Abstract
Background:
The addition of trastuzumab (T) and lapatinib (L) to neoadjuvant chemotherapy increases the pathological complete response (pCR) rate in patients with human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. We investigated the efficacy of T or L with neoadjuvant chemotherapy and specific efficacy biomarkers.
Methods:
Patients with stages I–III (including inflammatory) HER2-positive breast cancer were randomised to receive epirubicin (E) plus cyclophosphamide (C) × 4 cycles followed by docetaxel (D) plus either T (EC-DT) or L (EC-DL). End points included pCR (primary), clinical response, toxicity, and pCR-predictive biomarkers.
Results:
We randomised 102 patients to EC-DT (50) and EC-DL (52). Median age was 48, 56% were premenopausal and 58% had oestrogen receptor (ER)-positive tumours. Pathological complete response in breast was 52.1% (95% CI:38.0–66.2%) for EC-DT and 25.5% (95% CI:13.5–37.5%) for EC-DL (P=0.0065). Pathological complete response in breast and axilla was 47.9% for EC-DT and 23.5% for EC-DL (P=0.011). Grade 3–4 toxicity did not differ across treatments, except for diarrhoea (2% in EC-DT vs 13.5% in EC-DL, P=0.030). Multivariate analyses showed that treatment (P=0.036) and ER (P=0.014) were the only predictors of pCR in both groups.
Conclusion:
EC-DT exhibited higher efficacy and lower toxicity than EC-DL. Of the different biomarkers studied, only the absence of ER expression was associated with increased pCR.
Keywords: HER2-positive breast cancer, lapatinib, neoadjuvant, trastuzumab, biomarkers
Breast cancer is a heterogeneous disease including distinct biological subtypes with distinct natural histories. Breast cancer exhibits a wide spectrum of clinical presentations as well as diverse pathologic and molecular features, each with its distinctive prognostic and therapeutic implications (Bertucci and Birnbaum, 2008).
Neoadjuvant chemotherapy (NAC) has been the preferred treatment for locally advanced and inflammatory breast cancer patients. A multimodality approach, including NAC, surgery, and radiation, is the most effective treatment option as shown by better overall survival outcomes (Kaufmann et al, 2007). NAC has shown high clinical and pathological response rates and it increases the chance for breast conservation; thus, it is currently the preferred treatment for large resectable tumours (Fisher et al, 1997). Irrespectively of the disease stage at diagnosis, patients achieving pathological complete response (pCR) exhibit better survival outcomes (Kuerer et al, 1999; Rouzier et al, 2002; Hennessy et al, 2005; Guarneri et al, 2006; Rastogi et al, 2008). With regards to tumour biology, the impact of pCR in patient prognosis has been recently defined according to the intrinsic subtypes in a retrospective analysis of several German neoadjuvant studies. According to this meta-analysis, pCR is a suitable surrogate end point for patients with human epidermal growth factor receptor 2 (HER2)-positive (nonluminal), triple negative, and luminal B/HER2-negative tumours but not, however, for luminal B/HER2-positive and luminal A tumours (von Minckwitz et al, 2012). Thus, the identification and evaluation of new drug regimens that improve pCR rates in operable tumours are becoming the main objectives of NAC protocols, with the ultimate goal of achieving better survival outcomes. Further, the neoadjuvant setting permits an in vivo evaluation of treatment efficacy and allows the identification of subgroups of patients with different prognoses.
The HER2 is overexpressed in 15–20% of breast cancer, and it is associated with a highly aggressive tumour behaviour and poor outcomes. The availability of the anti-HER2 monoclonal antibody (mAb) trastuzumab has significantly improved the prognoses of patients with HER2-positive breast cancer both in early and advanced disease (Slamon et al, 2001; Marty et al, 2005; Piccart-Gebhart et al, 2005; Romond et al, 2005; Joensuu et al, 2006; Slamon et al, 2011). Lapatinib is a dual tyrosine kinase inhibitor of HER1 and HER2, currently approved for the treatment of patients with HER2-positive advanced breast cancer who fail to respond to trastuzumab therapy (Spector et al, 2005; Geyer et al, 2006; Konecny et al, 2006; Cameron et al, 2008; Di Leo et al, 2008; Gomez et al, 2008). Recent studies show that, in the preoperative setting, the combination of trastuzumab with sequential chemotherapy with taxanes and anthracyclines results in a high pCR rate (Buzdar et al, 2007; Gianni et al, 2010; Untch et al, 2010).
