous to chorionic villi in human), which is composed of highly branched fetal vasculature and trophoblast-lined maternal blood spaces (Figure S1A) (Rossant and Cross, 2001; Watson and Cross, 2005; Maltepe et al., 2010). Trophoblasts are epithelial cells that develop from the trophectoderm (Te), the outermost layer of the blastocyst. Mitotic activity is limited to polar Te (pTe) that differentiates into chorionic trophoblasts and the ectoplacental cone (EPC). Chorionic trophoblasts form the labyrinth, while the EPC gives rise to the junctional zone (JZ) consisting of spongiotrophoblasts (Sp) and trophoblast giant cells (TGC) that provide structural support and enable invasion to the uterus (Figure 1A; Figure S1A). Morphogenesis of the labyrinth occurs after fusion of the allantoic mesoderm with chorionic trophoblasts (E8.5), which undergo extensive branching (Figure 1A). The labyrinth consists of two layers of multinucleated syncytiotrophoblasts (SynT-I and -II) that control fetal-maternal transport, and sinusoidal trophoblast giant cells (sTGCs) that have endocrine functions and act as hematopoietic signaling centers (Chhabra et al., 2012). Fgf4-dependent trophoblast stem (TS) cells that generate all trophoblast subtypes can be established from the blastocyst and early post-implantation embryos, and are the in vitro equivalents of Te (Tanaka et al., 1998). However, TS cell potential disappears after chorioallantoic fusion (Uy et al., 2002) suggesting that yet unidentified precursors downstream of TS cells form the placenta (Figure 1A) (Simmons and Cross, 2005). Recent studies identified an EPC derived Blimp1+ precursor that generates multiple types of trophoblast giant cells in the spongiotrophoblast (Sp) layer (Mould et al., 2012). However, the precursors that generate the exchange interface in placental labyrinth are unknown.
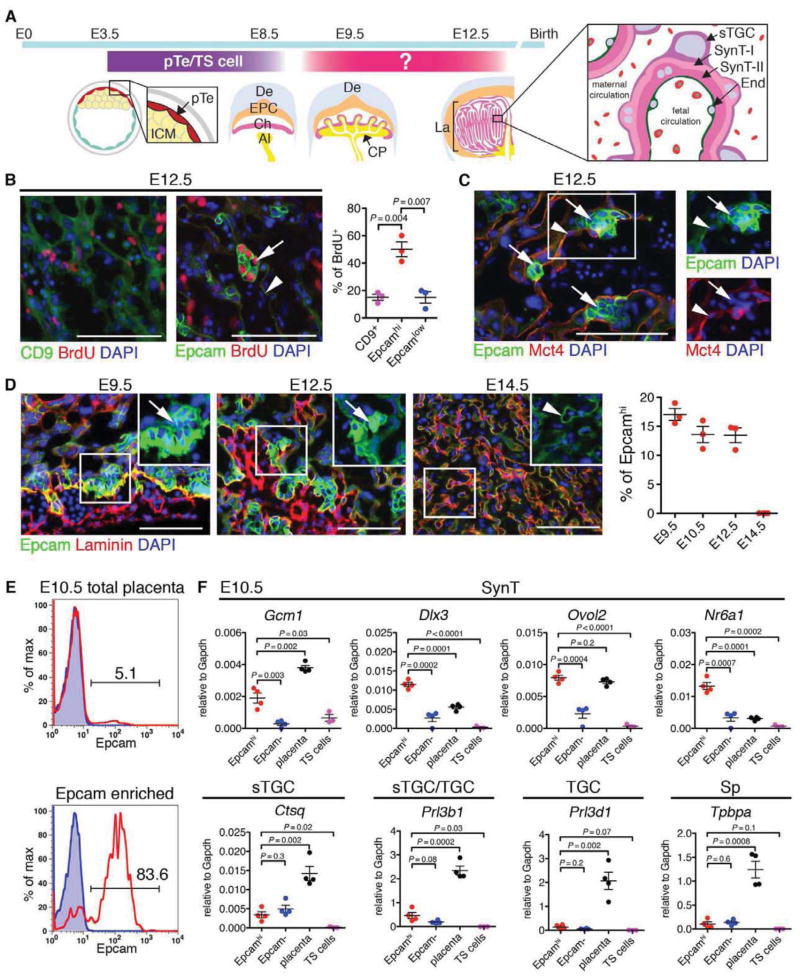
Figure 1. Epcamhi cells are candidate labyrinth trophoblast progenitor cells.
(A) Schematic depicting the development of the mouse placenta. Polar trophectoderm (pTe) develops into ectoplacental cone (EPC) and chorion (Ch). Trophoblast stem (TS) cells can established from trophoectoderm/placenta before E8.5, however, TS cells disappear after chorioallantoic fusion, suggesting that yet unidentified progenitors are responsible for labyrinth development. The labyrinth (La) contains three trophoblast cell types; syncytiotrophoblasts (SynT) I and II, and sinusoidal trophoblast giant cells (sTGC). The SynT-II layer is facing fetal endothelium (End), and the sTGC is facing maternal blood. ICM, Inner Cell Mass; De, decidua; Al, allantois; CP, chorionic plate.
(B) Identification of Epcam as a marker for proliferating trophoblasts. Sections from E12.5 placental labyrinth were stained with CD9 or Epcam. DNA synthesis was visualized by BrdU incorporation. Arrow, Epcamhi cluster. Arrowhead, SynT. Scale bar 100 μm.
(C) Correlation of SynT-II differentiation marker, Mct4, with low Epcam expression in SynT-II (arrowhead). Arrow, Epcamhi cluster. Scale bar 100 μm.
(D) Kinetic analysis of the frequency of Epcamhi cells in midgestation placentas. Scale bar 100 μm.
(E) Enrichment of Epcamhi cells from E10.5 placenta using magnetic bead separation.
(F) Quantitative analysis of the expression of trophoblast subtype-specific genes in Epcamhi enriched cells.
All error bars indicate SEM (Standard error of mean).
See also Figure S1.
Targeted mutagenesis in mice has provided clues to mechanisms regulating key stages of placental development (Rossant and Cross, 2001; Watson and Cross, 2005). c-Met receptor tyrosine kinase and its ligand, hepatocyte growth factor (Hgf) have been identified as regulators of labyrinth morphogenesis. Hgf and c-Met knockout (KO) embryos exhibit IUGR and smaller placenta and die by E14.5 (Bladt et al., 1995; Schmidt et al., 1995; Uehara et al., 1995). c-Met signaling governs various morphogenetic events in development, tissue repair and cancer metastasis by regulating cell growth and motility and stem/progenitor cells in multiple tissues express c-Met (Boccaccio and Comoglio, 2006). Nevertheless, little is known about the cellular and molecular mechanisms of how c-Met signaling governs placental development, and the extent to which placental dysfunction underlies the defective development of c-Met deficient embryos.
Here we identify an Hgf/c-Met signaling dependent Epcamhi multipotent labyrinth progenitor that gives rise to all labyrinth trophoblast subtypes. Trophoblast-specific loss of c-Met abrogated the proliferation of LaTP and terminal differentiation and polarization of syncytiotrophoblasts, compromising both placental and fetal development. These discoveries advance our understanding of placental stem/progenitor cell hierarchy and provide an in vivo model for studying how dysfunctional placental exchange compromises pregnancy.
Results
Epcam marks proliferative, undifferentiated trophoblasts in placental labyrinth
To identify candidate trophoblast progenitor cells in placental labyrinth, proliferative cells were labeled with bromodeoxyuridine (BrdU) 1 h before dissection, and immunofluorescence (IF) was performed for BrdU and CD9 or Epcam (Trop1) (Figure 1B; Figure S1A). CD9, a trophoblast marker upregulated at E10.5 during labyrinth morphogenesis (Wynne et al., 2006), was expressed broadly in syncytiotrophoblasts (SynT) in E12.5 labyrinth (Figure 1B, Figure S1A). In contrast, Epcam, a marker of many epithelial stem cells (McQualter et al., 2010), was expressed highly in a subset of labyrinth trophoblasts, while SynT showed low expression (Figure 1B, Figure S1A). Moreover, Epcamhi cells were arranged in clusters and showed much higher frequency of BrdU incorporation than CD9+ cells (Figure 1B). To assess whether Epcamhi cells are undifferentiated trophoblasts, sections were co-stained for monocarboxylate transporter (Mct) 4, which is expressed in the basal plasma membrane of SynT-II (Nagai et al., 2010) adjacent to fetal vasculature. Mct4 was undetectable by IF at E10.5 (data not shown), whereas by E12.5, Mct4 co-localized with Epcam in SynT-II (Epcamlow, Figure 1C). In contrast, Epcamhi cells were devoid of Mct4 expression (Figure 1C). At E9.5, Epcamhi cells resided at the site of chorioallantoic fusion (Figure 1D), and by E12.5, they formed clusters adjacent to laminin+ stromal cells in the labyrinth. No Epcamhi cells were detectable after completion of labyrinth morphogenesis at E14.5 (Figure 1D). These data nominated Epcamhi cells as candidate progenitor cells in placental labyrinth.
To define the identity of Epcamhi cells, magnetic bead selection was used to isolate them from E10.5 placenta (Figure 1E; Figure S1B). FACS analysis confirmed the enrichment of Epcamhi cells without significant contribution of Epcamlow CD9hi SynT (Figure S1B). QRT-PCR indicated that the expression of genes required for labyrinth trophoblast development, including transcription factors Gcm1, Dlx3 and Ovol2, (Morasso et al., 1999; Anson-Cartwright et al., 2000; Unezaki et al., 2007) and orphan nuclear factor Nr6a1 (Morasso et al., 1999), was enriched in Epcamhi fraction in E10.5 placenta as compared to Epcam− cells or TS cells (Figure 1F). Low expression of sTGC markers (Ctsq and Prl3b) was detected in both Epcamhi and Epcam− cells, but not in TS cells, whereas TGC (Prl3d1) and Sp (Tpbpa) markers were absent from both Epcamhi and TS cells (Figure 1F). These data suggested that Epcamhi cells are primed for differentiation to labyrinth trophoblasts.
