Abstract
Background:
Invasive pulmonary aspergillosis (IPA) is an important infection in critically ill patients including patients of intensive care units (ICU). Different diagnostic tools are available and since its mortality is high, it is vital to start the antifungal therapy as soon as possible. Knowing the epidemiology of this disease in each ICU and area will help to better and more rapid management of such patients. The aim of this study is to determine the frequency of IPA based on the level of galactomannan in bronchoalveolar lavage fluid in ICU of Al-Zahra hospital, Isfahan, Iran.
Materials and Methods:
This was a cross sectional study, which was conducted in Al-Zahra hospital, Isfahan, Iran, between 2010 to 2011. The study population was all the patients admitted to ICU and were suspected to have invasive Aspergillus spp pneumonia. The level of galactomannan in bronchoalveolar lavage was measured and demographic data were gathered by the questionnaire.
Results:
The frequency of IPA in this study was calculated as 2.43% while galactomannan level in bronchoalveolar lavage fluid of this patient (2.50) was significantly higher than others (0.03 ± 0.02).
Conclusion:
Larger studies are required to determine the exact frequency of IPA and the best antifungal therapy for it.
Keywords: Aspergillosis, bronchoalveolar lavage, galactomannan, intensive care unit, pneumonia
INTRODUCTION
Invasive Aspergillosis is one of the most important, life-threatening infections in immunodeficiency patients or those at risk; while, pulmonary aspergillosis is the most common form of them.[1] According to previous studies, pneumonia has commonly been observed in hospitalized patients with fungal infections including aspergillus spp. On the other side, pneumonia development together with factors such as aspergillus spp is common in hospitalized patients undergoing mechanical ventilation in Intensive Care Units (ICU), even in the absence of other obvious immunodeficiency factors. The reason for that is the damage in the respiratory mucous membranes of these patients due to intubation consequently followed by colonization with endogenous flora or exogenous pathogens.[2]
Although conducted studies on the epidemiology and causes of aspergillus spp infection have focused more on the patients with severe immunodeficiency including patients developing blood disorders or organ transplant recipients and those undergoing chemotherapy, in recent studies, a special place is given to IPA without the risk of classic immunodeficiency factor in patients hospitalized in ICU.[3,4,5,6]
Final diagnosis of IPA is usually based on the isolation and identification of aspergillus spp in culture or histological sample under the microscope. However, tissue biopsy in critically-ill patients is not risk-free and its sensitivity would not be clear. Conventional diagnostic methods such as culturing and investigating the respiratory tract sample under the microscope have the sensitivity and specificity of only about 50%.[7,8] Radiology such as high-resolution CT (HRCT) is one of the other methods applied for diagnosis, one of the deficiencies of which is its impossibility to be done in critically-ill patients including those undergoing mechanical ventilation.[9]
Galactomannan (GM) is a fungal cell wall polysaccharide released from fungal hyphae at the time of aspergillus spp invasion, level of which can be measured in body's fluids.[10,11] Level of GM in serum has been measured to a higher extent in neutropenic patients and stem cell or allogeneic bone marrow transplant recipients. A limited number of studies have investigated GM level of the serum in non-neutropenic patients, results of which are representative of the low value of serum's GM in the diagnosis of IA.[10,11,12,13] Another method is the measurement of GM level in bronchoalveolar lavage fluid which seems to have a greater diagnostic value than GM level of the serum due to greater abundance of fungal burden in the bronchial tree.[14,15]
Regarding the prevalence of IPA in critically-ill patients hospitalized in ICU and the vital importance of its early diagnosis, determining the frequency of this disease in each district and ICU center is a strikingly significant issue. Since the value of measuring GM level in Bronchoalveolar lavage fluid as a new diagnostic method has been proven by previous studies and no study has already been conducted on determining the frequency of this disease in critically-ill patients hospitalized in ICU in Iran, we have attempted to investigate GM level in bronchoalveolar lavage fluid in patients hospitalized in ICU in hospital “Al Zahra”, Isfahan, in order to determine the frequency of IPA in this health center.
