Abstract
Background:
The Autologous Chondrocytes Transplantation (ACT) method is being studied for repair of cartilage diseases. As the chondrocytes dedifferentiated during monolayer culture, three-dimensional cultures are suggested to redifferentiate them. The aim of this study was investigation of the effect of TGF-β3 growth factor on chondrocytes in pellet culture system.
Materials and Methods:
The chondrocytes were isolated from three human articular cartilages by enzymatic digestion. The cells of the second passage were transferred to pellet culture system. We determined the chondrogenic medium with TGF-β3 as the experimental group and without it as the control group. After 2 weeks, the aggrecan production was investigated using histological and immunohistochemical (IHC) methods.
Results:
The presence of glycosaminoglycans was proved through Toluiden blue staining. Comparison of IHC results using MATLAB software showed that aggrecan in the experimental group was significantly higher than in the control group (P ≤ 0.05).
Conclusion:
The presence of TGF-β3 in the chondrogenic medium could lead to the production of more aggrecan in chondrocytes cultivated in pellet culture system.
Keywords: Aggrecan, chondrocyte, growth factor, pellet system, TGF-β3
INTRODUCTION
Articular cartilage has few chondrocytes, approximately 1-2% of its volume, and 70-80% of water, 15% of collagens (especially type II), 9% of aggrecan and 3% of other kinds of macromolecules.[1,2] Proteoglycans, especially aggrecan, make the cartilages flexible.[3]
Articular cartilage damage leads to joint dysfunction, and the healed tissue does not have appropriate structural and biomechanical features.[4,5,6] Various therapies have been used in this regard. Microfracture method was satisfactory in some studies,[7,8,9] but with some deficiencies.[10] Other therapies, including drugs, debridement of tissue and joint lavage, arthroscopy, surgery and arthroplasty, not only does not heal the tissue but may also cause fibrosis, apoptosis and destruction of the tissue.[11]
In the Autologous Chondrocyte Transplantation (ACT) method, isolated chondrocytes from articular cartilage were proliferated in a monolayer culture and they gradually dedifferentiated in a way that the expression of type II collagen and protein core of aggrecan genes decreased and the expression of type I collagen gene increased.[12,13] The studies have shown that dedifferentiation improves by increasing the number of passages and using serum in the culture medium.[14,15,16,17] Today, three-dimensional culture systems (scaffolds or pellet) are suggested to redifferentiate of dedifferentiate chondrocytes and maintain chondrocytes’ phenotype.[18,19,20,21,22] The mechanism of pellet system is similar to the process occurring in the embryonic period, the formation of limb buds and condensation of mesenchymal cells.[23,24]
Growth factors are effective in properties and performance of chondrocytes as lack of growth factors and imbalance between anabolic and catabolic factors is considered as highly influencing factors in osteoarthritis progression.[25] Many studies have been performed on the effect of growth factors like BMP-6, TGF-β3, EGF, IGF-I, PDGF-AB and bFGF under “in vitro” conditions on stem cells and chondrocytes with different results.[26,27,28,29,30,31,32] The TGF-β family is mainly produced in bones and cartilages. The TGF-β 2 and 3 induce production of type II collagen and proteoglycans in chondrocytes and are effective in chondrogenesis of mesenchymal stem cells.[30] Although the use of TGF-β1 under “in vivo” conditions improves cartilaginous damage,[31] it causes side-effects like formation of osteophyte, synovial membrane inflammation and hyperplasia.[32]
Lots of challenges exist about the growth factors during the chondrogenic process due to the inconsistencies among studies. Therefore, in this study, the effect of TGF-β3 growth factor on natural chondrocytes for production of aggrecan was studied in pellet culture system using immunohistochemical (IHC) method.
MATERIALS AND METHODS
Isolation of chondrocytes from articular cartilage
Samples of articular cartilage were removed from three patients’ knees. The patients had signed written consent form before the start of experiment. The cartilaginous samples were cut to 1-2-mm pieces. Type II collagenase enzyme solution (Sigma,(Germany) 350 μg/mL) was applied to digest the tissue for 4 h at 37°C. The resultant suspension was centrifuged at 1400 rpm for 10 min and the chondrocytes were cultivated in DMEM/F12, Penicillin/streptomycin 1% and FBS 10% medium.