Epcamhi cells represent multipotent labyrinth trophoblast progenitors
To define the ability of Epcamhi cells to differentiate into labyrinth trophoblasts, MACS sorted Epcamhi cells were cultured on OP9 stroma. OP9 cells highly express Vcam1, a ligand for integrin α4 (Itga4), which is expressed both in Epcamhi cells (Figure 2A) and on the basal surface of the chorion at the time of chorio-allantoic fusion (Stecca et al., 2002). The differentiation potential of Epcamhi cells was compared to TS cells, which up-regulate markers for the SynT, Sp and TGC lineages after removal of Fgf4 (Tanaka et al., 1998). After 7 days culture on OP9, Epcamhi cells maintained the expression of SynT transcription factors Dlx3 and Ovol2 and up-regulated the mature SynT markers Mct1 (SynT-I) and Mct4 (SynT-II) (Nagai et al., 2010) (Figure 2B) to greater levels than differentiated TS cells, confirming the ability of Epcamhi cells to efficiently generate SynT. Moreover, after 7 days in culture, E-cadherin+ (Cdh1) cells with multiple nuclei (>20) were observed (Figure 2C), suggesting that SynT derived from Epcamhi cells can form syncytia in vitro. Co-staining with CD9 confirmed that the multinucleated cells were trophoblasts (Figure 2D). Interestingly, expression of sTGC markers Ctsq and Prl3b1 also increased by 7 days of culture suggesting that Epcamhi cells also differentiate into labyrinth sTGC in vitro (Figure 2B). In contrast, cultured Epcamhi cells evidenced minimal expression of TGC and Sp markers, implying that their differentiation potential is restricted to labyrinth trophoblasts. These data nominated Epcamhi cells as labyrinth trophoblast progenitor cells (LaTP) that can give rise to SynT-I, II and sTGC.
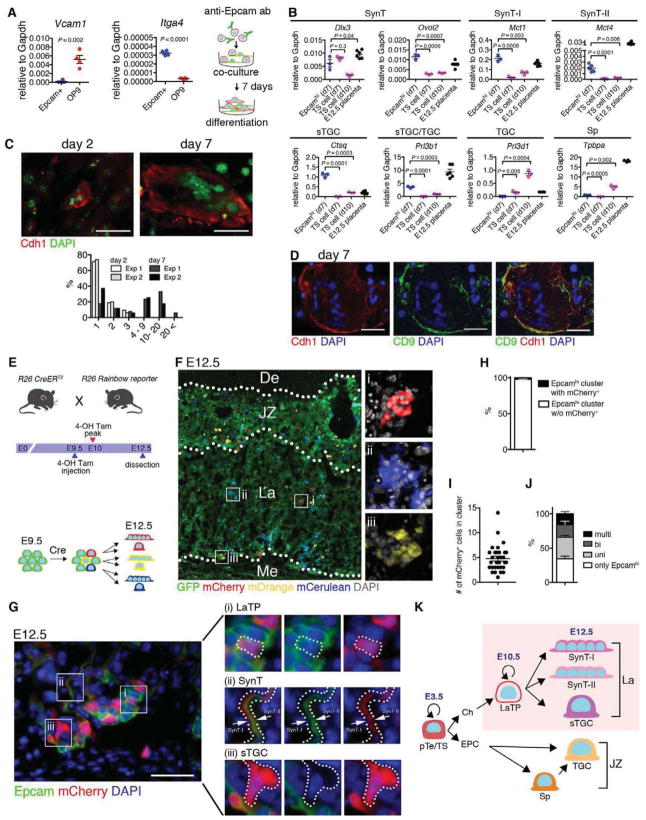
Figure 2. Epcamhi cells represent multipotent labyrinth trophoblast progenitors.
(A) Schematic for analysis of the developmental potential of Epcamhi cells in vitro. Epcamhi cells from E10.5 placenta were enriched using magnetic beads and co-cultured with OP9 for 7 days. QRT-PCR documents the expression of Itga4 in Epcamhi cells and Vcam1 in OP9.
(B) QRT-PCR showing the maintenance of SynT specific genes and up-regulation of markers for differentiated SynT-I and -II and sTGC in Epcamhi cultures after 7 days.
(C) Documentation of increased frequency of Cdh1+ SynT with multiple nuclei after 7 days in culture.
(D) Documentation that Cdh1+ multinucleated cells co-express trophoblast marker CD9.
(E) Schematic for in vivo clonality analysis. Rosa26 (R26) Rainbow reporter mice were mated with R26 CreERT2 mice. At E9.5, Cre-mediated gene deletion was induced by injection with 4OH-tamoxifen, and embryos were dissected at E12.5.
(F) Fluorescence image indicating that Cre-mediated gene recombination induces multi-color labeling and establishment of clones of labeled cells in the labyrinth. JZ, junctional zone. La, labyrinth. Me, mesenchyme.
(G) Documentation of multi-lineage differentiation in a cluster of mCherry+ labeled trophoblasts. (i) Epcamhi (LaTP), (ii) SynT (Epcamlow/neg) and (iii) sTGC (Epcamneg with large nuclei).
(H) Analysis of the frequency of clusters containing mCherry-labeled Epcamhi cells documenting infrequent labeling of clusters with same fluorescent color.
(I) Average number of mCherry+ cells in a labeled cluster documenting the establishment of multicellular, labeled clones.
(J) Differentiation potential of mCherry-labeled cells documenting the presence of clones with Epcamhi LaTP and all labyrinth trophoblast subtypes.
(K) Schematic representing the hierarchy of trophoblast-lineage differentiation. LaTP gives rise to all types of labyrinth trophoblasts, but not Sp or TGC.
All error bars indicate SEM (Standard error of mean).
See also Figure S2.
We next asked if Epcamhi cells are clonally linked to SynT and sTGC also in vivo. Tamoxifen-inducible Rosa26-CreERT2 mice were crossed with Rosa26-Rainbow reporter strain (Rinkevich et al., 2011), in which all cells express GFP until Cre-mediated recombination induces one of the three other fluorescent proteins (mCherry, mOrange and mCerulean) (Figure 2E and F). 4-OH tamoxifen was injected at E9.5 when labyrinth morphogenesis begins, and placentas were analyzed at E12.5 when Epcamhi cells and differentiated SynT and sTGC are present. To facilitate clonal analysis, the dose of 4-OH tamoxifen was titrated to induce rare recombination in trophoblasts. While individual cells marked with mCherry, mOrange or mCerulean were found in all parts of the fetal placenta, the labyrinth contained several clusters of cells marked with the same color (Figure 2F). To investigate differentiation potential of cells in individual clones, mCherry labeled clusters were chosen for further analysis (Figure 2G). Of all Epcamhi cell clusters, 2.2% (+/− 0.4) harbored mCherry+ Epcamhi cells (Figure 2H), with each cluster containing on average 4.8 (+/− 2.6) mCherry labeled trophoblasts (Figure 2I). Quantitative analysis indicated that 15.8% (+/− 4.1) of mCherry labeled clusters were multipotent and contained labeled clones with Epcamhi LaTP, Epcamlow/neg SynT and Epcamneg sTGC (Figure 2G and J). Comparable results of clonal association of Epcamhi LaTP with SynT and sTGC were obtained using Rosa26-YFP reporter mice (Figure S2A–F). Co-staining for Epcam, cytokeratin and Mct4 further confirmed labeling of all labyrinth trophoblast subtypes within YFP+ labeled Epcamhi LaTP clusters (Figure S2G). Together, these data suggest that Epcamhi cells are labyrinth trophoblast progenitors that generate all labyrinth trophoblast subtypes in vivo (Figure 2K).
c-Met signaling directly regulates labyrinth trophoblast development
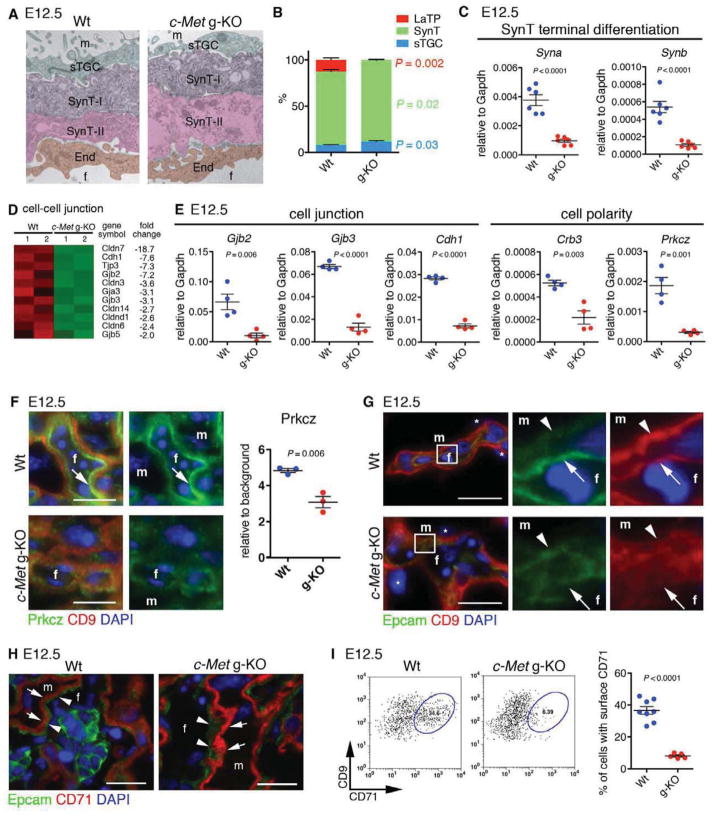
To identify regulators for LaTP, we sought for mouse models with labyrinth defects; the hepatocyte growth factor (Hgf) receptor c-Met was identified as a candidate. We first analyzed the placental defect in c-Met knockout embryos generated by deleting the conditionally targeted c-Met locus (Huh et al., 2004) in the germline (g-KO). Lethality of c-Met g-KO embryos occurred by E14.5, as reported for Hgf and c-Met KO embryos (Bladt et al., 1995; Schmidt et al., 1995; Uehara et al., 1995). No macroscopic defects were observed in placentas or embryos until E12.5, when c-Met g-KO placentas and embryos exhibited decreased size and hypocellular fetal liver (FL) (Figure S3A–D). c-Met g-KO placentas had thinner labyrinth (La) while the JZ composed of Sp and TGC was unaffected (Figure 3A), confirming that placental defects were limited to the labyrinth.
