Abstract
The melanocortin system is a neuroimmunoendocrine hormone system that constitutes the fulcrum in the homeostatic control of a diverse array of physiological functions, including melanogenesis, inflammation, immunomodulation, adrenocortical steroidogenesis, hemodynamics, natriuresis, energy homeostasis, sexual function and exocrine secretion. The kidney is a quintessential effector organ of the melanocortin hormone system with melanocortin receptors abundantly expressed by multiple renal paranchymal cells, including podocytes, mesangial cells, glomerular endothelial cells and renal tubular cells. Converging evidence unequivocally demonstrates that the melanocortin based therapy by using the melanocortin peptide adrenocorticotropic hormone (ACTH) is prominently effective in inducing remission of steroid resistant nephrotic syndrome caused by a variety of glomerular diseases, including membranous nephropathy and podocytopathies such as minimal change disease and focal segmental glomerulosclerosis, suggesting a steroidogenic independent melanocortin mechanism. Mechanistically, ACTH and other melanocortin peptides as well as synthetic melanocortin analogues possess potent proteinuria reducing and renoprotective effects that could be attributable to both direct protection of glomerular cells and systemic immunomodulation. Thus, leveraging melanocortin signaling pathways by using either the existing U.S. Food and Drug Administration approved melanocorin peptide ACTH or novel synthetic melanocortin analogues represents a promising and pragmatic therapeutic strategy for glomerular diseases. This review article introduces the biophysiology of melanocortin hormone system with emphasis on the kidney as the target organ, discusses the existing clinical and experimental data on melanocortin treatments for glomerular diseases, elucidates the potential mechanisms of action, and describes the potential side effects of melanocortin based therapy.
Keywords: glomerulopathy, nephrotic syndrome, melanocortin, adrenocorticotropic hormone, podocyte, proteinuria, inflammation, immunomodulation
Glomerular disease is the third leading cause of end stage kidney failure in the US1. Treatment of glomerular disease depends on its cause and type, but currently is limited largely to the use of blockades of the renin-angiotensin-aldosterone system (RAAS) and immunosuppressants, including glucocorticoids, alkylating agents, calcinurin inhibitors, antimetabolites and more2–4. These treatments are, however, of limited utility with unsatisfying therapeutic efficacy. Indeed, although 95% children5 and 50–75% adults6 with minimal change disease (MCD) achieve complete remission of proteinuria after an 8-week course of prednisone therapy, more than 70% of all patients who are initially prednisone responsive go on to experience relapses of the nephrotic syndrome, and almost half of them show frequent relapses or steroid dependence and require further immunosuppression5,6. For other glomerular disease such as idiopathic membranous nephropathy (iMN)7,8 and focal segmental glomerulosclerosis (FSGS)9, initial immunosuppressive treatments usually have much lower response rates than for MCD, additional immunosupressants are essential to induce remission. In actuality, neither the target cells nor the mechanism of action of these immunosuppressants for glomerular disease has been clearly understood4. Most of these therapeutic strategies were borrowed from transplant immunosuppressive regimens and thus are believed to be effective in glomerular diseases also via immunosuppression; however, some such as cyclosporine A10, are found to function, at least in part, through nonimmune mechanisms10, while some others, such as levamisole11, might be effective via immune stimulatory mechanisms. Furthermore, a considerable number of patients suffer from the complications of over-immunosuppression, including opportunistic infection, neoplasia formation and growth retardation2,3. Therefore, it is imperative to develop novel and more effective therapeutic modalities with minor side effects to satisfactorily ameliorate glomerular injury and induce remission of proteinuria in patients with refractory glomerular disease. Recently, a plethora of evidence suggests that melanocortins possess potent anti-proteinuric and renoprotective activities and might serve in this role12–17.
Melanocortin system: a multitasking neuroimmunnoendocrine hormone system
The melanocortin system is a set of hormonal, neuropeptidergic, and immune signaling pathways that play an integral role in the homeostatic control of a diverse array of physiological functions, including melanogenesis, inflammation, immunomodulation, adrenocortical steroidogenesis, hemodynamics, natriuresis, energy homeostasis, sexual function and exocrine secretion17. The melanocortin hormone system is comprised of multiple components, including the five guanine protein-coupled melanocortin receptors; peptide ligands derived from the proopiomelanocortin preprohormone precursor; and endogenous antagonists, agouti signaling protein and agouti-related protein (Table 1)18,19.
Table 1.
Components of the melanocortin hormone system.
| The melanocortin system | |
|---|---|
| Ligands | ACTH; α-MSH, β-MSH, γ-MSH |
| Receptors | melanocortin receptor type 1~5 (Class A G-protein coupled receptors) |
| Antagonists | Agouti signaling protein (ASIP); Agouti related protein (AgRP) |
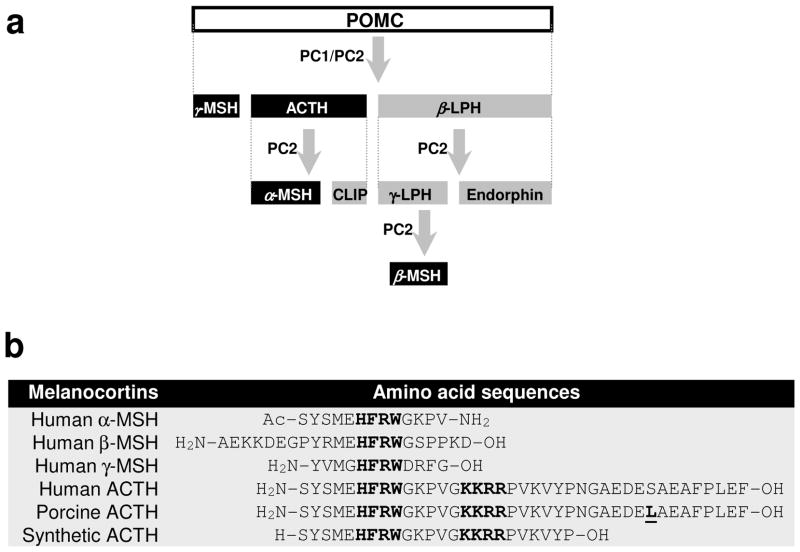
The endogenous agonist ligands of melanocortin hormone system, also known as melanocortins, are a group of hormonal neuropeptide that include adrenocorticotropic hormone (ACTH) and the different forms of melanocyte-stimulating hormone (MSH). Melanocortin peptides are produced by corticotrophs in the anterior lobe of pituitary gland, constituting 15–20% of the cells in the anterior lobe of the pituitary gland20. Melanocortins are synthesized from the precursor peptide pre-pro-opiomelanocortin (pre-POMC), which is encoded by a single-copy gene on chromosome 2p23.3 that is over 8 kb in length20. The removal of the signal peptide during translation produces the 267 amino acid polypeptide POMC, which undergoes a series of post-translational modifications such as phosphorylation and glycosylation before it is proteolytically cleaved by prohormone convertase (PC) enzymes PC1 and PC2 to yield a chemically and biogenetically related family of polypeptides with varying physiological activity, including endorphin, lipotropins and melanocortins20 (ACTH, α, β, γ-MSH) (Figure 1a).
Figure 1. Biosynthesis and molecular structures of melanocortin peptides.
(a) Pituitary biosynthesis of melanocortins. Melanocortins are synthesized in the anterior pituitary gland from the precursor peptide pre-pro-opiomelanocortin (pre-POMC), which undergoes a series of post-translational modifications such as phosphorylation and glycosylation before it is proteolytically cleaved by prohormone convertase (PC) enzymes PC1 and PC2 to yield a chemically and biogenetically related family of melanocortin polypeptides, including ACTH, α-melanocyte stimulating hormone (MSH), β-MSH and γ-MSH. CLIP, corticotropin-like intermediate peptide; LPH, lipotropin; (b) Molecular sequences of natural human melanocortin peptides, porcine ACTH and synthetic ACTH. ACTH is a linear nonatriacontapeptide straight-chain polypeptide containing 39 amino acids. The sequence of amino acid occupying positions 25 to 33 may vary among species. However, the N-terminal 24-amino acid segment is highly conserved and actually identical in all species. The only one different amino acid between porcine and human ACTH is underscored (residual 31 is serine for human and lysine for porcine). All melanocortin peptides share the same core tetrapeptide sequence His-Phe-Arg-Trp (HFRW), which is the minimal sequence required for selectivity and stimulation of the cognate MCRs (except MC2R). The Lys-Lys-Arg-Arg (KKRR) motif is the address sequence that permits MC2R recognition and is unique to ACTH.
Plasma melanocortins have a diurnal variation in normal subjects21,22 and can be induced by either physical or psychological stress, via hypophysiotropic hormones including corticotropin-releasing hormone and arginine vasopressin secreted by hypothalamus. Conversely, melanocortin synthesis and release are negatively controlled by slow/intermediate or fast feedbacks by many substances secreted within the hypothalamic-pituitary-adrenal (HPA) axis. Glucocorticoids (cortisol in human) secreted from the adrenal cortex in response to ACTH stimulation generate a negative feedback21. Thus patients treated with a high dose of synthetic glucocorticoids for a long period are likely to have a very low plasma level of melanocortins and develop a clinical constellation of symptoms that highly mimic the phenotypes of POMC deficiency syndrome, a rare genetic disease, including hyperphagia, central obesity, pale skin and adrenal insufficiency23.
The melanocortins exert their biological functions by binding to and activating the cognate melanocortin receptors (MCRs), with different affinity24. So far five MCRs have been cloned and characterized. All of the five MCRs are highly conservative across different species and share many homologs.19,25 The MCRs are all members of the rhodopsin family (class A) of seven-transmembrane guanine protein-coupled receptors, which intracellularly mediate their effects mainly by activating adenylate cyclase leading to stimulation of the cAMP-dependent cell signaling pathways24. The five MCRs have distinct tissue distribution, convey signaling of different melanocortins and exert varying biological activities24.
MC1R exhibits high affinity for ACTH and most MSH. It is highly expressed in melanocytes and is the principal melanocortin receptor in the skin where it mediates pigmentation as one of the major biological functions of most melanocortin peptides19,25. MC1R is also widely expressed in other organ systems, including adrenals, lung, lymph node, ovary, testis, brain, placenta, spleen and uterus19,25. It is also present in vascular endothelial cells and immune competent cells including leukocytes, dendritic cells and macrophages, suggesting a role of MC1R in the regulation of inflammatory reaction and immune response19,25. Indeed, α-MSH26 or ACTH27 treatment has been shown to prevent acute and chronic inflammation in animal models of multiple diseases, including acute kidney inflammation27 and injury26,28. Direct evidence of the important role of MC1R in inflammation and immunomodulation was recently shown in mice with a nonfunctional MC1R29. These mice demonstrated a dramatic exacerbation of experimental inflammation29, confirming a general anti-inflammatory effect of the MC1R signaling pathway.
The MC2R is the primary and exclusive receptor for ACTH that is expressed mainly in the adrenal gland and binds to ACTH with strong affinity but does not bind to the MSH peptides19,25. Activation of the MC2R initiates a cascade of events affecting multiple steps in steroidogenesis and growth of adrenal cortex. Of note, even though MC2R is predominantly expressed in adrenal cortex, recent studies indicate that it is also present in some other tissues including adipocytes where MC2R mediates stress-induced lipolysis via central ACTH release19,25.
