Abstract
Biosensors are small devices that employ biological/biochemical reactions for detecting target analytes. Basically, the device consists of a biocatalyst and a transducer. The biocatalyst may be a cell, tissue, enzyme or even an oligonucleotide. The transducers are mainly amperometric, potentiometric or optical. The classification of biosensors is based on (a) the nature of the recognition event or (b) the intimacy between the biocatalyst and the transducer. Bioaffinity and biocatalytic devices are examples for the former and the first, whereas second and third generation instruments are examples for the latter. Cell-based biosensors utilizing immobilized cells, tissues as also enzyme immunosensors and DNA biosensors find variegated uses in diagnostics. Enzyme nanoparticle-based biosensors make use of small particles in the nanometer scale and are currently making a mark in laboratory medicine. Nanotechnology can help in optimizing the diagnostic biochips, which would facilitate sensitive, rapid, accurate and precise bedside monitoring. Biosensors render themselves as capable diagnostic tools as they meet most of the above-mentioned criteria.
Keywords: Biocatalyst transducer, biosensor, DNA biosensor, nanotechnology
INTRODUCTION
The field of biosensors has crossed lots of leaps and bounce and now become as one of the essential state of the art technology in laboratory medicine especially in point of care testing. The idea of biosensors has revolutionized the concept of self-testing by the patient in many clinical conditions especially diabetes mellitus. In this review we have discussed about biosensors, their classifications, mechanisms involved in their function and their diagnostic applications.
Historical perspectives
Professor Clark father of biosensor, has published his monumental paper on oxygen electrode using the enzyme glucose oxidase (GOX) in transducer in measurement of glucose. Where he describes different methods of electro-chemical sensors made analytically viable,[1] Guilbault and Montalvo elaborated the potentiometric enzyme electrode based on the urease immobilization technique. This remains the trigger for the development of thermal enzyme probes and enzyme thermistors.[2,3] The idea of incorporating bacteria as the biological element in microbial electrodes revolutionized the biosensor industry.[4,5] In 1975, fiberoptic sensors (optodes) with immobilized indicators were also released for a commercial purpose, and are presently proving their worth as regards in vivo measurement of pH, pCO2, and pO2 (blood gas parameters).[6] Subcutaneous implantation of the needle-type in vivo enzyme electrode of glucose biosensors was reported successfully, and its utility has immense value in diabetic management.[7] Liedberg et al. elaborated on the basis of fixing antibodies to a piezoelectric or potentiometric transducer in direct immunosensors.[8,9] Involving the nucleic acid biosensor as a microchip and enzyme nanoparticle-based biosensor has also been making rapid strides in clinical diagnostics and therapeutics as well.[10,11] These biosensors of recent years, e.g., horseradish peroxidase nanoparticles, afford reagent less electronic biosensors, which are simple, sensitive and accurate, and could be extended to clinical chemistry assays.[12]
WHAT IS BIOSENSOR?
Biosensors are small devices that utilize biological reactions for detecting target analytes.[13] The analytical device essentially consists of a biocatalyst and a transducer.
The biocatalyst in biosensor can be an enzyme, cell, tissue or even an oligonucleotide, etc.
The transducer effectively converts the biological or biochemical signal produced by the catalyst into a quantifiable signal. The common transducing elements including optical and electrochemical generate light and current signals, respectively.[14]
Analyte → Bioreceptor …Recognition → Biocatalyst → Transducer→ Measurement.
LOGISTICS
For a biosensor to be pragmatic, efficient and capable, it must inherently possess the following features and the biocatalyst must be highly specific for the analyte under investigation:
It must not be labile when subjected to normal storage conditions
It should exhibit good and uniform stability over a significant number of assays
The reaction should be independent of various physical parameters
The cofactors/coenzymes must be co-immobilized with the biocatalyst
Accurate, precise and linear results must be obtained without the need to resort to dilution/concentration
While using the biosensor for invasive monitoring, care must be taken to ensure that the probe is tiny and biocompatible and should eschew toxic or antigenic effects.
TRANSDUCERS
The key component of a biosensor is the transducer, which utilizes a physical change accompanying the given reaction. The change may be heat absorbed or released by the reaction: Calorimetric biosensors, or may refer to the distribution of charges leading to an electrical potential to be produced: Potentiometric biosensors. Alternatively, it may represent the movement of electrons produced in a redox reaction: Amperometric biosensors. In addition, transducers also signify light output during the course of the reaction or difference in light absorbance between the reactants and products (optical biosensors), and those denoting effects are attributable to the mass of the reactants or products (piezo – electric biosensors).[14]
Table 1 enumerates the various transducers and their modes of functioning.
Table 1.
Transducers in biosensors
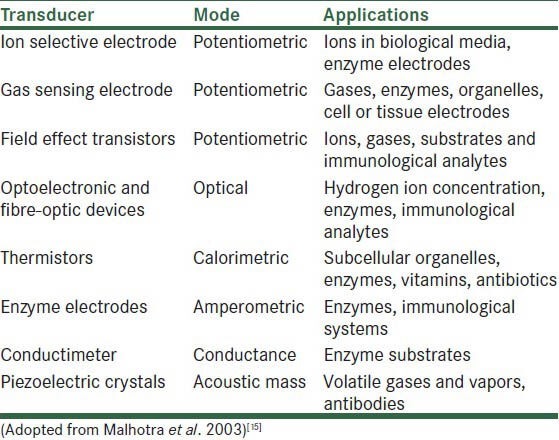
Some of the well-documented applications are also depicted therein.