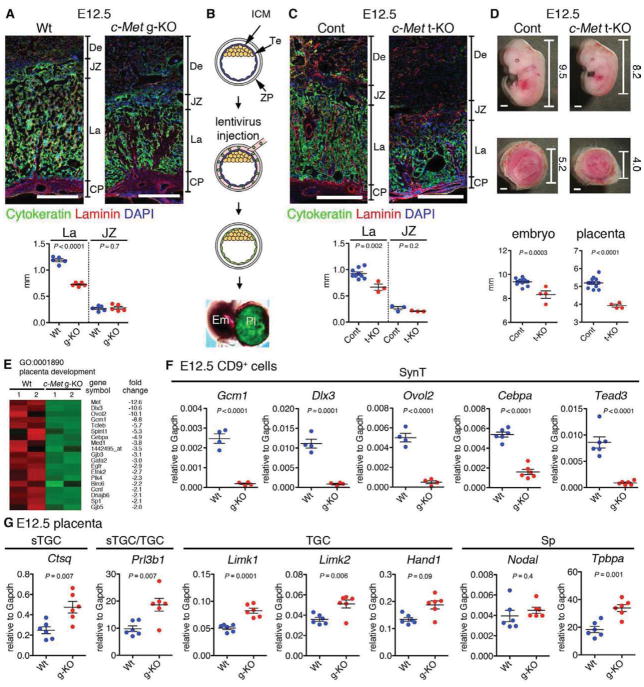
Figure 3. Loss of c-Met signaling in trophoblasts induces placental labyrinth hypoplasia and IUGR.
(A) IF for Cytokeratin (green, trophoblast), Laminin (red, mesenchymal cell), and DAPI (blue, nuclei) on Wt and c-Met germline KO (g-KO) placenta at E12.5 showing labyrinth (La)-specific hypoplasia in c-Met g-KO placenta. Scale bar 500 μm.
(B) Generation of trophoblast-specific c-Met KO (t-KO) embryos. Injection of Cre-GFP lentivirus under the zona pellucida (ZP) induces Te-specific gene deletion. ICM, inner cell mass; Te, trophectoderm.
(C) Trophoblast–specific c-Met gene deletion induces placental labyrinth (La) hypoplasia.
(D) Representative images of c-Metfl/fl and c-Met trophoblast-specific KO (t-KO) embryos and placentas. Numbers indicate size (mm) of each embryo and placenta. Scale bar 1 mm.
(E) Heatmap representing differential expression of genes related to placental development (>2.0 fold, p<0.05) in Wt vs. c-Met g-KO CD9+ trophoblasts.
(F) QRT-PCR for SynT specific genes on E12.5 Wt and c-Met g-KO CD9+ trophoblasts. The mean of biological replicates normalized to Gapdh is shown.
(G) QRT-PCR for sTGC, TGC and Sp-specific genes in E12.5 placenta.
All error bars indicate SEM (Standard error of mean).
To assess whether loss of Hgf/c-Met signaling in placental trophoblasts alone is sufficient to cause the defects in the placenta and the fetus, we generated trophoblast-specific c-Met KO (t-KO) embryos by deleting the c-Met gene in trophoblasts using a lentiviral Cre (Figure 3B) (Chhabra et al., 2012). UbiC-Cre-GFP lentiviral vector was injected under the zona pellucida (ZP) of c-Metfl/fl blastocysts, while c-Metfl/+ blastocysts and untransduced c-Metfl/fl blastocysts served as controls. Strikingly, trophoblast-specific c-Met deletion mimicked the phenotype of c-Met g-KO mutants, resulting in reduced placental labyrinth size and poorly developed branching structure (Figure S3E), IUGR of the embryo (Figure 3C and D) and lethality by E14.5 (data not shown). Moreover, loss of c-Met in placental trophoblasts alone was sufficient to cause hypocellularity of the FL (Figure S3F). Importantly, transduction of c-Metfl/+ blastocysts did not show any phenotype in the embryo or the placenta (data not shown). These findings indicate that trophoblast-specific loss of c-Met causes both labyrinth and FL hypoplasia and IUGR of the embryo.
To examine how c-Met regulates labyrinth development we conducted Affymetrix microarray analysis on wild type (Wt) and c-Met g-KO CD9+ labyrinth trophoblasts. 1,294 genes were identified as downregulated and 1,221 genes as upregulated in c-Met deficient trophoblasts (>2-fold, p-value<0.05) (Tables S1 and 2, Figure S3G). The GO category “placenta development” included genes regulating labyrinth development (Figure 3E; Figure S3G). QRT-PCR confirmed reduced expression of SynT genes (Gcm1, Dlx3, Ovol2, Cebpa, and Tead3) (Natale et al., 2006) in c-Met g-KO labyrinth trophoblasts (Figure 3F). QRT-PCR of total E12.5 placentas showed that there was no reduction in labyrinth sTGC- (Ctsq and Prl3b1), TGC- (Prl3b1, Limk1, Limk2, and Hand1) or Sp-specific genes (Tpbpa and Nodal) (Figure 3G) (Watson and Cross, 2005; Natale et al., 2006). These data further indicated that c-Met signaling regulates specifically labyrinth trophoblast development.
c-Met sustains proliferation of LaTP in midgestation placenta
The selective suppression of SynT genes and the morphological defects in placental labyrinth raised the hypothesis that c-Met signaling regulates the emergence, maintenance and/or differentiation of LaTP. QRT-PCR documented the expression of c-Met in both Epcamhi LaTP and CD9hi Epcamlow/neg SynT, whereas the mature SynT marker Mct4 was expressed at higher level in SynT (Figure 4A). The frequency of Epcamhi cells in c-Met g-KO placenta was comparable to Wt at E9.5, but dramatically decreased from E10.5 onward (Figure 4B). These data suggested that c-Met signaling is not required for specification of LaTP, but rather for their maintenance through midgestation.
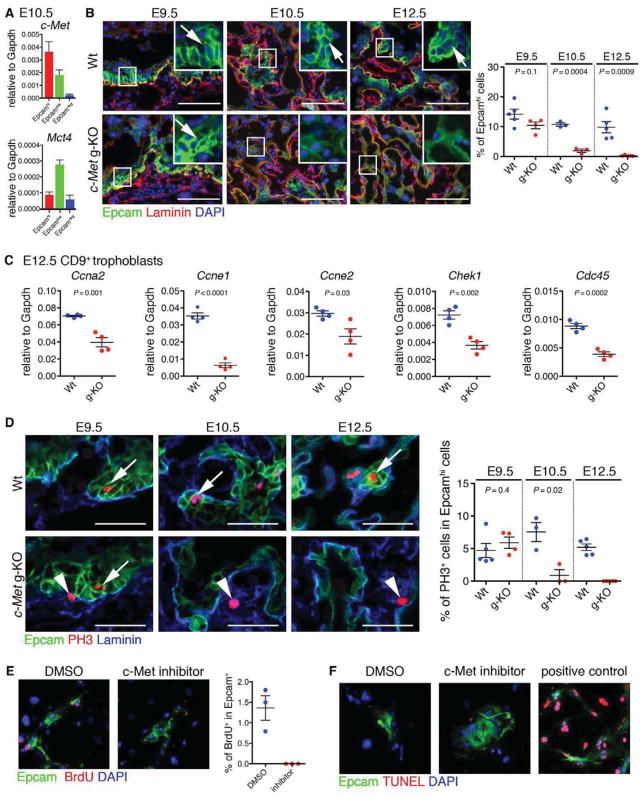
Figure 4. c-Met signaling regulates the maintenance of LaTP.
(A) QRT-PCR documenting the expression of c-Met in both Epcamhi LaTP and Epcamlow SynT.
(B) IF for Epcam (green) and Laminin (red) on Wt and c-Met g-KO placenta at E9.5, 10.5 and 12.5 documenting loss of Epcamhi cells in c-Met deficient placenta after E9.5. Arrows, Epcamhi cells adjacent to Laminin+ mesenchymal cells. Scale bar 100 μm.
(C) QRT-PCR analysis of gene expression in CD9+ trophoblast from Wt and c-Met g-KO placentas verifying down-regulation of cell cycle regulators.
(D) IF for Epcam (green), phospho Histone-H3 (PH3, red) and Laminin (blue) on Wt and c-Met g-KO placenta at E9.5, 10.5 and 12.5 documenting premature loss of proliferative LaTP in c-Met deficient placenta. Arrows, PH3+ Epcamhi cells. Arrowheads, PH3+ Epcam− cells. Scale bar 50 μm.
(E) Treatment of cultured LaTP with c-Met inhibitor documents reduced BrdU incorporation in Epcam+ cells. Epcam (green), BrdU (red) DAPI (blue).
(F) Treatment of cultured LaTP with c-Met inhibitor documenting no difference in cell death of Epcam+ cells. Epcam (green), TUNEL (red) DAPI (blue). Positive control, cells treated with DNase I.
All error bars indicate SEM (Standard error of mean).
See also Figure S4.
The most highly enriched GO categories among genes downregulated in c-Met deficient placentas were related to “cell cycle” (Figure S3G; Figure S4A). QRT-PCR confirmed downregulation of several genes required for cell division, including Ccna2, Ccne1, Ccne2, Chek1, and Cdc45 (Figure 4C). BrdU incorporation assay verified significant reduction in actively proliferating Cytokeratin+ trophoblasts in both c-Met g-KO and t-KO placentas at E12.5 (Figure S4B) whereas no difference in DNA fragmentation was observed (Figure S4C), suggesting that defective proliferation rather than apoptosis underlies labyrinth hypoplasia in c-Met deficient placentas. Co-staining for phospho-Histone H3 (PH3), a marker of mitosis, and Epcam showed no significant difference in mitotic activity between Wt and c-Met g-KO Epcamhi LaTP at E9.5, whereas their proliferation was dramatically decreased E10.5 onward (Figure 4D). These data implied that c-Met signaling is required to sustain the proliferation of LaTP in midgestation.