Pellet culture system
25 × 104 cells of passage 2 were transferred into 15 mm polypropylene conical tubes and were centrifuged with 0.5 mL of chondrogenic medium, including high glucose DMEM (Gibco, USA, Grand island), Penicillin/Streptomycine 1%, ITS 1%, Ascorbate-2-phosphate 50 μg/mL, dexamethason 10-8M, Linoleic acid 5 μg/mL and TGF-β3 10 ng/mL (Sigma) and cultured at 37°C and 5% CO2.
This medium was also used without TGF-β3 growth factor for the control group. The medium was replaced every 3 days and the samples were studied at the 14th day using histological and IHC methods.
Histological study of the pellets
The samples were fixed in 10% formaldehyde for 24 h, dehydrated in ascending ethanol, clarified with xylol and embedded in paraffin, sectioned into 4-μm thickness and stained by H and E and Toluidine blue. The samples were studied using a light microscope (Nikon, Japan).
Immunohistochemical method
For antigen retrieval, hyaluronidase enzyme (8 mg/mL) was used for 120 min at 37°C and block endogenous peroxidase activity with use of 3% hydrogen peroxide in absolute ethanol for 10 min. The anti-aggrecan primary antibody (Abcam, England) with concentration of 1:50 was added at 4°C for 24 h. The secondary antibody was conjugated with horse radish peroxidase (Abcam) for 60 min, followed by diaminobenzidine (DAB) (DakoCytomation, Denmark), and stained by H and E. The samples were studied using a light microscope (Nikon).
Semi quantitative analysis of the immunohistochemical images
The IHC images were analyzed by MATLAB software. Useless details of the image were eliminated and thickness of the original edges of the image was strengthened. The image holes were filled using suitable morphological operators and the desired area was specified to be studied after eliminating small areas [Figure 1]. The intensity of the brown color of the desired area was quantitatively obtained by extracting the red, blue and green values and combining them.
Figure 1.
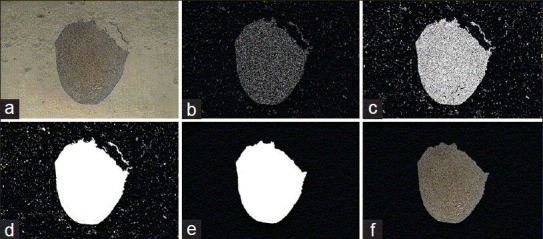
(a-f) Example of operations to analyze pictures by MATLAB software
RESULTS
Monolayer cultivation of chondrocytes
The isolated chondrocytes were gradually attached to the flasks in 24 h. Before adhesion, the cells were round and, after that, they were fibroblastic like [Figure 2].
Figure 2.
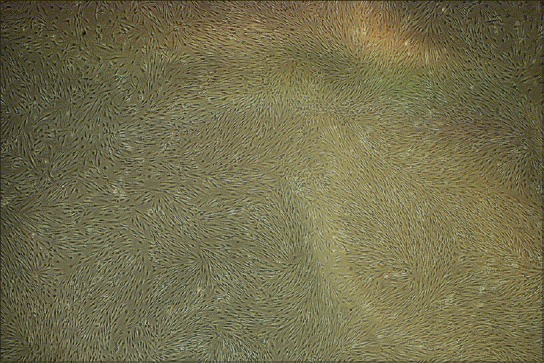
Monolayer culture of articular chondrocytes in passage 2 × 40
Pellet culture results
In pellet culture, passage 2 cells gradually aggregated and separated from the falcon's bottom during the second and third days. After removing, on the 14th day, cellular aggregates were round to oval, with a diameter of about 1 mm [Figure 3].
Figure 3.