CLASSIFICATION OF BIOSENSORS
Broad classification for biosensors includes the following criteria:
-
Based on the nature of the recognition event
- Bioaffinity devices: These rely on the selective binding of target analyte to a surface-restricted ligand partner (e.g., antibody, oligonucleotide, DNA, cell…)
- Biocatalytic devices: An immobilized enzyme is used herein to help recognize target substrate. e.g., Sensor strips with immobilized GOX have been in vogue for personal/home monitoring of diabetes mellitus.[12]
Glucose biosensors account for 90% of the market worldwide. In this review, we briefly summarize the principles of biosensors, the current commercial devices available for glucose and glycol-hemoglobin measurements and the recent work in the area of artificial receptors, and the potential for the development of new devices for diabetes specifically connected with in vitro monitoring of glucose.[16]
-
Based on different generation instruments
- First generation instruments: Herein, the two components, viz. biocatalyst and transducer, may be resolved and both may in turn remain functional in the absence of the other
- Second generation instruments: Enzyme membrane electrodes incorporating with mediators in detection are available, e.g., GOX association with peroxide detection are available as regards diluted samples.[17]
However, rapidly responding sensors are prepared by covering metal electrodes with a porous enzyme layer. The key feature as regards the second generation biosensors is that auxiliary enzymes and/or co-reactants are co-immobilized with the principal enzyme, thereby enhancing the analytical quality. The oxidizable interferences are limited by using a GOX/peroxidase complex that communicates with the electrode at a low working potential. Enzymatic recycling of the analyte and/or accumulation of intermediates increases the sensitivity several folds. For instance, the inclusion of Nicotinamide Adenine Dinucleotide NAD bound to polyethylene glycol in the glucose dehydrogenase layer allows a reagentless glucose measurement. Second generation biosensors use an artificial electron mediator that replaces O2 as the electron shuttle. Ferrocene, quinones, quinoid-like dyes, etc., have been used as mediators[18,19] [Figure 1]. Diabetes is a chronic disorder resulting from insulin deficiency and hyperglycemia, and has a high-risk of development of complications for the eyes, kidneys, peripheral nerves, heart, and blood vessels. There is a large population in the world suffering from this disease, and the healthcare costs increase every year. Quick diagnosis and early prevention are critical for the control of the disease status. Traditional biosensors such as glucose meters and glycohemoglobin test kits are widely used in vitro for this purpose because they are the two major indicators directly involved in diabetes diagnosis and long-term management. The market size and huge demand for these tests makes it a model disease to develop new approaches to biosensors.
Figure 1.
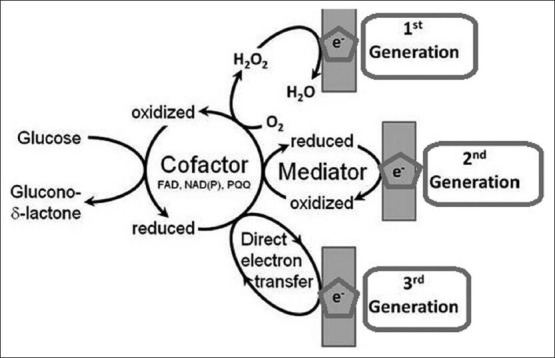
Schematic representation of three generations of glucose biosensor: Adapted from Ferri et al.[30]
Most oxidase enzymes are not choosy with respect to oxidizing agent, and allow substitution of a variety of artificial oxidizing agents, as depicted in the following reaction with GOX.
GOX − FADH2 + M ediatorOX ↔ GOX − FAD + M ediatorred
In this category, the biocatalyst and transducer interact in a more intimate fashion, and the removal of either of the two components affects the functioning of the other.
Third generation instruments: Herein, the biochemistry and electrochemistry are more closely associated without mediators. [Figure 1] When the electrochemistry occurs at a semi-conductor, the biochip may be applied to such instruments. Third generation sensors are reagentless, characterized by the progression from use of a freely diffusing mediator (O2 or artificial) to a system wherein the biocatalyst and mediator are coimmobilized at an electrode surface, thereby making the bio-recognition component an integral part of the electro de transducer. The process of coimmobilization of enzyme and mediator could be achieved by redox mediator labeling of the enzyme followed by enzyme immobilization, enzyme immobilization in a redox polymer or even enzyme and mediator immobilization on a suitably conducting polymeric surface.[16,20]
There has been an explosion of research into the physical and chemical properties of carbon-based nanomaterials since the discovery of carbon nanotubes by Iijima in 1991.[21,22] Carbon nanomaterials offer unique advantages in several areas, like high surface-volume ratio, high electrical conductivity, chemical stability and strong mechanical strength, and are thus frequently being incorporated into sensing elements. Carbon nanomaterial-based sensors generally have higher sensitivities and a lower detection limit than conventional ones.[21]
Mechanism of first generation instruments
Step 1
The biocatalyst within a biosensor responds to the substrate in solution by catalyzing the specific reaction.