On the basis of the existing evidence, we designed a phase II randomised study of standard chemotherapy with epirubicine (E), cyclophosphamide (C), and docetaxel (D) in combination with either trastuzumab or lapatinib. The main goal of the study was to evaluate the efficacy and safety of these two neoadjuvant treatments for HER2-positive breast cancer patients. Additionally, as an exploratory end point we examined the putative predictive role of various biomarkers on pathological response. These biomarkers, present in pretreatment tumour biopsies, were selected based on reported in vitro and clinical trial data as well as on their potential to mediate growth factor-induced changes in tumour growth, including hormonal receptors, proliferation and activation of ERK and PI3K/AKT signalling pathways (Okano et al, 2000; Xia et al, 2002; Song et al, 2005; Spector et al, 2005; Dave et al, 2011; Luporsi et al, 2012).
Patients and Methods
Eligibility criteria
Female subjects with histologically proven stages I, II, III or inflammatory breast cancer (by breast core biopsy) and HER2-positive status, by local results, were included in this study. HER2 amplification was confirmed by Pathvysion FISH probes in a central laboratory, following the ASCO/CAP guidelines (Wolff et al, 2007). Patients were eligible only if they were at least 18 years of age; had a Karnofsky performance status (PS) ⩾80; had adequate bone marrow, liver, renal, and cardiac functions; and were treatment-naive. For women of childbearing age, a negative pregnancy test and use of adequate contraception were also required. Patients were excluded if they had the following: bilateral invasive or metastatic breast cancer, a pre-existing neurotoxicity grade ⩾2 (based on the National Cancer Institute-Common Terminology Criteria for adverse events version 3.0 (NCI-CTCAE v3.0) score system (Cancer Therapy Evaluation Program (CTEP), 2006)), a previous history of cancer other than cervical or non-melanoma skin cancer adequately treated, or other malignant tumours treated more than 10 years before the study entry; or any other severe or uncontrolled systemic disease. All patients provided written informed consent before study entry.
Study design and treatment plan
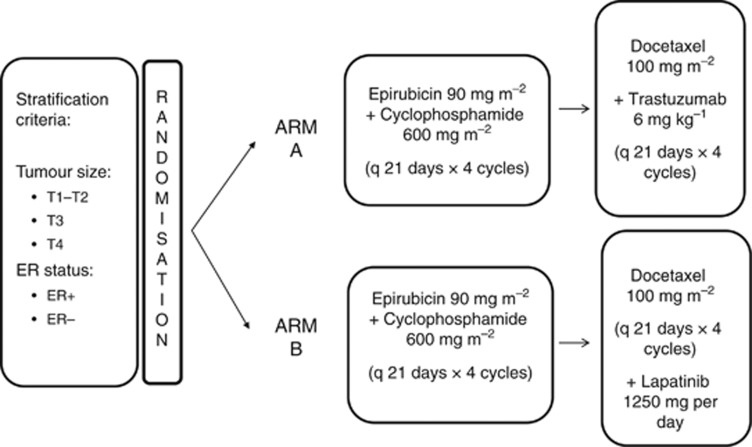
This was a multicentre, open-label, randomised phase II trial. All eligible patients were randomly assigned in a 1 : 1 ratio to NAC treatment either with EC × 4 cycles→docetaxel+trastuzumab × 4 (standard arm, EC-DT) or EC × 4→docetaxel+lapatinib × 4 (experimental arm, EC-DL). Randomisation was centralised at the headquarters of the Spanish Breast Cancer Research Group (GEICAM for its Spanish acronym). Patients were stratified according to tumour size (T1–T2 vs T3 vs T4) and oestrogen receptor (ER) status (ER-positive vs ER-negative).