MATERIALS AND METHODS
This study is a cross-sectional one conducted in “Al Zahra” hospital in Isfahan during a one-year period from 2010 to 2011. The population includes all the patients suspected to develop invasive pulmonary aspergillosis pneumonia hospitalized in ICU. It has to be mentioned that the study is conducted as a double-blind one in such a way that the data associated with the disease and patients’ identities would separately be registered by a researcher; while, the main researcher is not informed of the data when interpreting the tests and interprets them regardless of the data.
Patients meeting the inclusion criteria were as followings:
Patients over 18 developing one type of hematologic malignancies (aside from those receiving anti-fungal treatment due to diagnosis of IPA)
Patients with cancer undergoing chemotherapy during three months before hospitalization
Organ transplant recipients
Patients using minimally 4 mgs or more of methylprednisolone or other kinds of Corticosteroid equal to the same amount of methylprednisolone for at least 7 days during 3 weeks before being hospitalized in ICU
Those receiving corticosteroid as the same amount as methylprednisolone for at least 5 days during the period of hospitalization in ICU
Patients receiving at least a 250-mg cumulative dose of methylprednisolone or the equal amount before entering the study
Recipients of different types of, immunosuppressive treatments including tacrolimus, cyclosporine, methotrexate, cyclophosphamide and sirolimus
Patients catching child's class C cirrhosis
Those with HIV
Patients hospitalized in ICU, for whom ventilator was applied and at least 48 h were passed after the ventilation and on the basis of CDC criteria were suspected to have pneumonia together with ventilator.
On the other hand, based on CDC criteria, patients were considered to develop ventilation associated pneumonia (VAP), if they had the following radiological documents, symptoms, signs and experiments:
-
Radiological documents including two or more consequent chest X-ray coupled with at least one of the following symptoms:
- New, progressive or chronic infiltrate
- Consolidation
- Cavitation
- The presence of air in the lungs of children under one.
Note: A final radiography of the chest would be acceptable in patients not developing the underlying heart or pulmonary diseases (such as respiratory distress syndrome, bronchial-pulmonary dysplasia, pulmonary edema or chronic obstructive pulmonary disease).
-
The presence of minimally one of the followings based on the symptoms, signs and experiments for all patients:
- Fever (body temperature of higher than 38°) without any other clear causes
- Leukopenia (less than 4000 white corpuscle per milliliter of blood) or Leukocytosis (12000 and/or more white corpuscle per milliliter of blood)
-
A change in the state of awareness in patients over 70 without any obvious reasons and the presence of at least two of the followings:
- Sudden onset of purulent sputum, change in the characteristics of the sputum, increased respiratory secretions and the increasing need for suctioning
- Sudden onset or worsened state of the cough, asthma or rapid breathing
- Rhonchus or bronchial breathing sound
- The gas exchange getting worse (such as O2 desaturation [PaO2/FiO ≤240], the increasing need for oxygen and ventilation).
Patients meeting each of the inclusion criteria entered the study provided that they met two of the three criteria below leastwise (except group 8, for which VAP criteria were separately cited):
Having fever resistant to at least an appropriate 3-day antibiotic treatment or recurrent after a 48-h remission period while taking antibiotics
The presence of clinical symptoms or signs of developing invasive pulmonary aspergillosis pneumonia, pleuritic chest pain, pleural rub and having one of the symptoms of lower respiratory system infection such as mucus secretions, asthma and hemoptysis
Formation of new pulmonary infiltrate on the chest X-ray.
On the other hand, patients excluded from the study due to any clinical conditions making bronchoscopy and BAL sampling impossible. To implement the study, consents were developed, filled in by patients or their parents.
Sampling was done as a census in such a manner that samples were obtained from patients not meeting the exclusion criteria and hospitalized in ICU for a year and the associated informational form was completed. It has to be mentioned that due to the low number of patients, sampling was not done and all of them entered the study. Data including age, gender, medical background of diseases, cause of hospitalization in ICU, laboratory findings of the patients such as the presence or lack of neutropenia and list of used medications suppressing the immune of antibiotics were recorded in the data collection form.