Step 2
The rate of the reaction is measured by various ways using different transducers.
e.g., Estimation of glucose using GOX
Glucose + O2− −−GOX → Gluconic acid
The rate of the reaction can be measured by the following below-mentioned steps:
The rate of consumption of O2 can be measured amperometrically by its reduction at a platinum cathode polarized at - 0.6 V as against the standard calomel electrode (Clark oxygen electrode)
The rate of production of H2O2 can be measured by its oxidation at a platinum anode polarized at + 0.7 V as against the standard calomel electrode
The rate of production of gluconic acid can be measured using a pH electrode to measure the accompanying decrease in pH. Several reports are available pertaining to the role of hydrogen peroxide and peroxidase in biosensors.[7,23,24]
Mechanism of second generation instruments
E.g., Glucometer (home monitoring of blood glucose).
In the above device, the intimacy between biocatalyst and transducer is increased by facilitating the design of an electrode surface that is competent of capturing electrons transferred in the redox reaction.
In this device, the rate of oxidation of glucose is measured not by the rate of disappearance of substrate or appearance of product but by the rate of electron flow from glucose to an electrode surface.
The reactions that occur in the device are sequentially described below:
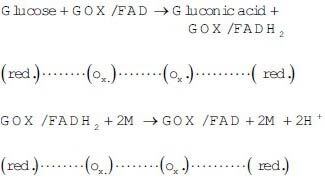
At the electrode

M → Mediator (e. g., Ferrocane)
The electrons donated to the electrode produce a current that is proportional to the rate of oxidation.[25,26,27]
Third generation instruments
Such instruments are essentially at the research level, primarily to eliminate complicated artificial mediators by adding metals, to overcome the difficulty to relate to electron transfer between electrode and enzyme. Presently, commercial viability is vague due temperature, pH, toxic chemical and metals. Although most biosensors in articles perform well in research labs, these could not be used for his/her sample. Therefore, it may take quite some time to make commercially available.[28,29]
CELL-BASED BIOSENSORS
These are based on the use of immobilized whole cells and tissues.[31]
Merits
More economical to manufacture as compared with those depending on enzymes
No requirement for complex biocatalyst isolation and laborious purification procedures.[32]
Demerits
TYPES OF CELL-BASED BIOSENSORS
Single cell type
Multicell type
Combination of cell and enzyme.
Single cell type
Estimation of cholesterol using microorganisms that act a source of cholesterol oxidase is immobilized to polyacrylamide or agar on an oxygen electrode.
Cholesterol + O2 → Cholest-4-en-3 one + H2O2
Cholesterol can be estimated by either using an oxygen electrode to measure the rate of O2 uptake or amperometric measurement of H2O2 production.[35]
Multicell type
It can be used to enhance the number of potential applications.
Combination of cell type and enzyme
Purified NAD are from Neurospora crassa in association with Escherichia coli (rich in nicotinamide deaminase activity), which would catalyze the following reactions.
NADase
NAD + H2O → Nicotinamide + ADP-ribose
Nicotinamide + H2O → Nicotinic acid + NH3 Nicotinamide deaminase
The ammonia released can be detected by gas sensing electrode (transducer) to produce an NAD+ sensitive biomarker.[36]
Cell types other than microorganisms
In addition to the above-mentioned procedures, immobilization of banana pulp in an oxygen electrode to design a dopamine-sensitive biosensor has also been attempted.
Banana pulp is rich in the enzyme polyphenol oxidase, and the enzyme found in banana pulp possesses a high selectivity for the neuroactive agent dopamine.[37]
Cell-based biosensors are variedly used in cancer research as well, for assessing the tumor cell sensitivity to pharmacological drugs, detection of toxins and chemical substances and clinical trial of new drugs.[38] A recent study by Ellis and Wolfgang has developed a cell-based biosensor to study fatty acid metabolism using malonyl CoA responsive element.[39]
Enzyme immunosensors
These biosensors combine the use of molecular recognition properties of antibodies with the high sensitivity of an enzyme-based analytical method.
A non-labeled immunosensor, whose selectivity depends on immunochemical affinity of an antigen for its corresponding antibody, has been developed as the basis for the potentiometric determination of an antigen with an antibody-bound membrane or electrode. These immunosensors are available for syphilis antibody, blood group typing, human chorionic gonadotropin (hCG) and human serum albumin. In contrast, the labeled immunosensors may be characterized by a pronounced increase of sensitivity. Of these labeled immunosensors, mention must be made of enzyme immunosensors that use the chemical amplification of a labeling enzyme for sensitivity. Enzyme immunosensors with an oxygen electrode have been fabricated to determine alpha-fetoprotein AFP, hCG, IgG and toxin. Bioaffinity sensors with a pre-formed metastable ligand-receptor complex that are homologus to the enzyme immunosensor have been used in the determination of thyroxine (T4), biotin and insulin.[40,41,42]
Mechanism
Competition between the enzyme-labeled antigen and unlabeled antigen for an antibody immobilized on an appropriate transducer forms the basis.