To verify that reduced proliferation of c-Met deficient LaTP is not caused by indirect effects from other tissues, c-Met signaling was blocked in LaTP culture using c-Met inhibitor PHA-665752 (Christensen et al., 2003). Inhibition of c-Met signaling impaired the proliferation of Epcam+ cells in culture without increasing apoptosis (Figure 4E and F), demonstrating a cell autonomous role for c-Met in sustaining the proliferation of LaTP and/or their progeny.
c-Met signaling is essential for the establishment of labyrinth exchange interface
As the expression analysis of c-Met deficient placentas had indicated a dramatic reduction of labyrinth trophoblast specific transcription factors, we investigated whether c-Met signaling is required for the differentiation of LaTP into SynT and/or sTGC. Electron microscopy indicated that the trilaminar trophoblast structure consisting of SynT-II, SynT-I and sTGC was established in c-Met g-KO placenta (Figure 5A). Co-staining for Mct4 (SynT-II) and CD9 (both SynT subtypes) confirmed the presence of both SynT-I and -II in c-Met g-KO placentas (Figure S5A). Quantification of the relative frequencies of labyrinth trophoblasts in c-Met g-KO placentas at E12.5 revealed a slight decrease of SynT and increase of sTGC, while Epcamhi cells were nearly undetectable (Figure 5B). Nevertheless, despite the presence of both SynT-I and SynT-II in c-Met g-KO placentas, the expression of Syncytin-a (Syna) and –b (Synb) that mark terminally differentiated SynT-I and SynT-II, respectively, was dramatically reduced, suggesting a defect in terminal differentiation (Figure 5C). Microarray analysis and QRT-PCR revealed reduced expression of genes encoding tight junction proteins Cldn3, 6, 7, and 14, and Tjp3, adherence junction proteins (Cdh1 (E-cadherin)), and gap junction proteins Gjb2 (Cx26) and Gjb3 (Cx31), which are highly expressed in differentiated SynT and critical for labyrinth development (Gabriel et al., 1998; Plum et al., 2001) (Figure 5D and E).
Figure 5. c-Met signaling is dispensable for SynT and sTGC specification but essential for SynT terminal differentiation and cell polarity.
(A) Electron microscopy showing tri-laminar structure of labyrinth trophoblasts (SynT-I, -II and sTGC) in Wt and c-Met g-KO placentas.
(B) Quantitative analysis for trophoblast subtypes.
(C) QRT-PCR showing decreased expression of Syna and Synb in E12.5 c-Met KO CD9+ trophoblasts.
(D) Heatmap of cell-cell junction genes differentially expressed between Wt and c-Met g-KO CD9+ trophoblasts.
(E) QRT-PCR showing reduced expression of cell-cell junction and cell polarity genes in c-Met g-KO trophoblasts.
(F) IF for Prkcz (green) and CD9 (red) showing polarized localization of Prkcz protein in fetal side of SynT-II in Wt placenta (arrow), and diffuse and decreased expression of Prkcz in c-Met g-KO placenta. DAPI (blue, nuclei)
(G) IF for Epcam (green), CD9 (red), and DAPI (blue) on Wt and c-Met g-KO placenta at E12.5. Arrows indicate fetal side of SynT-II and arrowheads indicate apical membrane of SynT-I. m, maternal blood space; f, fetal vascular lumen.
(H) IF for Epcam (green), CD71 (red), and DAPI (blue) on placental section at E12.5. Expression of cytoplasmic CD71 in SynT-I (arrowheads) is observed in both Wt and c-Met g-KO placenta.
(I) FACS analysis indicating reduced surface expression of CD71 on SynT in c-Met g-KO placentas.
All error bars indicate SEM (Standard error of mean).
See also Figure S5.
Epithelial cells exhibit apical-basolateral polarity that is essential for organ development and function (Martin-Belmonte and Perez-Moreno, 2012). Closer examination of CD9 staining pattern in c-Met deficient trophblasts revealed that although CD9 was localized on both the fetal side of SynT-II and maternal (apical) side of SynT-I in Wt placentas (Figure S5A), it was diffusely localized in c-Met g-KO SynT, suggesting that c-Met signaling regulates SynT cell polarity. Microarray analysis and QRT-PCR revealed downregulation of polarity genes Crb3 and aPKCζ (Prkcz) in c-Met g-KO trophoblasts (Figure 5E). No statistically significant reduction was observed by QRT-PCR for Pard3 and Pard6b (data not shown), which form a complex with Prkcz to establish cell polarity in many cell types (Martin-Belmonte and Perez-Moreno, 2012). Quantitative IF analysis documented reduction of Prkcz protein in c-Met KO placenta (Figure 5F). Moreover, while Prkcz was expressed at the fetal side of SynT-II in Wt placenta (Figure 5F), a more diffuse expression of the residual Prkcz protein was observed in c-Met g-KO SynT-II. Likewise, although Epcam expression in SynT in Wt placenta was confined to the membrane of the fetal (basal) side of SynT-II (Figure 5G), in c-Met g-KO placenta, Epcam was diffusely expressed. In the yolk sac, Epcam was distributed on the basolateral membrane and CD9 was abundantly expressed at the apical surface in both Wt and c-Met g-KO (Figure S5B), indicating that cell polarity was disrupted only in the placenta. These data demonstrated a pivotal role for c-Met signaling in the establishment of apical-basolateral cell polarity during SynT differentiation.
Transplacental iron transport from the mother is critical for normal fetal development. Serum iron is bound to transferrin (Trf), and is up-taken by transferrin receptor (CD71) via endocytosis. After iron dissociation, Trf-CD71 complex is recycled to the cell surface (Grant and Donaldson, 2009). CD71 is necessary for fetal development (Levy et al., 1999), and the endocytic recycling of CD71 is tightly regulated by cell-polarity machinery (Golachowska et al., 2010). IF and FACS analysis showed that in Wt placenta, CD71 is expressed in both the cytoplasm and cell surface of SynT-I (Figure 5H and I). However, although IF evidenced abundant cytoplasmic expression of CD71 in c-Met deficient SynT-I (Figure 5H), FACS analysis revealed drastic reduction of surface CD71 (Figure 5I). These data suggested that impaired cell polarity in c-Met KO placenta causes abnormal subcellular distribution of CD71 and other proteins required for fetal-maternal transport.
c-Met signaling is required for the maintenance of Gcm1 expression in midgestation placenta
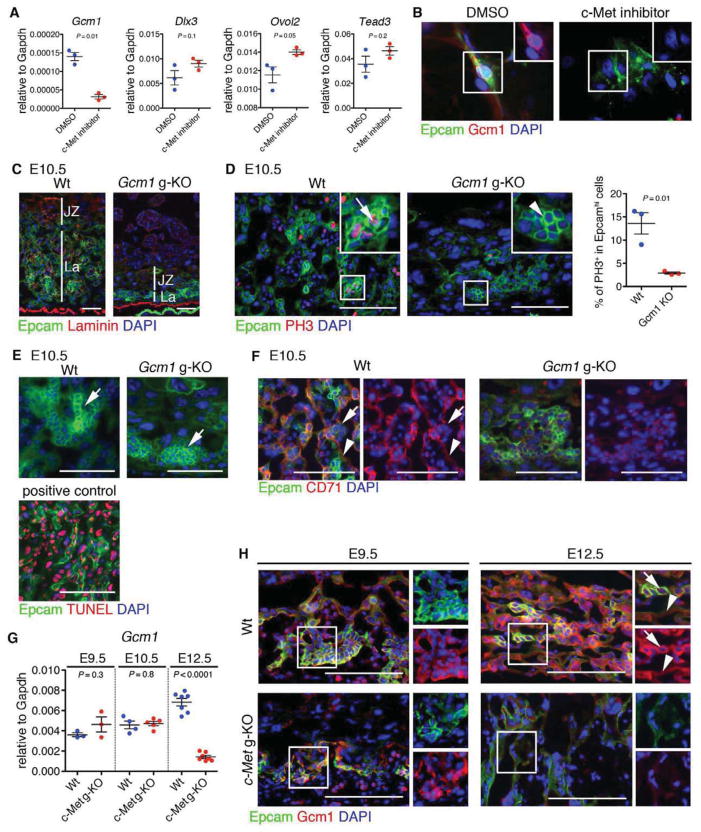
Expression analysis of c-Met deficient trophoblasts had indicated a dramatic reduction of transcription factors regulating SynT development; the analysis of trophoblast subtypes in the labyrinth indicated that both SynT-I and SynT-II were specified in the absence of c-Met, but their terminal differentiation was compromised. To investigate whether c-Met signaling in trophoblasts is directly required for the expression of transcription factors regulating labyrinth morphogenesis, cultured Epcamhi cells were treated with c-Met inhibitor. QRT-PCR revealed that blocking c-Met signaling in vitro in LaTP and their progeny resulted in a drastically reduced expression of Gcm1, but not Ovol2, Dlx3 or Tead3 (Figure 6A). IF verified the reduction of Gcm1 LaTP cultures treated with the c-Met inhibitor (Figure 6B).
Figure 6. c-Met signaling is required to maintain Gcm1 expression in midgestation placenta.
(A) QRT-PCR demonstrating loss of Gcm1 expression upon treatment of cultured LaTP with c-Met inhibitor.
(B) IF documenting loss of Gcm1 in Epcam+ cells upon treatment with c-Met inhibitor. Epcam (green), Gcm1 (red) DAPI (blue)
(C) IF documenting reduced labyrinth size in Gcm1 g-KO placenta. Scale bar 100 μm. Epcam (green), Laminin (red) DAPI (blue)
(D) Phospho-Histone H3 staining documenting reduction of mitotic Epcamhi cells (arrow) in Gcm1 KO placentas. Arrowheads, PH3 negative Epcamhi cluster. Epcam (green), PH3 (red), DAPI (blue). Scale bar 100 μm.
(E) TUNEL staining indicating apoptosis is not induced in Gcm1 g-KO placenta at E10.5. Positive control for Tunel staining is shown below. Epcam (green), TUNEL (red) DAPI (blue). Scale bar 100 μm.
