Abstract
Background:
Granulocyte colony-stimulating factor (G-CSF) is a pro-inflammatory cytokine that stimulates myeloid stem cell maturation, proliferation, and migration into circulation. Despite being a known growth factor, the impact of G-CSF on solid tumours has not been well examined. G-CSF receptor (G-CSFR) is expressed by some tumours, and thus the aim of this study was to examine the expression and impact of G-CSF and G-CSFR on gastrointestinal tumours.
Methods:
In this study, G-CSF expression was examined in human gastric and colon tumours and by tumour-derived stromal myofibroblasts and carcinoma cells. G-CSFR expression was examined on carcinoma cells isolated from human tissues. The effects of G-CSF on gastric and colon carcinoma cell proliferation, migration, and signalling were examined.
Results:
G-CSFR was highly expressed in 90% of human gastric and colon carcinomas. G-CSF was also found to be highly produced by stromal myofibroblasts and carcinoma cells. Exposure of carcinoma cells to G-CSF led to increased proliferation and migration, and expansion of a sub-population of carcinoma cells expressing stem-like markers. These processes were dependent on ERK1/2 and RSK1 phosphorylation.
Conclusions:
These data suggest that the G-CSF/R axis promotes gastric and colorectal cancer development and suggest they are potential tumour targets.
Keywords: G-CSF, G-CSFR, gastric cancer, colon cancer, stem cells
Gastric and colorectal cancers account for more than 600 000 deaths in the world each year. Gastric cancer is the fourth most prevalent cancer in the world and the second most common cause of cancer-related deaths (Torpy et al, 2010), whereas colon cancer is the second most common cancer in men and the third most common cancer in women (Jemal et al, 2010). Chronic inflammation is one of the major risk factors associated with the development of both of these gastrointestinal (GI) cancers. There is a known link between gastric cancer and Helicobacter pylori infection, which is known to induce inflammatory cytokines in the stomach. Also, patients with chronic inflammatory bowel disease have an increased incidence of colon cancer that is ∼18–19-fold compared with the general population (Gillen et al, 1994). Although a link between chronic inflammation and carcinogenesis is well established, the underlying mechanisms remain incompletely understood. Inflammatory mediators are thought to have a role in carcinogenesis by altering cell proliferation and inducing mutations that lead to the resistance of cancer cells to apoptosis. A greater understanding of the underlying mechanisms linking inflammation and GI cancers is likely to lead to improved therapeutics.
Granulocyte colony-stimulating factor (G-CSF) is a pro-inflammatory cytokine with the well-studied function of inducing differentiation and mobilisation of neutrophils. However, some tumours also express G-CSF and G-CSF receptor (G-CSFR) (Ninci et al, 2000; Hirai et al, 2001; Sunaga et al, 2001). Of concern is that activation of G-CSFR is known to stimulate common proliferation associated pathways such as MAPK and JAK/STAT (Sampson et al, 2007; Marino and Roguin, 2008), and thus the effects of G-CSFR expression on tumours should be considered. One group has demonstrated that G-CSF stimulates proliferation and migration of head and neck squamous cell carcinoma cell lines (Gutschalk et al, 2006). In addition, there are reports of highly aggressive solid tumours ranging from bladder to gastric cancers that secrete excessive G-CSF. These studies have shown that high levels of tumour-produced G-CSF are associated with poor patient outcomes (Tachibana and Murai, 1998; Yokoyama et al, 2005), and that G-CSF may stimulate tumour growth and progression.
Recently, research on the involvement of stromal cells in the initiation and development of GI cancers has progressed, and it is now acknowledged that fibroblasts/myofibroblasts (MF or α-smooth actin+ (α-SMA) positive, CD90+ stromal fibroblasts) have a critical role in the initiation, growth, and metastasis (Worthley et al, 2010). These cells are thought to facilitate tumour growth and stromal invasion of tumour cells by releasing pro-metastatic factors that act on tumours in a paracrine manner. One study suggests that human fibroblasts can produce G-CSF (Seelentag et al, 1989), but the ability of tumour-associated MF to produce G-CSF has not been examined. As MF are key regulators of chronic inflammation, tumour growth, and metastasis, one aim of this study was to examine if G-CSF is highly produced by these cells in the tumour microenvironment leading to a potential role in tumour growth and development.
As the most well-known function of G-CSF is to mobilise bone marrow-derived stem cells, it is important to examine its effects on tumour cells with stem-like properties. Tumour stem marker expressing cells are thought to be initiators of tumour growth, inducers of metastasis, and a cause of chemotherapy resistance. In gastric and colon tumours, several studies have shown that one marker indicating a stem-like population is CD44 (Wang et al, 2011; Su et al, 2011; Ohata et al, 2012). These studies further suggest that CD44 is not only a marker of cancer initiating cells, but also of invasive cells. The enzyme aldehyde dehydrogenase has also proved useful for identification of cancer stem cells for epithelial cancers, as cells that are high expressers of aldehyde dehydrogenase usually correlate with expression of other stem markers, metastasis, and poor clinical outcome (Deng et al, 2010; Sullivan et al, 2010). One study has also demonstrated that there is a direct correlation between expression of this enzyme and colon stem cells that transition from colitis to cancer, thereby demonstrating the importance of this marker in colon cancer (Carpentino et al, 2009). As G-CSF is known to increase proliferation and mobilisation of bone marrow-derived hematopoietic stem cells (Liongue et al, 2009), a possible role of G-CSF in growth and mobilisation of stem-like cancer cells expressing aldehyde dehydrogenase and CD44 was examined here.
Results of this study indicated that G-CSFR was highly expressed in ∼90% of human gastric and colorectal tumours examined. Tumour-derived stromal fibroblasts/myofibroblasts also produced increased G-CSF. Treatment of gastric and colorectal carcinoma cells with G-CSF or MF supernatants increased their proliferation and migration. Further, G-CSF expanded a stem-like sub-population of carcinoma cells. These processes were found to be dependent on ERK1/2 and RSK phosphorylation. Our findings suggest that G-CSF has a tumour promoting capacity in gastric and colorectal cancers, thus warranting more consideration for its pro-tumorigenic activity.
Materials and Methods
Tissue and cell culture
All human samples were collected under IRB approved human protocols at the University of New Mexico Health Sciences Center and the Legacy Research Institute Tumor Bank where patients gave written consent. Epithelial cells and primary cultures of MF from matched normal and cancer tissues were attained by a series of EDTA treatments and enzymatic cell dissociation using the GentleMACS system (Miltenyi Biotech, Bergisch Gladbach, Germany) and cultured as previously described (Saada et al, 2006). Epithelial cells were used for the flow cytometry. MFs were isolated according to the protocol routinely used in our laboratory (Saada et al, 2006; Pinchuk et al, 2013). The purity of isolated CD90+ and α-SMA MFs (98%–99%) was confirmed by flow cytometry, as previously described (19). Cells were cultured in complete Modified Eagle Medium (MEM) with 10% FBS. MKN-45 cells were obtained from Dr Yoshio Yamaoka at Baylor College of Medicine and AGS cells were obtained from American Type Tissue Culture (ATCC, Manassas, VA, USA) and maintained in RPMI with 10% FBS and 2 mM L-glutamine. Caco-2 and DLD1 cells were obtained from ATCC and maintained in DMEM with 10% FBS and 2 mM L-glutamine.
