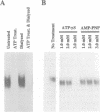
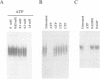
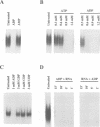
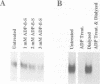
Abstract
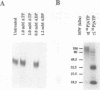
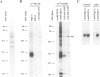
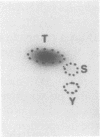
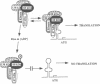
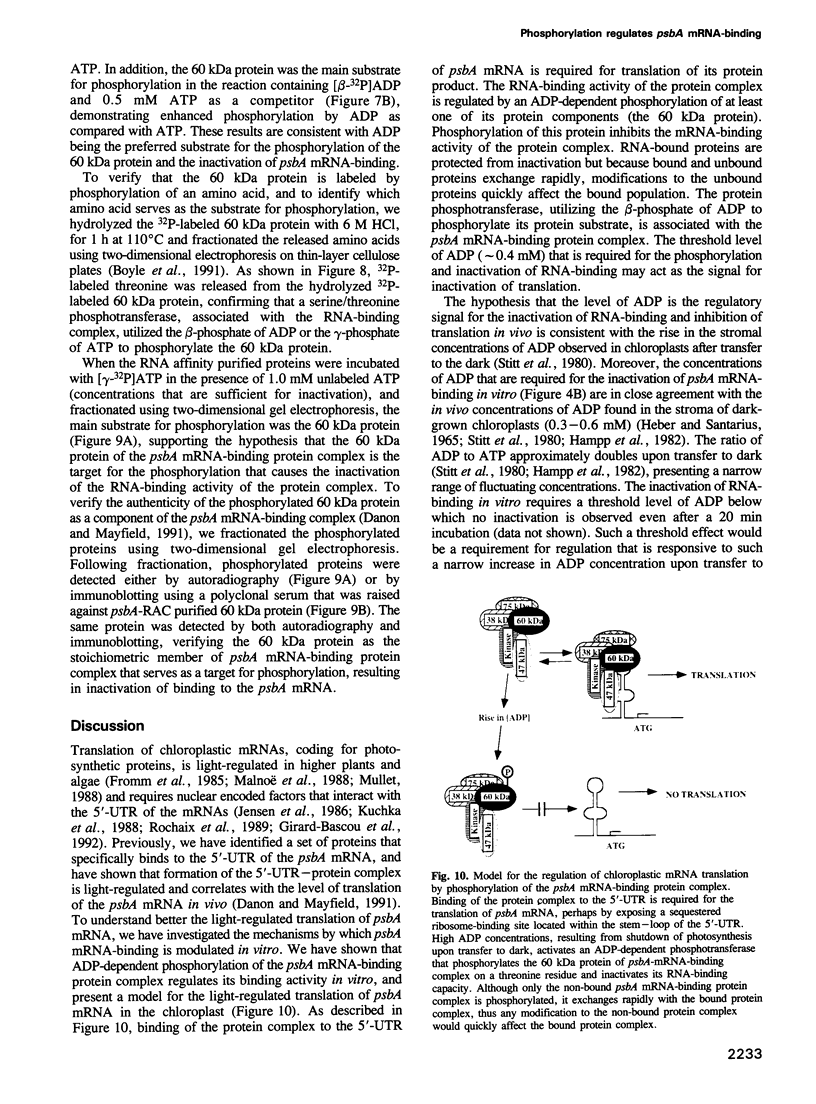
Light-regulated translation of chloroplastic mRNAs in the green alga Chlamydomonas reinhardtii requires nuclear encoded factors that interact with the 5'-untranslated region (5'-UTR) of specific mRNAs to enhance their translation. We have previously identified and characterized a set of proteins that bind specifically to the 5'-UTR of the chloroplastic psbA mRNA. Accumulation of these proteins is similar in dark- and light-grown cells, whereas their binding activity is enhanced during growth in the light. We have identified a serine/threonine protein phosphotransferase, associated with the psbA mRNA-binding complex, that utilizes the beta-phosphate of ADP to phosphorylate and inactivate psbA mRNA-binding in vitro. The inactivation of mRNA-binding in vitro is initiated at high ADP levels, levels that are attained in vivo only in dark-grown chloroplasts. These data suggest that the translation of psbA mRNA is attenuated by phosphorylation of the mRNA-binding protein complex in response to a rise in the stromal concentration of ADP upon transfer of cells to dark.