Both MC3R and MC4R regulate energy expenditure19,25. The MC3R is expressed in the brain predominantly in the arcuate nucleus in the hypothalamus and limbic system and in few regions of the brain stem, as well as in the periphery where it has been found in the placenta and gut tissues; it is also abundantly expressed in the heart and in renal distal tubules and thus has been postulated to regulate hemodynamics, natriuresis and cardiovascular functions. Indeed, γ-MSH promotes urinary salt excretion via MC3R in the kidneys30. Consistently, mice either with MC3R knockout or lacking γ-MSH due to a defect in PC2, when fed a high-salt diet, developed a severe hypertension30. The MC4R is predominately found in the central nervous system, where it is represented in almost every brain region, including the cortex, thalamus, hypothalamus, brain stem and spinal cord19,25. MC3R and MC4R both can bind to ACTH or α-MSH with high affinity, but evidence suggests that they are not redundant in regulating food intake behavior and energy homeostasis such as hunger and satiety signaling, because the MC4R receptor knockout mice manifest hyperphagia, obesity, insulin resistance, and increased linear growth while the MC3R receptor knockout animals are not hyperphagic but still obese19,25. In addition, MC3R has been found to have thermoregulatory and immunomodulatory effects, whereas MC4R could additionally govern mood effects, control erectile function and mediate aphrodisiac actions19,25.
The MC5R was the last of the MCR gene family to be discovered by homology screening from genomic DNA in early 1990s19,25. High expression levels of MC5R can be found in exocrine glands in addition to various other organs including adrenal glands, fat cells, liver, lung, lymph nodes, bone marrow, thymus, mammary glands, testis, ovary, pituitary testis, uterus, esophagus, stomach, duodenum, skin, lung, skeletal muscle, exocrine glands and kidney19,25. The MC5R mediated physiological functions are not well understood. Targeted disruption of the MC5R gene in mice resulted in a severe defect in water repulsion and thermoregulation due to decreased production of sebaceous lipids19,25. The MC5R has similar affinity to ACTH and α-MSH and is believed to be responsible for the biological functions such as sebaceous gland secretion, theromoregulation and immunomodulation19,25. It has been suggested that MC5R agonists may perhaps alleviate conditions such as dry eyes and mouth, and MC5R antagonists might be useful in the treatment of acne. In addition, MC5R widely expressed on B and T lymphocytes might mediate immunomdulatory effects of melanocortin peptides like α-MSH and ACTH19,25.
Receptor binding studies revealed that ACTH is able to target all of the five types of MCR, implying a close association between multiple melanocortin-directed physiological processes, including stress response, regulation of food intake, control of energy balance, immunoregulation and exocrine gland functions19,25. Other three MSH peptides are pan agonists of the MCRs except MC2R with different potency. The molecular basis for varying affinities of melanocortins to different MCRs lies in the molecular structures of the melanocortin peptides19,25. ACTH and all of the three MSH share the same core tetrapeptide sequence His-Phe-Arg-Trp (HFRW), which is the minimal sequence required for selectivity and stimulation of the cognate MCRs (except MC2R)19,25. The Lys-Lys-Arg-Arg (KKRR) motif is the address sequence that permits MC2R recognition and is unique to ACTH. This explains why ACTH is the primary melanocortin that binds to MC2R (Figure 1b)19,25. Recent data consistently proved the presence of various MCRs in kidney parenchymal cells, signifying kidney as a prototypical target organ of the melanocortin system.
Kidney is an important effector organ of the melanocortin hormone system
As small peptides with very low molecular weights (2.2~4.5 kDa), melanocortins could circulate through out the whole body and exert biological activities on most organ systems expressing the cognate MCRs. It has been known for a long time that melanocortin peptides possess a significant effect in the kidney30. As a matter of fact, ACTH has been used since a half century ago for the treatment of nephrotic glomerular disease31. In addition, the kidney protective activity possessed by melanocortins, in particular α-MSH and ACTH, has been reproducibly demonstrated in multiple animal models of kidney diseases, including MN32, FSGS33, acute kidney injury (AKI) due to ischemia or sepsis26,28, renal toxicity34 and ureteral obstruction27,35. Moreover, γ-MSH has been found to have a prominent natriuretic and diuretic effect on the kidney30. These extensive renal effects suggest that kidney serves as an important effector organ of the melanocortin hormone system. However, although MCR have been extensively investigated in many organ systems in animals and humans, their expression in the kidney has been less studied and what kind of MCR the renal parenchymal cells actually express remains controversial (Table 2). For instance, while Ni et al36 found that MC3R, MC4R and MC5R are expressed in both cortex and medulla of murine kidneys; Lee et al37 only demonstrated MC1R and MC3R expression in rat kidney. Our group recently found that MC1R is expressed abundantly and predominantly by renal tubules and weakly by glomeruli, whereas MC5R is expressed weakly and sporadically by renal interstitial cells but strongly by podocytes in rodents both in vivo and in vitro.17,28 Moreover, strong expression of MC5R and weak expression of MC2R have been demonstrated in human kidney by PCR amplification of human kidney specific cDNA.38 This, to a certain extent, is consistent with the observation in sea bass where MC5R was abundantly expressed in both the anterior and posterior kidneys39. In contrast, in a lately published study32, only MC1R was detected by RT-PCR as the major MCR in human kidney and also in cultured human podocytes, glomerular endothelial cells, mesangial cells and tubular epithelial cells. In depth studies, however, indicate that basal expression of MC1R is low in murine podocytes and upon stimulation by selective MC1R agonists cAMP response was not significantly induced40. These discrepancies could reflect species difference in renal expression of MCR but is more likely due to the potential pitfalls in the nature of the detection technologies. Future studies should adopt more conclusive assays like proteomic identification and results should be cross-validated by multiple traditional detection technologies. Nevertheless, despite the existing controversy on the exact subtypes of MCR expressed in the kidney, a growing body of evidence has clearly and consistently proved that the kidney is indeed an important target organ of the melanocortin hormone system (Table 2). In line with this view, deficiency of melanocortins such as ACTH has been found to be associated with nephrotic syndrome caused by FSGS41, inferring that the melanocortin hormone system might be essential for renal homeostasis and melanocortin deficiency might predispose to kidney disease41. Thus, renal parenchymal tissues and cells, including podocytes, mesangium, glomerular endotheia, tubular epithelia and tubulointerstitium, are potential targets of melanocortin-mediated actions. Accordingly, melanocortin based therapeutic regimens might have potential effects on disease or injuries of these target renal tissues or cells, including podocytopathies like minimal change disease and FSGS, mesangial diseases like IgA nephropathy and mesangial proliferative glomerulonephritis (MsPGN), glomerular endotheliosis caused by transplant glomerulopathy, pre-eclampsia, or thrombotic microangiopathy due to hemolytic-uremic syndrome or thrombocytopenic purpura, and possibly tubular injuries like acute tubular necrosis.
Table 2.
The kidney is an important effector organ of the melanocortin hormone system with evident expression of various MCRs in parenchymal kidney cells.
| MCRs | |||||
|---|---|---|---|---|---|
| MC1R | MC2R | MC3R | MC4R | MC5R | |
| Agonist preference and affinity | ACTH=α-MSH > β-MSH >γ-MSH | ACTH only | ACTH=α-MSH = β-MSH=γ-MSH | ACTH=α-MSH > β-MSH>γ-MSH | ACTH=α-MSH> β-MSH>γ-MSH |
| Signaling pathways |
|
|
|
|
|
| Biological functions |
|
|
|
|
|
| Documented renal expression | Human kidney; Mouse kidney; Rat kidney |
Human kidney (weak) | Human kidney; Mouse kidney; Rat kidney |
Rat kidney | Human kidney; Mouse kidney; Rat kidney |
| Distribution in the kidney | Glomeruli; Tubular epithelia |
Unknown | Inner medullary collecting duct | Renal cortex and medulla | Glomeruli; Interstitium |
| Expression in kidney cells in vitro | Podocytes; Mesangial cells, Glomerular endothelial cells; Tubular epithelial cells |
Unknown | Inner medullary collecting duct cell | Glomerular endothelial cells; Tubular epithelial cells; Inner medullary collecting duct cells |
Podocytes; Inner medullary collecting duct cells |
| Renal effects |
|
Unknown | Enhanced sodium excretion in collecting ducts | Unknown |
|
| Clinical effects on kidney disease |
|
Unknown |
|
Unknown |
|
Abbreviations: ACTH, adrenocorticotropic hormone; AKI, acute kidney injury; cAMP, cyclic adenosine monophosphate; IP3, Inositol 1,4,5-triphosphate; MAPK/ERK, mitogen-activated protein kinase/extracellular signal- regulated kinase; MCR, melanocortin receptors; MSH, melanocyte stimulating hormone.
Currently available melanocortin receptor agonists
A couple of natural and synthetic melanocortin peptides are already available for clinical use. Some novel synthetic melanocortin analogues have been successfully developed and are under clinical or pre-clinical trials for the purposes of sunless tanning, potential anti-obesity, aphrodisiac, improvement of erectile dysfunction and possibly treatment of kidney diseases (Table 3).
Table 3.
Currently available melanocortin receptor agonists, their biological functions and potential benefits for kidney diseases.
| MCR Agonists | Classification | MCR affinity | Biological functions | Benefits for kidney diseases | Proprietor |
|---|---|---|---|---|---|
| Acthar gel | Long-acting natural ACTH gel; FDA approved | Pan agonist |
|
|
Questcor Pharmaceuticals |
| Synacthen | Long-acting synthetic ACTH; Approved outside the US | Pan agonist |
|
|
Questcor Pharmaceuticals |
| α-MSH | Synthetic α-MSH peptide | Pan agonist (no MC2R) |
|
|
Generic API |
| Afamelanotide (Melanotan I) | Synthetic α-MSH analogue | Pan agonist (no MC2R) |
|
Unknown | Clinuvel Pharmaceuticals |
| Melanotan II | Synthetic α-MSH analogue | Pan agonist (no MC2R) |
|
Unknown | mondoBiotech Laboratories AG |
| Bremelanotide | Synthetic α-MSH analogue | MC1R MC4R |
|
Unknown | Palatin Technologies |
| AP214 | Synthetic α-MSH analogue | Pan agonist (no MC2R) |
|
|
Abbott Pharmaceuticals |
| RM-493 | Synthetic peptide agonist | MC4R |
|
Unknown | Rhythm Pharmaceuticals |
| MS05 | Synthetic α-MSH analogue | MC1R |
|
|
Action Pharma A/S |
Abbreviations: MCR, melanocortin receptors; ACTH, adrenocorticotropic hormone; FDA, food and drug administration; iMN, idiopathic membranous nephropathy; MCD, minimal change disease; FSGS, focal segmental glomerulosclerosis; IgAN, IgA nephropathy; LN, lupus nephritis; DN, diabetic Nephropathy; TG, transplant glomerulopathy; MsPGN, mesangial proliferative glomerulonephritis; MPGN, membranoproliferative glomerulonephritis; AKI, acute kidney injury; MN, membranous nephropathy; α-MSH, α-melanocyte stimulating hormone; API, active pharmaceutical ingredients.
New approaches with available drugs
ACTH is a linear straight-chain nonatriacontapeptide containing 39 amino acids42. The sequence of amino acids occupying positions 25 to 33 may vary among species42. However, the N-terminal 24-amino acid segment is highly conserved and actually identical in all species. In addition, this segment is required and sufficient for the adrenocorticotropic activity. As a key component of the HPA axis, ACTH is the only melanocortin peptide that is capable of activating MC2R and executing the steroidogenic action. Thus, ACTH possesses dual physiological functions, namely MC2R mediated steroidogenesis in the adrenal glands and a potent melanocortin effect in extra-adrenal organ systems (Figure 2). Currently there are two forms of ACTH that are commercially available, natural and synthetic ones. The natural ACTH, Acthar gel, is a proprietary mixture isolated from porcine pituitary extracts that is formulated with 16% gelatin gel. The major component of ACTH gel is porcine ACTH1-39, which is only different from human ACTH in one amino acid residual (residual 31 is serine for human and lysine for porcine) (Figure 1b). The ACTH gel has been approved by the FDA since 1952 to induce diuresis or remission of proteinuria in the nephrotic syndrome without uremia of the idiopathic type or that due to lupus erythematosus43. An active synthetic analogue of ACTH(1–24) that is not naturally occurring consists of the first 24 amino acids of the native ACTH and is available in the form of tetracosactide, including Cosyntropin as short acting formulation and Synacthen (aqueous suspension of tetracosactide with zinc hydroxide) as long acting formulation43. So far Cosyntropin has been used solely to assess adrenal gland function, whereas repository ACTH, either ACTH gel or Synacthen has been commonly applied to treat infantile spasm, multiple sclerosis, gouty arthritis, autoimmune diseases and nephrotic glomerular diseases42,43. It is generally believed that short acting ACTH has strong and acute steroidogenic activities while slow release long acting ACTH, either ACTH gel or Synacthen, disrupts the diurnal rhythm in adrenal steroidogenesis and thus has minor steroidogenic effects44 but potentially greater melanocortin effects in extra-adrenal organ systems, including the kidney.