Real-time (RT) PCR
RNA was isolated using trizol (Life Technologies, Grand Island, NY, USA) according to the manufacturer's instructions. RNA concentrations were measured using a Nanodrop instrument (Thermo Scientific, Wilmington, DE, USA). RT–PCR was performed according to Applied Biosystems' (Foster City, CA, USA) two-step protocol. The RT reaction mixture includes random 2.5 μM hexamers, 500 μM dNTPs, 0.4 U μl−1 of the RNase inhibitors, 5.5 mM MgCl2, MultiScribe Reverse Transcriptase (3.125 U μl−1) and its buffer, and 1 μg of cellular RNA. The RT step was performed according to the following protocol: 10 min at 25 °C, 60 min at 37 °C, 5 min at 95 °C. Obtained cDNA samples were stored at −80 °C and used for the PCR reaction step. The PCR reaction mix was prepared using the Assays-on-Demand gene expression assay mix (Applied Biosystems) for human 18S, G-CSF, and G-CSFR (a 20 × mix of unlabelled PCR primers and TaqMan MGB probe, FAM dye-labelled) and 2 μl of cDNA were added to the PCR reaction mix. The reaction was carried out according to the following protocol: 2 min at 50 °C, 10 min at 95 °C (1 cycle), and 15 s at 95 °C, and 1 min at 60 °C (45 cycles) on Applied Biosystem's StepOnePlus instrument. The end point used in RT–PCR quantification, CT, was defined as the PCR cycle number that crossed the signal threshold. Quantification of cytokine gene expression was performed using the comparative CT method (Sequence Detector User Bulletin 2; Applied Biosystems) and reported as the fold difference relative to the human housekeeping gene, 18S mRNA.
Flow cytometry
Single- and multi-color immunostaining was performed according to standard surface and intracellular FACS staining Biolegend protocols (Biolegend, San Diego, CA, USA). Anti-Ep-cam Alexa-Fluor 488 (Biolegend 9c4) was used for identification of epithelial cells. The purity of MF was analysed by staining for CD90-PE (Biolegend Thy1) and α-smooth actin-Alexa Fluor 488 (R&D Systems 1C1420G, Minneapolis, MN, USA) for flow cytometry compared with isotype controls. Anti-G-CSFR-PE (clone lmm741 Biolegend) and G-CSF-FITC (clone 85FSCSF eBioscience, San Diego, CA, USA) were used for the analysis of G-CSFR surface expression and intracellular G-CSF after 4 h exposure to Brefeldin A compared with the isotype controls. Anti-proliferating cell nuclear antigen (PCNA) (clone PCNA01, Biolegend) was used as a proliferation marker. Cancer stem-like populations in carcinoma cell lines were examined by staining with Aldefluor (Stem Cell Technologies, Vancouver, BC, Canada) and anti-CD44-APC (Biolegend clone BJ18) using the manufacturer's Aldefluor staining protocol compared with isotype control. All samples were analysed on a Guava easyCyte 8HT flow cytometer (EMD Millipore, Bellerica, MA, USA), and analysed using FCS Express software (DeNovo Software, Los Angeles, CA, USA).
Luminex arrays
G-CSF levels were measured in cell culture supernatants by singleplex fluorescent bead-based array (Life Technologies) according to the manufacturer's instructions. ERK1/2 and RSK phosphorylation were measured by Milliplex MAPmates phospho-ERK1/2 and RSK arrays (EMD Millipore) according to the manufacturer's protocol. All samples were analysed on a Luminex 200 machine.
Proliferation
MKN-45, AGS, Caco-2, and DLD1 cells (2500 per well) were added to wells of a 96-well plate and incubated with 5–25 ng ml−1 of recombinant G-CSF (eBioscience) or conditioned media from MF for 48 h. Conditioned media included a mixture of 50% media from cultured myofibroblasts and 50% fresh media for nutrients. Media from MF was taken when cells were 80% or greater confluent before passage (from ∼1 × 106 cells). Proliferation was measured using CyQuant dye for DNA content (Life Technologies). Other samples were incubated with 10 μM ERK activation inhibitor peptide 1 or RSK inhibitor SL0101 (EMD Millipore) for 30 min before addition of recombinant G-CSF. Samples were read on a Tecan GENios fluorescent plate reader (Tecan, Mannedorf, Switzerland).
Migration
MKN-45 and Caco-2 cells were stained with carboxyfluorein succinimidyl ester (CFSE) (Life Technologies) and added to the top of Fluoroblok 96-well plates with 8.0 μM pores. Serum-free media, media with 10% FBS, recombinant G-CSF, or MF supernatants were added to the bottom of the wells. G-CSF neutralising antibodies (1 μg ml−1) were added to some cultures. The fluorescence of migrated cells was measured after 4 h of incubation at 37 °C on a Tecan plate reader.
Statistical analysis
Results were expressed as the mean±s.e. of data obtained from at least three independent experiments done with triplicate sets in each experiment. Differences between means were evaluated by ANOVA using Student's t-test for multiple comparisons in GraphPad Prism 5. Values of P<0.05 were considered statistically significant.
Results
Human gastric and colon epithelial cells and tumour-derived fibroblasts produce G-CSF
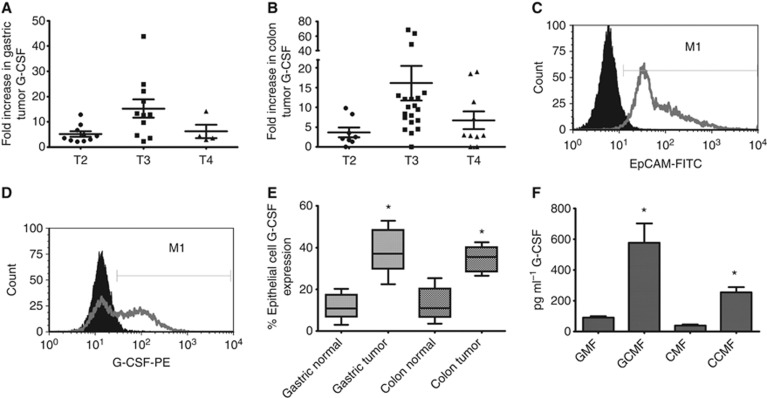
The pro-inflammatory nature of G-CSF and its ability to induce proliferation pathways led us to examine expression in human tumours. The mRNA levels were first examined in human gastric and colon tumours compared with matched normal tissues from the same individual. 26 gastric tumours were examined with 25 of the 26 exhibiting a greater than two-fold increase in G-CSF mRNA levels (Figure 1A). These data were further examined with regards to tumour stage. Tumour stage 3 (T3) samples were found to have significantly higher G-CSF expression than T2 and T4. The mean fold increase in G-CSF mRNA levels over matched normal tissues for T2 was 5.13, T3 was 15.25, and T4 was 6.22. Similar results were seen with colon tumours (Figure 1B) where T3 had significantly higher G-CSF mRNA levels with the mean for T2 at 3.65, T3 at 16.14, and T4 at 7.16-fold increase over normal tissues. Demographic and grade data were available for 5 of the gastric cancer patients and 14 of the colon cancer patients. There were three males and two females all with high-grade tumours in the gastric cancer group. The colon cancer group had eight males and six females, with three patients having high-grade tumours, eight having intermediate-grade tumours, and three having low-grade tumours. There were no significant differences between the fold increases in G-CSF and G-CSFR tumour expression when stratified by sex or tumour grade. To further examine G-CSF production, epithelial cells were isolated from tissues as previously described (Saada et al, 2006). After isolation, >95% of cells were epithelial cells as evidenced by staining with Ep-Cam for flow cytometry (Figure 1C). Isolated epithelial cells from T3 samples were stained for G-CSF intracellularly (Figure 1D) and data from both gastric and colon tumours reveal a significant increase in G-CSF expression (up to 30% increase) was seen compared with matched normal tissues from the same individuals (Figure 1E). In addition to epithelial cells isolated from tumours, tumour-associated fibroblasts/myofibroblasts were also examined. These cells are known to produce inflammatory cytokines and growth factors and our group routinely cultures these cells from human tissues (Saada et al, 2006; Pinchuk et al, 2013). Thus, we found cultured tumour-derived fibroblasts/myofibroblasts (GMF for gastric and CMF for colon) to produce significant amounts of G-CSF (Figure 1F). By Luminex fluorescent singleplex bead array, GMF was found to produce up to 600 pg ml−1 of G-CSF in supernatants compared with 50 pg ml−1 for normal tissue-derived cells, and CMF was found to produce up to 300 pg ml−1 of G-CSF in supernatants, whereas normal colon tissue-derived cells were found to produce 25 mg ml−1 on average. These data suggest that both gastric and colon tumours produce G-CSF, by both carcinoma cells and tumour- associated fibroblasts/myofibroblasts.