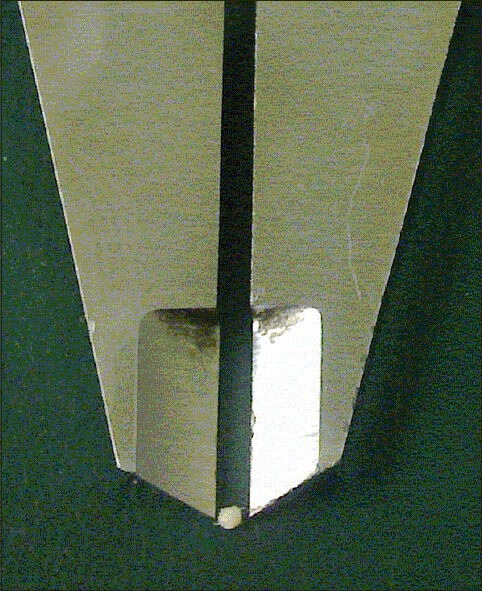
A sample of pellet in day 14 that formed as a 1-mm diameter spherical mass
Histological analyses of samples
Morphological study of the pieces resulting from pellet system was carried out by H and E staining [Figure 4]. The central part of the samples contained more or less round chondrocytes, which were confined in their own lacunas and surrounded by basophilic extracellular matrix. In the peripheral part of the samples, a layer similar to natural perichondrium was formed.
Figure 4.
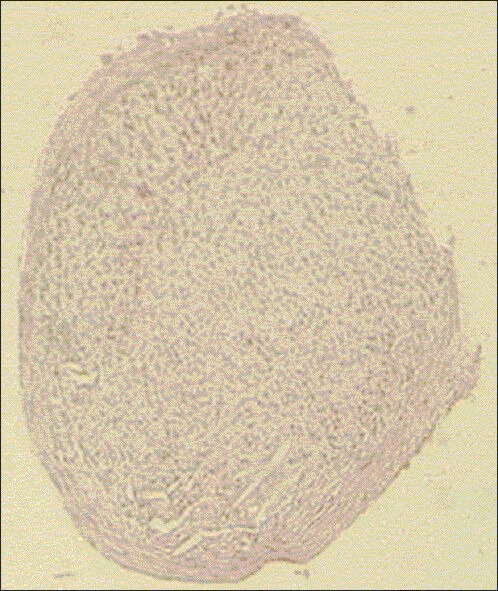
Hematoxylin and eosin staining of pellet
Toluidine blue staining
Toluidine blue is a cationic dye that satins sulfated glycosaminoglycans with negative charge metachromatically. The existence of sulfated glycosaminoglycans in the extracellular matrix of the tissue formed in pellet system was identified by the staining [Figure 5].
Figure 5.
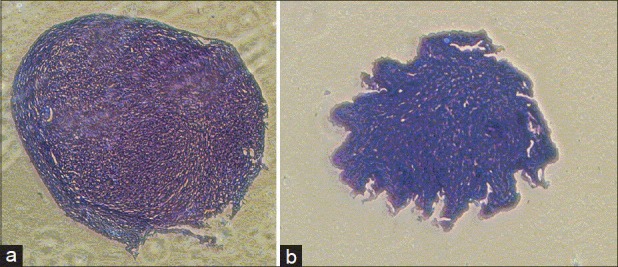
(a) Toluidine blue staining of pellet. Group + TGF-β3 (b) Toluidine blue staining of pellet. Group - TGF-β3
Immunohistochemical analyses
The brown color resulted from the interaction between DAB and peroxidase conjugated to the secondary antibody, observed in both groups, showing the production of aggrecan in the control and experimental groups [Figure 6]. Then, semiquantitative analysis was performed on the images obtained by immunohistocemical staining of the groups using MATLAB software. Figure 7 shows that color intensity in samples whose medium contained TGF-β3 growth factor was significantly higher than that of the control group.
Figure 6.
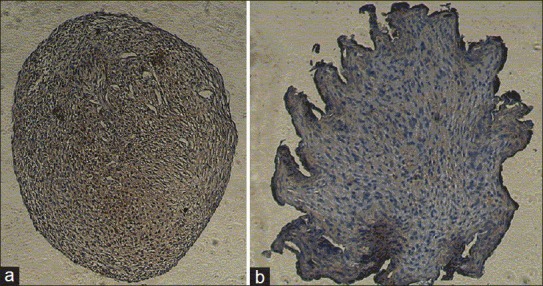
(a) Immunohistochemistry staining of pellet. Group + TGF-β3; (b) Immunohistochemistry staining of pellet. Group - TGF-β3
Figure 7.