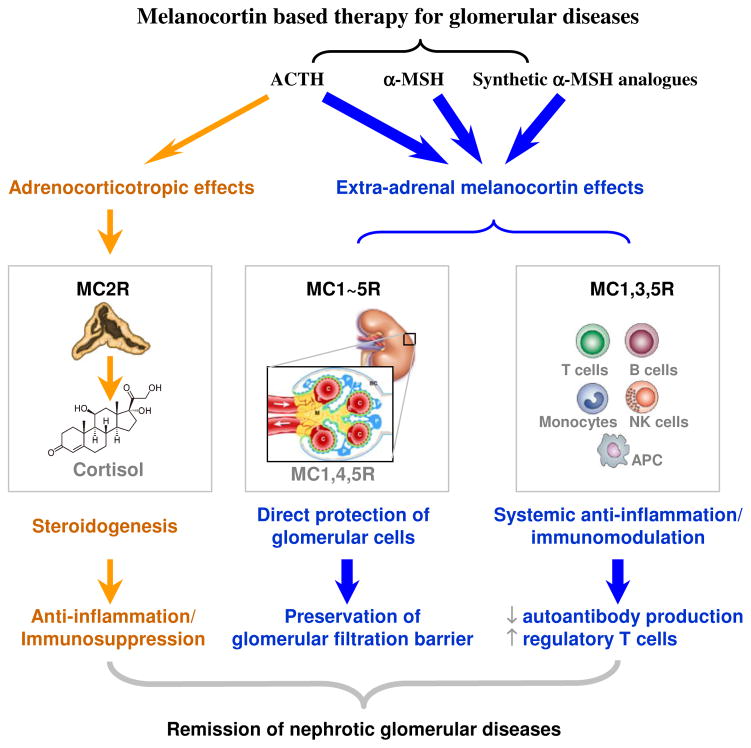
Figure 2. The melanocortin based therapy is effective in glomerular diseases via both direct protection of glomerular cells and systemic immunomodulation.
Melanocortins and their synthetic analogues induce remission of glomerular diseases mainly by targeting the glomerular cells (podocytes, mesangial cells and glomerular endothelial cells) and immune competence cells via the expressed melanocortin receptors (pathways in blue) and thereby exerting both a direct cellular protective effect on glomerular cells and a systemic immunomodulatory and anti-inflammatory effect. ACTH, the only melanocortin peptide with steroidogenic activities, exerts an additional cortisol dependent effect that might also contribute to the remission of glomerular diseases (pathways in yellow). P, podocytes; M, mesangium; E, glomerular endothelial cells; C, glomerular capillary lumen; BC, Bowman’s capsule; APC, antigen presenting cells. Schematic of a normal glomerulus is reproduced with permission from Quaggin SE and Kreidberg JA. Development 2008;135(4):609-620. doi:10.1242/dev.001081
Due to the high cost as well as potential concerns of anaphylactic reactions, natural ACTH as compared with synthetic ACTH has been less frequently chosen for various clinical or experimental purposes. However, even though it is claimed that synthetic ACTH (1–24) possesses the same efficacy as natural ACTH with regards to all its biological activities, a growing body of evidence suggests that they might be distinguishably different in terms of pharmacokinetics and pharmacodynamics. Of note, the biological function of the 15 amino acids (25–39) in the C-terminus of ACTH is uncertain and has been largely ignored for a long time. Recent data suggests that amino acids 25–39 primarily confer stability of the circulating ACTH, thus determining the half-life and efficacy of ACTH42. In support of this, site-specific amino acid substitution of phenylalanine (F) 39 of ACTH by cysteine (C), which has a free sulfhydryl group that can react specifically with iodoacetamide derivatives of lipophilic groups, significantly enhanced the biological activities of lipophilized ACTH(F39C) as compared with native ACTH45. Lipophilized ACTH(F39C) bound more tightly to serum albumin and cell membranes in vitro and had longer serum half-lives in vivo than native ACTH45. The documented plasma elimination half-life times of different types of ACTH has been shown to vary remarkably between 4.9 and 28.6 minutes. A study in healthy man indicated that the half-life of native ACTH is about 15 min46. By contrast, the half-life for tetracosactide (the active ingredient of Synacthen) is significantly shortened to 7 min. Consistently, in another study, the half-life of tetracosactide in the circulation of foetal sheep was only 0.27 min47, suggesting that the elimination rate of Synacthen in vivo is substantially accelerated as compared to that of natural ACTH. Therefore, it is speculated that natural ACTH might have more potent extra-adrenal actions than synthetic ACTH due to longer duration of action. In addition, post-translational modification is an important mechanism that regulates the biological activity of endocrine hormones, including ACTH. Such modifications as glycosylation, phosphorylation, C-terminal amidation and N-terminal acetylation, have been found to be present in natural ACTH48, but totally absent from the synthetic ones. These distinct molecular compositions and peptide structures might incur different biological activities and efficacies48. Moreover, ACTH is known to stimulate lipostasis49 and insulin secretion50,51. The β-cell tropic and insulin releasing activity has been confirmed to reside in the C-terminal part of ACTH51. Therefore, it is conceivable that synthetic ACTH in which the C-terminal amino acids 25–39 have been truncated would substantially lack the β-cell tropic and insulin-releasing activities. Despite these differences in pharmacokinetics and pharmacodynamics, both ACTH gel and Synacthen have recently been used respectively in the US and Europe for long-term treatment of steroid resistant nephrotic glomerulopathies.
Novel MCR agonists
α-MSH
α-MSH is a naturally occurring melanocortin peptide with a tridecapeptide structure52. It is the most important melanocyte-stimulating hormone involved in stimulating melanogenesis, a process that is responsible for hair and skin pigmentation in mammals. It also plays a key role in the control of feeding behavior, energy homeostasis, and sexual activities52. α-MSH is a nonselective pan agonist of the MC1, 3, 4, 5R, but not MC2R, which is exclusive for ACTH. Activation of the MC1R receptor is responsible for its effect on pigmentation, whereas its regulation of appetite, metabolism, and sexual behavior is mediated through both the MC3R and MC4R. α-MSH is generated as a proteolyic cleavage product from ACTH (1–13). Natural α-MSH, unfortunately, has too short a half life in the body to be practical as a therapeutic drug53. Moreover, the potential impurity of the product of natural α-MSH due to contamination by other dominant melanocortins or neuropeptides is another concern. So far synthetic α-MSH (acetate salt) has been mainly used in pre-clinical studies and demonstrated remarkable beneficial effects that ameliorate acute, chronic or systemic inflammation and injuries in various organ systems, including skin, lung, liver and kidney26,54. Since 1999, the FDA Office of Orphan Products Development has been sponsoring a phase I clinical trial (ClinicalTrials.gov identifier number NCT00004496) to assess the maximal tolerated dose and effects of α-MSH on acute renal failure and to determine the safety and pharmacokinetics of α-MSH in patients either with established ischemic acute renal failure or at high risk of acute renal failure following kidney transplantation.
Synthetic α-MSH analogues
Several synthetic analogues of α-MSH have been developed and investigated as medicinal drugs. These include afamelanotide55 (previously known as melanotan I), melanotan II56, bremelanotide57, AP21426,58, RM-49359, MS0532 and more. Most of these chemical compounds are derived from the chemical modification of the molecular structure of α-MSH and most are also pan agonists of the MCRs (no MC2R) except RM-493 and MS05, which respectively target MC4R and MC1R with high specificity. All of these α-MSH mimetics have significantly greater potencies than α-MSH, along with improved pharmacokinetics and distinctive MCR selectivity profiles. Because of the difference in their molecular structures, these analogues possess different agonizing activities for different MCRs and thus display distinct biological functions and clinical effects.
Afamelanotide or Melanotan I is a potent and longer-lasting synthetic analogue of naturally occurring α-MSH with the molecular structure modified ([Nle4-D-Phe7]-α-MSH) to increase binding affinity to its main receptor MC1R55. Afamelanotide is approximately 1,000 times more potent than natural α-MSH. Currently, Afamelanotide is in phase II clinical trials in Europe and in phase III in the US for skin diseases including vitiligo, erythropoietic protoporphyria, polymorphic light eruption and prevention of actinic keratoses in organ transplant recipients55.
Melanotan II is a cyclic lactam analog of α-MSH. It has weaker melanogenic activities as compared to Melanotan I but exhibits much greater aphrodisiac effects56. Melanotan II was found to enhance libido and erections in most male test subjects and sexual arousal with corresponding genital involvement in most female test subjects56.
Bremelanotide, a metabolite of melanotan II that lacks the C-terminal amide functional group, is under drug development as a treatment for female sexual dysfunction, hemorrhagic shock and ischemia/reperfusion injury60. It functions by activating MC1R and MC4R to modulate inflammation and limit ischemia associated injury. It was originally tested for intranasal administration in treating female sexual dysfunction but this application was temporarily discontinued due to concerns of side effects of elevated blood pressure60.
AP214, another synthetic analogue of α-MSH and a pan MCR agonist (no MC2R), was developed by Action Pharma and is now owned by Abbott Pharmaceuticals. AP214 has been shown by numerous preclinical studies26,58 to have potent anti-inflammatory activities in experimental sepsis and arthritis. A phase-II clinical trial has been completed using AP214 to prevent acute kidney injury in patients undergoing cardiac surgery.
In summary, more and more synthetic α-MSH mimetics have been developed for the purpose of melanocortin-based therapies for a variety of human diseases. As the kidney expresses all MCRs targeted by these synthetic agonists, the melanocortin-based treatments by using these novel drugs may also be a promising therapeutic modality for glomerular diseases.
