Abstract
Background:
It is uncertain whether synchronous colorectal cancers (S-CRCs) preferentially develop through widespread DNA methylation and whether they have a prognosis worse than solitary CRC. As tumours with microsatellite instability (MSI) may confound the effect of S-CRC methylation on outcome, we addressed this issue in a series of CRC characterised by BRAF and MS status.
Methods:
Demographics, clinicopathological records and disease-specific survival (DSS) were assessed in 881 consecutively resected CRC undergoing complete colonoscopy. All tumours were typed for BRAFc.1799T>A mutation and MS status, followed by search of germ-line mutation in patients with MSI CRC.
Results:
Synchronous colorectal cancers (50/881, 5.7%) were associated with stage IV microsatellite-stable (MSS) CRC (19/205, 9.3%, P=0.001) and with HNPCC (9/32, 28%, P<0.001). BRAF mutation (60/881, 6.8%) was associated with sporadic MSI CRC (37/62, 60%, P<0.001) but not with S-CRC (3/50, 6.0%, P=0.96). Synchronous colorectal cancer (HR 1.82; 95% CI 1.15–2.87; P=0.01), synchronous advanced adenoma (HR 1.81; 95% CI 1.27–2.58; P=0.001), and BRAFc.1799T>A mutation (HR 2.16; 95% CI 1.25–3.73; P=0.01) were stage-independent predictors of death from MSS CRC. Disease-specific survival of MSI CRC patients was not affected by S-CRC (HR 0.74; 95% CI 0.09–5.75; P=0.77).
Conclusion:
Microsatellite-stable CRCs have a worse prognosis if S-CRC or synchronous advanced adenoma are diagnosed. The occurrence and the enhanced aggressiveness of synchronous MSS advanced neoplasia are not associated with BRAF mutation.
Keywords: synchronous colorectal cancer, advanced adenoma, microsatellite instability, BRAF mutation, prognosis
Two or more primary colorectal carcinomas are detected in 2–5% of all patients newly diagnosed with colorectal cancer (CRC) (Adloff et al, 1989; Passman et al, 1996; Chen and Sheen-Chen, 2000; Oya et al, 2003; Papadopoulos et al, 2004; Latournerie et al, 2008; Nosho et al, 2009; Mulder et al, 2011). The recognition of synchronous colorectal cancer (S-CRC) as a clinical entity has enhanced the awareness that an accurate perioperative exploration of the entire colon is mandatory in patients undergoing CRC resection. However, it remains uncertain whether S-CRC has prognostic and molecular features distinct from those of solitary CRC.
Case–control studies examining characteristics and outcome of patients with S-CRC have provided discrepant results. Male gender (Oya et al, 2003; Latournerie et al, 2008; Mulder et al, 2011), older age (Passman et al, 1996; Papadopoulos et al, 2004; Wang et al, 2004; Mulder et al, 2011), coexisting adenomas (Chen and Sheen-Chen, 2000; Latournerie et al, 2008), and worse survival (Oya et al, 2003; Nosho et al, 2009; Mulder et al, 2011) were associated with S-CRC in some series but not in others. These inconsistencies likely reflect a bias in the selection of index cases as well as in the recruitment of controls with solitary CRC. In particular, different series may have variable prevalences of cancers with microsatellite instability (MSI), which are associated with multiple lesions (Pedroni et al, 1999; Dykes et al, 2003; Nosho et al, 2009; Bae et al, 2012) but also with a better prognosis (Gryfe et al, 2000; Malesci et al, 2007).
It is also uncertain whether S-CRC is the result of a stochastic oncogenic event or, alternatively, of an increased susceptibility of the colonic mucosa to neoplastic transformation, as supported by the established association of S-CRC with metachronous CRC (Mulder et al, 2011). Beside the controversial association of S-CRC with conventional adenomas, evidence also exists that S-CRC might be more frequent among cancers of the serrated neoplastic pathway, which is characterised by a CpG island methylation (CIMP) phenotype, as opposed to tumours of the chromosomal instability pathway. Two studies found a higher degree of gene promoter methylation in tumour samples from multiple lesions than from solitary CRC, thus suggesting an epigenetic field effect as the basis for S-CRC (Konishi et al, 2009; Gonzalo et al, 2010; Moon et al, 2010). More recently, Nosho et al (2009) found that S-CRCs more frequently exhibited the BRAFc.1799T>A mutation, an established marker of CIMP phenotype (Kambara et al, 2004; Samowitz et al, 2005a; Spring et al, 2006), and that they had a worse outcome. Conversely, Bae et al (2012) failed to detect any association of BRAF mutation with S-CRC. The discrepancy might reflect the heterogeneity of CIMP-positive cancers, a subclass encompassing tumours that do not undergo MLH1-methylation, remain microsatellite-stable (MSS), and have a poor prognosis but also MLH1-deficient MSI CRC, which have a much more favourable prognosis.
Given the complex interactions between outcome and distinct molecular profiles, it is obvious that the prognostic significance of S-CRC can be safely assessed only through the analysis of single molecular subgroups of CRC. In addition, the analysis should be extended to synchronous advanced adenomas that are also associated with metachronous CRC (Moon et al, 2010). We retrospectively searched for synchronous adenomas or S-CRC the records of perioperative colonoscopies performed in 881 consecutive patients, whose resected CRC had been originally classified in molecular subclasses according to microsatellite and BRAF status. Clinicopathological features and outcome of patients with synchronous advanced neoplasia were then assessed.