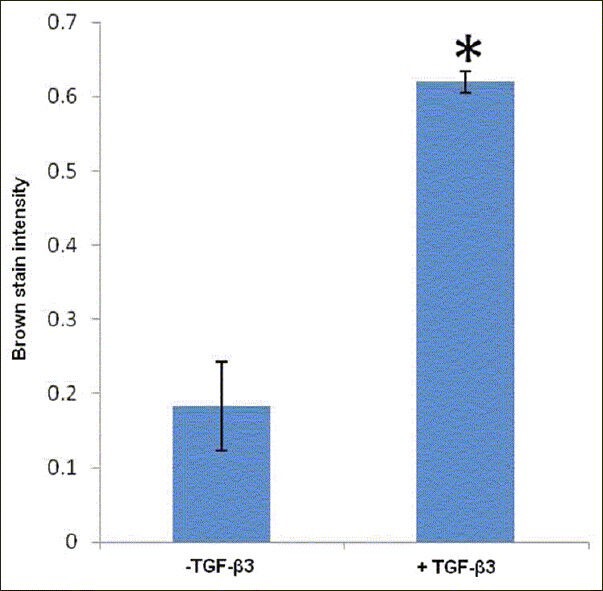
Diagram of analysis of immunohistochemical pictures
DISCUSSIONS
In our study, pellet system was used in order to redifferentiate chondrocytes that lost their phenotype during monolayer culture. Today, pellet culture as a three-dimensional culture system is largely used in chondrogenic studies that provide appropriate cell to cell interactions like cellular communications during cartilage formation in the embryonic period.[33]
Bernstein et al. showed that the expression of type II collagen and SOX9 genes in pellet system was higher than that in alginate scaffold in pig knee joint chondrocytes.[34]
Despite many studies on the effect of growth factors such as HGF, EGF, PDGF-AB, bFGF, IGF-I and TGF-β on chondrogenic process, there is still not enough information for choosing appropriate growth factors. It seems that TGF-β is more suitable for more synthesis of extracellular matrix.[35,36] In vitro studies on the effect of TGF-β in chondrocytes have shown quite opposite results. Some researchers found the increase of proliferation in the presence of TGF-β,[37,38,39,40] while some others considered this growth factor as an inhibitor to proliferation.[41] This contradiction has also been observed about the effects of this growth factor on the synthesis of extracellular matrix by chondrocytes. As some studies showed that TGF-β increased the synthesis of glycosaminoglycans, both in vivo and in vitro,[42,43] another study showed that adding this growth factor to chondrocytes inhibited the synthesis of glycosaminoglycans.[44] In Gruber's study, the use of TGF-β increased proteoglycan biglycan and decreased proteoglycan decorin simultaneously.[38] Our results showed that mean aggrecan in the treated group with TGF-β3 was significantly higher than in the control group in the pellet system.
An important point that is usually ignored in studies conducted on the effect of TGF-β on chondrocytes and stem cells is that this growth factor exists by different values (from 2.7 ± 0.37 to 20.9 ± 2.2 ng/mL) in FBS used in the culture medium,[45] as the presence or absence and serum concentration can disrupt the results of the experiment. Gunja et al. examined concurrent effect of TGF-β and mechanical pressure on cells removed from a rabbit's meniscus tear and tried to minimize FBS concentration in experiments to solve the problem. In the first stage, he reduced the concentration of FBS from 10% to 1% in the medium, which resulted in the reduction of synthesis of collagen and glycosaminoglycan by 50% and the number of cells by 60%. In the second stage, he used TGF-β1 with a concentration of 10 ng/mL in the medium, which resulted in the increase of collagen by 15-times and glycosaminoglycan by eight times.[46] In the present study, the effect of TGF-β in the chondrogenic induction stage in synthesis of aggrecan was examined using pellet system and chondrogenic medium without FBS.
The results of the present study confirm the results of the studies showing a positive effect of TGF-β on the synthesis of extracellular matrix, as the produced proteoglycan in the experimental group was more than that of the control group. However, an important question on the use of growth factors is the mechanism with which these factors increase synthesis of proteoglycans. In normal conditions, synthesis of proteoglycans by chondrocytes is controlled using a negative feedback mechanism, as the chondrocytes are sensitive to proteoglycan concentrations in extracellular matrix and, when these concentrations become adequate, synthesis of proteoglycans reduces. Therefore, without the interference of another factor, the amount of cell proliferation determines the content of proteoglycan of matrix.[47] Because the amount of cell proliferation probably was decreased in pellet system, it could be concluded that the increase of proteoglycan aggrecan was mainly due to the increase of synthetic activity of chondrocytes than the increase of their proliferation activity. In other words, TGF-β3 increased the cell activity probably through stimulating them, which resulted in higher production of aggrecans in extracellular matrix; therefore, the cell proliferation was less effective in this process.