Melanocortin based therapy for glomerular diseases: clinical and experimental evidence
A plethora of experimental evidence lately supports that melanocortins or their synthetic analogues have a great beneficial effect in animal models of kidney diseases, including MN, FSGS and AKI17,61. Clinical experience of melanocortin based therapy for kidney diseases is, however, largely limited to the use of ACTH, because so far all of the MSH and MSH analogues have not been approved for clinical use yet and ACTH is the only FDA approved drug with melanocortin activities. ACTH has been used since a half century ago for the treatment of nephrotic glomerular disease and exhibited great clinical benefits in inducing remission of proteinuria and nephrotic syndrome17,61. Since ACTH has both steroidogenic effects in the adrenal gland and melanocortin activities in extra-adrenal organs, the clinical effects of ACTH could be easily confounded and masked by the glucocorticoid mediated effects. Indeed, in early times, it was believed that ACTH exerts these proteinuria-reducing effects indirectly through the adrenal steroidogenic action because corticosteroids sometimes also induce similar response17. Recently, some clinical studies applied ACTH therapy in patients with glucocorticoid resistant glomerular diseases and shed light on the mechanism of action of the anti-proteinuric effect of ACTH. It seems that ACTH might have a protective effect on the kidney that is independent of its steroidogenic activity. This notion is based on the following clinical observations: 1) the plasma levels of cortisols detected in ACTH treated patients13,14,44 were much lower than circulating glucocorticoid levels in patients undergoing standard glucocorticoid therapy62. Yet, the ACTH therapy still resulted in comparable or even better outcome than steroid treatments in terms of remission of proteinuria; 2) For some glomerular diseases like iMN, corticosteroids as sole treatment even when administered at high doses, are unable to modify the natural disease course and has unproven benefit, as demonstrated by multiple randomized controlled clinical trials7,8; in contrast, ACTH monotherapy demonstrated striking beneficial effects and induced remission of proteinuria and nephrotic syndrome16, arguing that the effects of ACTH is not mediated via the steroid mechanisms; 3) In some nephrotic patients that had been resistant to previous glucocorticoid therapy, ACTH treatment could still produce satisfactory improvement in proteinuria, again suggesting a steroid independent mechanism of action. Collectively, a burgeoning body of evidence supports that the renoprotective effect of ACTH cannot be fully explained by its steroidogenic action. The extra-adrenal melanocortin signaling mechanism might be, at least in part, responsible for the anti-proteinuric effect of ACTH in nephrotic glomerular diseases.
iMN
After decades of neglect, the therapeutic effect of ACTH on nephrotic syndrome was first reexamined by Berg et al13. In their first study to optimize the dose of ACTH for adults with nephrotic syndrome, 14 patients with biopsy proven iMN received intramuscular injection of synthetic ACTH (1–24) at increasing dose during 8 weeks. The optimal dose was estimated to be 1 mg twice per week, taking both the therapeutic effects and the modest side effects of ACTH into consideration. ACTH therapy attained a concomitant improvement of dyslipidemia, proteinuria and kidney dysfunction. Among the 14 patients, 5 who had severe steroid resistant nephrotic syndrome responded remarkably well to ACTH therapy, implying that the therapeutic effect of ACTH might be ascribed not to steroidogenesis but to its melanocortin activities. Noteworthily, a quick relapse was observed in all cases when ACTH was discontinued after a short treatment duration of 8 weeks. By contrast, patients who received ACTH therapy for 1 year were still in remission even 18 months after cessation of treatment, suggesting that an extended term of ACTH treatment is required in order to achieve a sustained remission. In support of this, Hofstra et al63 recently reported a high incidence of relapse of nephrotic syndrome after ACTH treatment for 9 months. In their nonrandomized study reported as a conference abstract, 75% of the iMN patients, who achieved a remission after twice weekly synthetic ACTH therapy for 9 months, experienced a relapse during 3 months follow-up after discontinuation of the therapy. To assess the effectiveness and safety of ACTH therapy for nephrotic syndrome, Poticelli et al16 conducted an open label, prospective, randomized, controlled trial to compare the 12-month course of synthetic ACTH monotherapy (1 mg, twice weekly intramuscular injection) with the 6-month course Ponticelli immunosuppressive regimen [methylprednisolone plus a cytotoxic agent (either chlorambucil or cyclophosphamide)] in 32 nephrotic patients with biopsy proven iMN. ACTH therapy resulted in a similar remission rate (83%) as compared with the Ponticelli immunosuppressive regimen (75%)16. Furthermore, there were no significant difference in the relapse rate between the two therapeutic regimens after a median follow-up of 24 months16. This study, at least, demonstrated that the therapeutic efficacy of ACTH monotherapy is not inferior to that of the standard Ponticelli immunosuppressive regimen for iMN. Consistently, Picardi et al64 reported a sustained remission of nephrotic syndrome in 5 iMN patients in a follow-up period up to 26 months after receiving a 12-month course of synthetic ACTH (1 mg twice weekly) monotherapy.
Similar anti-proteinuric and renoprotective effects were also observed lately with natural ACTH in patients with refractory iMN. In a retrospective case series study, Bomback et al15 recruited 21 patients with refractory nephrotic syndrome due to a variety of glomerular diseases. Among the 21 patients, 11 were diagnosed as iMN and most of them were previously treated and failed to respond to a mean of 2 immunosuppressive regimens. After ACTH gel treatment at a most commonly used dose of 80IU twice a week for 5 to 12 months, 9 of the 11 patients (82%) with iMN achieved either partial (55%) or complete (27%) remission of proteinuria15.
To understand the mechanism of action underlying the proteinuria reducing effect of ACTH in MN, Lindskog et al32 tested the effect of synthetic ACTH in a rat model of passive Heymann’s nephritis (PHN). Synthetic ACTH (10μg/d) therapy for 4 weeks significantly reduced proteinuria (about 50% reduction in urine albumin to creatinine ratio). This anti-proteinuric effect was mimicked with an equal efficacy by the non-steroidogenic melanocortin peptide α-MSH or its analogue MS05, a highly selective MC1R agonist, accompanied with improved glomerular morphology and podocyte ultrastructure as well as diminished urine excretion of thiobarbituric acid-reactive substances, a marker of oxidative stress32, suggesting that the proteinuria reducing and kidney protective effects of ACTH is mediated mainly by MC1R signaling but less likely due to steroid effects. Surprisingly, glomerular deposition of the complement C5b-9 membrane attack complex remained unchanged as estimated by fluorescent immunohistochemistry, inferring that the proteinuria reducing effect was achieved via direct podocyte protection32. This hypothesis was further supported by successful detection of MC1R in podocytes in human and rat glomeruli32. Thus, melanocortins including ACTH and α-MSH as well as synthetic melanocortin analogues could potentially protect the podocytes from injury possibly via activating the MC1R signaling in podocytes.
In addition to a direct podocyte protective effect, ACTH and other melanocortins might induce improvement of proteinuria in iMN also via systemic immunomodulatory mechanisms. This has been supported by multiple clinical studies. In a prospective study by Bomback et al14, 5 patients with resistant iMN, who previously failed 2 to 4 immunosuppressive regimens, were recruited as one of the studied groups and received ACTH gel therapy (80 IU twice a week) for 6 months. By the end of the treatment, 2 of 5 patients (40%) attained partial remission of proteinuria. Mechanistically, by 4 months of ACTH gel therapy 3 of 5 patients (60%) demonstrated the disappearance in the serum of the anti-phospholipase A2 receptor (PLA2R) autoantibody14, an etiological factor recently characterized for the pathogenesis of iMN, thus denoting an immunologic remission. Consistently, ACTH-induced remission of proteinuria in iMN is also observed to correlate with a reduction in anti-PLA2R levels by another two collaborative studies reported as conference abstracts65,66. One study examined serum samples from iMN patients treated with synthetic ACTH by Berg et al65. Of the 9 patients who were anti-PLA2R positive at baseline, all had either absent or decreased anti-PLA2R in their post-treatment serum samples, with a mean decrease of 86% (range 19–100%) from the initial anti-PLA2R levels. In 7 patients, a disappearance or a substantial decrease in anti-PLA2R correlated respectively with complete (4 patients) or partial (3 patients) remission of proteinuria65. In the other study66, 12 patients that were anti-PLA2R positive at baseline experienced a reduction in anti-PLA2R autoantibody (17–100%) after 6 months of ACTH gel treatment with a complete disappearance of anti-PLA2R in 5 cases. Another pilot study67 lately reported as an abstract was designed to determine the dose and effectiveness of ACTH gel in nephrotic patients with iMN and also revealed a similar effect of ACTH on the depletion of anti-PLA2R autoantibody: ACTH gel treatment induced a significant remission of proteinuria along with improvement in both serum albumin and total cholesterol levels67; Anti-PLA2R was positive at baseline in 15 of the total 20 patients and the levels were found to change prior to and in correlation with the proteinuria response to ACTH gel therapy67. Taken together, it seems that ACTH therapy may exert the therapeutic effects additionally by suppressing autoantibody production.
In agreement with the above clinical evidence, immunological research has evidently indicated that B lymphocytes express abundant MCRs, including MC1R and MC3R. Melanocortins like α-MSH possesses a potent inhibitory effect on human immune responses to exogenous antigens likely via activating these MCRs on B lymphocytes68. Moreover, this melanocortin induced immunomodulatory effects on B lymphocytes seems distinct from the immunosuppressive effect conferred by glucocorticoids, because most of the patients with refractory iMN recruited in the aforementioned studies had been previously treated with other immunosuppressive regimens, including glucocorticoids, and failed to achieve clinical or immunologic remissions. Thus the autoantibody suppressing activity of ACTH is apparently not steroidogenic dependent but possibly mediated by a unique MCR directed mechanism.
Collectively, clinical and experimental evidence proves that melanocortin therapy is able to induce remission of proteinuria in patients with iMN through both a direct podocyte protective mechanism and a steroidogenic independent systemic autoantibody suppressing mechanism that might be ascribed to the MCR mediated immunomodulatory effects on B lymphocytes (Figure 2).
MCD and FSGS
As one of the 4 native melanocortin peptides, ACTH has been widely used since early 1950s for the treatment of nephrotic syndrome possibly caused by lipoid nephrosis, an older term for nil lesion or MCD31,69,70. ACTH therapy was particularly provided for childhood nephrotic syndrome in lieu of steroids with the hope of less growth retardation since it does not suppress the adrenal glands31. ACTH therapy was quite effective for both pediatric and adult patients with nephrotic syndrome in 1950s and 1960s, but later has been largely abandoned and replaced by the synthetic glucocorticoid therapy, due to the following reasons: 1) the mechanism of action of ACTH was believed at that time to be mediated solely via steroidogenesis; and 2) ACTH, either naturally or synthetically derived, is a peptide and has to be administered by subcutaneous or intramuscular injection, which has been considered less convenient as compared with the oral synthetic glucocorticoid therapy. Recent clinical studies using ACTH in patients with steroid resistant MCD and FSGS revealed extra benefit of ACTH and shed light on the melanocortin mechanisms. In an observational study by Berg et al12, 2 patients with MCD and 1 patient with FSGS had nephrotic range proteinuria and demonstrated no response to previous steroid and cytotoxic treatments. After the patients were converted to synthetic ACTH monotherapy for 2 to 7 months, the 2 MCD patients achieved complete remission and the FSGS patient attained partial remission. The patients all remained in remission after they were followed up for 4 to 28 months following cessation of ACTH therapy12. In another retrospective case series abstract report71, 12 patients with primary FSGS and nephrotic syndrome resistant to prior immunosuppressive therapies (median 3 therapies) were administered ACTH gel (median dose 80 IU subcutaneous injection twice weekly) for a median of 26 (range 12–56) weeks. Five of 12 patients (42%) experienced partial remission at last follow-up (median follow-up time 58 weeks after stopping ACTH). The median time-to-remission was 6 (range 6–24) weeks. This ACTH induced remission rate in patients with refractory FSGS is consistent with the finding by another prospective trial14, in which 5 patients with either MCD or FSGS failed 2 to 4 previous immunosuppressive treatments and were converted to ACTH gel monotherapy. After 6-month treatment, 2 of 5 patients (40%), 1 MCD and 1 FSGS, achieved partial remission. Because the development of FSGS might involve multiple pathogenic mechanisms, one would assume that combination of ACTH and a second immunosuppressive agent that simultaneously targets different pathogenic pathways might confer better benefit for refractory FSGS. Indeed, in a recent study reported as a conference abstract, Berg et al72 recruited 10 patients with severe FSGS that failed to respond to prior treatments with prednisolone in combination with other immunosuppressants, including cyclosporine, tacrolimus, mycophenolate mofetil and/or cyclophosphamide. After prednisolone was replaced with synthetic ACTH for a median 18 months while continuing therapy with a second immunosuppressive agent, 8 of 10 patients reached either complete (3 patients) or partial remission (5 patients), again suggesting a steroidogenic independent mechanism mediating such a robust therapeutic efficacy.