Patients meeting the inclusion criteria underwent fiberoptic bronchoscopy by a specialist and the sample of bronchoalveolar lavage was obtained (BAL 2 × 20 mm). The area of sampling was determined in terms of infiltration region on the chest X-ray. Three CCS of the sample obtained from alveolar washing by bronchoalveolar lavage was kept in Tropical Infectious Disease Research Center at –20° up to the experiment time. Then, the “Sandwich ELISA” test was used to determine GM level in samples in Infectious Disease Research Center in Isfahan, based on the procedures of the factory (BIO-RAD, France) in charge. The optical density of 0.5 or higher in lavage sample and the results with the index of I ≥0.5 would be considered positive in regard to aspergillus spp and antigen of the GM, respectively. Serum sample index of lower than 0.5 is considered negative in respect to antigen of the GM. Positive results have to be interpreted considering the microbial culture, histological examinations or the biopsy of the samples and X-rays.
The beginning of the anti-fungal treatment for the patients, similar to that of other studies, does not follow any certain pre-determined protocol and the fungal treatment would be prescribed at the right time according to the doctor in charge.
Finally, like other similar studies, relative frequency of GM level of the lavage sample in patients was reported separately, in terms of taking or not taking on of the two drugs- “piperacillin - tazobactam” and “amoxicillin clavulanate”, for, on the basis of the previous studies conducted, the consumption of these two antibiotics have been in association with false-positive results.
Statistical analysis has been performed with SPSS-20. Data are presented as mean ± 1SD or number (%) based on the variables. Variables were comparing between groups by One-Sample T-test and Chi-square or Fisher exact test. Statistical significance was accepted at P < 0.05.
RESULTS
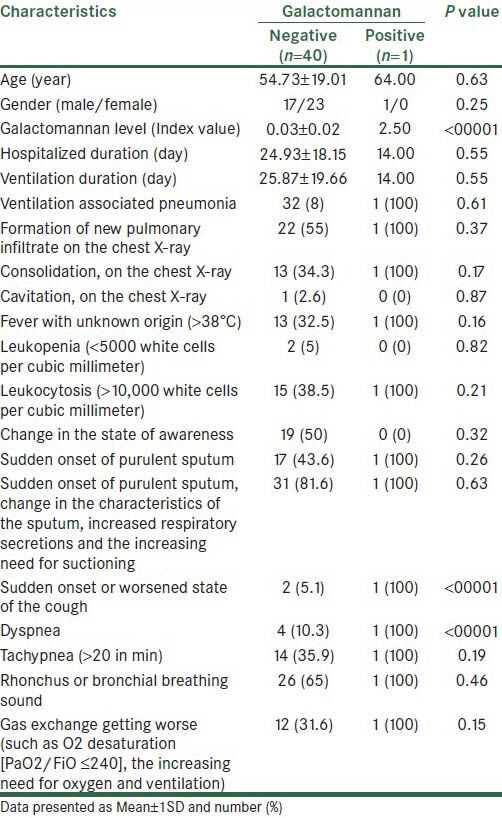
Studied patients had the mean age of 54.95 ± 18.82. Youngest and oldest patients had 20 and 86 years old, respectively. Twenty-three (56%) were male and 18 (44%) of them were female. Of the 41 studied patients, one patient (2.4%) was invasive pulmonary aspergillosis (IPA) positive based on the GM level in bronchoalveolar lavage fluid. Table 1 showed patients characteristics based on GM value. As showed in this table, patient with IPA positive was male and was 64 years old. Mean of GM level in this patient was 2.5. Based on results, only GM level, sudden onset or worsened state of the cough and rapid breathing in studied patients were statistically significant in regard to IPA positive or negative (P < 0.0001).
Table 1.