Regarding the positive effect of TGF-β3 in production of aggrecan by chondrocytes in pellet culture system, it should be considered the other effects of TGF-β3 in this culture condition like production of type I and II collagen and SOX9.
CONCLUSION
The presence of TGF-β3 in the chondrogenic medium leads to higher production of aggrecan by chondrocytes cultivated in pellet system, but we suggest higher evaluation for another effects of this growth factor in pellet system.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Stewart MC, Saunders KM, Wurster NB, Macleod JN. Phenotypic stability of articular chondrocytes in vitro: The effects of culture models, bone morphogenetic protein-2, and serum supplementation. JBMR. 2000;15:166–74. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- 2.Aigner T, Stove J. Collagens-major component of the physiological cartilage matrix, major target of cartilage degeneration, major tool in cartilage repair. Adv Drug Deliv Rev. 2003:1569–93. doi: 10.1016/j.addr.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Temenoff JS, Mikos AG. Tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21:431–40. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Camarillo MA, Almonte-Becerril M, Vasquez Tort M, Tapia-Ramirez J, Kouri Flores JB. Chondrocyte proliferation in a new culture system. Cell Prolif. 2009;42:207–18. doi: 10.1111/j.1365-2184.2008.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickey DG, Frenkel SR, DiCesare PE. Clinical applications of growth factors for articular cartilage repair. Am J Orthop (Belle Mead NJ) 2003;32:70–6. [PubMed] [Google Scholar]
- 6.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: Roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7–16. [PubMed] [Google Scholar]
- 7.Mithofer K, Williams EJ, III, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: A prospective cohort study. J Bone Joint Surg Am. 2005;87:1911–20. doi: 10.2106/JBJS.D.02846. [DOI] [PubMed] [Google Scholar]
- 8.Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: Surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170–6. [PubMed] [Google Scholar]
- 9.Sterett WI, Steadman JR. Chondral resurfacing and high tibial osteotomy in the varus knee. AmJ Sports Med. 2000;32:1243–9. doi: 10.1177/0363546503259301. [DOI] [PubMed] [Google Scholar]
- 10.Gobbi A, Nunag P, Malinowski K. Treatment of full thicknesschondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc. 2005;13:213–21. doi: 10.1007/s00167-004-0499-3. [DOI] [PubMed] [Google Scholar]
- 11.Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: Role of apoptosis. Arthraitis Rheum. 2000;43:215–25. doi: 10.1002/1529-0131(200001)43:1<215::AID-ANR26>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Hering T, Kollar J, Huynh TD, Varelas JB, Sandell LJ. Modulation of extracellular matrix gene expression in bovine high density chondrocyte cultures by ascorbic acid and enzymatic resuspension. Arch Biochem Biophys. 1994;314:90–8. doi: 10.1006/abbi.1994.1415. [DOI] [PubMed] [Google Scholar]
- 13.Lefebvre V, Garofalo S, Zhou G, Metsaranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocyte from type 2 collagen/β-galactosidase transgenic mice. Matrix Biol. 1994;14:329–35. doi: 10.1016/0945-053x(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 14.Hiraki Y, Inoue H, Shigeno C, Sanma Y, Bentz H, Rosen DM, et al. Bone morphogenetic proteins (BMP-2 and BMP-3) promote growth and expression of differentiated phenotype of rabbit chondrocyte and osteoblastic MC3T3-E1 cells invitro. J Bone Miner Res. 1994;12:1373–85. doi: 10.1002/jbmr.5650061215. [DOI] [PubMed] [Google Scholar]
- 15.Luyten FP, Chen P, Paralkar V, Reddi AH. Recombinant human bone morphogenetic protein -4, transforming growth factor beta-1, and activin A enhance the cartilage phenotype of articular chondrocytes in vitro. Exp Cell Res. 1994;210:224–9. doi: 10.1006/excr.1994.1033. [DOI] [PubMed] [Google Scholar]