(F) IF showing absence of CD71+ SynT in Gcm1 g-KO placenta. Epcam (green), CD71 (red) DAPI (blue). Scale bar 100 μm.
(G) QRT-PCR and (H) IF documenting loss of Gcm1 expression by E12.5 in c-Met g-KO placentas. Epcam (green), Gcm1 (red) DAPI (blue). Scale bar 100 μm.
All error bars indicate SEM (Standard error of mean).
See also Figure S6.
Gcm1 is a transcription factor essential for labyrinth morphogenesis and SynT differentiation (Anson-Cartwright et al., 2000)(Simmons et al., 2008). While Gcm1 is highly expressed in differentiated, post-mitotic Synt-II, Gcm1 expression has also been reported in the chorion at E8.5 and in putative precursors of the labyrinth trophoblasts at E9.5) (Basyuk et al., 1999; Hunter et al., 1999; Stecca et al., 2002). QRT-PCR analysis of FACS sorted Epcamhi LaTP and Epcamlow CD9hi SynT cells from E10.5 placenta documented Gcm1 expression in both fractions (Figure S6A). In situ hybridization evidenced the expression of Gcm1 also in Epcamhi LaTP at E10.5 (Figure S6B), whereas by E14.5, Gcm1 expression was largely confined to SynT-II (Figure S6C). IF suggested that Gcm1 is expressed in some Epcamhi LaTP that are undergoing mitosis (Figure S6D). These data imply that Gcm1 expression is not restricted to differentiated, post-mitotic SynT but begins already at the level of LaTP.
To investigate whether Gcm1 expression is required for the emergence and/or differentiation of LaTP, the expression of Epcam was assessed in Gcm1 KO placentas at E10.5, before embryonic death. As reported previously (Anson-Cartwright et al., 2000), the labyrinth layer was much thinner, and no branching morphogenesis was observed (Figure 6C). Notably, Gcm1 KO placentas harbored Epcamhi cells; however, the frequency of PH3+ mitotic Epcamhi cells was drastically reduced (Figure 6D), while no increase in apoptosis was reveled by TUNEL assay (Figure 6E). Moreover, no CD71+ differentiating SynT were found in Gcm1 KO placentas (Figure 6F). These data suggested that complete lack of Gcm1 expression compromises both the proliferation and differentiation of Epcamhi LaTP in vivo.
As the placental phenotype in Gcm1 KO embryos was much more severe than in c-Met KO embryos, we investigated the kinetics of Gcm1 expression in c-Met deficient placentas. At E9.5, there was no difference in the expression of Gcm1 between c-Met deficient and Wt placentas, whereas by E12.5, Gcm1 expression in the mutant trophoblasts was nearly undetectable both by IF and by QRT-PCR (Figure 6G and H). This data suggested that c-Met is not essential for inducing Gcm1 expression, but rather for maintaining its expression in LaTP and SynT through midgestation. Altogether, these findings identify Gcm1 as a key downstream effector of c-Met signaling, and suggest that its continued expression in LaTP and/or SynT in midgestation placenta is required for the establishment of functional placental exchange interface.
Discussion
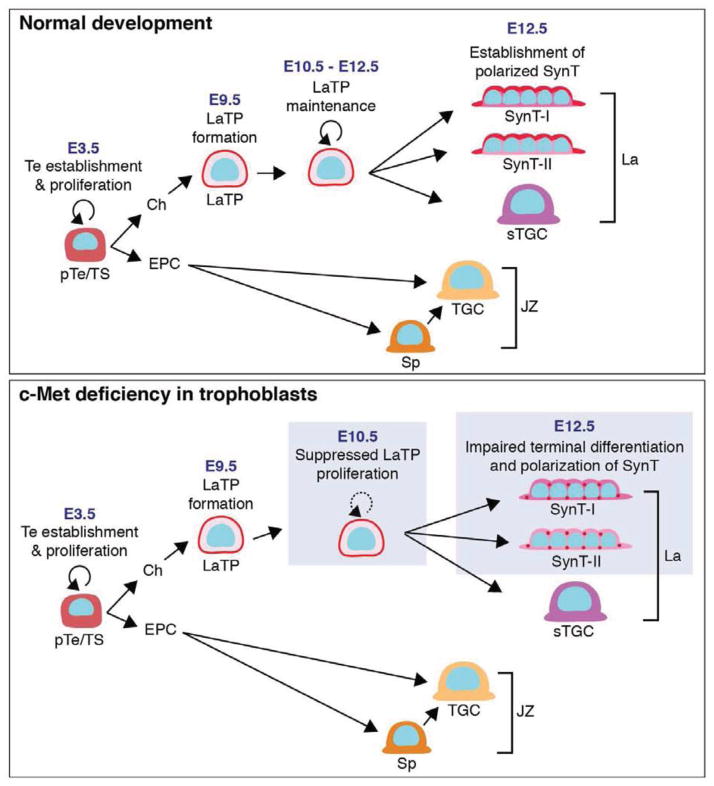
The stem/progenitor cell hierarchy of the trophoblasts responsible for placental labyrinth morphogenesis and exchange function has been unknown. We discovered that the midgestation mouse placenta harbors Epcamhi multipotent labyrinth trophoblast progenitors (LaTP) that differentiate into all labyrinth trophoblast subtypes, SynT-I, SynT-II and sTGC, in vitro and in vivo. We discovered that c-Met signaling is required for sustained proliferation of LaTP during midgestation and for terminal differentiation and polarization of SynT, which is a pre-requisite for a functional placental exchange interface and healthy fetal development (Fig. 7).
Figure 7. Model for function of Hgf/c-Met signaling in the development of placental exchange interface.
Labyrinth trophoblast progenitors (LaTP) are responsible for the generation of SynT (syncytiotrophoblasts) I and II and sTGC (sinusoidal trophoblast giant cells) that compose the placental exchange interface. While LaTP can be specified independent of c-Met signaling, c-Met is essential for sustained proliferation of LaTP. c-Met signaling is also required for the establishment of SynT cell polarity. Loss of c-Met signaling results in placental hypoplasia, compromised exchange interface and defective fetal development.
Clonal analysis using multi-color Rainbow reporter mouse (Rinkevich et al., 2011) revealed that marking individual cells in the placenta results in the generation of multicellular clusters that contain Epcamhi LaTP, SynT-I, SynT-II and sTGC. Although 15.8% of the labeled trophoblast clusters identified at E12.5 (labeled at around E9.5) were multipotent, 34.5% of all labeled the clusters contained only undifferentiated Epcamhi LaTP, indicating that they had not started to differentiate at this stage, while the remaining labeled clones were composed of Epcamhi LaTP and SynT or sTGC only. Future studies using marking at different time points will reveal whether the differentiation potential of LaTP changes during development. Thus, studies should focus on creating a Cre line that confers LaTP specific labeling to lineage trace the progeny of LaTP throughout development.
Our data show that both LaTP and SynT express c-Met, while mesenchymal cells derived from the allantois secrete Hgf (Uehara et al., 1995), suggesting paracrine signaling. Abrogation of c-Met signaling disrupted the proliferation of Epcamhi cells both in vivo and in vitro. Nevertheless, our data suggests that Hgf/c-Met signaling is necessary, but not sufficient to support LaTP proliferation and/or SynT differentiation without other niche factors; culture of Epcamhi cells in TS cell conditions or on matrigel with Hgf failed to support SynT differentiation and maintain Gcm1 expression. However, culture of Epcamhi cells on OP9 mesenchymal stroma, which both secretes Hgf and expresses Vcam1 (Malhotra and Kincade, 2009) that is essential for maintaining Gcm1 expression in placental explant cultures (Stecca et al., 2002), facilitated the maintenance/induction of SynT genes and formation of multinucleated syncytia. Cultured Epcamhi LaTP also induced genes specific for labyrinth sTGC, which have been implicated as key hematopoietic niche cells (Chhabra et al., 2012). These data imply that the Epcamhi cell culture may be used to study the trophoblast subtypes that establish the exchange interface and the hematopoietic niche in placental labyrinth. Future work will be needed to define distinct niche components to direct the differentiation of LaTP to specific labyrinth trophoblast subtypes, and to investigate whether LaTP can be sustained in undifferentiated state in culture.
Although prior studies had associated defective c-Met signaling with disrupted labyrinth development, the underlying mechanisms were unknown. Our data revealed a critical role for c-Met in the maintenance of Gcm1 expression in midgestation placenta while the initial induction of Gcm1 occurs independent of c-Met. This finding helps explain the less drastic placental defect in c-Met KO embryos than in Gcm1 KO embryos, as complete lack of Gcm1 disrupted labyrinth morphogenesis and SynT differentiation entirely, causing embryonic death by E10.5 (Anson-Cartwright et al., 2000). Analysis of Gcm1 KO placentas suggested that Epcamhi LaTP can be specified in the absence of Gcm1, but their proliferation and differentiation is disturbed. In contrast, loss of c-Met did not abolish the expression of Gcm1 or cause macroscopic defects in c-Met KO labyrinth until E12.5, when LaTP were extinguished and Gcm1 expression dropped to undetectable levels. Altogether, these data suggest that the specification of LaTP is induced by c-Met and Gcm1 independent mechanism; however, c-Met is required for SynT terminal differentiation and establishment of placental exchange interface, which may at least in part be linked to the requirement of c-Met signaling to maintain Gcm1 expression.
Previous studies have shown that Gcm1 is highly expressed in post mitotic cells in the labyrinth, and that overexpression of Gcm1 induces cell cycle arrest (Hughes et al., 2004). Our data also confirmed high expression of Gcm1 in differentiated SynT-II. In addition, the ability to distinguish undifferentiated LaTP in labyrinth by Epcamhi staining identified a small population of proliferative LaTP that also express Gcm1. As TS cells do not express Gcm1, it is plausible that expression of Gcm1 is important for LaTP commitment to the SynT lineage. To verify if Gcm1 governs the differentiation of LaTP to SynT, and/or is directly involved in maintaining LaTP proliferation, further studies deleting Gcm1 in cell type specific and temporal manner will be required.