Materials and Methods
Study population and CRC subgrouping by synchronous neoplasia
The study intended to include 1000 consecutive patients who had undergone resective surgery for CRC at the Humanitas Clinical and Research Center between 2 February 1998 and 6 April 2006. Exclusion criteria were limited to the following: (1) absence of submucosal invasion at pathology; (2) anastomotic recurrence of a previously resected colorectal tumour; (3) diagnosis of familial adenomatous polyposis; (4) CRC associated to inflammatory bowel disease. The protocol was approved by the Ethical Committee of the Institution and the informed consent of patients regarding the treatment of their personal data was obtained by the referring physician or by other clinicians involved in the study. At preliminary analysis of records, 119 patients (none of which with S-CRC) were excluded because of incomplete or poor-quality perioperative colonoscopy, leading to a final study population of 881 subjects.
Demographic and clinicopathological records were obtained for each patient from the hospital's intranet system. Synchronous colorectal cancers were defined as the simultaneous detection of two or more invasive (at least pT1) tumours, separated by at least 5 cm of normal colorectal mucosa, at the time of diagnosis or within 6 months for obstructing tumours. The most invading lesion (greatest pT) was taken as the reference lesion for pathological and molecular classification of S-CRC. By combining macroscopic and histological findings, the following CRC subsets were defined: (I) no synchronous neoplasia (n=548); (II) synchronous not-advanced adenoma (n=177); (III) synchronous advanced adenoma (tubular adenoma 10 mm or greater in diameter, and/or >25% villous component, and/or high-grade dysplasia), (n=106); (IV) S-CRC (n=50). To define stage IV disease, pathological reports were combined with surgical findings and with perioperative imaging. The disease-specific survival (DSS) was calculated from diagnosis until death, or until data were censored, as of 30 September 2011. On this date, each patient was confirmed to be alive by direct phone call or by formal enquiry at the local registry of vital statistics.
Tumour molecular subtyping
Tumour samples from all patients, and all cancers from patients with S-CRC, were screened for MSI. BAT-26 mononucleotide, and BAT-25 mononucleotide in patients fulfilling the Amsterdam Criteria II and/or the Bethesda Criteria (n=279), were used as molecular markers of MSI (Hatch et al, 2005; Laghi et al, 2008). DNA was obtained from paraffin-embedded sections of tumours containing at least 50% tumour cells or from tumour micro-dissections. BAT-25 and BAT-26 loci were amplified by fluoresceinated primers and analysed by capillary gel electrophoresis (ABI PRISM 310 Genetic Analyzer, Applied Biosystems, Monza, Italy) (Aaltonen et al, 1998; Malesci et al, 2007).
In all MSI CRC, a defect in mismatch repair (MMR) protein was assessed by the lack of nuclear expression of hMLH1 (clone G-168–15, BD Bio sciences, Buccinasco, Milan, Italy), hMSH2 (clone FE11, Calbiochem, Merk Millipore, Darmstadt, Germany), or hMSH6 (clone 44, BD Bio sciences) at immunohistochemistry (Truninger et al, 2005). Mismatch repair protein expression was also checked in MSS tumours from patients fulfilling the Amsterdam Criteria (n=10). Patients with MSI CRCs underwent germ-line genetic testing for MLH1, MSH2 and MSH6 mutations by sequencing according to the MMR defect in the primary cancer (Wahlberg et al, 2002). Multiple ligation probe amplification analysis (SALSA MLPA P003 MLH1-MSH2 probemix, P248 MLH1-MSH2 probemix, P072 MSH6 probemix, Medical Research Council-Holland, Amsterdam, The Netherland) was performed in mutation-negative patients.
All CRC samples were scrutinised for BRAFc.1799T>A mutation by Real Time–PCR using a TaqMan SNP Genotyping Assay (Applied Biosystem). TaqMan MGB probes were designed using the Custom TaqMan Assay Design Tool (Applied Biosystem). The chosen reporter fluorophores were VIC for detecting the wild-type allele and FAM for the mutant allele.
Sequencing of all exons of the MYH gene was performed to assess germ-line mutations in all patients with MSS S-CRC. We also investigated all tissues from MSS S-CRC for germ-line or somatic mutations in the exon 13 of the POLE gene and in the exon 11 of the POLD1 gene.
Statistical analysis
Associations between synchronous neoplasia and clinicopathological or molecular features of the index CRC were tested using χ2-test or, if appropriate, Fisher's Exact test for categorical variables and by Student's t-test for continuous variables. Pathological and molecular factors significantly associated with S-CRCs at univariate analysis were entered into a multivariate logistic regression analysis. Survival curves were drawn according to the Kaplan–Meier method to comparatively evaluate the DSS of patients with synchronous colorectal neoplasia. To better assess the prognostic role of S-CRCs, as well as of synchronous adenomas, and of tumour MS/BRAF status, Cox proportional-hazard models were also used. For all statistical tests, P<0.05 was considered statistically significant.
Results
Out of 1000 patients undergoing resective surgery for newly diagnosed CRC, 50 (5%) were found to have S-CRC (two cancers in 47 patients and three cancers in three patients). At full colonoscopy, 17 of 50 (34.0%) patients with S-CRC and 283 of 831 (34.1%, P>0.5) patients with solitary cancer had at least one distinct concomitant adenoma.