As more and more physician scientists and renal pathologists agree, regardless of the original etiology, the pathological basis of MCD and FSGS is essentially a disease of podocytes or podocytopathy, characterized by massive podocyte foot process effacement, microvillous transformation and podocytopenia as revealed by electron microscopy of the diseased glomeruli73. In an effort to understand if the anti-proteinuric activity of ACTH observed in steroid resistant MCD and FSGS is attributable to a possible melanocortin mediated podocyte protection, ACTH gel therapy was performed in rats subtotal nephorectomy33, a standard model of postadaptive FSGS (classic variant) and progressive chronic kidney disease74. After subtotal renal ablation, rats received ACTH gel treatment every other day for 5 weeks. ACTH markedly diminished proteinuria (over 50% reduction in 24 hour urine protein excretion) and significantly preserved kidney function as measured by increased renal plasma flow, improved inulin clearance rate and lowered serum creatinine levels. Histologically, glomerulosclerosis and tubulointerstitial fibrosis, renal inflammation, tubular atrophy, and tubular epithelial to mesenchymal transdifferentiation were all reduced after ACTH therapy. Of note, ACTH had no significant effects on mean arterial pressure or kidney-to-body weight ratio, suggesting that the effect of ACTH is less likely due to hemodynamic regulatory activities or anti-hypertrophic mechanisms. Because glucocorticoid therapy exacerbates proteinuria and glomerulosclerosis in this model75, steroidogenesis is also unlikely to contribute to the protective effect of ACTH. Instead, evidence for direct podocyte protection was discovered in remnant glomeruli. This included reduction in foot process effacement, diminished podocytic apoptosis, and lesser reductions in glomerular expression of podocyte markers including vimentin, nephrin, podocin and WT-1 in ACTH treated rats. These data suggest that ACTH reduces proteinuria and injury in progressive glomerulosclerosis via direct podocyte protection. As MC1R and MC5R have been evidently identified in podocytes both in vivo and in vitro, ACTH and α-MSH as well as synthetic α-MSH analogues that possess agonizing activities on these MCRs might be useful for the treatment of podocytopathic glomerular diseases like MCD and FSGS (Figure 2).
Other glomerular diseases
Experimental evidence proves that human glomerular capillary endothelial cells and mesangial cells also express abundant MCR both in vivo and in vitro32, suggesting that glomerular endothelium and mesangium may serve as targets of the melanocortin system, and melanocortin therapy might have effects on mesangial disease, glomerular endothelial injuries and mesangiocapillary diseases. So far pre-clinical data are very scarce on the effects of melanocorins in these glomerular diseases. But clinical experience from ACTH therapy in steroid resistant glomerular diseases, including mesangial proliferative glomerulonephritis (MsPGN), IgA nephropathy (IgAN), mesangiocapillary (also known as membranoproliferative) glomerulonephritis (MPGN) and glomerular endotheliosis suggests that melanocorin based therapy is indeed beneficial for these glomerular diseases.
Both Berg et al12 and Bomback et al14,15 reported in their case series studies that patients with steroid resistant MsPGN, IgAN or MPGN responded very well to ACTH therapy in terms of remission of proteinuria, inferring steroidogenic-independent melanocortin mechanisms mediating a renoprotective benefit. In a prospective study by Bomback et al14, 5 patients with IgAN, 3 of whom previously failed other immunosuppressive treatment regimens including prednisone, were recruited as one of the studied groups and received ACTH gel therapy (80 IU twice a week). After 6 months treatment, 2 of 5 patients, one previously untreated and one steroid resistant, both achieved remarkable proteinuria remission. The experience of melanocortin-based therapy in glomerular endotheliosis is even scarcer and only limited to a couple of case reports as conference abstracts. Patel et al76 tried ACTH gel therapy in a patient with biopsy proven transplant glomerulopathy, a prototypical glomerular endotheliosis possibly caused by antibody mediated rejection, hepatitis C infection or calcinurin inhibitor toxicity. After 4-month ACTH treatment, the patient demonstrated 50% reduction in proteinuria, associated with improvement in serum albumin levels and kidney function. Noteworthily, as early as 1950s ACTH was proposed as a promising therapy for thrombotic microangiopathy77. More experimental as well as clinical studies are, however, required to validate if melanocortin therapeutics is really beneficial for diseases of the glomerular endothelium, including thrombotic microangiopathy, transplant glomerulopathy and preeclampsia.
In addition to direct melanocortin effects on kidney parenchymal cells, melanocortins including ACTH are also able to target leukocytes and generate potent immunomodulatory effects. Therefore, besides direct protection of podocytes, mesangial cells and glomerular endothelial cells in primary glomerular diseases, melanocortin based therapy might also have a beneficial effect in glomerular diseases that are secondary to systemic autoimmune disorders, such as lupus nephritis. In fact, one of the FDA approved indications for ACTH therapy is to induce remission of nephrotic syndrome caused by lupus erythematosus. In a recent study by Berg et al78 that was presented as a conference abstract, 5 patients with lupus nephropathy received synthetic ACTH treatment for average 6 months. Urinary albumin excretion decreased from average 4278 μg/ml to 285μg/ml, associated with significant improvement in hypoalbuminemia and stabilization of kidney function. Three of the 5 patients previously failed multiple immunosuppressive regimens including glucocorticoids, again suggesting a steroidogenic independent melanocortin mechanism mediating the renoprotective effect in lupus nephritis. To further verify the efficacy of melanocortin based therapy in systemic autoimmune diseases, more randomized controlled studies are merited to evaluate the improvements in not only renal parameters but also systemic autoimmune activities.
Molecular mechanisms of melanocotin based therapy for glomerular diseases
The MCRs are prototypical G protein coupled receptors18. Upon activation by melanocortins, MCRs will activate the two principal signal transduction pathways involving MCRs: cAMP signal pathway and phosphatidylinositol signal pathway79. These two signal pathways will ignite many other downstream signaling cascades that are responsible for various cellular effects, ultimately leading to renoprotection and remission of proteinuria.
1. Anti-apoptosis and pro-survival activities
Evidence suggests that the cAMP signaling pathway triggered by the MCR, in particular MC1R, interacts and cross-talks with the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway80–83, which is a key signaling cascade that relays pro-survival/anti-apoptotic signals84. Podocytes have been proved to express MCRs. In cultured murine podocytes expressing MC1R, selective agonist of MC1R strongly triggered cAMP signaling pathway40. Thus MCR agonists like ACTH and α-MSH might have a direct anti-apoptosis/pro-survival effect on podocytes and protect podocytes from death through the crosstalk between the MCR/cAMP signaling pathway and the MAPK/ERK pathway (Figure 3). This mechanism presumably explains why ACTH treatment had a direct podocyte protective effect and prevented podocytopenia in animal models of postadaptive FSGS33.
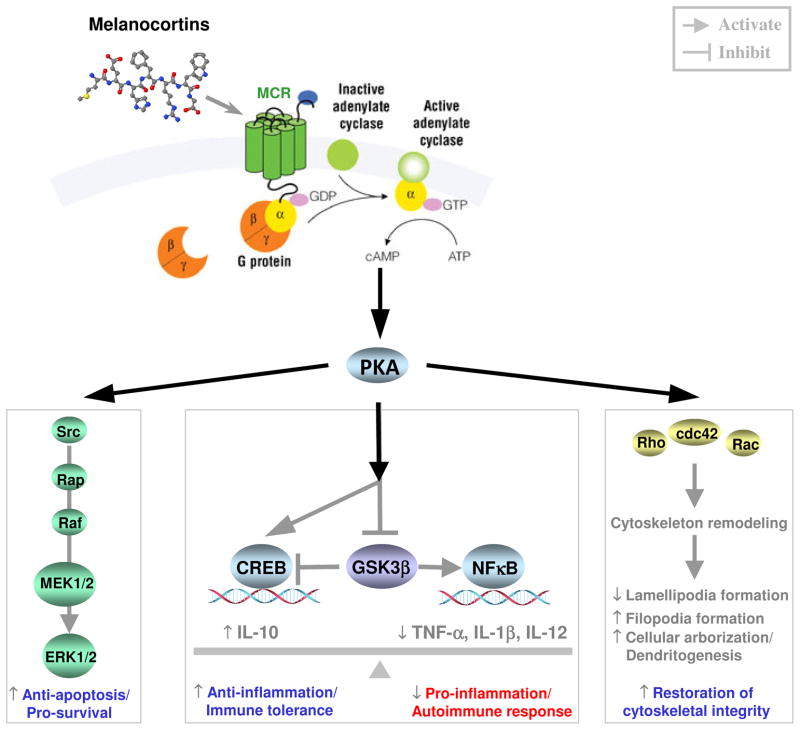
Figure 3. Molecular mechanisms responsible for the effect of melanocortin based therapy in glomerular diseases.
When melanocortin peptide ligands bind to MCRs, which are prototypical G protein coupled receptors, they causes a conformational change in the MCR, which allows it to act as a guanine nucleotide exchange factor. The MCR can then activate an associated G-protein by exchanging its bound GDP for a GTP. The G-protein’s α subunit, together with the bound GTP, can then dissociate from the β and γ subunits to further affect intracellular signaling proteins, including adenylyl cyclase and phospholipase C, thus trigger the two principal signal transduction pathways involving MCRs: cAMP signal pathway and phosphatidylinositol signal pathway. The cAMP-PKA pathway regulates the downstream signaling cascades, including the MAPK/ERK mediated pro-survival/anti-apoptosis pathway that might protect the glomerular cells, such as podocytes and glomerular endothelial cells, from injury and death; the CREB/NFκB pathway that balances the pro- and anti-inflammatory reaction as well as the immune tolerance and autoimmune response; and the cytoskeleton regulatory signaling pathway that might be involved in cytoskeleton remodeling in glomerular cells such as podocytes. MEK, MAPK kinase; MCR, melanocortin receptor; PKA, protein kinase A; CREB, cAMP response element binding protein; GSK3β, glycogen synthase kinase 3β; ERK, signal-regulated kinase; NFκB, nuclear factor κB.
2. Anti-inflammation and immunomodulation
As neuroimmunopeptides, melanorcortins possess a potent immunomodulatory activity in circulating leukocytes, including monocyte, T and B lymphocytes, NK cells and antigen presenting cells85–87. In addition, in parenchymal cells of most organ systems, including the kidney, melanocortins also have a prominent anti-inflammatory effect88. This anti-inflammatory/immunomodulatory effect could be mediated by multiple MCRs, including MC1R, MC3R and MC5R, and achieved through suppression of the transactivation activities of NFκB54,88, a pivotal transcription factor that governs the expression of numerous proinflammatory mediators implicated in immune response and inflammatory reaction, such as chemokines, lymphokines, adhesion molecules and more89. The exact mechanisms for NFκB inhibition induced by MCR signaling pathways remain unclear, but evidence suggests that the MCR activated cAMP signaling pathway is involved88. Protein kinase A (PKA) is substantially activated following the MCR triggered induction of intracellular cAMP levels. As an important substrate of PKA, glycogen synthase kinase (GSK) 3β will be phosphorylated and its activity suppressed. GSK3β has been shown to be an important signaling transducer that controls phosphorylation specificity of NFκB RelA/p65 and directs the selective transcription of NFκB dependent proinflammatory molecules90. Thus, inhibition of GSK3β activity by the MCR-cAMP-PKA signaling pathway will selectively obliterate the expression of proinflammatoty cytokines responsible for inflammatory reactions and Th1 mediated immune response, including TNF-α, IL-1β and IL-12 (Figure 3)87. In addition, both activation of PKA and inhibition of GSK3β could increase the activity of cAMP response element binding protein (CREB), a transcription factor that controls the expression of anti-inflammatory cytokines like IL-10 that are essential for immunotolerance91. Besides, CREB also accounts for differential regulation of Th1, Th2, and Th17 responses86,87,91. CREB activation directs regulatory T cell lineage commitment and thus favors immune tolerance91 (Figure 3). Indeed, treatment of primed T cells with α-MSH could induce tolerance/anergy in primed CD4+ T helper cells and mediate induction of CD25+CD4+ regulatory T cells through an MC5R dependent mechanism, thus resulting in tolerance to experimental autoimmune injuries92. Furthermore, α-MSH has been demonstrated to have immunosuppressive activities also in humans, likely acting in part via MC1R on monocytes and MC1,3R on B lymphocytes68. It is tempting to speculate that the therapeutic effects of melanocortin treatments in immune mediated glomerular diseases like iMN and podocytopathies like MCD and FSGS might be attributable, at least in part, to systemic immunomodulation, which could: 1) reinstate the immune balance between the subsets of T helper lymphocytes, 2) reduce the production of autoantibody, and 3) diminishes the release of pathogenic lymphokines that possess glomerular permeability activities93,94.