Following are the steps that need to be carried out while using an immunosensor for IgG using an amperometric oxygen electrode.[43]
Step 1
Oxygen electrode that contains a membrane onto which will be bound an anti-IgG antibody.
Step 2
Free IgG is labeled with the enzyme catalase.
Step 3
A known amount of this labeled IgG is mixed with a sample containing an unknown amount of unlabeled IgG.
Step 4
This mixture is then placed into the chamber of the oxygen electrode and the labeled and the unlabeled IgG compete for the antibody on the membrane.
Step 5
Rinse the sensor to remove any non-specifically associated IgG.
Step 6
The sensor is then filled with H2O2 solution, which acts as a substrate for catalase. The more unlabeled IgG that is present, lower the amount of the labeled IgG and lower the rate of oxygen evolution.
Regeneration
The biosensor is regenerated by rinsing with an appropriate acidic buffer such as glycine – HCl to dissociate the antigen from the antibody.
Transducers in immunosensors
Limitations
The time taken for each assay (antigen-antibody coupling) takes several hours. To overcome the limitations, binding and rinsing processes are carried out as separate stages and then the membrane is introduced to the transducer. This will allow one sensor to be used with several antibody-linked membranes and measure numerous samples over a short period of time.
The time taken for each assay (antigen-antibody coupling) takes several hours. To overcome the limitations, binding and rinsing processes are carried out as separate stages and then the membrane is introduced to the transducer. This will allow one sensor to be used with several antibody-linked membranes and measure numerous samples over a short period of time.
DNA BIOSENSORS
DNA biosensors are based on nucleic acid recognition processes whose task is to envisage, rapid, simple and economical testing of genetic and infectious diseases and also for enabling the detection of DNA damage and interactions. A DNA biosensor microchip that is suited clinically has also been developed.[45]
Advantages
The greatest merit is that nucleic acid recognition layers can be readily synthesized and regenerated for multiple uses. This is unlike enzymes or immunoglobulins.[44,46,47]
Types of DNA biosensors
Sequence-specific hybridization biosensor:
Optical biosensors
Electrochemical biosensors
Mass sensitive devices
DNA microarrays.
SEQUENCE-SPECIFIC HYBRIDIZATION BIOSENSORS
They depend on the immobilization of a single stranded ss DNA probe onto the transducer surface. The duplex formation can be aptly detected following the association of an appropriate hybridization indicator or through other changes resulting from the binding event. The immobilization step should lead to a well-defined probe orientation that is readily accessible to the target. Depending upon the nature of the physical transducer, various schemes can be used for attaching the DNA probe to the surface.[48]
The following possibilities exist:
Use of thiolated DNA for self-assembly into gold transducers and covalent linkage to the gold surface through a functional alkanethiol-based monolayer are examples of schemes that could be utilized for attaching the DNA probes[49]
Use of biotinylated DNA for complex formation with a surface-confined avidin is another possible scheme. Covalent coupling to functional groups on carbon electrodes and, alternately, a simple adsorption onto the carbon surface are other instances of available schemes.[50]
Nucleic acid recognition is the basis on which the DNA sensors are constructed. The advent of peptide nucleic acid (PNA) has opened up new vistas in biosensors. In PNA, the sugar phosphate backbone is replaced with a pseudopeptide. The features of solution phase PNA could be extended onto the transducer surfaces while designing the uniquely selective DNA biosensors. Surface-confined PNA recognition layers attribute sequence specificity onto DNA biosensors that could detect even single base mismatch. The ramified superstructures possessing numerous single stranded arms that could hybridize to the complementary DNA sequence are the DNA dendrimers. Immobilizing these dendritic nucleic acids onto the physical transducers imparts greatly increased hybridization capacity and is synonymous with a substantially amplified response.[51]
Optical DNA biosensors
Fiberoptics are essential devices that carry light form one place to another through a series of internal inflections, and DNA optical biosensors employ such devices. The ss DNA probe is placed at one end of the fiber and fluorescent changes resulting from the association of a (fluorescent) indicator with the double stranded ds DNA hybrid is measured. The first developed DNA optical biosensor centered around the use of ethidium bromide as an indicator. Extremely low concentrations (femtomolar) could be detected in comparison with other conventional fluorescent indicators. In general, the hybridization of fluorescently labeled complementary oligonucleotides is monitored by observing the increase in fluorescence that accompanied the binding. Optical transduction based on evanescent wave devices provides real-time label-free optical detection of DNA hybridization. These rely on monitoring changes in surface optical properties.[52,53]
The coupling of chemi-luminescence with sandwich hybridization, magnetic bead capture and flow injection operation have been used in the rapid tests for detection of hepatitis B virus DNA.