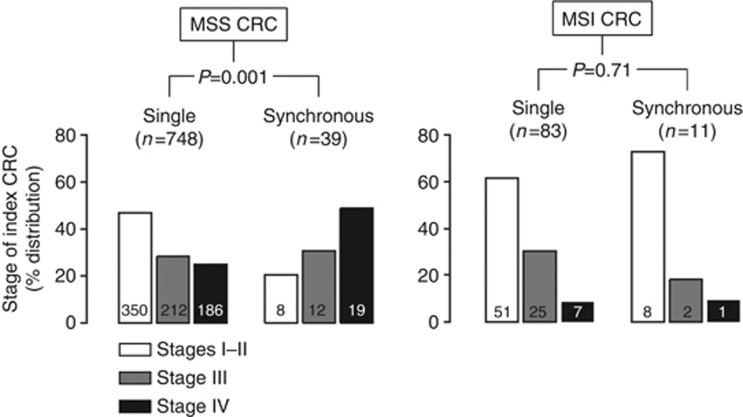
Table 1 reports the clinicopathological and molecular features of the index CRC stratified by the absence or the presence of a synchronous colorectal neoplasia. As compared with patients with no synchronous neoplasia, subjects with synchronous adenoma or cancer were older (66.4±9.9 vs 63.9±11.8 years; P=0.001), were more frequently men (66.4% vs 53.3% P<0.001), and more frequently had a right-sided CRC (41.3% vs 31.8% P=0.003). Synchronous colorectal cancer was strongly associated with stage IV disease and with MSI hereditary cancer (HNPCC); however, a statistically significant (P=0.03) interaction of the two variables was detected at multivariate analysis. Accordingly, Figure 1 details how only MSS S-CRCs were associated with stage IV (P=0.001), whereas MSI CRC presented a low prevalence of metastatic disease even in the presence of synchronous invasive cancer (P=0.88). A full concordance in the MS status was observed in all pairs and triplets of S-CRC, except in one patient carrying an MSI-sporadic index CRC and a second MSS cancer. An interaction was also observed between the MSI status and BRAFc.1799T>A mutation in determining the association of these two variables with synchronous non-advanced adenoma, whereas no association was detected between the BRAF status and synchronous advanced adenomas and S-CRC. Figure 2 shows that BRAFc.1799T>Amutation was strongly associated with MSI-sporadic CRC (37/62, 59.7% vs 23/787, 2.9% in MSS CRC; P<0.001), and that the prevalence of the mutation was higher in MSI-sporadic CRC with synchronous lesions than in those with no concurrent neoplasia (21/26, 80.8% vs 16/36, 44.4% P=0.005). Conversely, in MSS CRC the presence of synchronous colorectal adenomas or cancer was not associated with BRAFc.1799T>A mutation. No MYH germ-line mutations and no POLE or POLD1 germ-line and/or somatic mutations were detected in patients with S-CRC.
Table 1. Demographics, pathology and molecular features of CRC with synchronous colorectal neoplasia.
|
Synchronous colorectal neoplasia |
|||||||
|---|---|---|---|---|---|---|---|
|
Nonea |
Not-advanced adenomab |
Advanced adenomac |
Invasive cancerd |
||||
| n=548, ref. | n=177 | P* | n=106 | P* | n=50 | P* | |
|
Age | |||||||
| Years, mean±s.d. |
63.9±11.8 |
66.8±9.7 |
0.003 |
66.6±10.0 |
0.02 |
64.6±10.8 |
0.70 |
|
Gender | |||||||
| Male | 292 (53.3) | 115 (65.0) | 77 (72.6) | 29 (53.4) | |||
| Female |
256 (46.7) |
62 (35.0) |
0.006 |
29 (27.4) |
<0.001 |
21 (46.6) |
0.52 |
|
Site | |||||||
| Distal | 374 (68.2) | 101 (57.1) | 64 (60.4) | 30 (61.2) | |||
| Proximal |
174 (31.8) |
76 (42.9) |
0.006 |
42 (39.6) |
0.11 |
19 (38.8) |
0.31 |
|
Stage | |||||||
| I–III | 419 (76.5) | 139 (78.5) | 80 (75.5) | 30 (60.0) | |||
| IV |
129 (23.5) |
38 (21.5) |
0.57 |
26 (24.5) |
0.83 |
20 (40.0) |
0.01e |
|
Histotype | |||||||
| Adenoca | 506 (92.3) | 162 (91.5) | 100 (94.3) | 48 (96.0) | |||
| Variant |
42 (7.7) |
15 (8.5) |
0.73 |
6 (5.7) |
0.47 |
2 (4.0) |
0.34 |
|
Gradef | |||||||
| G1–G2 | 406 (79.3) | 143 (83.6) | 78 (77.2) | 38 (77.6) | |||
| G3 |
106 (20.7) |
28 (16.4) |
0.22 |
23 (22.8) |
0.64 |
11 (22.4) |
0.77 |
|
Vein invasion | |||||||
| No | 423 (77.2) | 140 (79.1) | 82 (77.4) | 36 (72.0) | |||
| Yes |
125 (22.8) |
37 (20.9) |
0.60 |
24 (22.6) |
0.97 |
14 (28.0) |
0.40 |
|
MS status | |||||||
| MSS | 495 (90.3) | 153 (86.4) | 100 (94.3) | 39 (78.0) | |||
| MSI-sporadic | 36 (6.6) | 19 (10.7) | 0.04g | 5 (4.7) | 0.44 | 2 (4.0) | 0.64 |
| HNPCC |
17 (3.1) |
5 (2.8) |
0.92 |
1 (0.9) |
0.20 |
9 (18.0) |
<0.001e |
|
BRAF | |||||||
| BRAF WT | 516 (94.2) | 158 (89.3) | 100 (94.3) | 47 (94.0) | |||
| BRAF c.1799T>A | 32 (5.8) | 19 (10.7) | 0.02g | 6 (5.7) | 0.94 | 3 (6.0) | 0.96 |
Abbreviations: CRC=colorectal cancer; MSI=microsatellite instability; MSS=microsatellite-stable.