As with mouse placenta, little is known about the stem/progenitor cell hierarchy in the human placenta. Despite similarities in molecular regulation, the mouse and human placentas are macroscopically distinct (Georgiades et al., 2002). Thus, the direct relevance of the findings of mouse LaTP to human placental progenitor biology needs to be explored separately. Recent studies documented that the chorionic membrane of the 1st trimester human placenta can serve as a source of trophoblast progenitor cell lines that highly express GCM1 and up-regulate SYNCYTIN after differentiation in vitro (Genbacev et al., 2011), raising the hypothesis that they originate from a precursor that has a parallel function in establishing placental exchange interface as the mouse LaTP.
Differentiated SynT form a “placental barrier” that tightly regulates the passage of substances between the maternal and fetal circulations (Kokkinos et al., 2010). We showed that while c-Met deficient LaTP can form all labyrinth trophoblast subtypes, c-Met is necessary for terminal differentiation into polarized SynT. Trophoblast cell lines have provided a useful tool to study cell polarity in vitro (Sivasubramaniyam et al., 2013); however, little is known about the direct functional consequences of disrupted SynT cell polarity in vivo. In addition to the low expression of key cell polarity molecules and the inability to achieve polarized localization of SynT surface proteins in c-Met KO placentas, the surface localization of transferrin receptor (CD71), required for transplacental-iron transfer, on SynT-I was impaired. Future studies are needed to define the mechanisms how the disturbed placental exchange c-Met deficient embryos causes fetal liver hypocellularity, IUGR and death of the fetus. Thus, c-Met deficient placentas not only offer a unique in vivo model to investigate how the apical-basolateral polarity and bi-directional transport in SynT is established, but may also help uncover the etiology of pregnancy complications related to placental transport function.
Reduced expression of HGF has been observed in human placentas from pre-eclampsia and IUGR pregnancies (Furugori et al., 1997; Somerset et al., 1998), and in vitro studies demonstrated that HGF activates invasion of human trophoblasts (Kauma et al., 1999; Nasu et al., 2000), providing evidence for MET signaling in placental development and disease in human. Moreover, similar to many human placenta-related disorders such as spontaneous abortion, premature delivery, IUGR and pre-eclampsia that are accompanied with placental inflammation (Young et al., 2010), c-Met deficiency in trophoblasts induced inflammation and macrophage infiltration in the placenta (unpublished data). Although the mechanisms that trigger inflammation in c-Met deficient trophoblasts are still unknown, this may be related to downregulation of cell-cell junction molecules and/or defective cell polarity in SynT that compromise fetal-maternal barrier function. Moreover, c-Met is constitutively activated in human choriocarcinoma, and HGF stimulates the proliferation of choriocarcinoma cells (Saito et al., 1995; Takayanagi T, 2000). c-Met also regulates the proliferation and migration of various other cancer cells and stem cells (Boccaccio and Comoglio, 2006). Thus, defining how Hgf/c-Met signaling governs the proliferation of stem/progenitor cells such as LaTP and the establishment of cell polarity in their differentiated progeny may have implications to understanding the pathophysiology of other common diseases.
Experimental procedures
Animals
All procedures with animals were conducted according to the guidelines of the UCLA Animal Research Committee. For LaTP culture, E10.5 pregnant mice (ICR, Taconic) were used. For the in vivo clonality experiments, Rosa26-Rainbow reporter or Rosa26-YFP reporter mice were mated with Rosa26-Cre-ERT2 mice. Pregnant females were injected with 0.5 mg 4-OH tamoxifen at E9.5, and tissues were collected at E12.5. c-Metfl/fl mice were provided by Dr. Snorri S. Thorgeirsson (NIH, Bethesda, MD, USA) (Huh et al., 2004). To generate trophoblast-specific c-Met KO embryos, lentiviral vector expressing Cre-Gfp was microinjected under the zona pellucida of the blastocyst and embryos were transplanted into pseudopregnant females, as shown earlier (Chhabra et al., 2012). For details, see Extended Experimental Procedures. Sections of Gcm1 KO placentas were provided by Dr. James C. Cross (University of Calgary, Calgary, Canada).
LaTP and TS Cell Culture
E10.5 placenta was digested with 1 mg/ml collagenase and 1 mg/ml dispase. Epcamhi cells were enriched using anti-Epcam antibody and anti-rat IgG magnetic beads. Epcamhi cells were co-cultured with Mitomycin C-treated OP9 stroma cell with TS medium (RPMI 1640 (Invitrogen) containing 20% FCS (HyClone), 2 mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, and 100 μM β-mercaptoethanol) with or without c-Met inhibitor, PHA665752 (4 μM, Sigma-Aldrich) for 7 days. TS cells were maintained as described (Tanaka et al., 1998). TS cells differentiation was induced by removing MEF conditioned medium, Fgf4 and heparin for 7 and 10 days.
Immunofluorescence and in situ hybridization
Histology, BrdU incorporation assay, electron microscopy, QRT-PCR, and immunofluorescence were performed as described (Chhabra et al., 2012). For details of the antibodies, see Extended Experimental Procedures. For visualization of vascular branching, thick placental sections (3 mm) were prepared by vibratome and 3D images were constructed using confocal microscopy. In situ hybridization was performed using digoxigenin (DIG)-labeled RNA antisense or sense probes for Gcm1 and the alkaline phosphatase reaction (Simmons et al., 2008).
Gene Expression Analysis
For microarray analysis of c-Met deficient trophoblasts, single cell suspension of E12.5 placentas was stained with PE-conjugated anti-CD9 antibody and CD9+ trophoblasts were enriched using anti-PE antibody-magnetic microbeads. Affymetrix MOE430_2.0 microarrays were performed on two independent Wt and two c-Met g-KO CD9+ trophoblasts. Details about microarray analysis and primer sequences (Table S3) are available in the Extended Experimental Procedures.
Statistical Analysis
For statistical analysis, Student’s unpaired two-tailed t-test was used for all comparisons.
Supplementary Material
Figure S1. Labyrinth trophoblasts express CD9 and Epcam. Related to Figure 1.
(A) Immunofluorescence and schematic representation of mouse placental structure. Sections from E12.5 placenta were stained with antibodies for CD9 or Epcam (green), and Cytokeratin (red, trophoblasts). DAPI (blue) indicates nuclei. The fully developed placenta is composed of three major layers: a decidual layer (De), which includes decidual cells of the uterus; a junctional zone (JZ) which attaches the fetal placenta to the decidua and contains trophoblast giant cells (TGC) and spongiotrophoblasts (Sp); and a labyrinth layer (La), composed of fetal endothelial cells and labyrinth trophoblasts (SynT-I and -II, and sinusoidal trophoblast giant cells (sTGC)) that establish the substance exchange interface between fetal and maternal circulations. CP, chorionic plate.
(B) FACS analysis of cells enriched by anti-Epcam antibody documents effective isolation of Epcamhi cells without significant contamination of differentiated Epcamlow cells.
Figure S2. Epcamhi LaTP cells are clonally linked to terminally differentiated SynT. Related to Figure 2.
(A) Schematic for in vivo clonality experiment. Rosa26 (R26) YFP reporter mice were mated with R26 CreERT2 mice. At E9.5 Cre-mediated gene deletion was induced by injection with 4OH-tamoxifen and embryos were dissected at E12.5.
(B) Frequency of Cre-mediated gene-recombination in different cell types in the placenta documenting rare marking of trophoblasts. (C) Frequency of Epcamhi clusters containing YFP-labeled cells.
(D) Average number of YFP+ cells in a labeled Epcamhi cell cluster.
(E) Differentiation potential of YFP labeled cells in individual clusters. Multi, Epcamhi LaTP with SynT-I, SynT-II and sTGC; bi, Epcamhi with SynT-I and SynT-II; uni, Epcamhi with sTGC, SynT-I or SynT-II.
(F) Documentation of multi-lineage differentiation in a cluster of YFP+ labeled trophoblasts. (i) Epcamhi Cytokeratinlow (LaTP), (ii) SynT-I (Epcam− Cytokeratin+) and SynT-II (Epcamlow Cytokeratin+) and sTGC (iii) (Epcam− Cytokeratin+), *, SynT layer.
(G) Documentation of terminally differentiated SynT cells (Epcamlow Mct4+) in the same YFP+ marked clusters as Epcamhi LaTP. Epcam (red), YFP (green), DAPI (blue), Mct4 (purple). Scale bar 100 μm.
All error bars indicate SEM (Standard error of mean).
Figure S3. c-Met regulates both placental and embryonic development. Related to Figure 3.
(A, B, C and D) Kinetic analysis documenting growth retardation of c-Met deficient embryos, fetal livers and placentas after E12.5. Scale bar 1 mm.
(E) IF for trophoblast specific marker (Cytokeratin) and ECM marker that marks blood vessels (Laminin) documenting poorly developed branching structure in c-Met g-and t-KO placenta.
(F) c-Met t-KO fetal livers show smaller size and low cellularity.
(G) Selected GO (gene ontology) categories representing genes down- or up-regulated in placental CD9+ cells in the absence of c-Met signaling.
All error bars indicate SEM (Standard error of mean).
Figure S4. c-Met directly regulates trophoblast proliferation. Related to Figure 4.
(A) Microarray analysis of CD9+ trophoblasts from Wt vs. c-Met g-KO placenta. The heat map presents expression of various genes that related to GO category “cell cycle phase” that are suppressed in CD9+ trophoblasts of c-Met g-KO placenta.
(B) BrdU incorporation assay. Sections from E12.5 Wt, c-Met g-KO and t-KO placentas were stained for Cytokeratin (green), BrdU (red), and DAPI (blue). Although BrdU+ Cytokeratin+ trophoblasts (arrows) were observed in Wt placenta, only Cytokeratin− cells (arrowheads) showed mitotic activity in c-Met g- and t-KO placenta. Scale bar 100 μm.
(C) Sections from Wt and c-Met g-KO placentas were stained for TUNEL (red), Cytokeratin (green) and DAPI (blue). Arrows indicate TUNEL and Cytokeratin double-positive cells. No significant difference was observed in apoptosis between Wt and c-Met g-KO trophoblasts. Scale bar 100 μm.