In recent years, yet another innovative technique based on molecular beacons (Mbs) has been developed. Mbs are oligonucleotides (stem and loop structures) labeled with a fluorophore and a quencher on the two extremities of the stem that acquire fluorescence upon hybridization. This procedure monitors capability and Mb offers high sensitivity and specificity in mutational analysis.[54]
Electrochemical biosensors
Electrochemical detection of DNA hybridization pertains to monitoring a current response under controlled potential conditions. The hybridization event is commonly detected through the increased current signal of a redox indicator (that recognize duplex DNA) or from other hybridization-induced changes in electrochemical parameters. The use of a threading intercalator, viz. ferrocenyl naphthalene diimide, in binding to the DNA tightly has also been launched. Use of enzyme labels for electrochemical detection of DNA hybridization is in vogue. A direct amperometric monitoring of the hybridization event could be achieved with regard to the use of horse radish peroxidase-labeled target. The hybridization event eventually resulted in the “Wiring” of the enzyme to the transducer, thereby letting in a continuous electroreduction current attributable to hydrogen peroxide.
Novel label-free electrochemical detection schemes affording rapid and simpler assays have come into existence. Changes occurring in the intrinsic electroactivity of DNA as a result of hybridization could be monitored.[55] One of the useful indicators of free detection scheme is provided by mass sensitive devices wherein quartz crystal microbalance (QCM) transducers are employed. These facilitate dynamic monitoring of hybridization events. Basically, QCM hybridization biosensors consist of an oscillating crystal with the DNA probe immobilized.[56]
A highly sensitive device has been developed to help detect the genetic disorder, Tay-Sach's disease. Complex DNA samples with reference to their sequence and information could be analyzed by the integration of multiple biosensors in association with DNA microarrays.[57]
NANOTECHNOLOGY
Nanotechnology is a term covering a wide range of technologies concerned with the structures and processes on the nanometer scale. Enzyme nanoparticle-based biosensors are being put to use, which are simple and sensitive, and effective methods are available, wherein an enzyme electronic biosensor could be fabricated by immobilizing enzyme nanoparticles onto the gold electrode surface. Horseradish peroxidase nanoparticles have been successfully mobilized to develop reagentless electronic biosensors for H2O2 detection without calling for promoters and mediators and hence offer a great potential to develop elegant enzyme-based and competent electronic biosensors.[58]
Application in diagnostics
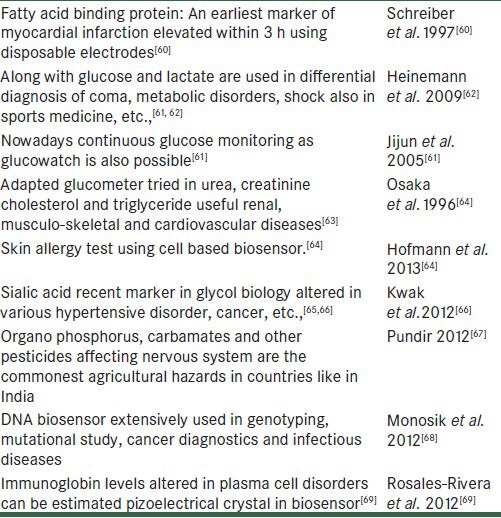
Nanotechnology can help in optimizing the diagnostics biochips. These diagnostic chips can be used to analyze thousands of genes simultaneously. For this purpose, the gene fragments with known properties synthesized in the laboratory are bonded to the chip surface, and the diagnosis depends on which gene pieces of the DNA from a patient's blood gets deposited on. Viruses and cell types can be identified on the basis of surface properties, which make them adhere to certain nanostructures.Re-generative medicine widely uses nanotechnology recently.[59,60] Also refer Table 2 for other applications of biosensor.
Table 2.
Overview applications of biosensor
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Heineman WR, Jensen WB, Leland C, Clark 1918-2005. Biosens Bioelectron. 2006;21:1403–4. [Google Scholar]
- 2.Weiner PH, Parcher JF. Improved urea electrode. Anal Chem. 1973;45:417–9. [Google Scholar]
- 3.Docolomanský P, Gemeiner P, Mislovicová D, Stefuca V, Danielsson B. Screening of concanavalin A-bead cellulose conjugates using an enzyme thermistor with immobilized invertase as the reporter catalyst. Biotechnol Bioeng. 1994;43:286–92. doi: 10.1002/bit.260430404. [DOI] [PubMed] [Google Scholar]
- 4.Timur S, Anik U, Odaci D, Gorton L. Development of a microbial biosensor based on carbon nanotube (CNT) modified electrodes. Electrochem commun. 2007;9:1810–5. [Google Scholar]
- 5.Turner AP, Karube I, Wilson GS. Oxford UK: Oxford University Press; 1987. Biosensors: Fundamentals and applications; p. 770. [Google Scholar]
- 6.Tusa JK, He H. Critical care analyzer with fluorescent optical chemosensors for blood analytes. J Mater Chem. 2005;15:2640–7. [Google Scholar]