At Fisher's exact test.
‘No adenoma'.
‘Low-grade dysplasia <10 mm tubular adenoma, no serrated adenoma.
Forty-five patients with low-grade dysplasia adenoma ⩾10 mm or with villous component >25%, 59 with high-grade dysplasia adenoma, 2 with ⩾10 mm low-grade dysplasia serrated adenoma.
Pathological and molecular characteristics of the most advanced cancer (‘index' lesion, by pT) were to be inserted. Of 23 pairs with identical pT (no ‘index' lesion assessable), 22 had fully concordant pathological and molecular features, whereas one pair with discordant tumour site was excluded from the analysis of this variable.
Interactions at multivariate analysis (logistic regression): ‘Stages I–II vs III–IV' * ‘MS status', P=0.03.
Not assessed in 48 cases (34, 6, 7 and 1 in the four subclasses, respectively).
‘BRAF status' * ‘MS status' (excluding HNPCC), P=0.04.
Figure 1.
Interaction of CRC microsatellite status and of synchronous colorectal malignancy in determining the association of these two variables with TNM stage. Stage distribution of synchronous CRC is compared with that of single CRC, stratifying by microsatellite status. Stage IV was significantly associated with synchronous CRC in patients with MSS cancer (19/39, 48.7% vs 186/748, 24.9% P=0.001). The frequency of stage IV disease was not different in MSS CRC patients with no concomitant adenoma (124/495, 25.1%, ref.), with synchronous not-advanced adenoma (36/153, 23.5% P=0.70), and with synchronous advanced adenoma (26/100; 26.0% P=0.84). The frequency of stage IV disease, in patients with MSI CRC, was not associated with S-CRC (7/83, 8.4% vs 1/11, 9.1% P=0.88). *P-values at χ2 or Fisher's exact test, as appropriate (stage IV vs others).
Figure 2.
BRAFc.1799T>A mutation was significantly (P<0.001) more frequent in MSI-sporadic (37/62, 59.7%) than in MSS CRC (23/787, 2.9%). In MSS CRC, no association was found between the occurrence of BRAF mutation in the index CRC and the presence of synchronous neoplasia. Conversely, the mutation in MSI-sporadic CRC was less frequent in the absence of synchronous neoplasia (16/36, 44.4%) than in (a) any synchronous neoplasia (21/26, 80.8%, P=0.005), (b) synchronous not-advanced adenoma (15/19, 78.9%, P=0.01), (c) synchronous advanced adenoma or CRC (6/7, 85.7%, P=0.05). HNPCC, which invariably carry no BRAF mutation, was excluded from analysis. P-values are from χ2 or Fisher's exact test, as appropriate.
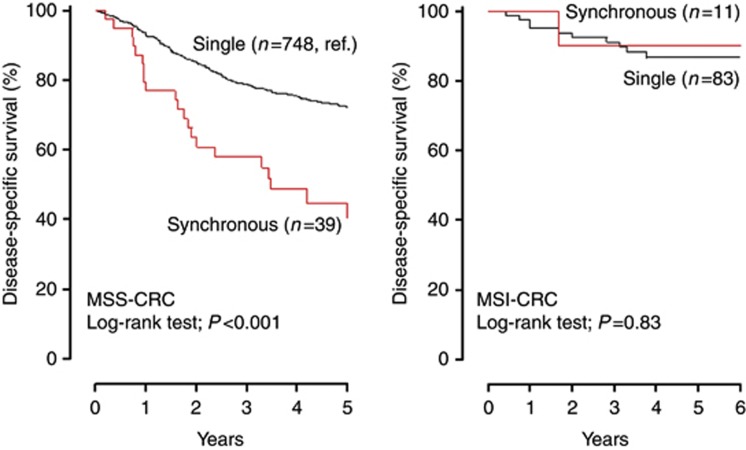
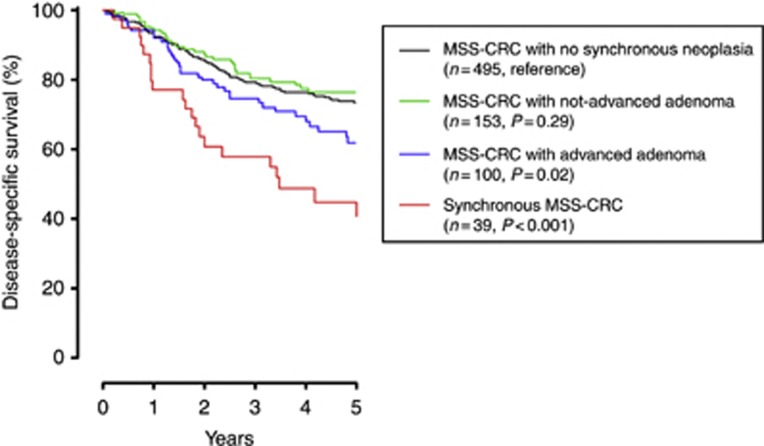
Over a mean post-surgical follow-up of 4.3±2.4 years, a total of 231 CRC-related deaths were registered, 220 (28.0%) among the 787 patients with MSS CRC and only 11 (11.7%) among the 94 patients with MSI cancer (P<0.001). At Kaplan–Meier curves, the presence of S-CRC significantly affected the DSS of patients with MSS CRC (P<0.001) but not that of patients with MSI cancer (P=0.83) (Figure 3). MSS CRC had a poorer prognosis also in the presence of a synchronous advanced adenoma (P=0.02) but not in the presence of a not-advanced adenoma (P=0.29) (Figure 4). The negative prognostic effect of S-CRC or synchronous advanced adenoma was limited to MSS cancers with no BRAFc.1799T>Amutation (P<0.001), whereas BRAF-mutated MSS CRC had a much poorer outcome independent of the presence of a synchronous advanced neoplasia (P=0.98) (Supplemental Material 1). At Cox proportional-hazard models (Table 2), the presence of synchronous advanced neoplasia was confirmed to be associated with a worse outcome of MSS CRC (S-CRC: HR 2.66; 95% CI, 1.69–4.19; P<0.001; synchronous advanced adenoma: HR 1.59; 95% CI, 1.11–2.26; P=0.01). Given the higher prevalence of stage IV disease in patients with MSS S-CRC but not in those with synchronous advanced adenoma (see Figure 1 and its legend), the incremental risk of death associated with synchronous advanced adenoma (HR 1.81; 95% CI, 1.27–2.58; P=0.001) and that conferred by the presence of synchronous