All error bars indicate SEM (Standard error of mean).
Figure S5. Loss of c-Met compromises cell polarity specifically in the placenta. Related to Figure 5.
(A) IF for Mct4 (green), CD9 (red) and DAPI (blue) in Wt and c-Met g-KO placentas at E12.5. Presence of both Mct4− CD9+ cells and Mct4+ CD9+ cells indicates that both SynT-I and II are present in Wt and c-Met g-KO placentas. Notably, in Wt placenta, Mct4 is specifically expressed on the fetal side in SynT-II and CD9 is expressed on both apical side of SynT-I and fetal side of SynT-II. However diffused localization of both CD9 and Mct4 was observed in c-Met g-KO placenta. Arrows indicate fetal side of SynT-II and arrowheads indicate apical membrane of SynT-I. f, fetal side. m, maternal side. Scale bar 25 μm.
(B) IF for Epcam (green), CD9 (red), and DAPI (blue) on Wt and c-Met g-KO yolk sac. No abnormal localization of Epcam or CD9 and was observed in c-Met g-KO yolk sac. Arrows indicate basolateral localization of Epcam and arrowheads indicate apical staining of CD9; a, apical; b, basal. Scale bar 25 μm.
Figure S6. Gcm1 is expressed in LaTP and SynT in the placenta. Related to Figure 6.
(A) QRT-PCR documenting the enriched expression of Gcm1 in both Epcamhi LaTP and Epcamlow SynT in E 10.5 placenta. Error bars indicate SEM (Standard error of mean)
(B) IHC with anti-Epcam antibody and in situ hybridization (ISH) with Gcm1 antisense probe indicates high expression of Gcm1 in Epcamhi LaTP (arrows) in E10.5 placenta. Scale bar 100 μm.
(C) ISH analysis showing high expression of Gcm1 in SynT-II layer (arrow in the inset) adjacent to maternal blood spaces in E14.5 placenta. Scale bar 100 μm.
(D) Sections from Wt and c-Met g-KO placentas at E12.5 were stained for Epcam, Gcm1, PH3, and DAPI. Arrows indicate Epcamhi and PH3+ cells that also express Gcm1. Scale bar 100 μm.
Related to Figure 3.
Related to Figure 3.
Highlights.
Epcamhi cells are multipotent labyrinth trophoblast progenitor (LaTP) cells
c-Met signaling sustains proliferation of LaTP in midgestation placenta
c-Met regulates terminal differentiation and polarization of syncytiotrophoblasts
Loss of c-Met in trophoblasts compromises fetal growth and development
Ueno et al. identify a multipotent placental labyrinth trophoblast progenitor (LaTP) that generates all trophoblast subtypes that form the fetal-maternal exchange interface. Loss of c-Met signaling in trophoblasts disrupts LaTP maintenance and terminal differentiation to polarized syncytiotrophoblasts, compromising fetal growth and hematopoiesis.
Acknowledgments
We thank Yanling Wang and Hirohito Shimizu for technical assistance, UCLA Vector Core for preparation of Cre-Gfp lentiviral vector, Marianne Cilluffo and Sirus A. Kohan for electron microscopy and UCLA Clinical Microarray Core for microarray analysis. We thank Dr. James Cross at University of Calgary for Gcm1 KO tissues. This work was supported by RO1 HL097766 for H.K.A.M and Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA. M.U. was supported by Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad, L.L. by American Association of Obstetricians and Gynecologists Foundation Scholarship and Fellowship from CIRM. A.C. was supported by JCCF at UCLA. B.V.H. was supported by the Ruth L. Kirschstein National Research Service Award NIH/NHLBI T32 HL69766.
Footnotes
Accession Number
Microarray data were deposited in the Gene Expression Omnibus database (accession number GSE38342).
The authors declare no competing financial interests.
M.U. conceived the hypothesis, designed and performed the experiments and analyzed the data. L.K.L., A.C., Y.J.K., Y.W., B.V.H., M.K., P.K., K-I.S. R.A. and M.J. carried out experiments, and R.S. carried out bioinformatics analyses. H.K.A.M. conceived the hypothesis and directed the project. M.U. and H.K.A.M. prepared the manuscript, which all authors edited and approved.
Supplemental Information including Extended Experimental Procedures, 6 Figures and 3 Tables can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anson-Cartwright L, Dawson K, Holmyard D, Fisher SJ, Lazzarini Ra, Cross JC. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat Genet. 2000;25:311–314. doi: 10.1038/77076. [DOI] [PubMed] [Google Scholar]
- Basyuk E, Cross JC, Corbin J, Nakayama H, Hunter P, Nait-Oumesmar B, Lazzarini RA. Murine Gcm1 gene is expressed in a subset of placental trophoblast cells. Dev Dyn. 1999;214:303–311. doi: 10.1002/(SICI)1097-0177(199904)214:4<303::AID-AJA3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. doi: 10.1038/nrc1912. [DOI] [PubMed] [Google Scholar]
- Chhabra A, Lechner AJ, Ueno M, Acharya A, Van Handel B, Wang Y, Iruela-Arispe ML, Tallquist MD, Mikkola HKA. Trophoblasts Regulate the Placental Hematopoietic Niche through PDGF-B Signaling. Dev Cell. 2012;22:651–659. doi: 10.1016/j.devcel.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen JG, Schreck R, Burrows J, Kuruganti P, Chan E, Le P, Chen J, Wang X, Ruslim L, Blake R, et al. A selective small molecule inhibitor of c-Met kinase inhibits c-Met-dependent phenotypes in vitro and exhibits cytoreductive antitumor activity in vivo. Cancer Res. 2003;63:7345–7355. [PubMed] [Google Scholar]
- Furugori K, Kurauchi O, Itakura A, Kanou Y, Murata Y, Mizutani S, Seo H, Tomoda Y, Nakamura T. Levels of hepatocyte growth factor and its messenger ribonucleic acid in uncomplicated pregnancies and those complicated by preeclampsia. J Clin Endocrinol Metab. 1997;82:2726–2730. doi: 10.1210/jcem.82.8.4176. [DOI] [PubMed] [Google Scholar]
- Gabriel HD, Jung D, Bützler C, Temme A, Traub O, Winterhager E, Willecke K. Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol. 1998;140:1453–1461. doi: 10.1083/jcb.140.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HKA. The placenta is a niche for hematopoietic stem cells. Dev Cell. 2005;8:365–375. doi: 10.1016/j.devcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Donne M, Kapidzic M, Gormley M, Lamb J, Gilmore J, Larocque N, Goldfien G, Zdravkovic T, McMaster MT, et al. Establishment of human trophoblast progenitor cell lines from the chorion (2011) Stem Cells. 2011;29:1427–1436. doi: 10.1002/stem.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades P, Ferguson-Smith AC, Burton GJ. Comparative developmental anatomy of the murine and human definitive placentae. Placenta. 2002;23:3–19. doi: 10.1053/plac.2001.0738. [DOI] [PubMed] [Google Scholar]
- Golachowska MR, Hoekstra D, van IJzendoorn SC. Recycling endosomes in apical plasma membrane domain formation and epithelial cell polarity. Trends Cell Biol. 2010;20:618–626. doi: 10.1016/j.tcb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Handel B, Prashad SL, Hassanzadeh-Kiabi N, Huang A, Magnusson M, Atanassova B, Chen A, Hamalainen EI, Mikkola HK. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood. 2010;116:3321–3330. doi: 10.1182/blood-2010-04-279489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M, Dobric N, Scott IC, Su L, Starovic M, St-Pierre B, Egan SE, Kingdom JC, Cross JC. The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol. 2004;271:26–37. doi: 10.1016/j.ydbio.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Hunter PJ, Swanson BJ, Haendel MA, Lyons GE, Cross JC. Mrj encodes a DnaJ-related co-chaperone that is essential for murine placental development. Development. 1999;126:1247–1258. doi: 10.1242/dev.126.6.1247. [DOI] [PubMed] [Google Scholar]
- Huh CG, Factor VM, Sánchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci USA. 2004;101:4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauma SW, Bae-Jump V, Walsh SW. Hepatocyte growth factor stimulates trophoblast invasion: a potential mechanism for abnormal placentation in preeclampsia. J Clin Endocrinol Metab. 1999;84:4092–4096. doi: 10.1210/jcem.84.11.6120. [DOI] [PubMed] [Google Scholar]
- Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta--epithelial-mesenchymal transition (EMT) and placental development. Placenta. 2010;31:747–755. doi: 10.1016/j.placenta.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Kincade PW. Canonical Wnt pathway signaling suppresses VCAM-1 expression by marrow stromal and hematopoietic cells. Exp Hematol. 2009;37:19–30. doi: 10.1016/j.exphem.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy JE, Jin O, Fujiwara Y, Kuo F, Andrews NC. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- Maltepe E, Bakardjiev AI, Fisher SJ. The placenta: transcriptional, epigenetic, and physiological integration during development. J Clin Invest. 2010;120:1016–1025. doi: 10.1172/JCI41211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno M. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer. 2012;12:23–38. doi: 10.1038/nrc3169. [DOI] [PubMed] [Google Scholar]
- McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA. 2010;107:1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasso MI, Grinberg A, Robinson G, Sargent TD, Mahon Ka. Placental failure in mice lacking the homeobox gene Dlx3. Proc Natl Acad Sci USA. 1999;96:162–167. doi: 10.1073/pnas.96.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould A, Morgan MA, Li L, Bikoff EK, Robertson EJ. Blimp1/Prdm1 governs terminal differentiation of endovascular trophoblast giant cells and defines multipotent progenitors in the developing placenta. Genes Dev. 2012;26:2063–2074. doi: 10.1101/gad.199828.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai A, Takebe K, Nio-Kobayashi J, Takahashi-Iwanaga H, Iwanaga T. Cellular expression of the monocarboxylate transporter (MCT) family in the placenta of mice. Placenta. 2010;31:126–133. doi: 10.1016/j.placenta.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Nasu K, Zhou Y, McMaster MT, Fisher SJ. Upregulation of human cytotrophoblast invasion by hepatocyte growth factor. J Reprod Fertil Suppl. 2000;55:73–80. [PubMed] [Google Scholar]