Figure 1.
G-CSF is highly expressed in human tumours and by GI epithelial cells. G-CSF mRNA levels are increased in (A) gastric and (B) colon tumours compared with normal tissues as shown by tumour stage by quantitative RT–PCR. (C) Epithelial cells isolated from human tumours express EpCAM and (D) and G-CSF in representative figures. (E) In compiled data from multiple experiments, G-CSF expression is increased in epithelial cells isolated from gastric and colon tumours compared with epithelial cells from matched normal tissues when analysed by flow cytometry. G-CSF is also produced in increased amounts by tumour-derived gastric and colon cancer fibroblasts/myofibroblasts as compared with matched normal tissue-derived fibroblasts/myofibroblasts as shown in the media from such cultured cells by (F) Luminex bead array. N=8 for E and F and the mean±s.e. values are shown as the results of multiple experiments. *P<0.05.
Human gastric and colon tumours express G-CSFR
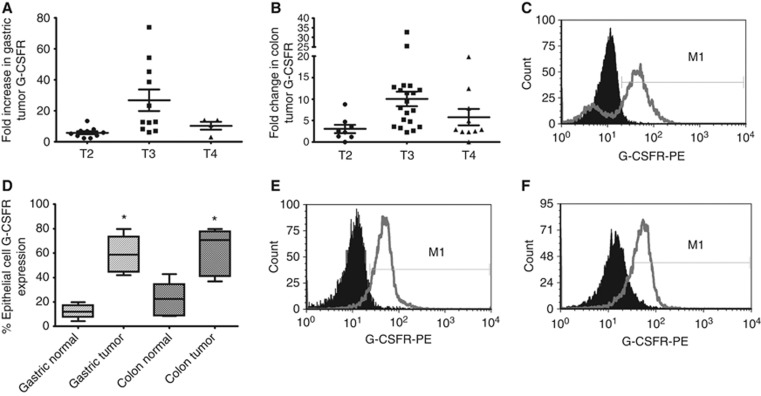
As G-CSF is known to induce proliferation of some cell types through induction of G-CSFR signalling, the expression of G-CSFR was examined in human gastric and colon tumours. The G-CSFR mRNA level in tumour samples was compared with that in matched normal gastric or colon tissues from the same patient by quantitative RT–PCR. 26 gastric tumours were examined with 25 exhibiting a greater than two-fold increase in G-CSFR mRNA levels. The fold increase in mRNA levels ranged from 2.3 to 73.9, with 15 samples having over a five-fold increase (Figure 2A). When samples were examined by tumour stage, T3 had a significantly higher mean than T2 and T4. The mean fold increase in G-CSFR levels in tumours over normal tissues for T2 was 7.96, T3 was 26.80, and T4 was 10.68. 39 colorectal cancer samples were also examined with 35 exhibiting a greater than two-fold increase in G-CSFR mRNA levels over the matched normal colon tissue. The increase ranged from 2.1 to 32.7-fold with 13 samples having over a five-fold increase (Figure 2B). When samples were examined by tumour stage, T3 had a significantly higher mean similar to the gastric tumour samples with T2 having a mean fold increase of 3.57, T3 was 10.06, and T4 was 5.8 in tumours over matched normal tissues. To assure the overexpression was on the epithelial cells as opposed to local infiltration of immune cells, epithelial cells were isolated from tissues as previously described (Saada et al, 2006). As described above, >95% of isolated cells were epithelial cells as evidenced by staining with Ep-Cam for flow cytometry (not shown). On staining epithelial cells for G-CSFR surface expression for flow cytometry, ∼60% of tumour-derived epithelial cells were found to express G-CSFR compared with 10% of normal gastric epithelial cells and 20% of normal colon epithelial cells (Figure 2C and D). As functional assays require the use of cell lines, G-CSFR expression was also examined on the surface MKN-45 gastric carcinoma cells and Caco-2 colorectal carcinoma cells. Figure 2E and F demonstrate the presence of G-CSFR on both MKN-45 and Caco-2 cell surfaces when analysed by flow cytometry. G-CSFR expression was also seen with AGS gastric carcinoma cells and DLD1 colon carcinoma cells (not shown). To compare expression of G-CSFR on human samples to cell lines, the mean fluorescence intensities (MFI) were compared in flow cytometry data from Figures 2C–E, and MFI of cell lines was within 12% of the median for cancer cells, indicating that these are relevant cell line models with similar expression levels as the primary tumour samples. We also examined the cell lines for production of G-CSF and found low basal levels of production, which was significantly increased when they were exposed to lipopolysaccharide (data not shown). The increased expression of the receptor in gastric and colon tumours and the expression on carcinoma cell lines suggest a need to examine the effects of G-CSF on GI tumour cells.
Figure 2.
G-CSFR is highly expressed in human tumours and by GI epithelial cells. G-CSFR mRNA levels are increased in (A) gastric and (B) colon tumours compared with normal tissues as shown by tumour stage by quantitative RT–PCR. (C) G-CSFR is expressed on epithelial cells isolated from a tumour sample and (D) expression is increased in epithelial cells isolated from gastric and colon tumours compared with epithelial cells from matched normal tissues when analysed by flow cytometry. G-CSFR is expressed on the surface of (E) MKN-45 and (F) Caco-2 cells in representative histograms compared with solid peak isotype controls. N=8 for D and the mean±s.e. values are shown as the results of multiple experiments, *P<0.05.