3. Regulation of cytoskeletons
G protein signaling ignited by MCRs, prototypical GPCR, is closely involved in fast acting cellular responses, including cytoskeleton rearrangement and cell shape changes. Activation of MC1R has been shown to regulate the activities of multiple cytoskeleton regulatory signaling transducers, including Rac, Cdc42 and Rho through the cAMP-PKA signaling pathway95,96. MC1R-cAMP-PKA signaling enhances the activity of GTP-binding protein Rac, quickly resulting in altered lamellipodia and filopodia formation and increased cellular arborization and dendritogenesis95 (Figure 3). This effect might protect the podocytes from cytoskeleton dysorganization induced by various immune or non-immune mediated injuries, and might also account for the improvement in podocyte foot process effacement and podocyte shape changes observed after ACTH treatment in animal models of postadaptive FSGS33.
Side effects of melanocortin based therapy
According to the existing experience of clinical use of ACTH as well as ongoing clinical trials of other melanocortin analogues, the side effects of melanocortin-based therapy seem mild, tolerable and reversible.
Allergy and anaphylaxis
All of the melanocortins that are currently available for medicinal use are peptides and require injection as a way of administration. Among these drugs, natural ACTH is prepared from porcine pituitary glands, while synthestic ACTH and other synthetic melanocortin analogues are oligopeptides structurally derived from natural ACTH or α-MSH. As foreign proteins or synthetic oligopeptides, these macromolecular drugs might potentially have antigenic/immunogenic activity. Thus, allergy and anaphylaxis are reasonable concerns for melanocortin based therapy. However, in the past 60 years there were only several case reports on mild allergic/anaphylactic reactions to clinical use of natural or synthetic ACTH and most were limited to pediatric patients97,98, suggesting the antigenicity and immunogenicity of the melanocortin peptides might be minor.
Cushingoid symptoms
The steroidogenic melanocortin peptide ACTH has been labeled to potentially generate steroid like side effects and incur cushingoid symptoms, including visceral obesity, hyperglycemia, osteoporosis, avascular osteonecrosis and more. However, multifaceted evidence indicates that ACTH as compared with glucocorticoids might play a distinct or even opposing role in the development of some of these side effects. It is known that glucocorticoid treatment or overproduction of endogenous cortisol could reduce bone mineral density and result in osteoporosis99. However, patients with adrenal Cushing’s syndrome, where ACTH levels are suppressed, experience greater bone loss than those with pituitary Cushing’s with high serum ACTH levels100. Likewise, patients with familial glucocorticoid deficiency with elevated ACTH levels, due to hypothalamic feedback, have a higher bone mass than age-matched controls101. Taken together, these findings are consistent with an anabolic effect of ACTH, which seemingly counteracts the bone loss due to cortisol. Lately, through an animal model of glucocorticoid-induced osteonecrosis, it is confirmed that ACTH treatment actually protected from methylprednisolone induced avascular osteonecrosis of femoral head102. And this effect was attributed to the ACTH promoted maintenance and regeneration of fine vascular networks surrounding the highly remodeling bone102.
In addition, hyperglycemia is another common side effect of glucocorticoid therapy due to impaired insulin production103 or insulin resistance104. By contrast, ACTH has been demonstrated to have opposing effects and prominently induce β cell production of insulin105–107. In actuality, natural ACTH possess a potent β-cell tropic and insulinogenic activity, which has been proved to reside in the C-terminal part ACTH(22–39) of ACTH51. Consistently, in a recent prospective randomized trial presented as a conference abstract, 18 patients with advanced diabetic nephropathy were treated with low doses of ACTH gel (16 or 32 IU daily subcutaneous injection) for 6 months. The average 24 hour urine protein excretion was significantly reduced from 7.8 to 2.3 gram, associated with stabilized kidney function and no deterioration of diabetes. Only 2 of 18 patients (11%) required reduction in ACTH dose secondary to hyperglycemia108, suggesting that ACTH therapy, totally different from glucocorticoid therapy, is safe for patients with diabetes, although large-scale trials with long-term follow-up are required to validate this finding. Collectively, the steroid like side effects or Cushingoid symptoms seem to be mild at the clinical doses of ACTH. It is also conceivable that clinical use of non-steroidogenic melanocortins such as α-MSH or its synthetic analogues should be able to avoid these steroid like side effects.
Hypertension
Hypertension is another occasional side effect observed in melanocortin based therapy. For non-steroidogenic melanocortin or analogues mildly elevated blood pressure has been reported during phase I or II clinical trials, possibly due to the hemodynamic actions subsequent to MC3R activation in cardiovascular system30 or due to the central nervous actions109. In addition, ACTH therapy through the cortisol effect might also induce mild hypertension. As a type of natural glucocorticoid, cortisol may elevate blood pressure directly by enhancing vascular tone110. On the other hand, cortisol has a fair amount of activity on the minerocorticoid receptor111, and may cause sodium and water retention, potassium wasting and expansion of effective circulating volume, thus resulting in mild hypertension or even congestive heart failure in some high-risk patients111. Uncontrollable malignant hypertension following ACTH therapy is very rare but could be seen in patients with glucocorticoid remediable aldosteronism (GRA)112. GRA is a rare (prevalence: 0.66%) autosomal dominant disorder113, in which the promoter region of the 11-hydroxylase gene is fused to the coding region of the aldosterone synthase due to an unequal gene crossing-over, so that adrenal production of aldosterone is not only regulated by the RAAS, but largely under the control of ACTH112. Following ACTH treatment, GRA patients may rapidly manifest with fatigue, a drastic increase in blood pressure and severe headache112. The most serious side effect of ACTH therapy in GRA patients is very severe hypertension resulting in hemorrhage stroke112. Therefore, for patients with uncontrollable hypertension following ACTH treatment, GRA should be suspected and ACTH therapy should be discontinued.
Other side effects
Other possible side effects of melanocortins include skin pigmentation and increased risk of melanoma due to MC1R activation114–116. Therefore, melanocortin based therapy should be avoided in patients with current or even antecedent malignant melanoma. Additional side effects may include anorexia, mood changes, sleep disorders and behavioral disturbances due to activation of MC3R and MC4R in the central nervous system18. Seborrheic dermatitis and acne vulgaris are also possible concerns due to over-function of sebaceous glands following activation of MC5R on sebocytes.117 Pan MCR agonists may potentially increase libido and activate the yawning, stretching and erection reflexes due to activation of MC4R in central nervous system118.
Conclusions
In the past several years, a growing body of clinical and experimental evidence consistently indicates that melanocortin treatments have prominent anti-proteinuric and kidney protective effects in a variety of glomerular diseases. The existing clinical findings are encouraging, however, most were made in single-center observational studies with small sample sizes, poor controls and short term follow up. Thus, additional large-scale multicenter randomized controlled trials are warranted to revalidate the safety and efficacy of the melanocorin based therapy for glomerular diseases. Moreover, an extended follow-up duration is merited to assess whether the melanocortin based therapy can actually retard the progression of CKD and stabilize kidney function, in addition to its proteinuria-reducing effect. In summary, the melanocortin based therapy might represent a novel, promising and pragmatic therapeutic strategy for glomerular diseases.
CLINICAL SUMMARY.
The melanocortin system is a set of hormonal, neuropeptidergic, and immune signaling pathways that play an integral role in the homeostatic control of a diverse array of physiological functions, including inflammation, immunomodulation, and adrenocortical steroidogenesis.
The kidney is a quintessential effector organ of the melanocortin hormone system with melanocortin receptors abundantly expressed by multiple renal paranchymal cells, including podocytes, mesangial cells, glomerular endothelial cells and renal tubular epithelial cells.
Converging clinical and experimental evidence suggests that the melanocortin based therapy by using melanocortin peptides, including adrenocorticotropic hormone (ACTH) and other melanocortin analogues confers potent proteinuria reducing and kidney protective effects in a variety of glomerular diseases.
The anti-proteinuric and renoprotective effects exerted by the melanocortin based therapy could be ascribed to both direct protection of glomerular cells and steroidogenic-independent systemic immunomodulation and anti-inflammation.
Acknowledgments
This work was made possible, in part, by support from the Foundation for Health, the Rhode Island Foundation and the US National Institutes of Health grant R01 DK092485.