Full text
PDF
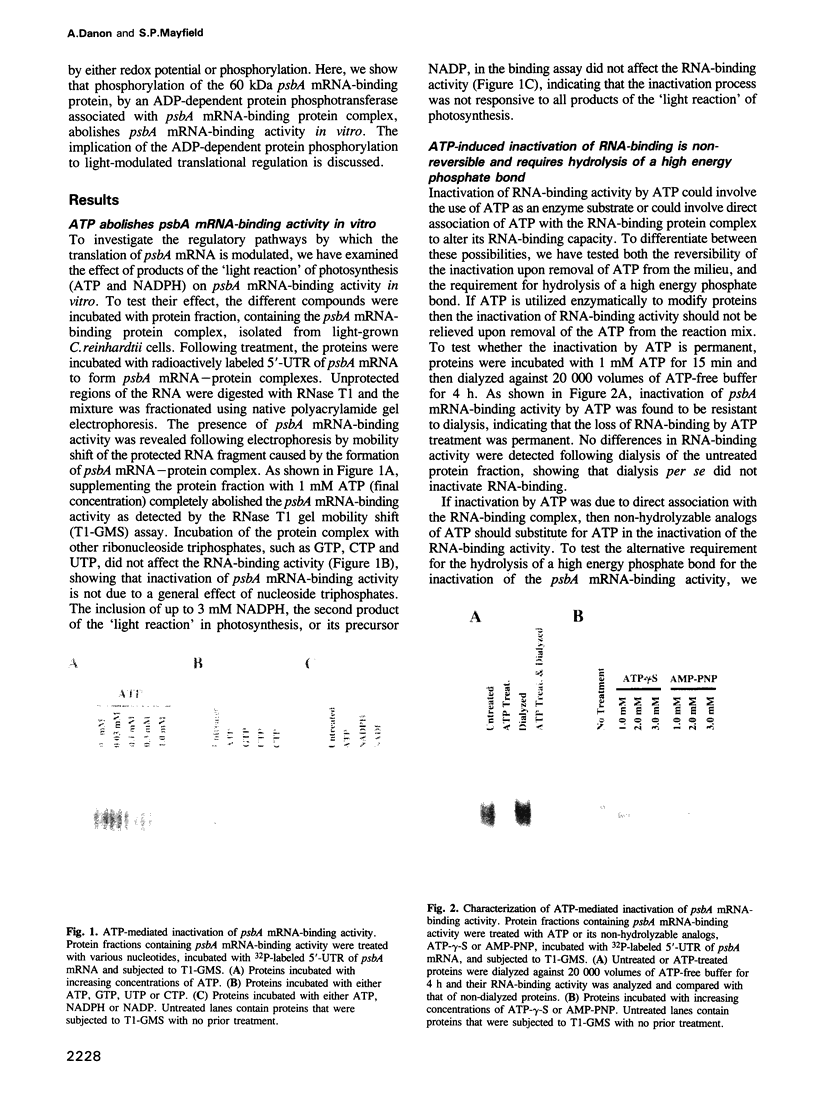
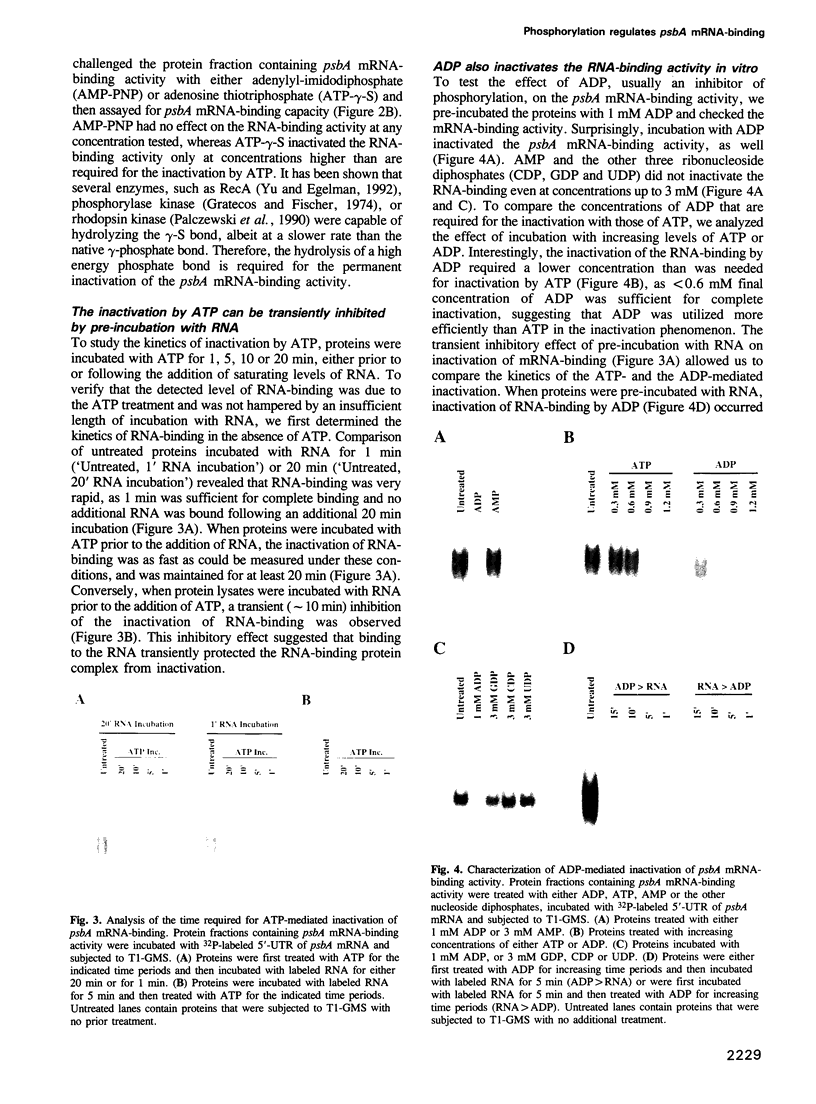
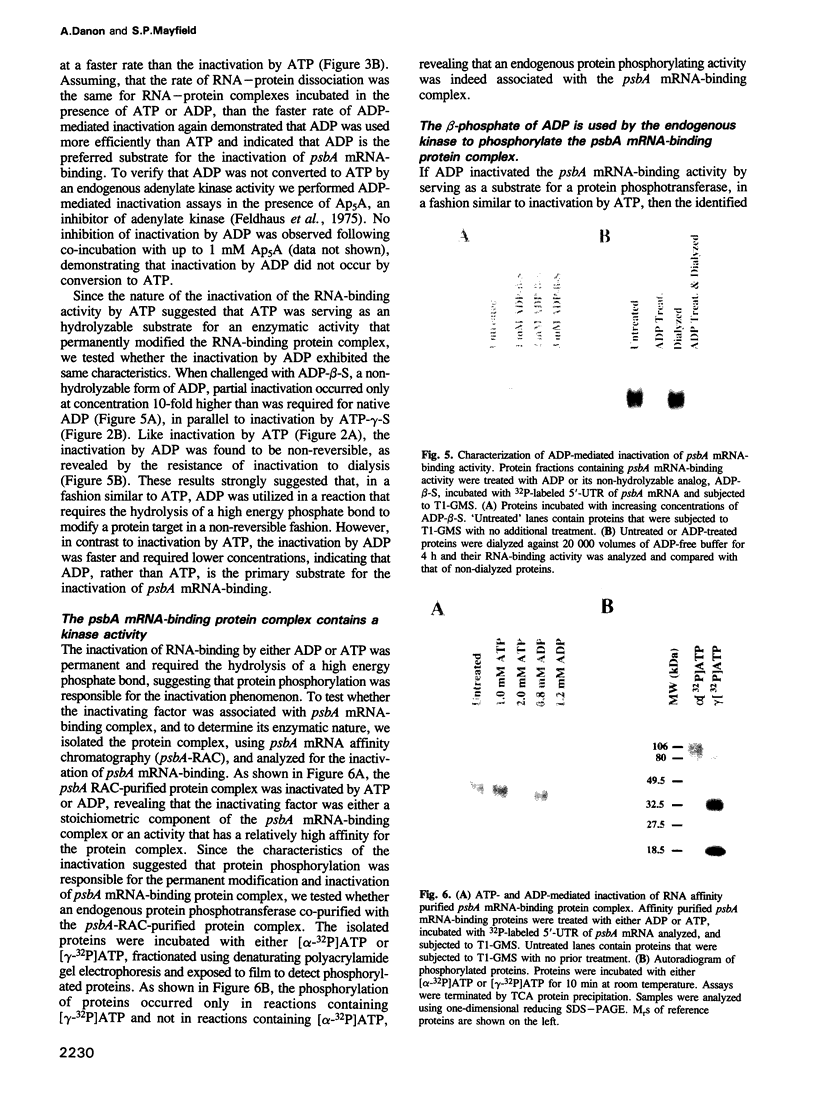
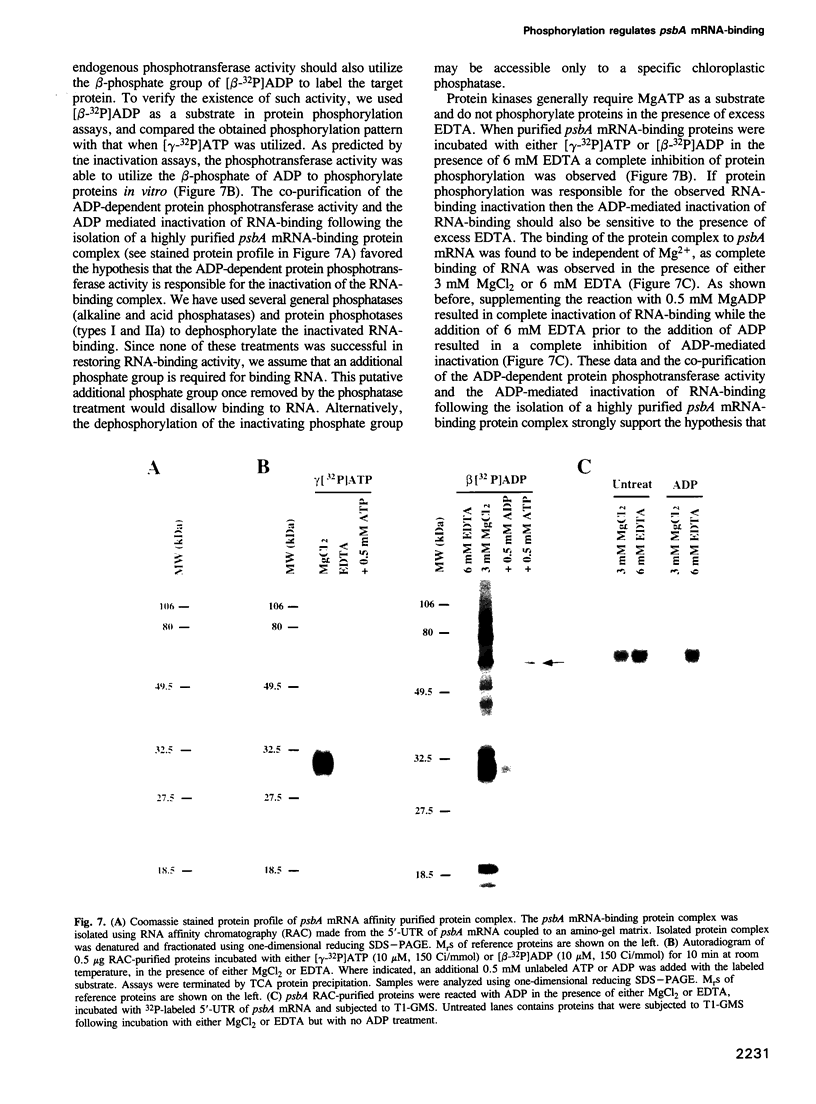



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottomley W., Spencer D., Whitfeld P. R. Protein synthesis in isolated spinach chloroplasts: comparison of light-driven and ATP-driven synthesis. Arch Biochem Biophys. 1974 Sep;164(1):106–117. doi: 10.1016/0003-9861(74)90012-5. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B. Regulation of CO2 assimilation in oxygenic photosynthesis: the ferredoxin/thioredoxin system. Perspective on its discovery, present status, and future development. Arch Biochem Biophys. 1991 Jul;288(1):1–9. doi: 10.1016/0003-9861(91)90157-e. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Ernst S. M., Chollet R. Substrate specificity and regulation of the maize (Zea mays) leaf ADP: protein phosphotransferase catalysing phosphorylation/inactivation of pyruvate, orthophosphate dikinase. Biochem J. 1986 Jun 1;236(2):579–584. doi: 10.1042/bj2360579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnell J. N., Hatch M. D. Dark-light regulation of pyruvate, Pi dikinase in C4 plants: evidence that the same protein catalyses activation and inactivation. Biochem Biophys Res Commun. 1983 Feb 28;111(1):288–293. doi: 10.1016/s0006-291x(83)80149-1. [DOI] [PubMed] [Google Scholar]