- 7.Abel PU, von Woedtke T. Biosensors for in vivo glucose measurement: Can we cross the experimental stage. Biosens Bioelectron. 2002;17:1059–70. doi: 10.1016/s0956-5663(02)00099-4. [DOI] [PubMed] [Google Scholar]
- 8.Morgan CL, Newman DJ, Price CP. Immunosensors: Technology and opportunities in laboratory medicine. Clin Chem. 1996;42:193–209. [PubMed] [Google Scholar]
- 9.Byfield MP, Abuknesha RA. Biochemical aspects of biosensors. Biosens Bioelectron. 1994;9:373–400. doi: 10.1016/0956-5663(94)80038-3. [DOI] [PubMed] [Google Scholar]
- 10.Vo-Dinh T. Development of a DNA biochip: Principle and applications. Sens Actuators B Chem. 1998;51:52–9. [Google Scholar]
- 11.Salah KA, Alrokyan SA, Khan MN, Ansari AA. Nanomaterials as analytical tools for genosensors. Sensors (Basel) 2010;10:963–93. doi: 10.3390/s100100963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thévenot DR, Toth K, Durst RA, Wilson GS. Electrochemical biosensors: Recommended definitions and classification. Biosens Bioelectron. 2001;16:121–31. doi: 10.1016/s0956-5663(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 13.García-Martinez G, Bustabad EA, Perrot H, Gabrielli C, Bucur B, Lazerges M, et al. Development of a mass sensitive quartz crystal microbalance (QCM)-based DNA biosensor using a 50 MHz electronic oscillator circuit. Sensors. 2011;11:7656–64. doi: 10.3390/s110807656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheller F, Schubert F. Structure and function of transducer. Amsterdam: Elsevier Science Ltd; 1992. [Last cited 2012 Jul 26]. Biosensors [Internet] pp. 10–34. Available from: http://www.books.google.co.in/books?hl=en and lr=and id=TF7AW4kSY1 gC and oi=fnd and pg=PP1 and dq=SCHELLER+, SCHUBERT++1992 and ots=s8RJn1F6ff and sig=gGpTJ2asLuRsXzsqIDcdJvCJwyY . [Google Scholar]
- 15.Malhotra BD, Chaubey A. Biosensors for clinical diagnostics industry. Sens Actuators B Chem. 2003;91:117–27. [Google Scholar]
- 16.Yoo EH, Lee SY. Glucose biosensors: An overview of use in clinical practice. Sensors (Basel) 2010;10:4558–76. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Wang J. A novel improved design for the first-generation glucose biosensor. Food Technol Biotechnol. 2001;39:55–8. [Google Scholar]
- 18.Turner AP, Chen B, Piletsky SA. In vitro diagnostics in diabetes: Meeting the challenge. Clin Chem. 1999;45:1596–601. [PubMed] [Google Scholar]
- 19.Cass AE, Davis G, Francis GD, Hill HA, Aston WJ, Higgins IJ, et al. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal Chem. 1984;56:667–71. doi: 10.1021/ac00268a018. [DOI] [PubMed] [Google Scholar]
- 20.Zafar MN, Safina G, Ludwig R, Gorton L. Characteristics of third-generation glucose biosensors based on Corynascus thermophilus cellobiose dehydrogenase immobilized on commercially available screen-printed electrodes working under physiological conditions. Anal Biochem. 2012;425:36–42. doi: 10.1016/j.ab.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Z, Garcia-Gancedo L, Flewitt AJ, Xie H, Moussy F, Milne WI. A critical review of glucose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors (Basel) 2012;12:5996–6022. doi: 10.3390/s120505996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carbon nanotube [Internet]. Wikipedia, the free encyclopedia. 2012. [Last cited 2012 Nov 14]. Available from: http://www.en.wikipedia.org/w/index.php?title=Carbon_nanotube and oldid=522624068 .
- 23.Newman JD, Turner AP. Home blood glucose biosensors: A commercial perspective. Biosens Bioelectron. 2005;20:2435–53. doi: 10.1016/j.bios.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Shichiri M, Kawamori R, Yamasaki Y, Hakui N, Abe H. Wearable artificial endocrine pancrease with needle-type glucose sensor. Lancet. 1982;2:1129–31. doi: 10.1016/s0140-6736(82)92788-x. [DOI] [PubMed] [Google Scholar]
- 25.Renard E. Implantable continuous glucose sensors. Curr Diabetes Rev. 2008;4:169–74. doi: 10.2174/157339908785294406. [DOI] [PubMed] [Google Scholar]
- 26.Koschwanez HE, Reichert WM. In vitro, in vivo and post explantation testing of glucose-detecting biosensors: Current methods and recommendations. Biomaterials. 2007;28:3687–703. doi: 10.1016/j.biomaterials.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song S, Xu H, Fan C. Potential diagnostic applications of biosensors: Current and future directions. Int J Nanomedicine. 2006;1:433–40. doi: 10.2147/nano.2006.1.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Zhang WD, Ye JS. Nonenzymatic electrochemical glucose sensor based on MnO2/MWNTs nanocomposite. Electrochem commun. 2008;10:1268–71. [Google Scholar]