invasive cancer (HR 1.82; 95% CI, 1.15–2.87; P=0.01) were almost identical at stage-adjusted multivariate analysis. The occurrence of BRAFc.1799T>Amutation in the index cancer also predicted a higher risk of death from MSS CRC, independently of the presence of a synchronous advanced neoplasia and of TNM stage (HR 2.16; 95% CI 1.25–3.73; P=0.01). Synchronous advanced neoplasia (HR 1.75, C.I. 95% 1.30–2.37, P<0.001) and BRAFc.1799T>A mutation (HR 1.74, C.I. 95% 1.00–3.03, P=0.05) remained stage-independent predictors of death when adjuvant 5-fluorouracil-based chemotherapy was entered into the multivariate analysis (Supplemental Material 2). On the contrary, neither the presence of synchronous advanced neoplasia nor the BRAF status of the tumour significantly affected the DSS of patients with MSI CRC.
Figure 3.
Disease-specific survival of patients with CRC by MS status and by synchronous invasive cancer. Synchronous CRC significantly affected disease-specific survival of patients with MSS cancer but not of those with MSI tumour (Kaplan–Meier curves, log-rank test).
Figure 4.
Disease-specific survival of patients with MSS CRC stratified by synchronous colorectal neoplasia. The presence of synchronous advanced neoplasia, but not that of synchronous not-advanced adenoma, negatively affected the survival of CRC patients (Kaplan–Meier curves, log-rank test).
Table 2. Synchronous advanced colorectal neoplasia and BRAF c.1799T>A mutation as predictors of death from CRC (Cox proportional-hazard models).
|
Death |
Univariate |
Stage-adjusted multivariate |
||||
|---|---|---|---|---|---|---|
| No | Yes | HR (95% CI) | P | HR (95% CI) | P | |
|
MSS CRC | ||||||
|
Synchronous advanced neoplasia | ||||||
| None | 487 | 161 | 1.00 Ref. | 1.00 Ref. | ||
| Advanced adenoma | 62 | 38 | 1.59 (1.11–2.26) | 0.01 | 1.81 (1.27–2.58) | 0.001 |
| Invasive cancer |
18 |
21 |
2.66 (1.69–4.19) |
<0.001 |
1.82 (1.15–2.87) |
0.01 |
|
BRAF
status | ||||||
| WT | 558 | 206 | 1.00 Ref. | 1.00 Ref. | ||
|
BRAF
c.1799T>A |
9 |
14 |
3.29 (1.91–5.70) |
<0.001 |
2.16 (1.25–3.73) |
0.01 |
|
MSI CRC | ||||||
|
Synchronous advanced neoplasia | ||||||
| None | 67 | 10 | 1.00 Ref. | |||
| Advanced adenoma | 6 | 0 | NA | 0.45 | ||
| Invasive cancer |
10 |
1 |
0.74 (0.09–5.75) |
0.77 |
|
|
|
BRAF
status by sporadic/HNPCCa | ||||||
| BRAF WT–sporadic CRC | 23 | 2 | 1.00 Ref. | |||
| BRAF WT–HNPCC | 30 | 2 | 0.75 (0.11–5.33) | 0.77 | ||
| BRAF c.1799T>A–sporadic CRC | 30 | 7 | 2.68 (0.55–12.9) | 0.22 | ||
Abbreviations: HR=hazard ratios <1 represent a decreased risk of death, whereas HR >1 represent an increased risk of death; MSI=microsatellite instability; MSS CRC=microsatellite-stable colorectal cancer; NA=not applicable.
No HNPCC exhibited the BRAF c.1799T>A mutation.
Discussion
In this large, hospital-based study, patients with MSS CRC had a significantly poorer outcome if originally diagnosed with a S-CRC or even with a synchronous advanced adenoma. The finding is important in that it contributes to a highly controversial issue generated by the fact that most studies failed to recognise any association between S-CRC and poor prognosis (Passman et al, 1996; Chen and Sheen-Chen, 2000; Papadopoulos et al, 2004; Latournerie et al, 2008), whereas the only prospective study reported a higher mortality in patients with multiple primary cancers (Nosho et al, 2009). Notably, our study is unique in having investigated the prognostic role of S-CRC in molecularly defined subgroups of CRC, so as to avoid the confounding effect of MSI cancers that more likely occur as synchronous malignancies but also have an overall better prognosis. In addition, BRAFc.1799T>A mutation, an established marker of CIMP and of poor prognosis (Samowitz et al, 2005b; Weisenberger et al, 2006), was not associated with MSS S-CRC, indicating that neither the occurrence nor the outcome of chromosomal-unstable synchronous cancers are likely due to an epigenetic field effect.