- Natale DRC, Starovic M, Cross JC. Phenotypic analysis of the mouse placenta. Methods Mol Med. 2006;121:275–293. doi: 10.1385/1-59259-983-4:273. [DOI] [PubMed] [Google Scholar]
- Plum A, Winterhager E, Pesch J, Lautermann J, Hallas G, Rosentreter B, Traub O, Herberhold C, Willecke K. Connexin31-deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation. Dev Biol. 2001;231:334–347. doi: 10.1006/dbio.2000.0148. [DOI] [PubMed] [Google Scholar]
- Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HKA. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2:252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–413. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Saito S, Sakakura S, Enomoto M, Ichijo M, Matsumoto K, Nakamura T. Hepatocyte growth factor promotes the growth of cytotrophoblasts by the paracrine mechanism. J of Biochem. 1995;117:671–676. doi: 10.1093/oxfordjournals.jbchem.a124761. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Scifres CM, Nelson DM. Intrauterine growth restriction, human placental development and trophoblast cell death. J Physiol. 2009;587:3453–3458. doi: 10.1113/jphysiol.2009.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284:12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Natale DRC, Begay V, Hughes M, Leutz A, Cross JC. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development. 2008;135:2083–2091. doi: 10.1242/dev.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasubramaniyam T, Garcia J, Tagliaferro A, Melland-Smith M, Chauvin S, Post M, Todros T, Caniggia I. Where polarity meets fusion: role of Par6 in trophoblast differentiation during placental development and preeclampsia. Endocrinology. 2013;154:1296–1309. doi: 10.1210/en.2012-1823. [DOI] [PubMed] [Google Scholar]
- Somerset DA, Li XF, Afford S, Strain AJ, Ahmed A, Sangha RK, Whittle MJ, Kilby MD. Ontogeny of hepatocyte growth factor (HGF) and its receptor (c-met) in human placenta: reduced HGF expression in intrauterine growth restriction. Am J Pathol. 1998;153:1139–1147. doi: 10.1016/S0002-9440(10)65658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecca B, Nait-Oumesmar B, Kelley KA, Voss AK, Thomas T, Lazzarini RA. Gcm1 expression defines three stages of chorio-allantoic interaction during placental development. Mech Dev. 2002;115:27–34. doi: 10.1016/s0925-4773(02)00095-3. [DOI] [PubMed] [Google Scholar]
- Takayanagi T, Aoki Y, Tanaka K. Expression of constitutively active c-MET receptor in human choriocarcinoma. Gynecol Obstet Invest. 2000;50:198–202. doi: 10.1159/000010310. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373:702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Unezaki S, Horai R, Sudo K, Iwakura Y, Ito S. Ovol2/Movo, a homologue of Drosophila ovo, is required for angiogenesis, heart formation and placental development in mice. Genes Cells. 2007;12:773–785. doi: 10.1111/j.1365-2443.2007.01084.x. [DOI] [PubMed] [Google Scholar]
- Uy GD, Downs KM, Gardner RL. Inhibition of trophoblast stem cell potential in chorionic ectoderm coincides with occlusion of the ectoplacental cavity in the mouse. Development. 2002;129:3913–3924. doi: 10.1242/dev.129.16.3913. [DOI] [PubMed] [Google Scholar]
- Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology. 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- Wynne F, Ball M, McLellan AS, Dockery P, Zimmermann W, Moore T. Mouse pregnancy-specific glycoproteins: tissue-specific expression and evidence of association with maternal vasculature. Reproduction. 2006;131:721–732. doi: 10.1530/rep.1.00869. [DOI] [PubMed] [Google Scholar]
- Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. doi: 10.1146/annurev-pathol-121808-102149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Labyrinth trophoblasts express CD9 and Epcam. Related to Figure 1.
(A) Immunofluorescence and schematic representation of mouse placental structure. Sections from E12.5 placenta were stained with antibodies for CD9 or Epcam (green), and Cytokeratin (red, trophoblasts). DAPI (blue) indicates nuclei. The fully developed placenta is composed of three major layers: a decidual layer (De), which includes decidual cells of the uterus; a junctional zone (JZ) which attaches the fetal placenta to the decidua and contains trophoblast giant cells (TGC) and spongiotrophoblasts (Sp); and a labyrinth layer (La), composed of fetal endothelial cells and labyrinth trophoblasts (SynT-I and -II, and sinusoidal trophoblast giant cells (sTGC)) that establish the substance exchange interface between fetal and maternal circulations. CP, chorionic plate.
(B) FACS analysis of cells enriched by anti-Epcam antibody documents effective isolation of Epcamhi cells without significant contamination of differentiated Epcamlow cells.
Figure S2. Epcamhi LaTP cells are clonally linked to terminally differentiated SynT. Related to Figure 2.
(A) Schematic for in vivo clonality experiment. Rosa26 (R26) YFP reporter mice were mated with R26 CreERT2 mice. At E9.5 Cre-mediated gene deletion was induced by injection with 4OH-tamoxifen and embryos were dissected at E12.5.
(B) Frequency of Cre-mediated gene-recombination in different cell types in the placenta documenting rare marking of trophoblasts. (C) Frequency of Epcamhi clusters containing YFP-labeled cells.
(D) Average number of YFP+ cells in a labeled Epcamhi cell cluster.
(E) Differentiation potential of YFP labeled cells in individual clusters. Multi, Epcamhi LaTP with SynT-I, SynT-II and sTGC; bi, Epcamhi with SynT-I and SynT-II; uni, Epcamhi with sTGC, SynT-I or SynT-II.
(F) Documentation of multi-lineage differentiation in a cluster of YFP+ labeled trophoblasts. (i) Epcamhi Cytokeratinlow (LaTP), (ii) SynT-I (Epcam− Cytokeratin+) and SynT-II (Epcamlow Cytokeratin+) and sTGC (iii) (Epcam− Cytokeratin+), *, SynT layer.
(G) Documentation of terminally differentiated SynT cells (Epcamlow Mct4+) in the same YFP+ marked clusters as Epcamhi LaTP. Epcam (red), YFP (green), DAPI (blue), Mct4 (purple). Scale bar 100 μm.
All error bars indicate SEM (Standard error of mean).
Figure S3. c-Met regulates both placental and embryonic development. Related to Figure 3.
(A, B, C and D) Kinetic analysis documenting growth retardation of c-Met deficient embryos, fetal livers and placentas after E12.5. Scale bar 1 mm.
(E) IF for trophoblast specific marker (Cytokeratin) and ECM marker that marks blood vessels (Laminin) documenting poorly developed branching structure in c-Met g-and t-KO placenta.
(F) c-Met t-KO fetal livers show smaller size and low cellularity.
(G) Selected GO (gene ontology) categories representing genes down- or up-regulated in placental CD9+ cells in the absence of c-Met signaling.
All error bars indicate SEM (Standard error of mean).
Figure S4. c-Met directly regulates trophoblast proliferation. Related to Figure 4.
(A) Microarray analysis of CD9+ trophoblasts from Wt vs. c-Met g-KO placenta. The heat map presents expression of various genes that related to GO category “cell cycle phase” that are suppressed in CD9+ trophoblasts of c-Met g-KO placenta.
(B) BrdU incorporation assay. Sections from E12.5 Wt, c-Met g-KO and t-KO placentas were stained for Cytokeratin (green), BrdU (red), and DAPI (blue). Although BrdU+ Cytokeratin+ trophoblasts (arrows) were observed in Wt placenta, only Cytokeratin− cells (arrowheads) showed mitotic activity in c-Met g- and t-KO placenta. Scale bar 100 μm.
(C) Sections from Wt and c-Met g-KO placentas were stained for TUNEL (red), Cytokeratin (green) and DAPI (blue). Arrows indicate TUNEL and Cytokeratin double-positive cells. No significant difference was observed in apoptosis between Wt and c-Met g-KO trophoblasts. Scale bar 100 μm.
All error bars indicate SEM (Standard error of mean).
Figure S5. Loss of c-Met compromises cell polarity specifically in the placenta. Related to Figure 5.
(A) IF for Mct4 (green), CD9 (red) and DAPI (blue) in Wt and c-Met g-KO placentas at E12.5. Presence of both Mct4− CD9+ cells and Mct4+ CD9+ cells indicates that both SynT-I and II are present in Wt and c-Met g-KO placentas. Notably, in Wt placenta, Mct4 is specifically expressed on the fetal side in SynT-II and CD9 is expressed on both apical side of SynT-I and fetal side of SynT-II. However diffused localization of both CD9 and Mct4 was observed in c-Met g-KO placenta. Arrows indicate fetal side of SynT-II and arrowheads indicate apical membrane of SynT-I. f, fetal side. m, maternal side. Scale bar 25 μm.
(B) IF for Epcam (green), CD9 (red), and DAPI (blue) on Wt and c-Met g-KO yolk sac. No abnormal localization of Epcam or CD9 and was observed in c-Met g-KO yolk sac. Arrows indicate basolateral localization of Epcam and arrowheads indicate apical staining of CD9; a, apical; b, basal. Scale bar 25 μm.
Figure S6. Gcm1 is expressed in LaTP and SynT in the placenta. Related to Figure 6.
(A) QRT-PCR documenting the enriched expression of Gcm1 in both Epcamhi LaTP and Epcamlow SynT in E 10.5 placenta. Error bars indicate SEM (Standard error of mean)
(B) IHC with anti-Epcam antibody and in situ hybridization (ISH) with Gcm1 antisense probe indicates high expression of Gcm1 in Epcamhi LaTP (arrows) in E10.5 placenta. Scale bar 100 μm.
(C) ISH analysis showing high expression of Gcm1 in SynT-II layer (arrow in the inset) adjacent to maternal blood spaces in E14.5 placenta. Scale bar 100 μm.
(D) Sections from Wt and c-Met g-KO placentas at E12.5 were stained for Epcam, Gcm1, PH3, and DAPI. Arrows indicate Epcamhi and PH3+ cells that also express Gcm1. Scale bar 100 μm.
Related to Figure 3.
Related to Figure 3.