- Coughlan S. J., Hind G. Protein kinases of the thylakoid membrane. J Biol Chem. 1986 Oct 25;261(30):14062–14068. [PubMed] [Google Scholar]
- Danon A., Mayfield S. P. Light regulated translational activators: identification of chloroplast gene specific mRNA binding proteins. EMBO J. 1991 Dec;10(13):3993–4001. doi: 10.1002/j.1460-2075.1991.tb04974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X. W., Gruissem W. Constitutive transcription and regulation of gene expression in non-photosynthetic plastids of higher plants. EMBO J. 1988 Nov;7(11):3301–3308. doi: 10.1002/j.1460-2075.1988.tb03200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhau P., Fröhlich T., Goody R. S., Isakov M., Schirmer R. H. Synthetic inhibitors of adenylate kinases in the assays for ATPases and phosphokinases. Eur J Biochem. 1975 Sep 1;57(1):197–204. doi: 10.1111/j.1432-1033.1975.tb02291.x. [DOI] [PubMed] [Google Scholar]
- Fromm H., Devic M., Fluhr R., Edelman M. Control of psbA gene expression: in mature Spirodela chloroplasts light regulation of 32-kd protein synthesis is independent of transcript level. EMBO J. 1985 Feb;4(2):291–295. doi: 10.1002/j.1460-2075.1985.tb03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Bascou J., Pierre Y., Drapier D. A nuclear mutation affects the synthesis of the chloroplast psbA gene production Chlamydomonas reinhardtii. Curr Genet. 1992 Jul;22(1):47–52. doi: 10.1007/BF00351741. [DOI] [PubMed] [Google Scholar]