Several studies have documented the association between MSI and S-CRC (Pedroni et al, 1999; Dykes et al, 2003; Nosho et al, 2009; Bae et al, 2012). The strong concordance in the MSI status among synchronous cancers also led to the concept that, for genetic and/or environmental reasons, some individuals may be prone to develop multiple cancers through the pathway of MSI secondary to widespread CIMP and to silencing of the mismatch repair gene MMR MLH1 (Leggett and Worthley, 2009). This concept was mainly based on the assumption that the majority of MSI S-CRCs were sporadic tumours, as suggested by the typically old age of patients with synchronous colorectal malignancies and by the established association between MSI-sporadic tumours and older age (Leggett and Worthley, 2009; Nosho et al, 2009). As a matter of fact, no previous study addressing the issue of synchronous cancers systematically screened patients with MSI S-CRC for germ-line mutations in the MMR genes. Therefore, it was a novel, and somehow unexpected finding of our series to see that HNPCC largely accounted for MSI S-CRC (9 of 11, 82%) so that about one out of five of all S-CRC were diagnosed in patients with hereditary cancer. Consistently, BRAFc.1799T>A mutation was associated only with sporadic MSI CRC, whereas no mutation was detected in any HNPCC. Overall, data cannot exclude the existence of an epigenetic field effect favouring the development of multiple neoplasia in patients with sporadic MSI CRC, but certainly contradict the idea that this mechanism may account for most synchronous MSI cancers. Rather, our results confirm the appropriateness of the Bethesda criteria, which recommend MSI testing of CRC in the presence of multiple primary tumours (Umar et al, 2004).
The interaction between the MS status and advanced stage in their association with S-CRC indicated the need to analyse separately the prognosis of MSI and MSS S-CRC. The analysis revealed that the prognosis of MSI cancers was not affected by any concurrent neoplasia, whereas MSS CRC had a significantly poorer outcome if S-CRC, or even a synchronous advanced adenoma, had been diagnosed. Interestingly, at stage-adjusted analysis, the negative prognostic effect of S-CRC equalled that of synchronous advanced adenoma, indicating that the worsened prognosis of synchronous advanced neoplasia likely reflects a more aggressive biological behaviour rather than a larger cancer burden. This concept is consistent with the well-recognized value of S-CRC, as well as of synchronous advanced adenomas, in predicting the future development of metachronous colorectal neoplasia (Balleste et al, 2007; Moon et al, 2010). Of note, both the presence of synchronous advanced neoplasia and the rare BRAFc.1799T>A mutation were unrelated and stage-independent predictors of poor prognosis for MSS CRC. These findings also contradict the hypothesis that an epigenetic field defect may predispose to MSS synchronous neoplasia, which account for the vast majority of multiple primary colorectal malignancies (Leggett and Worthley, 2009). In this respect, the study by Nosho et al (2009) may have failed to recognise the existing interactions of S-CRC with MSI and BRAFc.1799T>A mutations due to the small number of tumours fully characterised for the MS/BRAF status.
Finally, our study failed to detect any MYH germ-line mutation, not confirming the previously reported association between homozygous or compound heterozygous mutations and S-CRC (Cleary et al, 2009), nor POLE or POLD1 germ-line/somatic/mutations, which have been recently associated with multiple CRCs or adenomas (Palles et al, 2013). Cleary et al (2009) found MYH mutations in <1% of the general population with CRC and in about 6% of patients with S-CRC, so that discrepancies might still reflect a type II statistical error. Alternatively, we might have been more selective in excluding mild polyposis syndromes from our colonoscopy-based clinical series.
Our study has the intrinsic limitation of being a case–control, retrospective analysis. This may have altered the relative contribution of different molecular and clinical subsets of CRC, but a bias in the selection of controls to S-CRC is unlikely, given the consecutive series and the use of complete colonoscopy are the only criteria for inclusion of patients with solitary CRC. Then, the correlations found between synchronous neoplasia and prognosis in single molecular subsets can hardly be interpreted as the result of selection artifacts. The analysis was also limited by the use of BRAFc.1799T>A mutation as the only marker of DNA methylation. Although the BRAF status is validated as a reproducible and very specific marker of cancers with methylator phenotype (Weisenberger et al, 2006), we might have missed a few methylated tumours potentially identifiable at analysis of multiple markers of CIMP. However, we believe that the lack of association between MSS S-CRC and tumour methylation status in our series cannot be disputed on the basis of this limitation. Finally, synchronous serrated adenomas, which may coexist with an advanced colorectal neoplasia (Álvarez et al, 2013), were surely underestimated in a series collected when there was little or no awareness for endoscopic removal of ‘hyperplastic' lesions.
In conclusion, our study demonstrates that MSS cancers presenting with the phenotype of multiple advanced lesions account for the vast majority of S-CRC, are not associated with BRAF mutation, and have a worse prognosis than corresponding solitary tumours. The association between non-hypermutated multiple advanced neoplasia and poor prognosis might have important implications for post-surgical surveillance and for adjuvant therapeutic strategies.