Specifically, NAC consisted of epirubicin 90 mg m−2 plus cyclophosphamide 600 mg m−2 both administered intravenously (IV) on day 1 every 21 days for four cycles followed by docetaxel 100 mg m−2 also administered IV on day 1 every 3 weeks for four cycles. The anti-HER2 therapy was added to docetaxel as follows: patients in the standard arm received T 6 mg kg−1 (after a loading dose of 8 mg kg−1) administered IV on day 1 every 21 days (EC-DT), whereas patients in the experimental arm were administered a daily dose of lapatinib 1250 mg orally (EC-DL) (Figure 1). Upon completion of the NAC treatment, patients underwent mastectomy or conservative surgery plus axillary lymph node dissection (unless previous negative sentinel lymph node biopsy). Postoperative treatment was left at the investigator's criteria.
Figure 1.
Trial design.
This trial was approved by the local Ethical Review Boards of the recruitment sites and the Spanish Ministry of Health. It is registered in ClinicalTrials.Gov with the number NCT00841828. The trial was conducted in compliance with Good Clinical Practices and the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients before study entry.
Assessments and end points
The primary end point of this study was to determine the pCR rate in the breast upon NAC treatment completion. Secondary end points included toxicity and clinical response rates (by a radiological method). Additionally, through analyses of the tumour samples prespecified in the study protocol, we explored potentially predictive associations between tumour biomarkers and pCR.
Before study entry, all patients underwent a breast and axillary disease assessment by ultrasound, mammography, or magnetic resonance imaging (MRI). In addition, patients had an ECOG PS evaluation, a core biopsy, HER2-positive assessment, a complete blood cell count, serum chemistry, an electrocardiogram, and a left ventricular ejection fraction (LVEF) measurement.
pCR was assessed at surgery based on the Miller and Payne criteria (Ogston et al, 2003). Clinical response defined as complete response (CR)+partial response (PR) was evaluated according to the Response Evaluation Criteria in Solid Tumours (RECIST) criteria (Therasse et al, 2000) after the fourth EC cycle and before surgery (upon NAC completion) using ultrasound, mammography, or MRI. Specific response end points evaluated include the following: pCR rate in the breast defined as the absence of any residual invasive tumour in the breast, residual DCIS permitted (grade 5 according to Miller and Payne classification); breast and axilla pCR defined as the absence of any residual invasive tumour in the breast and axilla at diagnosis in node-negative patients (grade 5-A) or in node-positive patients (grade 5-D). Adverse events were graded according to the NCI-CTCAE version 3.0 (Cancer Therapy Evaluation Program (CTEP), 2006). The worst grade for each patient was reported. LVEF was evaluated after the fourth EC cycle and, again, at the end of NAC and before surgery.
Putative predictive biomarker assessment
Biomarker analysis by immunohistochemistry was carried out at a central laboratory. Immunostaining was performed using 3-μm formalin-fixed, paraffin-embedded tissue sections in Dako Autostainer platforms. Briefly, after deparaffinisation, heat antigen retrieval was performed in a pH9 EDTA-based buffered solution. Endogenous peroxidase was blocked by immersing the sections in 0.03% hydrogen peroxide for 5 min. Sections were incubated with primary mAbs for ER (clone EP1, Dako, Glostrup, DK, USA), PR (PgR636, Dako), Ki67 (MIB1, Dako), PTEN (6H2.1, Dako), rabbit mAb total ERK1/2 (137F5, Cell Signalling, Danvers, MA, USA), phosphorylated (p) ERK1/2 at Thr202/Tyr204 (D13.14.4E, Cell Signalling), AKT (11E7, Cell Signalling), and p-AKT at Ser473 (D9E, Cell Signalling). Detection was performed by EnVision FLEX system (Dako). The same sections incubated with non-immunised serum were used as negative controls, whereas sections of a human tumour with a known marker expression were assayed as positive controls.