- 29.Ferri S, Kojima K, Sode K. Review of glucose oxidases and glucose dehydrogenases: A bird's eye view of glucose sensing enzymes. J Diabetes Sci Technol. 2011;5:1068–76. doi: 10.1177/193229681100500507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P, Liu Q. Definition of cell based biosensor: Artech House; 2009. Cell-Based Biosensors: Principles and applications; pp. 01–5. [Google Scholar]
- 31.Pancrazio JJ, Whelan JP, Borkholder DA, Ma W, Stenger DA. Development and application of cell-based biosensors. Ann Biomed Eng. 1999;27:697–711. doi: 10.1114/1.225. [DOI] [PubMed] [Google Scholar]
- 32.Aravamudhan S, Kumar A, Mohapatra S, Bhansali S. Sensitive estimation of total cholesterol in blood using Au nanowires based micro-fluidic platform. Biosens Bioelectron. 2007;22:2289–94. doi: 10.1016/j.bios.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 33.Riechel T, Rechnitz G. Hybrid bacterial and enzyme membrane electrode with nicotinamide adenine dinucleotide response. J Memb Sci. 1978;4:243–50. [Google Scholar]
- 34.Singh S, Solanki PR, Pandey MK, Malhotra BD. Cholesterol biosensor based on cholesterol esterase, cholesterol oxidase and peroxidase immobilized onto conducting polyaniline films. Sens Actuators B Chem. 2006;115:534–41. [Google Scholar]
- 35.Hikuma M, Obana H, Yasuda T, Karube I, Suzuki S. A potentiometric microbial sensor based on immobilized Escherichia coli for glutamic acid. Anal Chim Acta. 1980;116:61–7. [Google Scholar]
- 36.Sidwell JS, Rechnitz GA. Bananatrode: An electrochemical biosensor for dompamine. Biotechnol Lett. 1985;7:419–22. [Google Scholar]
- 37.Baranauskas G, Gusmeroli R, Spinelli AS, Giordano C, Raimondi MT. Cell-based biosensors: Current trends of the development. J Appl Biomater Biomech. 2006;4:125–34. [PubMed] [Google Scholar]
- 38.Ellis JM, Wolfgang MJ. A genetically encoded metabolite sensor for malonyl-CoA. Chem Biol. 2012;19:1333–9. doi: 10.1016/j.chembiol.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bojorge Ramírez N, Salgado AM, Valdman B. The evolution and developments of immunosensors for health and environmental monitoring: Problems and perspectives. Brazilian J Chem Eng. 2009;26:227–49. [Google Scholar]
- 40.Tomassetti M, Martini E, Campanella L, Favero G, Carlucci L, Mazzei F. Comparison of three immunosensor methods (surface plasmon resonance, screen-printed and classical amperometric immunosensors) for immunoglobulin G determination in human serum and animal or powdered milks. [Last cited 2012 Jul 30];J Pharm Biomed Anal. 2013 73:90–8. doi: 10.1016/j.jpba.2012.03.020. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22561059 . [DOI] [PubMed] [Google Scholar]
- 41.Aizawa M. Immunosensors. Philos Trans R Soc Lond. B Biol Sci. 1987;316:121–34. doi: 10.1098/rstb.1987.0022. [DOI] [PubMed] [Google Scholar]
- 42.Campanella L, Attioli R, Colapicchioni C, Tomassetti M. New amperometric and potentiometric immunosensors for anti-human immunoglobulin G determinations. Sens Actuators B Chem. 1999;55:23–32. [Google Scholar]
- 43.Palecek E, Fojta M, Tomschik M, Wang J. Electrochemical biosensors for DNA hybridization and DNA damage. Biosens Bioelectron. 1998;13:621–8. doi: 10.1016/s0956-5663(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 44.Borgmann S, Schulte A, Neugebauer S, Schuhmann W. Amperometric biosensors. In: Alkire RC, Kolb DM, Lipkowski J, editors. Advances in Electrochemical Science and Engineering. [Internet] Weinheim: Wiley-VCH Verlag GmbH and Co, KGaA; 2011. [Last cited 2012 Jul 31]. pp. 1–83. Available from: http://www.onlinelibrary.wiley.com/doi/10.1002/9783527644117.ch1/summary . [Google Scholar]
- 45.Fojta M. Electrochemical sensors for DNA interactions and damage. Electroanalysis. 2002;14:1449–63. [Google Scholar]
- 46.Teles FR, Fonseca LP. Trends in DNA biosensors. Talanta. 2008;77:606–23. [Google Scholar]
- 47.Hashimoto K, Ito K, Ishimori Y. Sequence-specific gene detection with a gold electrode modified with DNA probes and an electrochemically active dye. Anal Chem. 1994;66:3830–3. doi: 10.1021/ac00093a045. [DOI] [PubMed] [Google Scholar]
- 48.Lucarelli F, Marrazza G, Turner AP, Mascini M. Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens Bioelectron. 2004;19:515–30. doi: 10.1016/s0956-5663(03)00256-2. [DOI] [PubMed] [Google Scholar]