Acknowledgments
The study was funded by the Italian Association for Cancer Research (AIRC), grant ‘Colorectal cancer metastasis: linking mesenchymal transition and inflammation to genetic instability' (AIRC, No. IG 5256), and by Ministero della Salute, Ricerca Finalizzata, grant ‘Molecular phenotypes of colorectal-cancer as potential prognostic markers. A joint study of genetic instability, cancer stem cells, immune response, and epidermal-to-mesenchymal transition' (RF-ICH-2007-665438).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Material
References
- Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomaki P, Chadwick RB, Kaariainen H, Eskelinen M, Jarvinen H, Mecklin JP, de la Chapelle A. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338:1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- Adloff M, Arnaud JP, Bergamaschi R, Schloegel M. Synchronous carcinoma of the colon and rectum: prognostic and therapeutic implications. Am J Surg. 1989;157:299–302. doi: 10.1016/0002-9610(89)90555-2. [DOI] [PubMed] [Google Scholar]
- Álvarez C, Andreu M, Castells A, Quintero E, Bujanda L, Cubiella J, Salas D, Lanas Á, Carballo F, Morillas JD, Hernández C, Jover R, Sarasqueta C, Enriquéz-Navascués JM, Hernández V, Estévez P, Macenlle R, Sala T, Balaguer F, Pellisé M, Moreira L, Gil I, Peris A, González-Rubio F, Ferrández A, Poves C, Ponce M, Grau J, Serradesanferm A, Ono A, Cruzado J, Pérez-Riquelme F, Alonso-Abreu I, Carrillo-Palau M, Santander C, Díaz Tasende J, Herreros A, Cacho G, Barranco LE, Bessa X. Relationship of colonoscopy-detected serrated polyps with synchronous advanced neoplasia in average-risk individuals. Gastrointest Endosc. 2013;78:333–341. doi: 10.1016/j.gie.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Bae JM, Cho NY, Kim TY, Kang GH. Clinicopathologic and molecular characteristics of synchronous colorectal cancers: heterogeneity of clinical outcome depending on microsatellite instability status of individual tumors. Dis Colon Rectum. 2012;55:181–190. doi: 10.1097/DCR.0b013e31823c46ce. [DOI] [PubMed] [Google Scholar]
- Balleste B, Bessa X, Pinol V, Castellvi-Bel S, Castells A, Alenda C, Paya A, Jover R, Xicola RM, Pons E, Llor X, Cordero C, Fernandez-Banares F, de Castro L, Rene JM, Andreu M. Detection of metachronous neoplasms in colorectal cancer patients: identification of risk factors. Dis Colon Rectum. 2007;50:971–980. doi: 10.1007/s10350-007-0237-2. [DOI] [PubMed] [Google Scholar]
- Chen HS, Sheen-Chen SM. Synchronous and ‘early' metachronous colorectal adenocarcinoma: analysis of prognosis and current trends. Dis Colon Rectum. 2000;43:1093–1099. doi: 10.1007/BF02236556. [DOI] [PubMed] [Google Scholar]
- Cleary SP, Cotterchio M, Jenkins MA, Kim H, Bristow R, Green R, Haile R, Hopper JL, LeMarchand L, Lindor N, Parfrey P, Potter J, Younghusband B, Gallinger S. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology. 2009;136:1251–1260. doi: 10.1053/j.gastro.2008.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes SL, Qui H, Rothenberger DA, Garcia-Aguilar J. Evidence of a preferred molecular pathway in patients with synchronous colorectal cancer. Cancer. 2003;98:48–54. doi: 10.1002/cncr.11445. [DOI] [PubMed] [Google Scholar]
- Gonzalo V, Lozano JJ, Munoz J, Balaguer F, Pellise M, Rodriguez de Miguel C, Andreu M, Jover R, Llor X, Giraldez MD, Ocana T, Serradesanferm A, Alonso-Espinaco V, Jimeno M, Cuatrecasas M, Sendino O, Castellvi-Bel S, Castells A. Aberrant gene promoter methylation associated with sporadic multiple colorectal cancer. PLoS One. 2010;5:e8777. doi: 10.1371/journal.pone.0008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- Hatch SB, Lightfoot HM, Jr., Garwacki CP, Moore DT, Calvo BF, Woosley JT, Sciarrotta J, Funkhouser WK, Farber RA. Microsatellite instability testing in colorectal carcinoma: choice of markers affects sensitivity of detection of mismatch repair-deficient tumors. Clin Cancer Res. 2005;11:2180–2187. doi: 10.1158/1078-0432.CCR-04-0234. [DOI] [PubMed] [Google Scholar]
- Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, Tanaka N, Higuchi T, Young J, Jass JR, Leggett BA. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi K, Shen L, Jelinek J, Watanabe Y, Ahmed S, Kaneko K, Kogo M, Takano T, Imawari M, Hamilton SR, Issa JP. Concordant DNA methylation in synchronous colorectal carcinomas. Cancer Prev Res (Phila) 2009;2:814–822. doi: 10.1158/1940-6207.CAPR-09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laghi L, Bianchi P, Malesci A. Differences and evolution of the methods for the assessment of microsatellite instability. Oncogene. 2008;27:6313–6321. doi: 10.1038/onc.2008.217. [DOI] [PubMed] [Google Scholar]
- Latournerie M, Jooste V, Cottet V, Lepage C, Faivre J, Bouvier AM. Epidemiology and prognosis of synchronous colorectal cancers. Br J Surg. 2008;95:1528–1533. doi: 10.1002/bjs.6382. [DOI] [PubMed] [Google Scholar]