The expression of the studied markers was assessed by a pathologist blinded to clinical parameters. ER and PR status were classified as positive according to the ASCO/CAP guidelines (Hammond et al, 2010) based on a threshold of 1%. HER2 amplification was confirmed by Pathvysion FISH probes (Vysis Abbott Molecular, Abbott Park, IL, USA) in a central laboratory following the ASCO/CAP guidelines (Wolff et al, 2007). The cut point considered for Ki67 expression was 14% based on the optimal threshold determined by Cheang et al (2009) to distinguish luminal B from luminal A tumours. PTEN was scored semiquantitatively using the immunoreactive score (IRS). IRS was defined as: IRS=Staining Intensity (SI) × Positivity Percentage (PP); where SI was categorised as 0=negative, 1=weak, 2=moderate, and 3=strong; and PP as 0=<1% 1=1–10% 2=11–50% 3=51–80% and 4=>80% positive cells. High PTEN was defined as an IRS ⩾6 (Nagata et al, 2004). For ERK1/2 and AKT, a semiquantitative HistoScore (Hscore) was calculated estimating the percentage of tumour cells positively stained at the nucleus with low, medium, or high staining intensity. A final score was determined applying a weighting factor to each estimate, following a formula: H-score=(low %) × 1+(medium %) × 2+(high %) × 3. The results ranged from 0 to 300, and the median values were considered as high expression cutoffs. The expression of phosphorylated forms of ERK1/2 and AKT was corrected with the total protein expression.
Statistical considerations
The sample size of the experimental arm was calculated using the 2-stage Simon method and with pCR as the primary study end point. Sample size was based on the null hypothesis of a pCR of 40% and an alternative hypothesis of a pCR of 60%. Assuming an alpha error of 0.05 and a test power of 80%, 92 evaluable patients were required to be recruited and retained in the study. Sixteen patients per arm were to be included in the first stage, for at least eight pCR per arm to be seen, and 30 additional patients per arm were to be included in the second stage for a total of 46 evaluable patients in each arm. Assuming a 10% drop-out rate, 102 patients were recruited.
The efficacy variables (pCR and clinical response) were analysed in the evaluable population (defined as the randomised and treated HER2-positive patients). The number and proportion of patients experiencing response in each treatment arm, and the corresponding two-sided 95% CIs, were calculated for the best overall response. For hypothesis generation, we used χ2 to compare the rate of response and adverse events between treatment arms. Univariate analyses and multivariate logistic regression analyses were used to study the association of biomarkers with clinical end points. Expression scores were analysed as dichotomous variables (that is, high/low expression groups) or entered as continuous variables. In the multivariate analysis we selected the variables associated with pCR using the stepwise method. All analyses were performed using the Statistical Analysis System (SAS) Enterprise Guide 4.3 software (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
Between February 2009 and October 2010, 102 patients from 16 participating centres were included and randomised (50 to EC-DT and 52 to EC-DL). HER2 status was retrospectively evaluated in a central laboratory by IHC and 3+ results were considered HER2-positive, whereas 2+ results were considered equivocal and further tested using FISH.
Three patients were FISH-negative in the central analysis (two patients in the EC-DT and one in the EC-DL arm), which rendered them non-evaluable for efficacy. Thus, 99 patients were evaluable for efficacy (48 and 51 in the EC-DT and EC-DL, respectively), however, all 102 patients were evaluable for safety (on an intention-to-treat analysis). Patients' and tumour characteristics are summarised in Table 1. No statistically significant differences were observed in any clinicopathological characteristics between treatment arms. The median age was 48 years (range: 30–79 years), 56% of the patients were premenopausal, 46% of tumours were grade III, 59% T2, and 69% of patients were node-positive. Regarding hormone receptor status (local assessment), 58% were ER+, 44% PR+, 42% were ER+PR+, and 16% ER+PR−. ER and PR expressions were also confirmed by a central laboratory, showing an agreement of 88.6% and 84.8%, respectively.