G-CSF induces GI carcinoma cell proliferation
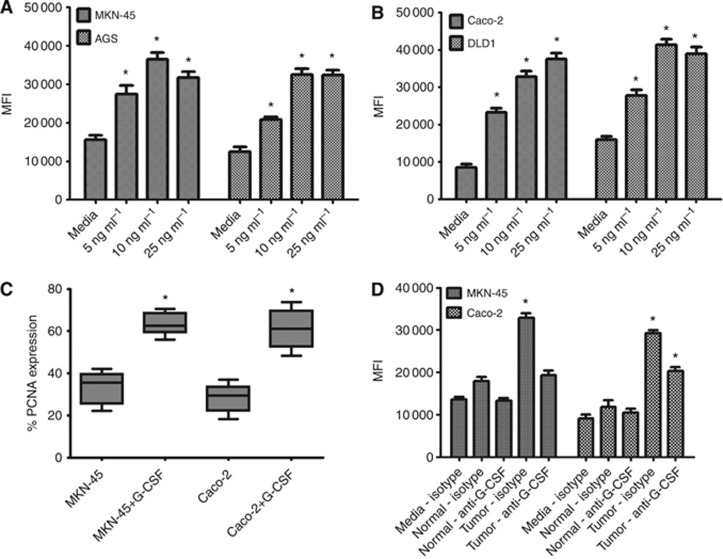
As G-CSF has been shown to induce the proliferation of myeloid cells as well as head and neck squamous cancer cells (Gutschalk et al, 2006), we examined the impact of G-CSF on gastric and colon carcinoma cells. MKN-45 and AGS gastric carcinoma cells and Caco-2 and DLD1 colon carcinoma cells were incubated with increasing doses of recombinant G-CSF (5–25 ng ml−1), which were chosen based on reports of serum concentrations in patients (Morstyn et al, 1989). After 4 days (∼2 doubling times), proliferation was measured using the CyQuant fluorescent proliferation assay for DNA content. G-CSF-treated cells were compared with untreated control cells. Both gastric and colon carcinoma cells exhibited a dose-dependent increase in proliferation compared with untreated cells, with up to a 2.5-fold increase in MKN-45 and AGS proliferation and up to 4.5-fold increase in Caco-2 and DLD1 colon carcinoma cell proliferation (Figure 3A and B). To further confirm G-CSF induction of cell proliferation, G-CSF-treated cells were stained intracellularly for PCNA. A greater than two-fold increase in PCNA staining was seen on flow cytometry for G-CSF-treated cells as compared with untreated cells for both cell types (Figure 3C). As tumour-derived fibroblasts/myofibroblasts were found to produce high levels of G-CSF, supernatants from these cells isolated from human tissues were incubated with MKN-45 and Caco-2 cells in proliferation assays (Figure 3D). Proliferation of carcinoma cells was found to increase significantly on incubation with conditioned media from cancer-derived fibroblasts/myofibroblasts, but was decreased on addition of G-CSF neutralising antibodies. These data suggest possible autocrine and paracrine activity of G-CSF on GI carcinoma cells.
Figure 3.
G-CSF induces proliferation of gastric and colon carcinoma cells. G-CSF treatment induces proliferation by CyQuant assay for DNA content of (A) MKN-45 and AGS gastric carcinoma cells, (B) Caco-2 and DLD1 colon carcinoma cells. Proliferation was further verified by (C) PCNA staining by flow cytometry. (D) Tumour-derived GMF and CMF supernatants induced proliferation of MKN-45 and Caco-2, which was decreased on adding anti-G-CSF neutralising antibodies. The mean±s.e. values are shown as the results of multiple experiments, N=8 *P<0.05 compared with untreated.
G-CSF induces GI carcinoma cell migration
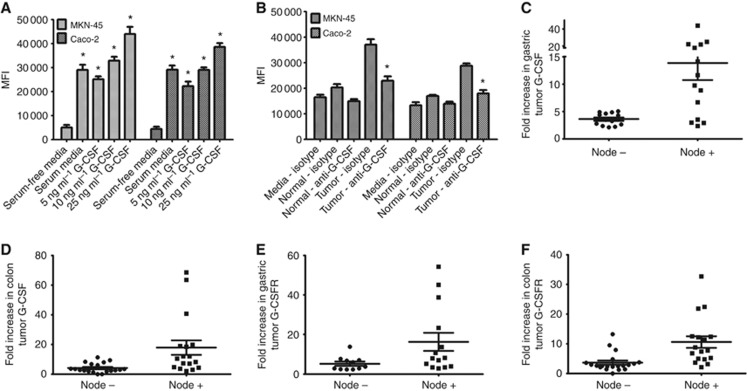
Given the ability of G-CSF to mobilise bone marrow-derived hematopoietic cells during systemic administration (Held and Gundert-Remy, 2010), we assessed whether it could increase the migratory ability, and therefore the malignant potential, of gastric and colon cancer cells. MKN-45 and Caco-2 were serum starved overnight, fluorescently labelled with CFSE, and plated on FluorBlok plates with 8 micron pores and serum-free media in the top of the wells. The bottom wells were filled with serum-free media, serum-free media containing 10 ng ml−1 of G-CSF or serum-containing media (positive control). As indicated in Figure 4A, G-CSF induced increased migration of both MKN-45 and Caco-2 cells. Both cell types migrated through the pores towards serum as a positive control, and at even higher rates towards G-CSF. Up to a four-fold increased migration was seen with G-CSF compared with serum-free media, and 1.5-fold increased migration was seen with G-CSF compared with serum-containing media. To further examine the potential for G-CSF to enhance the migratory ability of GI tumour cells, supernatants from tumour-derived gastric and colon MF were placed in the bottom wells and compared with wells with supernatants from normal tissue-derived MF and fresh media with serum. In Figure 4B, supernatants from tumour-derived MF increased migration of MKN-45 and Caco-2 cells by at least two-fold compared with normal MF supernatants. This effect was decreased by neutralising G-CSF with monoclonal antibodies. As MFs represent a major component of tumour stroma, their production of G-CSF could be very significant in inducing carcinoma cell mobilisation and contributing to the invasive potential of the tumour.
Figure 4.
G-CSF induces migration of gastric and colon carcinoma cells. Fluorescently labelled MKN-45 and Caco-2 cells were added to the top of Fluorblock plates with 8 μm pores with (A) recombinant G-CSF and (B) normal and tumour MF supernatants with G-CSF neutralisation by monoclonal antibodies. Migration was assessed by mean fluorescence intensity. The mean±s.e. values are shown as the results of multiple experiments, N=8 *P<0.05 compared with serum-free media. Human tumour tissues from individuals that had cells migrate to lympn nodes have higher (C) G-CSF in gastric cancer, (D) G-CSF in colon cancer, (E) G-CSFR in gastric cancer, and (F) G-CSFR in colon cancer.