- Gratecos D., Fischer E. H. Adenosine 5'-O(3-thiotriphosphate) in the control of phosphorylase activity. Biochem Biophys Res Commun. 1974 Jun 18;58(4):960–967. doi: 10.1016/s0006-291x(74)80237-8. [DOI] [PubMed] [Google Scholar]
- Guitton C., Dorne A. M., Mache R. In organello and in vitro phosphorylation of chloroplast ribosomal proteins. Biochem Biophys Res Commun. 1984 May 31;121(1):297–303. doi: 10.1016/0006-291x(84)90722-8. [DOI] [PubMed] [Google Scholar]
- Guitton C., Mache R. Phosphorylation in vitro of the large subunit of the ribulose-1,5-bisphosphate carboxylase and of the glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1987 Jul 1;166(1):249–254. doi: 10.1111/j.1432-1033.1987.tb13509.x. [DOI] [PubMed] [Google Scholar]
- Hampp R., Goller M., Ziegler H. Adenylate Levels, Energy Charge, and Phosphorylation Potential during Dark-Light and Light-Dark Transition in Chloroplasts, Mitochondria, and Cytosol of Mesophyll Protoplasts from Avena sativa L. Plant Physiol. 1982 Feb;69(2):448–455. doi: 10.1104/pp.69.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Jensen K. H., Herrin D. L., Plumley F. G., Schmidt G. W. Biogenesis of photosystem II complexes: transcriptional, translational, and posttranslational regulation. J Cell Biol. 1986 Oct;103(4):1315–1325. doi: 10.1083/jcb.103.