Table 1. Patient characteristics by treatment regimen.
| EC-DT | EC-DL | |
|---|---|---|
| Characteristics | n =50 | n =52 |
|
Age, median (range) |
48.5 (32–74) |
48 (30–79) |
|
ECOG PS, n (%) | ||
| 0 | 46 (92.0) | 47 (90.4) |
| 1 |
4 (8.0) |
5 (9.6) |
|
Menopausal status, n (%) | ||
| Premenopausal | 29 (58.0) | 28 (53.9) |
| Postmenopausal |
21 (42.0) |
24 (46.1) |
|
Estrogen receptor | ||
| Positive | 30 (60.0) | 29 (55.8) |
| Negative |
20 (40.0) |
23 (44.2) |
|
Progesterone receptor | ||
| Positive | 21 (42.0) | 24 (46.2) |
| Negative |
29 (58.0) |
28 (53.8) |
|
Estrogen receptor/progesterone receptor | ||
| ER+/PR+ | 21 (42.0) | 22 (42.3) |
| ER+/PR- |
9 (18.0) |
7 (13.5) |
|
Histologic type, n (%) | ||
| Ductal | 48 (96.0) | 48 (92.3) |
| Lobular | 0 (0.0) | 1 (1.9) |
| Other |
2 (4.0) |
3 (5.8) |
|
Tumor grade, n (%) | ||
| 1 | 5 (10.0) | 2 (3.8) |
| 2 | 15 (30.0) | 17 (32.7) |
| 3 | 22 (44.0) | 25 (48.1) |
| Unknown |
8 (16.0) |
8 (15.4) |
|
Tumour size, n (%) | ||
| T1 | 6 (12.0) | 8 (15.4) |
| T2 | 31 (62.0) | 29 (55.8) |
| T3 | 4 (8.0) | 8 (15.4) |
| T4 | 9 (18.0) | 7 (13.4) |
| Median tumour size, cm (range) |
3.3 (1.0–10.0) |
3.5 (1.0–15.8) |
|
Nodal status, n (%) | ||
| N0 | 13 (26.0) | 19 (36.5) |
| N1 | 35 (70.0) | 32 (61.6) |
| N2 | 2 (4.0) | 1 (1.9) |
Abbreviations: EC-DL=epirubicin plus cyclophosphamide x 4 cycles followed by docetaxel plus lapatinib; EC-DT=epirubicin plus cyclophosphamide x 4 cycles followed by docetaxel plus trastuzumab; ECOG=Eastern Cooperative Oncology Group.
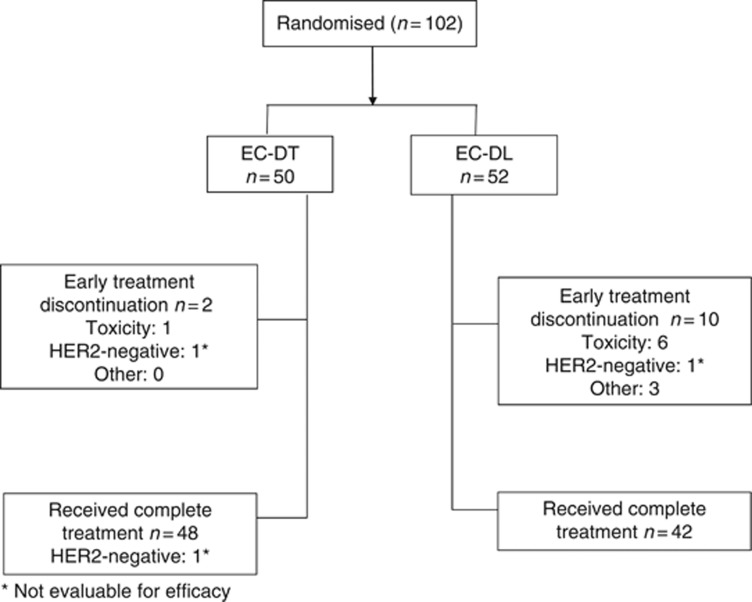
Figure 2 shows the CONSORT study flow chart (Schulz et al, 2010). Twelve patients, 2 and 10 in the EC-DT and EC-DL arms, respectively, discontinued treatment early (P=0.0143). The main reason for discontinuation was as follows: toxicity in seven patients (one in EC-DT and six in EC-DL, P=0.0551). Out of the six patients who withdrew from the lapatinib arm due to toxicity, four cases were related to lapatinib as follows: one grade 3 supraventricular arrhythmia; one grade 4 mucositis–estomatitis plus grade 4 skin rash; one grade 3 diarrhoea; and one grade 3 diarrhoea plus grade 3 skin rash. Other reasons for discontinuation included HER2 negativity in two patients (one in each arm), and three patient withdrawals. Thus, 90 patients, 48 in the EC-DT arm (one of them HER2-negative) and 42 in the EC-DL arm, completed the treatment as planned.