To support this data, the presence of G-CSF and G-CSFR in human gastric and colon tumours was analysed by comparing primary tumour samples from lymph node-negative vs lymph node-positive individuals. Figures 4C–F indicate that the majority of samples from node positive individuals were higher in both G-CSF and G-CSFR expression than node negative samples. For gastric tumours, G-CSF mRNA had a mean of 3.48-fold increase in samples from node negative individuals and 14.13 in samples from node positive individuals. G-CSFR mRNA was increased by 5.25-fold in samples from node negative individuals and 16.27 with node positive individuals. Similar results were seen with colon tumours where G-CSF mRNA levels were increased by 4.17-fold in tissues from node negative individuals and 17.97 in tissues from node positive individuals, whereas G-CSFR mRNA levels were increased by 3.62-fold in node negative and 10.56-fold in tissues from node positive individuals. These results suggest that increased G-CSF in human colon and gastric cancers is linked to cell migration from primary tumours from gastric and colon cancer patients in which cancer cells have migrated to a lymph node.
G-CSF expands a population of carcinoma cells expressing stem-like markers
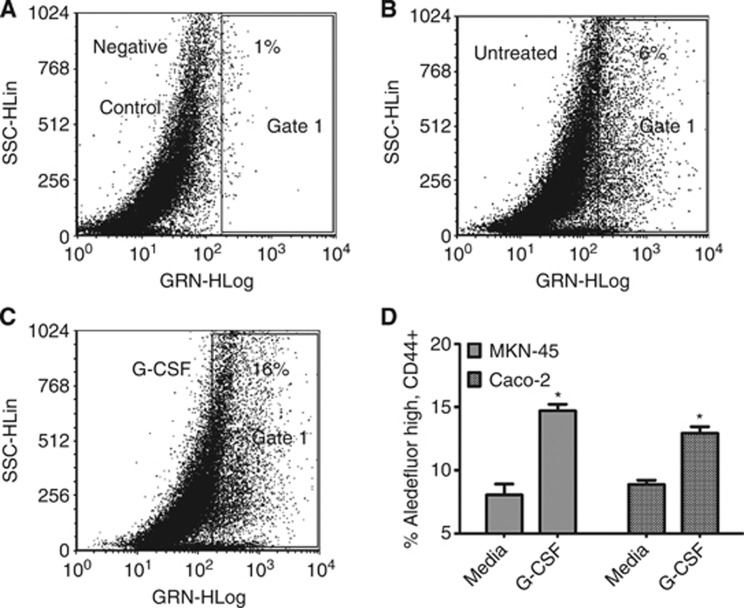
Recently, sub-populations of cells within GI cell lines have been shown to express stem markers such as CD44 and aldehyde dehydrogenase (Huang et al, 2009; Takaishi et al, 2009; Su et al, 2011). Therefore, assessment of MKN-45 and Caco-2 cells for a similar population was undertaken. Cells were stained with Aldefluor, which has been shown to identify cells with stem-like characteristics that stain for aldehyde dehydrogenase (Huang et al, 2009; Katsuno et al, 2012). Cells were also stained with CD44, as in gastric and colon cancers CD44 has also been shown on cells with stem-like features (Huang et al, 2009; Takaishi et al, 2009; Su et al, 2011; Katsuno et al, 2012; Wakamatsu et al, 2012). To examine Aldefluor staining, cells were gated on the CD44 positive population. Then, another gate was set using the diethylaminobenzaldehyde (DEAB) inhibitor of aldehyde dehydrogenase as a negative control (Figure 5A). MKN-45 cells were found to contain stem-like populations as identified by Aldefluor when compared with cells treated with DEAB (Figure 5A and B). G-CSF treatment expanded this population from 6 to 16% of the cell population (Figure 5C). Further, 48 h treatment of MKN-45 and Caco-2 cells with 10 ng ml−1 of G-CSF led to an ∼10% increase in the stem-like AldefluorhighCD44+ population (Figure 5D) with both cell types. These data suggest that G-CSF may not only expand the numbers of hematopoietic stem cells, but also cells that express markers that may indicate stem-like characteristics.
Figure 5.
G-CSF increases a population of cells expressing stem-like markers in MKN-45 and Caco-2 cells. Aldefluor staining of MKN-45 cells gated on the CD44 positive population in representative dot plots as shown with (A) DEAB aldehyde dehydrogenase negative control inhibitor, (B) untreated cells, (C) G-CSF-treated cells, and (D) compiled data for MKN-45 and Caco-2 cells. For D, the mean±s.e. values are shown as the results of multiple experiments. N=8, *P<0.05.
G-CSF induces proliferation through ERK1/2 and RSK1 signalling
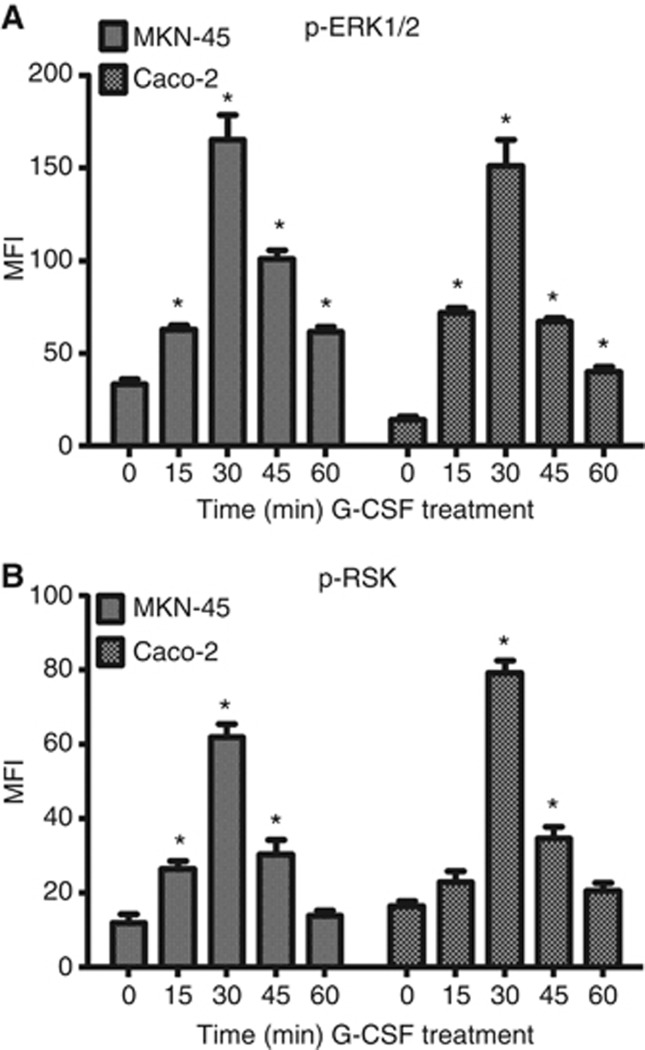
Some studies have shown that G-CSFR signalling induces signalling pathways that lead to cell proliferation (Kamezaki et al, 2005; Wang et al, 2008). However, most of the studies have been performed in myeloid cells or neutrophils. Thus, we sought to examine G-CSFR signalling in MKN-45 and Caco-2 cells. Cells were preincubated with serum-free media for 2 h before treatments and recombinant G-CSF (10 ng ml−1) was added to wells in 15 min intervals. Cells were lysed and protein levels normalised. Lysates were run on Luminex signalling bead arrays where ERK1/2 and RSK1 were found to be phosphorylated, peaking at 30 min of incubation time. A five-fold increase in phosphorylation was found in MKN-45 and a 10-fold increase in phosphorylation was found in Caco-2 cells (Figure 6A and B). The other signalling molecules tested showed no change in phosphorylation after G-CSF treatment. As we found phosphorylation of these signalling molecules after G-CSF treatment, we further sought to characterise the role of these pathways in the increased cell proliferation resulting from G-CSF treatment observed in MKN-45 and Caco-2 carcinoma cells shown in Figure 3. ERK1/2 or RSK inhibitors were added to some wells of cells before addition of G-CSF, and proliferation examined by CyQuant proliferation assay for DNA content. Figure 7A indicates that inhibiting either ERK1/2 or RSK drastically inhibited G-CSF induced proliferation, back to basal levels or below, indicating a mechanism of G-CSF-induced proliferation of cancer epithelial cells.