- 49.Wang J. DNA biosensors based on peptide nucleic acid (PNA) recognition layers. A review. Biosens Bioelectron. 1998;13:757–62. doi: 10.1016/s0956-5663(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 50.Stiriba SE, Frey H, Haag R. Dendritic polymers in biomedical applications: From potential to clinical use in diagnostics and therapy. Angew Chem Int Ed Engl. 2002;41:1329–34. doi: 10.1002/1521-3773(20020415)41:8<1329::aid-anie1329>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 51.Graham CR, Leslie D, Squirrell DJ. Gene probe assays on a fibre-optic evanescent wave biosensor. Biosens Bioelectron. 1992;7:487–93. doi: 10.1016/0956-5663(92)80005-v. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Tan W. A fiber-optic evanescent wave DNA biosensor based on novel molecular beacons. Anal Chem. 1999;71:5054–9. doi: 10.1021/ac990561c. [DOI] [PubMed] [Google Scholar]
- 53.Kerman K, Kobayashi M, Tamiya E. Recent trends in electrochemical DNA biosensor technology. Meas Sci Technol. 2004;15:R1–11. [Google Scholar]
- 54.Hang TC, Guiseppi-Elie A. Frequency dependent and surface characterization of DNA immobilization and hybridization. Biosens Bioelectron. 2004;19:1537–48. doi: 10.1016/j.bios.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 55.Cagnin S, Caraballo M, Guiducci C, Martini P, Ross M, Santaana M, et al. Overview of electrochemical DNA biosensors: New approaches to detect the expression of life. Sensors (Basel) 2009;9:3122–48. doi: 10.3390/s90403122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rippel RA, Seifalian AM. Gold revolution: Gold nanoparticles for modern medicine and surgery. J Nanosci Nanotechnol. 2011;11:3740–8. doi: 10.1166/jnn.2011.4170. [DOI] [PubMed] [Google Scholar]
- 57.Godin B, Sakamoto JH, Serda RE, Grattoni A, Bouamrani A, Ferrari M. Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Trends Pharmacol Sci. 2010;31:199–205. doi: 10.1016/j.tips.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Becker E, Navarro-López F, Francino A, Brenner B, Kraft T. Quantification of mutant versus wild-type myosin in human muscle biopsies using nano-LC/ESI-MS. Anal Chem. 2007;79:9531–8. doi: 10.1021/ac701711h. [DOI] [PubMed] [Google Scholar]
- 59.Malima A, Siavoshi S, Musacchio T, Upponi J, Yilmaz C, Somu S, et al. Highly sensitive microscale in vivo sensor enabled by electrophoretic assembly of nanoparticles for multiple biomarker detection. Lab Chip. 2012;12:4748–54. doi: 10.1039/c2lc40580f. [DOI] [PubMed] [Google Scholar]
- 60.Schreiber A, Feldbrügge R, Key G, Glatz JF, Spener F. An immunosensor based on disposable electrodes for rapid estimation of fatty acid-binding protein, an early marker of myocardial infarction. Biosens Bioelectron. 1997;12:1131–7. doi: 10.1016/s0956-5663(97)00003-1. [DOI] [PubMed] [Google Scholar]
- 61.Jijun T, Jie H, Zhongchao H, Min P, Yuquan C. A novel lactate biosensor. Conf Proc IEEE Eng Med Biol Soc. 2005;1:252–4. doi: 10.1109/IEMBS.2005.1616391. [DOI] [PubMed] [Google Scholar]
- 62.Heinemann L. Continuous glucose monitoring and clinical trials. J Diabetes Sci Technol. 2009;3:981–5. doi: 10.1177/193229680900300447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osaka T, Komaba S, Seyama M, Tanabe K. High-sensitivity urea sensor based on the composite film of electroinactive polypyrrole with polyion complex. Sens Actuators B Chem. 1996;36:463–9. [Google Scholar]
- 64.Hofmann U, Michaelis S, Winckler T, Wegener J, Feller KH. A whole-cell biosensor as in vitro alternative to skin irritation tests. Biosens Bioelectron. 2013;39:156–62. doi: 10.1016/j.bios.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 65.Babu MS, Bobby Z, Habeebullah S. Increased inflammatory response and imbalance in blood and urinary oxidant-antioxidant status in South Indian women with gestational hypertension and preeclampsia. Clin Biochem. 2012;45:835–8. doi: 10.1016/j.clinbiochem.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 66.Kwak BS, Kim HO, Kim JH, Lee S, Jung HI. Quantitative analysis of sialic acid on erythrocyte membranes using a photothermal biosensor. Biosens Bioelectron. 2012;35:484–8. doi: 10.1016/j.bios.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 67.Pundir CS, Chauhan N. Acetylcholinesterase inhibition-based biosensors for pesticide determination: A review. Anal Biochem. 2012;429:19–31. doi: 10.1016/j.ab.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 68.Monosik R, Stredansky M, Strurdik E. Application of electrochemical biosensor in clinical diagnosis in clinical diagnosis. J Clin Lab Anal. 2012;26:22–34. doi: 10.1002/jcla.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosales-Rivera LC, Acero-Sánchez JL, Lozano-Sánchez P, Katakis I, O’Sullivan CK. Amperometric immunosensor for the determination of IgA deficiency in human serum samples. Biosens Bioelectron. 2012;33:134–8. doi: 10.1016/j.bios.2011.12.040. [DOI] [PubMed] [Google Scholar]