Figure 2.
Consort study flowchart.
Dose administration
The EC regimen has been described in detail elsewhere (Alba et al, 2012). A total of 195 cycles of DT and 181 cycles of DL were administered. In the standard arm, 8.7% of the DT cycles were delayed; 6.2% of docetaxel doses were omitted or reduced, and all trastuzumab doses were administered as planned. In the experimental arm, 8.8% of the DL cycles were delayed; 7.7% of docetaxel doses were omitted or reduced, and the lapatinib doses were omitted or reduced in 18.2% of the cycles.
The main reasons for dose modification with the DT combination were neutropenia (4.1%), fatigue (2.5%), infection (2.1%), and transaminase elevation (2.1%), whereas in the DL combination were diarrhoea (8.8%), rash (7.7%), and neutropenia (2.2%). The median relative dose intensity for docetaxel was 99.8% in both treatment arms, 99.8% for trastuzumab, and 96.4% for lapatinib.
Toxicity
Table 2 summarises all grade 3–4 adverse events in the study. The most frequent grade 3–4 toxicity was neutropenia, observed in 23 cases (22%), and two patients (2%) suffered febrile neutropenia. Grade 3–4 toxicity rates were similar across arms except for diarrhoea, which was more frequent among the EC-DL than the EC-DT arm patients (13.5% vs 2% P=0.03). More patients in the EC-DL arm discontinued treatment due to toxicity than in the EC-DT arm (6 vs 1, respectively; P=0.055).
Table 2. Grade 3–4 Adverse events based on the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 3.0.
| |
EC-DT |
EC-DL |
|
|---|---|---|---|
| Adverse eventa, n (%) | n =50 | n =52 | P-value |
| Leukocytes, (total WBC) |
5 (10.0) |
10 (19.2) |
0.0961 |
| Lymphopenia |
1 (2.0) |
6 (11.5) |
0.0551 |
| Neutropeniab |
10 (20.0) |
13 (25.0) |
0.5458 |
| Rash/desquamation |
0 (0.0) |
4 (7.7) |
0.0637 |
| Diarrhea |
1 (2.0) |
7 (13.5) |
0.0305 |
| Nausea and vomiting |
0 (0.0) |
3 (5.8) |
0.1287 |
| ALT, SGPT |
3 (6.0) |
0 (0.0) |
0.1142 |
| Infection |
0 (0.0) |
2 (3.8) |
0.2574 |
| Fatigue |
6 (12.0) |
2 (3.8) |
0.0961 |
| Anyc | 21 (42.0) | 28 (53.8) | 0.2313 |
Abbreviations: EC-DL=epirubicin plus cyclophosphamide x 4 cycles followed by docetaxel plus lapatinib; EC-DT=epirubicin plus cyclophosphamide x 4 cycles followed by docetaxel plus trastuzumab.
Grade 3–4 adverse events ⩾3% in total.
One patient per treatment arm experienced febrile neutropenia.
Patients with any grade 3–4 adverse event.
No cases of symptomatic congestive heart failure (CHF) (grade 3–4 LVEF decline) were observed. The median LVEF was 63% (range: 52–88%) at baseline and 63% (range: 40–85%) at the end of treatment. Only three patients had asymptomatic grade 2 LVEF decline during treatment (two patients on EC-DT arm and one patient on EC-DL arm) and none of them discontinued treatment for this reason.
Efficacy
Table 3 shows treatment efficacy data. The attained pCR rate in the breast (grade 5 according to Miller and Payne classification) was significantly higher in the EC-DT group (52.1% of patients, 95% CI: 38–66.2%) compared with the EC-DL-treated patients (25.5% of cases, 95% CI: 13.5–37.5%) (P=0.0065). Moreover, breast and axilla pCR in node-negative patients (grade 5-A) and in node-positive patients (grade 5-D) was also superior in the EC-DT regimen (47.9%, 95% CI: 33.8–62.0%) than in the EC-DL one (23.5, 95% CI: 11.9–35.1%, P=0.0112).