Patients characteristics based on galactomannan
DISCUSSION
IPA is one of the major and known causes of mortality in transplant recipients or those developing blood disorders. In recent studies, IPA has been cited as an opportunistic fungal disease in critically-ill patients, even in the absence of organ transplant or blood malignancies, diagnosis of which is most of the time neglected.[16] The studies conducted on the investigation of the autopsy of the patients hospitalized in ICU approve everything stated above and account for invasive pulmonary fungal infections as one of the most common undiagnosed cases in patients hospitalized in ICU.[3,5,17]
In the present study, the frequency of IPA was estimated applying the method of the investigation of GM level in bronchoalveolar lavage fluid of the patients hospitalized in ICU of the hospital “Al Zahra” in Isfahan to be 2.43% (1 patient out of 41 participants). GM rate in bronchoalveolar lavage fluid of this patient was 2.50, statistically and significantly higher than the mean GM of other patients (0.03 ± 0.02). Also, in other studies conducted in ICU in other countries, various frequencies of the disease have been reported.
In a study conducted by Morseman et al., 2007, entitled “IPA in ICU”, IA has been observed in 67 percent of the hospitalized patients, 64% of whom have been without immunodeficiency and/or blood malignancy. Furthermore, mortality rate has been 90%.[5,6] In another study entitled “Aspergillosis Isolation from Airways in Critically-ill Patients” and conducted by Wandwood, 2006, occurrence rate of IA in ICU has been reported to be 5.8%.[3] In a study by Cournil in 2006 named “the comparison of IA epidemiology in neutropenic and non-neutropenic patients”, it has been claimed that a large number of IA cases occur in non-neutropenic patients, underlying cause of hospitalization of which have been COPD, asthma and vasculitis.[4]
The gold standard for the diagnosis of the disease includes: Histopathology and cytopathology evidences accessible only through invasive methods and unlikely to be done in critically ill patients or those with thrombocytopenia.[1]
In the present study, the method of the investigation of GM level in bronchoalveolar lavage fluid has been applied for the diagnosis of IPA, value of which has been proven to be a noninvasive method for the diagnosis of IPA in critically-ill patients by other studies. For instance, Acosta et al. have stated the effect, sensitivity and specificity of this method to be higher than 80% in diagnosing the foregoing disease and claimed that the method is far more effective than the investigation of GM level and (1 → 3)–b–D-glucan of the serum in patients hospitalized in ICU.[18] In another study by Lee Young et al., this method has been cited to be a rapid and fast one in the patients at risk and developing a hematologic disease. The method has showed a greater sensitivity than investigation of GM level in serum.[19] Further, Morseman et al. have introduced the investigation of GM level in the BAL of hospitalized patients in ICU as a new method for the quicker diagnosis of invasive aspergillosis.[20]
Low number of positive cases, the method for bronchoalveolar lavage fluid sampling to measure GM level not been completely known and the simultaneous measure of serum GM not been carried out have been among limitations of this study.
The frequency of IPA was estimated to be 2.43% applying the method of the investigation of GM level in bronchoalveolar fluid. Greater studies are recommended to be conducted in order for more accurate determination of this frequency and its relationship with other factors and also effective treatment regimens.
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Guo YL, Chen YQ, Wang K, Qin SM, Wu C, Kong JL. Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: A bivariate metaanalysis and systematic review. Chest. 2010;138:817–24. doi: 10.1378/chest.10-0488. [DOI] [PubMed] [Google Scholar]