Figure 6.
G-CSF induces ERK1/2 and RSK signalling. Recombinant G-CSF treatment induces (A) ERK1/2 and (B) RSK1 phosphorylation. The mean±s.e. values are shown as the results of multiple experiments. N=8, *P<0.05.
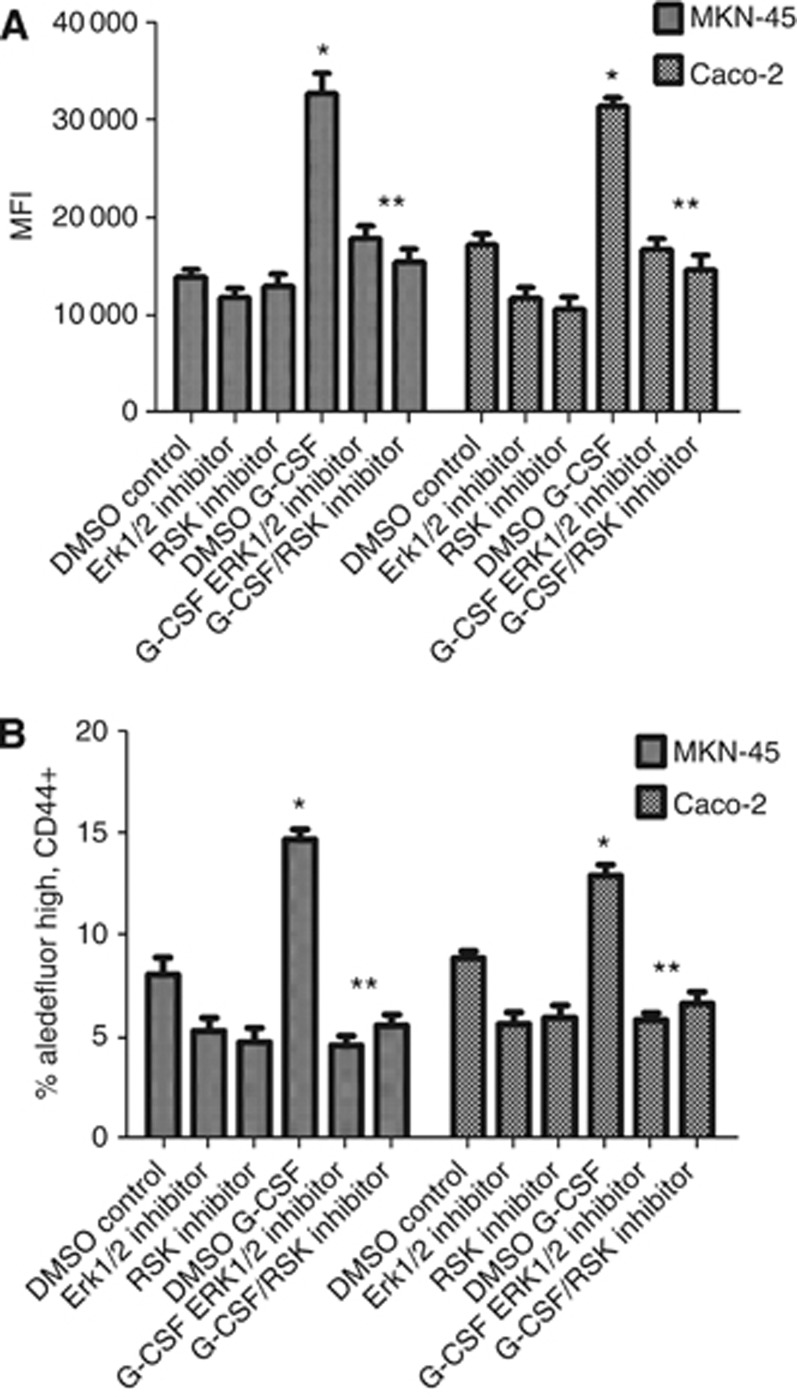
Figure 7.
Inhibition of ERK1/2 and RSK pathways reduces G-CSF-induced proliferation and the increased population expressing stem-like markers. (A) G-CSF induced cell proliferation is inhibited by ERK1/2 and RSK inhibitors as shown by fluorescent cell proliferation assay for DNA content (CyQuant) and (B) G-CSF-induced expansion populations expressing CD44 and aldehyde dehydrogenase is inhibited by ERK1/2 and RSK inhibitors. The mean±s.e. values are shown as the results of multiple experiments. N=8, *P<0.05 with G-CSF treated compared to control and **P<0.05 with inhibitors compared to G-CSF treated.
In parallel, ERK1/2 and RSK inhibition were also examined for effects on stem-like populations. Similar to the general decrease in gastric and colon carcinoma cell proliferation observed in Figure 7A, inhibiting ERK1/2 or RSK also decreased the G-CSF-induced expansion of the stem marker expressing cells as indicated in Figure 7B when the population expressing high levels of aldehyde dehydrogenase and CD44 was examined. The G-CSF-induced stem marker expressing cell expansion was completely abrogated by treatment with either ERK1/2 or RSK inhibitors, indicating activation of these signalling pathways is key for the tumour stimulatory effects observed after G-CSF treatment.
Discussion
As Virchow initially hypothesised a link between inflammation and cancer in 1863 (Balkwill and Mantovani, 2001), possible underlying mechanisms have been investigated, yet still not fully understood. G-CSF is a cytokine known to be involved in multiple cell survival, proliferation, and invasion related pathways, but its role in GI cancers has not been examined. The data presented here are similar to the findings for other tumour types in that we found increased amounts of G-CSF and G-CSFR in gastric and colorectal cancer specimens as well as in epithelial cells isolated from these human tumours. These results, along with reports in the literature of significant changes in the expression of over 300 genes, including increase in such pro-tumorigenic factors as VEGF and TGF-β after administration of G-CSF (McGuire et al, 2001; Fujii et al, 2004; Amariglio et al, 2007), led us to further investigate the potential for pro-tumorigenic effects of G-CSF on gastric and colorectal cancers. The data presented here suggest that G-CSF treatment may promote GI tumour growth, as it induces both the proliferation and migration of gastric and colon carcinoma cells. Our data are in agreement with the previous findings from the head and neck cancer field, where it has been demonstrated that G-CSF stimulates proliferation and migration of squamous carcinoma cells (Gutschalk et al, 2006). Further, using xenograft animal models, the same research group demonstrated that G-CSF expressing tumours exhibit higher invasive capacity.