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. R., Mason H. S., Mullet J. E. Light-regulated translation of chloroplast proteins. I. Transcripts of psaA-psaB, psbA, and rbcL are associated with polysomes in dark-grown and illuminated barley seedlings. J Cell Biol. 1988 Feb;106(2):289–301. doi: 10.1083/jcb.106.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchka M. R., Mayfield S. P., Rochaix J. D. Nuclear mutations specifically affect the synthesis and/or degradation of the chloroplast-encoded D2 polypeptide of photosystem II in Chlamydomonas reinhardtii. EMBO J. 1988 Feb;7(2):319–324. doi: 10.1002/j.1460-2075.1988.tb02815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin Z. F., Lucero H. A., Racker E. Protein kinases from spinach chloroplasts. I. Purification and identification of two distinct protein kinases. J Biol Chem. 1982 Oct 25;257(20):12153–12156. [PubMed] [Google Scholar]
- Malnoë P., Mayfield S. P., Rochaix J. D. Comparative analysis of the biogenesis of photosystem II in the wild-type and Y-1 mutant of Chlamydomonas reinhardtii. J Cell Biol. 1988 Mar;106(3):609–616. doi: 10.1083/jcb.106.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel H., Hunt D. F., Shabanowitz J., Bennett J. Tandem mass spectrometry reveals that three photosystem II proteins of spinach chloroplasts contain N-acetyl-O-phosphothreonine at their NH2 termini. J Biol Chem. 1988 Jan 25;263(3):1123–1130. [PubMed] [Google Scholar]