Footnotes
Financial disclosure:
Rujun Gong received research grants and speaker honoraria from Questcor and served on scientific advisory boards for Questcor.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meyers CM, Geanacopoulos M, Holzman LB, et al. Glomerular disease workshop. J Am Soc Nephrol. 2005;16:3472–3476. doi: 10.1681/ASN.2005090899. [DOI] [PubMed] [Google Scholar]
- 2.Kher A, Kher V. Immunotherapy in renal diseases. Med Clin North Am. 96:545–564. x. doi: 10.1016/j.mcna.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC, Falk RJ. Diagnosis and management of glomerular diseases. Med Clin North Am. 1997;81:653–677. doi: 10.1016/s0025-7125(05)70538-1. [DOI] [PubMed] [Google Scholar]
- 4.Greenbaum LA, Benndorf R, Smoyer WE. Childhood nephrotic syndrome--current and future therapies. Nat Rev Nephrol. 8:445–458. doi: 10.1038/nrneph.2012.115. [DOI] [PubMed] [Google Scholar]
- 5.Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 1981;20:765–771. doi: 10.1038/ki.1981.209. [DOI] [PubMed] [Google Scholar]
- 6.Colattur SN, Korbet SM. Long-term Outcome of Adult Onset Idiopathic Minimal Change Disease. Saudi J Kidney Dis Transpl. 2000;11:334–344. [PubMed] [Google Scholar]
- 7.Cattran DC, Delmore T, Roscoe J, et al. A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med. 1989;320:210–215. doi: 10.1056/NEJM198901263200403. [DOI] [PubMed] [Google Scholar]
- 8.Cameron JS, Healy MJ, Adu D. The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. The MRC Glomerulonephritis Working Party. Q J Med. 1990;74:133–156. [PubMed] [Google Scholar]
- 9.Pei Y, Cattran D, Delmore T, et al. Evidence suggesting under-treatment in adults with idiopathic focal segmental glomerulosclerosis. Regional Glomerulonephritis Registry Study. Am J Med. 1987;82:938–944. doi: 10.1016/0002-9343(87)90155-0. [DOI] [PubMed] [Google Scholar]
- 10.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Par A. Levamisole as immunostimulant in the clinical practice. Ther Hung. 1983;31:161–171. [PubMed] [Google Scholar]
- 12.Berg AL, Arnadottir M. ACTH-induced improvement in the nephrotic syndrome in patients with a variety of diagnoses. Nephrol Dial Transplant. 2004;19:1305–1307. doi: 10.1093/ndt/gfh110. [DOI] [PubMed] [Google Scholar]
- 13.Berg AL, Nilsson-Ehle P, Arnadottir M. Beneficial effects of ACTH on the serum lipoprotein profile and glomerular function in patients with membranous nephropathy. Kidney Int. 1999;56:1534–1543. doi: 10.1046/j.1523-1755.1999.00675.x. [DOI] [PubMed] [Google Scholar]
- 14.Bomback AS, Canetta PA, Beck LH, Jr, et al. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 36:58–67. doi: 10.1159/000339287. [DOI] [PubMed] [Google Scholar]
- 15.Bomback AS, Tumlin JA, Baranski JJ, et al. Treatment of resistant nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Design, Development and Therapy. 2011;5:147–153. doi: 10.2147/DDDT.S17521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponticelli C, Passerini P, Salvadori M, et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 8:122–128. doi: 10.1038/nrneph.2011.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gantz I, Fong TM. The melanocortin system. Am J Physiol Endocrinol Metab. 2003;284:E468–474. doi: 10.1152/ajpendo.00434.2002. [DOI] [PubMed] [Google Scholar]
- 19.Cone RD. Studies on the physiological functions of the melanocortin system. Endocr Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 20.Hadley ME, Haskell-Luevano C. The proopiomelanocortin system. Ann N Y Acad Sci. 1999;885:1–21. doi: 10.1111/j.1749-6632.1999.tb08662.x. [DOI] [PubMed] [Google Scholar]
- 21.Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16:265–271. doi: 10.1159/000216184. [DOI] [PubMed] [Google Scholar]
- 22.Veldhuis JD, Iranmanesh A, Johnson ML, et al. Amplitude, but not frequency, modulation of adrenocorticotropin secretory bursts gives rise to the nyctohemeral rhythm of the corticotropic axis in man. J Clin Endocrinol Metab. 1990;71:452–463. doi: 10.1210/jcem-71-2-452. [DOI] [PubMed] [Google Scholar]
- 23.Krude H, Biebermann H, Luck W, et al. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Malek ZA. Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell Mol Life Sci. 2001;58:434–441. doi: 10.1007/PL00000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cone RD, Lu D, Koppula S, et al. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res. 1996;51:287–317. discussion 318. [PubMed] [Google Scholar]
- 26.Doi K, Hu X, Yuen PS, et al. AP214, an analogue of alpha-melanocyte-stimulating hormone, ameliorates sepsis-induced acute kidney injury and mortality. Kidney Int. 2008;73:1266–1274. doi: 10.1038/ki.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong R, Dworkin LD. Adrenocorticotropin (ACTH) gel suppresses renal tubulointerstitial inflammation and injury by direct stimulation of the melanocortin 1 receptor (MC1R) J Am Soc Nephrol. 2011;22:136A. [Google Scholar]
- 28.Si J, Ge Y, Zhuang S, et al. Adrenocorticotropic hormone ameliorates acute kidney injury by steroidogenic-dependent and -independent mechanisms. Kidney Int. 83:635–646. doi: 10.1038/ki.2012.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maaser C, Kannengiesser K, Specht C, et al. Crucial role of the melanocortin receptor MC1R in experimental colitis. Gut. 2006;55:1415–1422. doi: 10.1136/gut.2005.083634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Humphreys MH. Cardiovascular and renal actions of melanocyte-stimulating hormone peptides. Curr Opin Nephrol Hypertens. 2007;16:32–38. doi: 10.1097/MNH.0b013e3280117fb5. [DOI] [PubMed] [Google Scholar]
- 31.Arneil GC, Wilson HE. A.C.T.H in nephrosis. Arch Dis Child. 1953;28:372–380. doi: 10.1136/adc.28.141.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindskog A, Ebefors K, Johansson ME, et al. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol. 2010;21:1290–1298. doi: 10.1681/ASN.2009101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong R, Dworkin LD. ACTH(Acthar Gel) prevents proteinuria and renal injury in the remnant kidney: Evidence for direct podocyte protection. J Am Soc Nephrol. 2010;21:548A. [Google Scholar]
- 34.Lee SY, Jo SK, Cho WY, et al. The effect of alpha-melanocyte-stimulating hormone on renal tubular cell apoptosis and tubulointerstitial fibrosis in cyclosporine A nephrotoxicity. Transplantation. 2004;78:1756–1764. doi: 10.1097/01.tp.0000144332.44435.ab. [DOI] [PubMed] [Google Scholar]
- 35.Li C, Shi Y, Wang W, et al. alpha-MSH prevents impairment in renal function and dysregulation of AQPs and Na-K-ATPase in rats with bilateral ureteral obstruction. Am J Physiol Renal Physiol. 2006;290:F384–396. doi: 10.1152/ajprenal.00282.2004. [DOI] [PubMed] [Google Scholar]
- 36.Ni XP, Bhargava A, Pearce D, et al. Modulation by dietary sodium intake of melanocortin 3 receptor mRNA and protein abundance in the rat kidney. Am J Physiol Regul Integr Comp Physiol. 2006;290:R560–567. doi: 10.1152/ajpregu.00279.2005. [DOI] [PubMed] [Google Scholar]
- 37.Lee YS, Park JJ, Chung KY. Change of melanocortin receptor expression in rat kidney ischemia-reperfusion injury. Transplant Proc. 2008;40:2142–2144. doi: 10.1016/j.transproceed.2008.07.101. [DOI] [PubMed] [Google Scholar]
- 38.Chhajlani V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int. 1996;38:73–80. [PubMed] [Google Scholar]
- 39.Sanchez E, Rubio VC, Cerda-Reverter JM. Characterization of the sea bass melanocortin 5 receptor: a putative role in hepatic lipid metabolism. J Exp Biol. 2009;212:3901–3910. doi: 10.1242/jeb.035121. [DOI] [PubMed] [Google Scholar]
- 40.Elvin J, Jonsson AL, Buvall LM, et al. The melanocortin receptor 1: A potential target for therapeutical drugs in nephrotic syndrome. J Am Soc Nephrol. 2012;23:825A. [Google Scholar]
- 41.Yamada S, Bandai S, Masutani K, et al. Focal segmental glomerulosclerosis in a patient with isolated ACTH deficiency and reversible hypothyroidism. Clin Exp Nephrol. 14:168–172. doi: 10.1007/s10157-009-0228-9. [DOI] [PubMed] [Google Scholar]
- 42.Schwyzer R. ACTH: a short introductory review. Ann N Y Acad Sci. 1977;297:3–26. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- 43.Gettig J, Cummings JP, Matuszewski K. H.p Acthar gel and cosyntropin review: clinical and financial implications. PT. 2009;34:250–257. [PMC free article] [PubMed] [Google Scholar]
- 44.Berg AL. Plasma cortisol levels after natural ACTH1–39 and synthetic ACTH1–24 in humans. National Kidney Foundation 2013 Spring Clinical Meetings; 2013. Poster 218. [Google Scholar]
- 45.Wan L, Chen YH, Chang TW. Improving pharmacokinetic properties of adrenocorticotropin by site-specific lipid modification. J Pharm Sci. 2003;92:1882–1892. doi: 10.1002/jps.10442. [DOI] [PubMed] [Google Scholar]
- 46.Schoneshofer M, Goverde HJ. Corticotropin in human plasma. General considerations. Surv Immunol Res. 1984;3:55–63. doi: 10.1007/BF02918598. [DOI] [PubMed] [Google Scholar]
- 47.Jones CT, Kendall JZ, Ritchie JW, et al. Adrenocorticotrophin and corticosteroid changes during dexamethasone infusion to intact and synacthen infusion to hypophysectomized foetuses. Acta Endocrinol (Copenh) 1978;87:203–211. doi: 10.1530/acta.0.0870203. [DOI] [PubMed] [Google Scholar]
- 48.Loh YP, Gainer H. Evidence that glycosylation of pro-opiocortin and ACTH influences their proteolysis by trypsin and blood proteases. Mol Cell Endocrinol. 1980;20:35–44. doi: 10.1016/0303-7207(80)90092-1. [DOI] [PubMed] [Google Scholar]
- 49.Schwandt P. Hypothalamic control of lipid metabolism. Acta Neurochir (Wien) 1985;75:122–124. doi: 10.1007/BF01406332. [DOI] [PubMed] [Google Scholar]
- 50.Beloff-Chain A, Billingham N, Cawthorne MA. Stimulation of insulin secretion from mouse pancreatic islets, maintained in tissue culture, by the pituitary neurointermediate lobe of the genetically obese mouse (ob/ob) Biochem Biophys Res Commun. 1980;95:792–795. doi: 10.1016/0006-291x(80)90856-6. [DOI] [PubMed] [Google Scholar]
- 51.Beloff-Chain A, Morton J, Dunmore S, et al. Evidence that the insulin secretagogue, beta-cell-tropin, is ACTH22–39. Nature. 1983;301:255–258. doi: 10.1038/301255a0. [DOI] [PubMed] [Google Scholar]
- 52.Voisey J, Carroll L, van Daal A. Melanocortins and their receptors and antagonists. Curr Drug Targets. 2003;4:586–597. doi: 10.2174/1389450033490858. [DOI] [PubMed] [Google Scholar]
- 53.Vessillier S, Adams G, Montero-Melendez T, et al. Molecular engineering of short half-life small peptides (VIP, alphaMSH and gamma(3)MSH) fused to latency-associated peptide results in improved anti-inflammatory therapeutics. Ann Rheum Dis. 71:143–149. doi: 10.1136/annrheumdis-2011-200100. [DOI] [PubMed] [Google Scholar]
- 54.Ichiyama T, Zhao H, Catania A, et al. alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation and IkappaBalpha degradation in human glioma cells and in experimental brain inflammation. Exp Neurol. 1999;157:359–365. doi: 10.1006/exnr.1999.7064. [DOI] [PubMed] [Google Scholar]
- 55.Fabrikant J, Touloei K, Brown SM. A review and update on melanocyte stimulating hormone therapy: afamelanotide. J Drugs Dermatol. 12:775–779. [PubMed] [Google Scholar]
- 56.Hadley ME, Hruby VJ, Blanchard J, et al. Discovery and development of novel melanogenic drugs. Melanotan-I and -II. Pharm Biotechnol. 1998;11:575–595. doi: 10.1007/0-306-47384-4_25. [DOI] [PubMed] [Google Scholar]
- 57.Safarinejad MR. Evaluation of the safety and efficacy of bremelanotide, a melanocortin receptor agonist, in female subjects with arousal disorder: a double-blind placebo-controlled, fixed dose, randomized study. J Sex Med. 2008;5:887–897. doi: 10.1111/j.1743-6109.2007.00698.x. [DOI] [PubMed] [Google Scholar]