The production of G-CSF by both tumour-derived epithelial cells and fibroblasts/myofibroblasts, and epithelial expression of G-CSFR suggests autocrine and paracrine loops leading to stimulation of GI carcinoma progression. The data shown here provide a potential mechanistic link between chronic inflammation and progression of GI cancers that has not yet been considered. These data suggest that tumour-derived fibroblasts/myofibroblasts respond to these malignant changes with increased secretion of G-CSF, thus enabling accelerated progression of malignancy. The observation of increased expression of both cytokine and receptor in higher tumour stage and nodal stage suggests a role for G-CSF in the progression and metastasis of human GI cancers. Interestingly, we found the highest ligand/receptor expression in T3 stage tumours, suggesting this cytokine may confer an advantage to tumours as far as migration and tumour progression. Elevated levels of G-CSF could facilitate this process in multiple ways illustrated by the findings of this study. Increased proliferation allows for more tumour heterogeneity, supporting the development of more invasive mutations within the tumour population. Increased migration towards elevated levels of G-CSF within the stroma could assist in initiating tumour cell mobilisation needed for metastasis. Thus, it is possible that suppression of this cytokine axis within tumours may decrease metastatic potential once cancers are diagnosed, or perhaps reverse progression from dysplasia to carcinoma in high risk patients. Clearly, further investigation into the role of this cytokine in human GI cancers is warranted.
G-CSF is most well-known for its ability to stimulate migration of bone marrow-derived granulocyte precursor stem cells, which results in increased white blood cell counts. This led us to consider its possible effects on cells that express stem-like markers as it has been postulated that cancer is a disease initiated and maintained by stem cells (Jiang et al, 2012). A substantial body of evidence supports the hypothesis that neoplasms are initiated and maintained by a small population of cells within a tumour that possess properties similar to those of normal adult stem cells (Lobo et al, 2007). These qualities include the ability to self-renew and generate differentiated progeny. According to this hypothesis, only a small subset of tumour cells are required to initiate and sustain malignant tumour growth and to give rise to the phenotypic heterogeneity observed in the original tumour; this is true for a wide variety of cancers, including gastric and colorectal cancer (Dalerba et al, 2007; Singh, 2013). Although markers of stem-like cells are debated in the field, many groups agree that high expression of aldehyde dehydrogenase is a strong indicator of stem-like characteristics (Huang et al, 2009). In addition, several studies have indicated that CD44 is highly expressed by cells with stem-like characteristics in both gastric and colon cancers (Takaishi et al, 2009; Wang et al, 2011; Wakamatsu et al, 2012). Thus, we chose to examine the effects of G-CSF on the subset of cells expressing these markers within gastric and colon carcinoma cells. Cells expressing these markers were found to be increased in number after G-CSF treatment. Similar data were found for prostate cancer (Ma et al, 2012), wherein stimulation with G-CSF of prostate cancer cells resulted in an increase in the number of cells expressing prostate cancer stem markers. Taken together with this report, our data suggest that G-CSF treatment may promote maintenance of higher levels of cells expressing markers representing stem-like characteristics. These findings raise additional concern about G-CSF in gastric or colon cancers. Indeed, if G-CSF increases the population of cells with stem-like qualities in vitro, it is possible it could increase risk of further metastasis or resistance to therapy.
The effects of G-CSF on proliferation of GI carcinoma cells are not surprising given the activation of such key proliferation pathways. We found significant activation in both the RSK and Erk1/2 pathways in gastric and colorectal cells after treatment with G-CSF. In addition, it was confirmed that the increased proliferation and migration in our studies were due to phosphorylation of these signalling molecules. Interestingly, the increased population of cells expressing stem-like markers after treatment with G-CSF was reduced on blockade of these pathways as well, suggesting that many of the potentially deleterious effects of G-CSF may be due to activation of RSK and ERK1/2. Others have also shown a role for G-CSF/G-CSFR in activating the JAK/STAT pathway along with the ERK pathway (Sampson et al, 2007; Marino and Roguin, 2008). In our study, we found the ERK and RSK pathways to be the major factors, which may reflect a difference in cell types examined.
Given the data presented here with gastric and colon tumour production of G-CSF and the resulting induction of proliferation and expansion of carcinoma cells, there is evidence that G-CSF may be an important link between inflammation and tumour progression. Thus, there is also the potential that this receptor and cytokine could be a prognostic marker. Further examination of these pathways could lead to changes in treatment for patients with this tumour attribute.
Acknowledgments
This work was supported by grants from the American Cancer Society (RSG-10-159-01-LIB) and (IRG-92-024) and National Institutes of Health 8UL1TR000041, The University of New Mexico Clinical and Translational Science Center. We would like to thank Dr Laurie Hudson, Department of Pharmaceutical Sciences at the University of New Mexico, for reviewing the article and providing helpful advice. We would also like to acknowledge Legacy Research Tumor Bank for generously supplying tumour samples.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Amariglio N, Jacob-Hirsch J, Shimoni A, Leiba M, Rechavi G, Nagler A. Changes in gene expression pattern following granulocyte colony-stimulating factor administration to normal stem cell sibling donors. Acta Haematol. 2007;117 (2:68–73. doi: 10.1159/000096856. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow. Lancet. 2001;357 (9255:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, Huang EH. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69 (20:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Deng S, Yang X, Lassus H, Liang S, Kaur S, Ye Q, Li C, Wang LP, Roby KF, Orsulic S, Connolly DC, Zhang Y, Montone K, Butzow R, Coukos G, Zhang L. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5 (4:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K, Ishimaru F, Kozuka T, Matsuo K, Nakase K, Kataoka I, Tabayashi T, Shinagawa K, Ikeda K, Harada M, Tanimoto M. Elevation of serum hepatocyte growth factor during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Br J Haematol. 2004;124 (2:190–194. doi: 10.1046/j.1365-2141.2003.04745.x. [DOI] [PubMed] [Google Scholar]
- Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35 (11:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschalk CM, Herold-Mende CC, Fusenig NE, Mueller MM. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor promote malignant growth of cells from head and neck squamous cell carcinomas in vivo. Cancer Res. 2006;66 (16:8026–8036. doi: 10.1158/0008-5472.CAN-06-0158. [DOI] [PubMed] [Google Scholar]