- Mullet J. E. The amino acid sequence of the polypeptide segment which regulates membrane adhesion (grana stacking) in chloroplasts. J Biol Chem. 1983 Aug 25;258(16):9941–9948. [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Kahn N., Hargrave P. A. Nucleoside inhibitors of rhodopsin kinase. Biochemistry. 1990 Jul 3;29(26):6276–6282. doi: 10.1021/bi00478a024. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Kuchka M., Mayfield S., Schirmer-Rahire M., Girard-Bascou J., Bennoun P. Nuclear and chloroplast mutations affect the synthesis or stability of the chloroplast psbC gene product in Chlamydomonas reinhardtii. EMBO J. 1989 Apr;8(4):1013–1021. doi: 10.1002/j.1460-2075.1989.tb03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Wirtz W., Heldt H. W. Metabolite levels during induction in the chloroplast and extrachloroplast compartments of spinach protoplasts. Biochim Biophys Acta. 1980 Nov 5;593(1):85–102. doi: 10.1016/0005-2728(80)90010-9. [DOI] [PubMed] [Google Scholar]
- Sun G., Bailey D., Jones M. W., Markwell J. Chloroplast thylakoid protein phosphatase is a membrane surface-associated activity. Plant Physiol. 1989 Jan;89(1):238–243. doi: 10.1104/pp.89.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Egelman E. H. Direct visualization of dynamics and co-operative conformational changes within RecA filaments that appear to be associated with the hydrolysis of adenosine 5'-O-(3-thiotriphosphate). J Mol Biol. 1992 May 5;225(1):193–216. doi: 10.1016/0022-2836(92)91036-o. [DOI] [PubMed] [Google Scholar]