- Leggett BA, Worthley DL. Synchronous colorectal cancer: not just bad luck. Gastroenterology. 2009;137:1559–1562. doi: 10.1053/j.gastro.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Malesci A, Laghi L, Bianchi P, Delconte G, Randolph A, Torri V, Carnaghi C, Doci R, Rosati R, Montorsi M, Roncalli M, Gennari L, Santoro A. Reduced likelihood of metastases in patients with microsatellite-unstable colorectal cancer. Clin Cancer Res. 2007;13:3831–3839. doi: 10.1158/1078-0432.CCR-07-0366. [DOI] [PubMed] [Google Scholar]
- Moon CM, Cheon JH, Choi EH, Kim ES, Park JJ, Han SY, Kim DH, Kim TI, Kim WH. Advanced synchronous adenoma but not simple adenoma predicts the future development of metachronous neoplasia in patients with resected colorectal cancer. J Clin Gastroenterol. 2010;44:495–501. doi: 10.1097/MCG.0b013e3181d6bd70. [DOI] [PubMed] [Google Scholar]
- Mulder SA, Kranse R, Damhuis RA, de Wilt JH, Ouwendijk RJ, Kuipers EJ, van Leerdam ME. Prevalence and prognosis of synchronous colorectal cancer: a Dutch population-based study. Cancer Epidemiol. 2011;35:442–447. doi: 10.1016/j.canep.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Nosho K, Kure S, Irahara N, Shima K, Baba Y, Spiegelman D, Meyerhardt JA, Giovannucci EL, Fuchs CS, Ogino S.2009A prospective cohort study shows unique epigenetic, genetic, and prognostic features of synchronous colorectal cancers Gastroenterology 1371609–1620.e1–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oya M, Takahashi S, Okuyama T, Yamaguchi M, Ueda Y. Synchronous colorectal carcinoma: clinico-pathological features and prognosis. Jpn J Clin Oncol. 2003;33:38–43. doi: 10.1093/jjco/hyg010. [DOI] [PubMed] [Google Scholar]
- Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I, Sherborne A, Chubb D, Carvajal-Carmona LG, Ma Y, Kaur K, Dobbins S, Barclay E, Gorman M, Martin L, Kovac MB, Humphray S, Lucassen A, Holmes CC, Bentley D, Donnelly P, Taylor J, Petridis C, Roylance R, Sawyer EJ, Kerr DJ, Clark S, Grimes J, Kearsey SE, Thomas HJ, McVean G, Houlston RS, Tomlinson I. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45:136–144. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Michalopoulos A, Basdanis G, Papapolychroniadis K, Paramythiotis D, Fotiadis P, Berovalis P, Harlaftis N. Synchronous and metachronous colorectal carcinoma. Tech Coloproctol. 2004;8 (Suppl 1:s97–s100. doi: 10.1007/s10151-004-0124-y. [DOI] [PubMed] [Google Scholar]
- Passman MA, Pommier RF, Vetto JT. Synchronous colon primaries have the same prognosis as solitary colon cancers. Dis Colon Rectum. 1996;39:329–334. doi: 10.1007/BF02049477. [DOI] [PubMed] [Google Scholar]
- Pedroni M, Tamassia MG, Percesepe A, Roncucci L, Benatti P, Lanza G, Jr, Gafa R, Di Gregorio C, Fante R, Losi L, Gallinari L, Scorcioni F, Vaccina F, Rossi G, Cesinaro AM, Ponz de Leon M. Microsatellite instability in multiple colorectal tumors. Int J Cancer. 1999;81:1–5. doi: 10.1002/(sici)1097-0215(19990331)81:1<1::aid-ijc1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Samowitz WS, Albertsen H, Herrick J, Levin TR, Sweeney C, Murtaugh MA, Wolff RK, Slattery ML. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–845. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slattery ML. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005;65:6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW, Appleyard M, Hewett D, Togashi K, Jass JR, Leggett BA. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Truninger K, Menigatti M, Luz J, Russell A, Haider R, Gebbers JO, Bannwart F, Yurtsever H, Neuweiler J, Riehle HM, Cattaruzza MS, Heinimann K, Schar P, Jiricny J, Marra G. Immunohistochemical analysis reveals high frequency of PMS2 defects in colorectal cancer. Gastroenterology. 2005;128:1160–1171. doi: 10.1053/j.gastro.2005.01.056. [DOI] [PubMed] [Google Scholar]
- Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, Fishel R, Lindor NM, Burgart LJ, Hamelin R, Hamilton SR, Hiatt RA, Jass J, Lindblom A, Lynch HT, Peltomaki P, Ramsey SD, Rodriguez-Bigas MA, Vasen HF, Hawk ET, Barrett JC, Freedman AN, Srivastava S. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlberg SS, Schmeits J, Thomas G, Loda M, Garber J, Syngal S, Kolodner RD, Fox E. Evaluation of microsatellite instability and immunohistochemistry for the prediction of germ-line MSH2 and MLH1 mutations in hereditary nonpolyposis colon cancer families. Cancer Res. 2002;62:3485–3492. [PubMed] [Google Scholar]
- Wang HZ, Huang XF, Wang Y, Ji JF, Gu J. Clinical features, diagnosis, treatment and prognosis of multiple primary colorectal carcinoma. World J Gastroenterol. 2004;10:2136–2139. doi: 10.3748/wjg.v10.i14.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberger DJ, Siegmund KD, Campan M, Young J, Long TI, Faasse MA, Kang GH, Widschwendter M, Weener D, Buchanan D, Koh H, Simms L, Barker M, Leggett B, Levine J, Kim M, French AJ, Thibodeau SN, Jass J, Haile R, Laird PW. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.