- 58.Montero-Melendez T, Patel HB, Seed M, et al. The melanocortin agonist AP214 exerts anti-inflammatory and proresolving properties. Am J Pathol. 179:259–269. doi: 10.1016/j.ajpath.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kievit P, Halem H, Marks DL, et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes. 62:490–497. doi: 10.2337/db12-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfaus J, Giuliano F, Gelez H. Bremelanotide: an overview of preclinical CNS effects on female sexual function. J Sex Med. 2007;4 (Suppl 4):269–279. doi: 10.1111/j.1743-6109.2007.00610.x. [DOI] [PubMed] [Google Scholar]
- 61.Bomback AS, Radhakrishnan J. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) Discov Med. 12:91–96. [PubMed] [Google Scholar]
- 62.Rouster-Stevens KA, Gursahaney A, Ngai KL, et al. Pharmacokinetic study of oral prednisolone compared with intravenous methylprednisolone in patients with juvenile dermatomyositis. Arthritis Rheum. 2008;59:222–226. doi: 10.1002/art.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofstra JM, Brink HS, Van de Kerkhof JJ, et al. Treatment with synthetic ACTH in patients with Idiopathic membranous nephropathy and high risk for renal failure. J Am Soc Nephrol. 2010;21:680A. [Google Scholar]
- 64.Picardi L, Villa G, Galli F, et al. ACTH therapy in nephrotic syndrome induced by idiopathic membranous nephropathy. Clin Nephrol. 2004;62:403–404. doi: 10.5414/cnp62403. [DOI] [PubMed] [Google Scholar]
- 65.Beck LH, Berg AL, Beck DM, et al. Treatment of idiopathic membranous nephropathy with ACTH is associated with a decline in anti-PLA2R antibody. J Am Soc Nephrol. 2009;20:306A. [Google Scholar]
- 66.Beck LH, Fervenza FC, Bomback AS, et al. Response of anti-PLA2R to adrenocorticotropic hormone (ACTH) gel in membranous nephropathy. J Am Soc Nephrol. 2011;22:33A. [Google Scholar]
- 67.Hladunewich MA, Fervenza FC, Beck LH, et al. Apilot study to determine dose, effectiveness and depletion of anti-PLA2R antibodies of adrenocorticotropic hormone (ACTH Acthar gel) in subjects with nephrotic syndrome and idiopathic membranous nephropathy (iMN) J Am Soc Nephrol. 2012;23:58A. [Google Scholar]
- 68.Cooper A, Robinson SJ, Pickard C, et al. Alpha-melanocyte-stimulating hormone suppresses antigen-induced lymphocyte proliferation in humans independently of melanocortin 1 receptor gene status. J Immunol. 2005;175:4806–4813. doi: 10.4049/jimmunol.175.7.4806. [DOI] [PubMed] [Google Scholar]
- 69.Farnsworth EB. Studies on the influence of adrenocorticotrophin in acute nephritis, in simple nephrosis and in nephrosis with azotemia. In: Mote JR, editor. Proceedings of theFirst Clinical ACTH Congress. The Blakiston Company; Philadelphia, PA: 1950. pp. 297–317. [Google Scholar]
- 70.Piel CF. Management of nephrosis; the use of long continued hormone therapy. Calif Med. 1956;85:152–156. [PMC free article] [PubMed] [Google Scholar]
- 71.Hogan JJ, Bomback AS, Canetta PA, et al. Treatment of resistant primary focal segmental glomerulosclerosis (FSGS) with adrenocorticotropic hormone (ACTH) gel. J Am Soc Nephrol. 2012;23:725A. [Google Scholar]
- 72.Berg AL, Dolinina J, Bakoush O. Treatment of focal segmental glomerulosclerosis with ACTH. J Am Soc Nephrol. 2012;23:941A. [Google Scholar]
- 73.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–3015. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 74.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, et al. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol. 2009;296:F213–229. doi: 10.1152/ajprenal.90421.2008. [DOI] [PubMed] [Google Scholar]
- 75.Garcia DL, Rennke HG, Brenner BM, et al. Chronic glucocorticoid therapy amplifies glomerular injury in rats with renal ablation. J Clin Invest. 1987;80:867–874. doi: 10.1172/JCI113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Patel AB, Markell MS. Use of ACTH gel in a patient with progressive nephrotic syndrome secondary to transplant glomerulopathy (TG) J Am Soc Nephrol. 2012;23:365A. [Google Scholar]
- 77.Symmers WS. Thrombotic microangiopathic haemolytic anaemia (thrombotic microangiopathy) Br Med J. 1952;2:897–903. doi: 10.1136/bmj.2.4790.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berg AL, Back SE. ACTH treatment in patients with lupus nephritis. National Kidney Foundation 2013 Spring Clinical Meetings; 2013. Poster 217. [Google Scholar]
- 79.Katritch V, Cherezov V, Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol. 53:531–556. doi: 10.1146/annurev-pharmtox-032112-135923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herraiz C, Journe F, Abdel-Malek Z, et al. Signaling from the human melanocortin 1 receptor to ERK1 and ERK2 mitogen-activated protein kinases involves transactivation of cKIT. Mol Endocrinol. 25:138–156. doi: 10.1210/me.2010-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chai B, Li JY, Zhang W, et al. Melanocortin-3 receptor activates MAP kinase via PI3 kinase. Regul Pept. 2007;139:115–121. doi: 10.1016/j.regpep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Rodrigues AR, Pignatelli D, Almeida H, et al. Melanocortin 5 receptor activates ERK1/2 through a PI3K-regulated signaling mechanism. Mol Cell Endocrinol. 2009;303:74–81. doi: 10.1016/j.mce.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 83.Vongs A, Lynn NM, Rosenblum CI. Activation of MAP kinase by MC4-R through PI3 kinase. Regul Pept. 2004;120:113–118. doi: 10.1016/j.regpep.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Andersen GN, Hagglund M, Nagaeva O, et al. Quantitative measurement of the levels of melanocortin receptor subtype 1, 2, 3 and 5 and pro-opio-melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scand J Immunol. 2005;61:279–284. doi: 10.1111/j.1365-3083.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 86.Delgado M, Ganea D. Anti-inflammatory neuropeptides: a new class of endogenous immunoregulatory agents. Brain Behav Immun. 2008;22:1146–1151. doi: 10.1016/j.bbi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Rey E, Chorny A, Delgado M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat Rev Immunol. 2007;7:52–63. doi: 10.1038/nri1984. [DOI] [PubMed] [Google Scholar]
- 88.Catania A, Gatti S, Colombo G, et al. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 89.Baeuerle PA. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998;95:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- 90.Gong R, Rifai A, Ge Y, et al. Hepatocyte growth factor suppresses proinflammatory NFkappaB activation through GSK3beta inactivation in renal tubular epithelial cells. J Biol Chem. 2008;283:7401–7410. doi: 10.1074/jbc.M710396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor A, Namba K. In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH) Immunol Cell Biol. 2001;79:358–367. doi: 10.1046/j.1440-1711.2001.01022.x. [DOI] [PubMed] [Google Scholar]
- 93.Shalhoub RJ. Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2:556–560. doi: 10.1016/s0140-6736(74)91880-7. [DOI] [PubMed] [Google Scholar]
- 94.Koyama A, Fujisaki M, Kobayashi M, et al. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40:453–460. doi: 10.1038/ki.1991.232. [DOI] [PubMed] [Google Scholar]
- 95.Scott GA, Cassidy L. Rac1 mediates dendrite formation in response to melanocyte stimulating hormone and ultraviolet light in a murine melanoma model. J Invest Dermatol. 1998;111:243–250. doi: 10.1046/j.1523-1747.1998.00276.x. [DOI] [PubMed] [Google Scholar]
- 96.Busca R, Bertolotto C, Abbe P, et al. Inhibition of Rho is required for cAMP-induced melanoma cell differentiation. Mol Biol Cell. 1998;9:1367–1378. doi: 10.1091/mbc.9.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forssman O, Mulder J. Hypersensitivity to different ACTH peptides. Acta Med Scand. 1973;193:557–559. doi: 10.1111/j.0954-6820.1973.tb10627.x. [DOI] [PubMed] [Google Scholar]
- 98.Lee TM, Grammer LC, Shaughnessy MA, et al. Evaluation and management of corticotropin allergy. J Allergy Clin Immunol. 1987;79:964–968. doi: 10.1016/0091-6749(87)90248-x. [DOI] [PubMed] [Google Scholar]
- 99.Reid IR. Glucocorticoid effects on bone. J Clin Endocrinol Metab. 1998;83:1860–1862. doi: 10.1210/jcem.83.6.4911. [DOI] [PubMed] [Google Scholar]
- 100.Minetto M, Reimondo G, Osella G, et al. Bone loss is more severe in primary adrenal than in pituitary-dependent Cushing’s syndrome. Osteoporos Int. 2004;15:855–861. doi: 10.1007/s00198-004-1616-3. [DOI] [PubMed] [Google Scholar]
- 101.Elias LL, Huebner A, Metherell LA, et al. Tall stature in familial glucocorticoid deficiency. Clin Endocrinol (Oxf) 2000;53:423–430. doi: 10.1046/j.1365-2265.2000.01122.x. [DOI] [PubMed] [Google Scholar]
- 102.Zaidi M, Sun L, Robinson LJ, et al. ACTH protects against glucocorticoid-induced osteonecrosis of bone. Proc Natl Acad Sci U S A. 107:8782–8787. doi: 10.1073/pnas.0912176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest. 1997;99:414–423. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ferris HA, Kahn CR. New mechanisms of glucocorticoid-induced insulin resistance: make no bones about it. J Clin Invest. 122:3854–3857. doi: 10.1172/JCI66180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sussman KE, Vaughan GD. Insulin release after ACTH, glucagon and adenosine-3′-5′-phosphate (cyclic AMP) in the perfused isolated rat pancreas. Diabetes. 1967;16:449–454. doi: 10.2337/diab.16.7.449. [DOI] [PubMed] [Google Scholar]
- 106.Al-Majed HT, Jones PM, Persaud SJ, et al. ACTH stimulates insulin secretion from MIN6 cells and primary mouse and human islets of Langerhans. J Endocrinol. 2004;180:155–166. doi: 10.1677/joe.0.1800155. [DOI] [PubMed] [Google Scholar]
- 107.Ohsawa N, Kuzuya T, Tanioka T, et al. Effect of administration of ACTH on insulin secretion in dogs. Endocrinology. 1967;81:925–927. doi: 10.1210/endo-81-4-925. [DOI] [PubMed] [Google Scholar]
- 108.Tumlin JA. Safety and efficacy of Acthar gel (ACTH) on albuminuria and progression of diabetic nephropathy in patients with nephrotic range proteinuria: A randomized prospective study. J Am Soc Nephrol. 2010;21:678A. [Google Scholar]
- 109.Greenfield JR, Miller JW, Keogh JM, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360:44–52. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 110.Ullian ME. The role of corticosteriods in the regulation of vascular tone. Cardiovasc Res. 1999;41:55–64. doi: 10.1016/s0008-6363(98)00230-2. [DOI] [PubMed] [Google Scholar]
- 111.Frey FJ, Odermatt A, Frey BM. Glucocorticoid-mediated mineralocorticoid receptor activation and hypertension. Curr Opin Nephrol Hypertens. 2004;13:451–458. doi: 10.1097/01.mnh.0000133976.32559.b0. [DOI] [PubMed] [Google Scholar]
- 112.Dluhy RG, Lifton RP. Glucocorticoid-remediable aldosteronism. J Clin Endocrinol Metab. 1999;84:4341–4344. doi: 10.1210/jcem.84.12.6256. [DOI] [PubMed] [Google Scholar]
- 113.Mulatero P, Tizzani D, Viola A, et al. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (Primary Aldosteronism in TOrino-GENetic forms) Hypertension. 58:797–803. doi: 10.1161/HYPERTENSIONAHA.111.175083. [DOI] [PubMed] [Google Scholar]
- 114.Sheppard JR, Koestler TP, Corwin SP, et al. Experimental metastasis correlates with cyclic AMP accumulation in B16 melanoma clones. Nature. 1984;308:544–547. doi: 10.1038/308544a0. [DOI] [PubMed] [Google Scholar]
- 115.Bennett DC, Dexter TJ, Ormerod EJ, et al. Increased experimental metastatic capacity of a murine melanoma following induction of differentiation. Cancer Res. 1986;46:3239–3244. [PubMed] [Google Scholar]
- 116.Williams PF, Olsen CM, Hayward NK, et al. Melanocortin 1 receptor and risk of cutaneous melanoma: a meta-analysis and estimates of population burden. Int J Cancer. 129:1730–1740. doi: 10.1002/ijc.25804. [DOI] [PubMed] [Google Scholar]
- 117.Smith KR, Thiboutot DM. Thematic review series: skin lipids. Sebaceous gland lipids: friend or foe? J Lipid Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 118.Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 31:506–543. doi: 10.1210/er.2009-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]