- Held TK, Gundert-Remy U. Pharmacodynamic effects of haematopoietic cytokines: the view of a clinical oncologist. Basic Clin Pharmacol Toxicol. 2010;106 (3:210–214. doi: 10.1111/j.1742-7843.2009.00514.x. [DOI] [PubMed] [Google Scholar]
- Hirai K, Kumakiri M, Fujieda S, Sunaga H, Lao LM, Imamura Y, Ueda K, Fukuda M. Expression of granulocyte colony-stimulating factor and its receptor in epithelial skin tumors. J Dermatol Sci. 2001;25 (3:179–188. doi: 10.1016/s0923-1811(00)00131-6. [DOI] [PubMed] [Google Scholar]
- Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69 (8:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19 (8:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- Jiang W, Peng J, Zhang Y, Cho WC, Jin K. The implications of cancer stem cells for cancer therapy. Int J Mol Sci. 2012;13 (12:16636–16657. doi: 10.3390/ijms131216636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamezaki K, Shimoda K, Numata A, Haro T, Kakumitsu H, Yoshie M, Yamamoto M, Takeda K, Matsuda T, Akira S, Ogawa K, Harada M. Roles of Stat3 and ERK in G-CSF signaling. Stem Cells. 2005;23 (2:252–263. doi: 10.1634/stemcells.2004-0173a. [DOI] [PubMed] [Google Scholar]
- Katsuno Y, Ehata S, Yashiro M, Yanagihara K, Hirakawa K, Miyazono K. Coordinated expression of REG4 and aldehyde dehydrogenase 1 regulating tumourigenic capacity of diffuse-type gastric carcinoma-initiating cells is inhibited by TGF-beta. J Pathol. 2012;228 (3:391–404. doi: 10.1002/path.4020. [DOI] [PubMed] [Google Scholar]
- Liongue C, Wright C, Russell AP, Ward AC. Granulocyte colony-stimulating factor receptor: stimulating granulopoiesis and much more. Int J Biochem Cell Biol. 2009;41 (12:2372–2375. doi: 10.1016/j.biocel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- Ma Y, Liang D, Liu J, Axcrona K, Kvalheim G, Giercksky KE, Nesland JM, Suo Z. Synergistic effect of SCF and G-CSF on stem-like properties in prostate cancer cell lines. Tumour Biol. 2012;33 (4:967–978. doi: 10.1007/s13277-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino VJ, Roguin LP. The granulocyte colony stimulating factor (G-CSF) activates Jak/STAT and MAPK pathways in a trophoblastic cell line. J Cell Biochem. 2008;103 (5:1512–1523. doi: 10.1002/jcb.21542. [DOI] [PubMed] [Google Scholar]
- McGuire TR, Kessinger A, Hock L, Sharp JG. Elevated transforming growth factor beta levels in the plasma of cytokine-treated cancer patients and normal allogeneic stem cell donors. Cytotherapy. 2001;3 (5:361–364. doi: 10.1080/146532401753277652. [DOI] [PubMed] [Google Scholar]
- Morstyn G, Campbell L, Lieschke G, Layton JE, Maher D, O'Connor M, Green M, Sheridan W, Vincent M, Alton K. Treatment of chemotherapy-induced neutropenia by subcutaneously administered granulocyte colony-stimulating factor with optimization of dose and duration of therapy. J Clin Oncol. 1989;7 (10:1554–1562. doi: 10.1200/JCO.1989.7.10.1554. [DOI] [PubMed] [Google Scholar]
- Ninci EB, Brandstetter T, Meinhold-Heerlein I, Bettendorf H, Sellin D, Bauknecht T. G-CSF receptor expression in ovarian cancer. Int J Gynecol Cancer. 2000;10 (1:19–26. doi: 10.1046/j.1525-1438.2000.99076.x. [DOI] [PubMed] [Google Scholar]
- Ohata H, Ishiguro T, Aihara Y, Sato A, Sakai H, Sekine S, Taniguchi H, Akasu T, Fujita S, Nakagama H, Okamoto K. Induction of the stem-like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer-initiating cells. Cancer Res. 2012;72 (19:5101–5110. doi: 10.1158/0008-5472.CAN-11-3812. [DOI] [PubMed] [Google Scholar]
- Pinchuk IV, Morris KT, Nofchissey RA, Earley RB, Wu JY, Ma TY, Beswick EJ. Stromal cells induce Th17 during Helicobacter pylori infection and in the gastric tumor microenvironment. PLoS ONE. 2013;8 (1:e53798. doi: 10.1371/journal.pone.0053798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC, Di Mari JF, Reyes VE, Powell DW. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol. 2006;177 (9:5968–5979. doi: 10.4049/jimmunol.177.9.5968. [DOI] [PubMed] [Google Scholar]
- Sampson M, Zhu QS, Corey SJ. Src kinases in G-CSF receptor signaling. Front Biosci. 2007;12:1463–1474. doi: 10.2741/2160. [DOI] [PubMed] [Google Scholar]
- Seelentag W, Mermod JJ, Vassalli P. Interleukin 1 and tumor necrosis factor-alpha additively increase the levels of granulocyte-macrophage and granulocyte colony-stimulating factor (CSF) mRNA in human fibroblasts. Eur J Immunol. 1989;19 (1:209–212. doi: 10.1002/eji.1830190135. [DOI] [PubMed] [Google Scholar]
- Singh SR. Gastric cancer stem cells: a novel therapeutic target. Cancer Lett. 2013;338 (1:110–9. doi: 10.1016/j.canlet.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YJ, Lai HM, Chang YW, Chen GY, Lee JL. Direct reprogramming of stem cell properties in colon cancer cells by CD44. EMBO J. 2011;30 (15:3186–3199. doi: 10.1038/emboj.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, Schuster K, Shao C, Larsen JE, Sullivan LA, Honorio S, Xie Y, Scaglioni PP, DiMaio JM, Gazdar AF, Shay JW, Wistuba II, Minna JD. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Res. 2010;70 (23:9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunaga H, Fujieda S, Tsuzuki H, Asamoto K, Fukuda M, Saito H. Expression of granulocyte colony-stimulating factor receptor and platelet-derived endothelial cell growth factor in oral and oropharyngeal precancerous lesions. Anticancer Res. 2001;21 (4B:2901–2906. [PubMed] [Google Scholar]
- Tachibana M, Murai M. G-CSF production in human bladder cancer and its ability to promote autocrine growth: a review. Cytokines Cell Mol Ther. 1998;4 (2:113–120. [PubMed] [Google Scholar]
- Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27 (5:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torpy JM, Lynm C, Glass RM. JAMA patient page. Stomach cancer. JAMA. 2010;303 (17:1771. doi: 10.1001/jama.303.17.1771. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y, Uraoka N, Anami K, Sentani K, Oue N, Yasui W. Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int. 2012;62 (2:112–119. doi: 10.1111/j.1440-1827.2011.02760.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Xue J, Zadorozny EV, Robinson LJ. G-CSF stimulates Jak2-dependent Gab2 phosphorylation leading to Erk1/2 activation and cell proliferation. Cell Signal. 2008;20 (10:1890–1899. doi: 10.1016/j.cellsig.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Ong CW, Shi J, Srivastava S, Yan B, Cheng CL, Yong WP, Chan SL, Yeoh KG, Iacopetta B, Salto-Tellez M. Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer. 2011;105 (5:658–665. doi: 10.1038/bjc.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthley DL, Giraud AS, Wang TC. Stromal fibroblasts in digestive cancer. Cancer Microenviron. 2010;3 (1:117–125. doi: 10.1007/s12307-009-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T, Hyodo M, Hosoya Y, Koinuma K, Kurashina K, Saitoh S, Hirashima Y, Arai W, Zuiki T, Yasuda Y, Nagai H, Kanai N, Saitoh K. Aggressive G-CSF-producing gastric cancer complicated by lung and brain abscesses, mimicking metastases. Gastric Cancer. 2005;8 (3:198–201. doi: 10.1007/s10120-005-0335-6. [DOI] [PubMed] [Google Scholar]