- 2.Slavin M, Fastenau J, Sukarom I, Mavros P, Crowley S, Gerth WC. Burden of hospitalization of patients with Candida and Aspergillus infections in Australia. Int J Infect Dis. 2004;8:111–20. doi: 10.1016/j.ijid.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Vandewoude KH, Blot SI, Depuydt P, Benoit D, Temmerman W, Colardyn F, et al. Clinical relevance of Aspergillus isolation from respiratory tract samples in critically ill patients. Crit Care. 2006;10:R31. doi: 10.1186/cc4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornillet A, Camus C, Nimubona S, Gandemer V, Tattevin P, Belleguic C, et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: A 6-year survey. Clin Infect Dis. 2006;43:577–84. doi: 10.1086/505870. [DOI] [PubMed] [Google Scholar]
- 5.Meersseman W, Vandecasteele SJ, Wilmer A, Verbeken E, Peetermans WE, Van WE. Invasive aspergillosis in critically ill patients without malignancy. Am J Respir Crit Care Med. 2004;170:621–5. doi: 10.1164/rccm.200401-093OC. [DOI] [PubMed] [Google Scholar]
- 6.Meersseman W, Van WE. Invasive aspergillosis in the ICU: An emerging disease. Intensive Care Med. 2007;33:1679–81. doi: 10.1007/s00134-007-0792-y. [DOI] [PubMed] [Google Scholar]
- 7.Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infect Dis. 2005;5:609–22. doi: 10.1016/S1473-3099(05)70238-3. [DOI] [PubMed] [Google Scholar]
- 8.Horvath JA, Dummer S. The use of respiratory-tract cultures in the diagnosis of invasive pulmonary aspergillosis. Am J Med. 1996;100:171–8. doi: 10.1016/s0002-9343(97)89455-7. [DOI] [PubMed] [Google Scholar]
- 9.Vandewoude KH, Vogelaers D. Medical imaging and timely diagnosis of invasive pulmonary aspergillosis. Clin Infect Dis. 2007;44:380–1. doi: 10.1086/509931. [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: A meta-analysis. Clin Infect Dis. 2006;42:1417–27. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 11.Mennink-Kersten MA, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect Dis. 2004;4:349–57. doi: 10.1016/S1473-3099(04)01045-X. [DOI] [PubMed] [Google Scholar]
- 12.Kwak EJ, Husain S, Obman A, Meinke L, Stout J, Kusne S, et al. Efficacy of galactomannan antigen in the Platelia Aspergillus enzyme immunoassay for diagnosis of invasive aspergillosis in liver transplant recipients. J Clin Microbiol. 2004;42:435–8. doi: 10.1128/JCM.42.1.435-438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husain S, Kwak EJ, Obman A, Wagener MM, Kusne S, Stout JE, et al. Prospective assessment of Platelia Aspergillus galactomannan antigen for the diagnosis of invasive aspergillosis in lung transplant recipients. Am J Transplant. 2004;4:796–802. doi: 10.1111/j.1600-6143.2004.00415.x. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Lee SO, Choi SH, Sung H, Kim MN, Choi CM, et al. Aspergillus galactomannan antigen assay in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. J Infect. 2010;61:492–8. doi: 10.1016/j.jinf.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Tabarsi P, Soraghi A, Marjani M, Zandian P, Baghaei P, Najafizadeh K, et al. Comparison of serum and bronchoalveolar lavage galactomannan in diagnosing invasive aspergillosis in solid-organ transplant recipients. Exp Clin Transplant. 2012;10:278–81. doi: 10.6002/ect.2011.0176. [DOI] [PubMed] [Google Scholar]
- 16.Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med. 2006;173:707–17. doi: 10.1164/rccm.200505-727SO. [DOI] [PubMed] [Google Scholar]
- 17.Garnacho-Montero J, Amaya-Villar R, Ortiz-Leyba C, Leon C, Alvarez-Lerma F, Nolla-Salas J, et al. Isolation of Aspergillus spp. from the respiratory tract in critically ill patients: Risk factors, clinical presentation and outcome. Crit Care. 2005;9:R191–9. doi: 10.1186/cc3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acosta J, Catalan M, del Palacio-Perez-Medel A, Lora D, Montejo JC, Cuetara MS, et al. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: Comparison with the diagnostic performance of galactomannan and of (1-->3)-beta-d-glucan chromogenic assay in serum samples. Clin Microbiol Infect. 2011;17:1053–60. doi: 10.1111/j.1469-0691.2010.03357.x. [DOI] [PubMed] [Google Scholar]
- 19.Hsu LY, Ding Y, Phua J, Koh LP, Chan DS, Khoo KL, et al. Galactomannan testing of bronchoalveolar lavage fluid is useful for diagnosis of invasive pulmonary aspergillosis in hematology patients. BMC Infect Dis. 2010;10:44. doi: 10.1186/1471-2334-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, et al. Galactomannan in bronchoalveolar lavage fluid: A tool for diagnosing aspergillosis in intensive care unit patients. Am J Respir Crit Care Med. 2008;177:27–34. doi: 10.1164/rccm.200704-606OC. [DOI] [PubMed] [Google Scholar]