- 16.Luyten FP, Yu YM, Yanagishita M, Vukicivic S, Hammonds RG, Reddi AH. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explants cultures. J Biol Chem. 1992;267:3691–95. [PubMed] [Google Scholar]
- 17.Sailor LZ, Wang JH, Morris EA, Hewick RH. Bone morphogenetic protein-2 (BMP-2) maintains the phenotype of articular chondrocytes in long term monolayer culture. J Orthop Res. 1996;14:937–45. doi: 10.1002/jor.1100140614. [DOI] [PubMed] [Google Scholar]
- 18.Guo J, Jourdian GW, McCallum DK. Culture and growth characteristics of chondrocyes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277–97. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 19.Hauselmann HJ, Masuda K, Hunziker EB, Neidhart M, Mok SS, Michel BA, et al. Adult human chondrocyte cultured in alginate form a matrix similar to native human articular cartilage. Am J Physiol. 1996;271:C742–52. doi: 10.1152/ajpcell.1996.271.3.C742. [DOI] [PubMed] [Google Scholar]
- 20.Lemare F, Steimberg N, Le Greil C, Demignot S, Adolphe M. Differentiated chondrocytes cultured in alginate beads: Restoration of the phenotype and metabolic responses to interlukin-1β. J Cell Physiol. 1998;176:303–13. doi: 10.1002/(SICI)1097-4652(199808)176:2<303::AID-JCP8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Banu N, Tsuchiya T. Markedly different effects of hyaluronic acid and chondroitin sulfate-A on the differentiation of human articular chondrocytes in micromass and 3-D honeycomb rotation cultures. J Biomed Mater Res A. 2007;80:257–67. doi: 10.1002/jbm.a.30931. [DOI] [PubMed] [Google Scholar]
- 22.Rahman MS, Tsuchiya T. Enhancement of chondrogenic differentiation of human articular chondrocytes by biodegradable polymers. Tissue Eng. 2001;7:781–90. doi: 10.1089/107632701753337726. [DOI] [PubMed] [Google Scholar]
- 23.Bradham DM, Horton WE., Jr In vivo cartilage formation from growth factor modulated articular chondrocytes. Clin Orthop Relat Res. 1998;352:239–49. [PubMed] [Google Scholar]
- 24.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. Invitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–72. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 25.Martin JA, Buckwalter JA. The role of chondrocyte-matrix interaction in maintaining and repairing articular cartilage. Biorheology. 2003;37:129–40. [PubMed] [Google Scholar]
- 26.HashemiBeni B, Razavi SH, Esfandiari E, Karbasi S, Mardani M, Sadeghi F, et al. Effect of transforming growth factor-β3 and bone morphogenetic protein-6 growth factors on chondrogenic differentiation of adipose-derived stem cells in alginate scaffold. J Isfahan Med Sch. 2010;28:607–20. [Google Scholar]
- 27.Hahemibeni B, Razavi SH, Esfandiary E, Karbasi S, Mardani M, Nasresfahani M. Induction of chondrogenic differentiation of human adipose-derived stem cells with TGF-β3 in pellet culture system. Iran J Basic Med Sci. 2008;11:10–7. [Google Scholar]
- 28.Ibold Y, Lubke C, Pelz S, Augst H, Kaps C, Ringe J, et al. Effect of different ascorbate supplementations on in vitro high-density pellet cultures. Tissue Cell. 2009;41:249–56. doi: 10.1016/j.tice.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Cheng C, Conte E, Pleshko-Camacho N, Hidaka C. Differences in matrix accumulation and hypertrophy in superficial and deep zone chondrocytes are controlled by bone morphogenetic protein. Matrix Biol. 2007;26:541–53. doi: 10.1016/j.matbio.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimaud E, Heymann D, Redini F. Recent advances in TGF-β effects on chondrocyte metabolism. Potetialtrapeutic roles of TGF-β in cartilage disorders. Cytokine Growth Factor Rev. 2002;13:241–57. doi: 10.1016/s1359-6101(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 31.Fan H, Irrgang JJ, Anderson AF, Boland AL, Horner CD, Kurosako M, et al. Porus gelatin-chondroitin-hyaluronatetricopolymer scaffold containing microspheres loaded with TGF-β1 induces differentiation of mesenchymal stem cells invivo for enhancing cartilage repair. J Biomed Mater Res A. 2006;77:785–94. doi: 10.1002/jbm.a.30647. [DOI] [PubMed] [Google Scholar]
- 32.Van Beuningen HM, Glansbeek HL, van der Kraan PM, van den Berg WB. differential effects of local application of BMP-2 or TGF-β 1 on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–17. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- 33.Yang IH, Kim SH, Kim YH, Sun HJ, Kim SJ, Lee JW. Comparison of phenotypic characterization between alginate bead and pellet culture system as chondrogenic differentiation models for human mesenchymal stem cells. Yonsei Med J. 2004;45:891–900. doi: 10.3349/ymj.2004.45.5.891. [DOI] [PubMed] [Google Scholar]
- 34.Bernstein P, Dong M, Corbeil D, Gelinsky M, Gunther KP, Fickert S. Pellet culture elicits superior chondrogenic redifferentiation than alginate-based systems. Biotechnol Prog. 2009;25:1146–52. doi: 10.1002/btpr.186. [DOI] [PubMed] [Google Scholar]
- 35.Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explants cultures derived from the knee joint meniscus. Osteoarthritis Cartilage. 1995;3:127–38. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 36.Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23:1184–90. doi: 10.1016/j.orthres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 37.De Hart WJ, Van Osch GJ, Verhaar JA. Optimization of chondrocyte expansion in culture. Effect of TGF beta-2, b FGF and L- ascorbic acid on bovine articular chondrocytes. Acta Orthop Scand. 1999;70:55–61. doi: 10.3109/17453679909000959. [DOI] [PubMed] [Google Scholar]
- 38.Gruber HE, Fisher EC, Jr, Desaei B, Stasky AA, Hoelscher G, Hanley EN., Jr Human invertebral disc cells from the annulus: Three dimensional culture in agaroseor alginate and responsiveness to TGF-beta 1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 39.Matsumura T, Whelan MC, Li XQ, Tripple SB. Regulation by IGF-1 of Swarm-rat chondrosarcoma chondrocytes. J Orthop Res. 2000;18:351–5. doi: 10.1002/jor.1100180305. [DOI] [PubMed] [Google Scholar]
- 40.Nixon AJ, Lillich JT, Burton-Wuster N, Lust G, Mohammed HO. Differential cellular function in fetal chondrocyte s cultured with insulin-like growth factor-1 and transforming growth factor-beta. J Orthop Res. 1998;16:531–41. doi: 10.1002/jor.1100160503. [DOI] [PubMed] [Google Scholar]
- 41.Boumediene K, Vivien D, Marco M, Bogdanovicz P, Lebrun E, Pujol JP. Modulation of rabbit articular chondrocyte (RAC) proliferation by TGF-beta isoforms. Cell Prolif. 1995;28:221–34. doi: 10.1111/j.1365-2184.1995.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 42.Richmond RS, Carlson CS, Register TC, Shunker G, Loeser RF. Functional esterogen receptors in adult articular cartilage. Arthritis Rheum. 2000;43:2081–90. doi: 10.1002/1529-0131(200009)43:9<2081::AID-ANR20>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Ushiyama T, Ueyama H, Inoue K, Ohkubo I, Hukuda S. Expression of genes for esterogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis and Cartilage. 1999;7:560–6. doi: 10.1053/joca.1999.0260. [DOI] [PubMed] [Google Scholar]
- 44.Van Osch GJ, Van der Veen SW, Buma P, Verwoerd-Verhoef HL. Effects of transforming growth factor-β on proteoglycan synthesis by chondrocytes in relation to differentiation stage and presence of pericellular matrix. Matrix Biol. 1998;17:413–24. doi: 10.1016/s0945-053x(98)90101-9. [DOI] [PubMed] [Google Scholar]
- 45.Wright M. TGF-β1 in bovine serum. Art Sci. 2001;19:1–3. [Google Scholar]
- 46.Gunja NJ, Uthamanthil RK, Athanasiou KA. Effects of TGF-β1 and hydrostatic pressure on meniscus cell-seeded scaffolds. J Biomater. 2009;30:565–73. doi: 10.1016/j.biomaterials.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lucas PA, Dziewiatkawski DD. Feedback control of selected biosynthetic activities of chondrocyte in culture. Connect Tissue Res. 1987;16:323–41. doi: 10.3109/03008208709005618. [DOI] [PubMed] [Google Scholar]