Table 3. Treatment efficacy—pathological response by treatment regimen.
| EC-DT | EC-DL | |
|---|---|---|
| Pathological responsea n (%) | n =48 | n =51 |
| Grade 1 |
2 (4.2) |
2 (3.9) |
| Grade 2 |
6 (12.5) |
6 (11.8) |
| Grade 3 |
9 (18.7) |
13 (25.5) |
| Grade 4 |
6 (12.5) |
11 (21.5) |
| Grade 5b |
25 (52.1) |
13 (25.5) |
| Unknown |
0 (0) |
6c (11.8) |
| pCR breast |
25 (52.1) |
13 (25.5) |
| (95% CI) |
(38.0–66.2) |
(13.5–37.5) |
| |
P-value=0.0065 | |
| pCR breast and axilla (Grade 5-A & 5-D)d |
23 (47.9) |
12 (23.5) |
| (95% CI) |
(33.8–62.0) |
(11.9–35.1) |
| P-value=0.0112 | ||
Abbreviations: EC-DL=epirubicin plus cyclophosphamide x 4 cycles followed by docetaxel plus lapatinib; EC-DT=epirubicin plus cyclophosphamide x 4 cycles followed by docetaxel plus trastuzumab; pCR=pathological complete response.
Grade 1-Grade 5: Miller and Payne classification of pathological response.
Absence of any residual invasive tumour in the breast, residual DCIS permitted.
All six patients were withdrawn from the study and treated with a different therapy. Four cases were related to lapatinib as follows: 1 grade 3 supraventricular arrhythmia; 1 grade 4 mucositis-estomatitis plus grade 4 skin rash; 1 grade 3 diarrhea; and 1 grade 3 diarrhea plus grade 3 skin rash.
The absence of any residual invasive tumour in the breast and axilla at diagnosis in node-negative patients (grade 5-A) or in node-positive patients (grade 5-D).
After the four EC cycles, the overall clinical response rate (ORR) was similar in both treatment arms (47.9% for EC-DT and 45.1% from EC-DL, P=0.7787). However, the differences in the ORR of the two groups before surgery, although numerically different, were also not significantly different statistically speaking (77% in the EC-DT arm and 63% in the EC-DL arm; P=0.1208).
All patients evaluable for efficacy underwent surgery. Breast conservation was achieved in 58 patients (58.6%) (28 patients in the EC-DT arm and 30 patients in the EC-DL arm). At the univariate level, pCR was associated with ER status (local results) regardless of treatment (34.3% in ER+ tumours vs 66.5% in ER− tumours; P=0.0008). Univariate analyses per arm, however, suggested that the ER status effect on pCR is found in the trastuzumab-treated patients (OR=7.5 (95% CI: 2.04–27.6; P=0.0024) for ER− vs ER+) but not in the lapatinib-treated group (OR=3.6 (95% CI: 0.9–14.0; P=0.0680) for ER− vs ER+).
Tumour markers and clinicopathological variables predictive of anti-HER2 efficacy
We performed biomarker central analyses in 79 patients (77.5%) with available pretreatment tumour samples. Sixteen cases (20.3%) showed the loss of PTEN expression, and 19 patients (24.1%) presented low expression of Ki67. ER and PR expressions were detected in the 64.6% and 43.0% of cases, respectively. Proportions of cases with ERK and AKT overexpression were 69.6% and 88.6%, respectively.
The results of the univariate and multivariate analyses are summarised in Tables 4 and 5. At the univariate level, only treatment (P=0.038), ER (P=0.014), and PR (P=0.019) were statistically associated with pCR in breast and axilla (grades 5-D and 5-A according to Miller and Payne classification; Table 4). AKT and ERK activation pathway markers were not associated with response (P=0.5799 and P=0.2873, respectively). The proportion of activated factors to total protein expression was not correlated with response (P=0.8477 and P=0.2873, respectively). Low Ki67 expression was not statistically associated with pCR in breast and axilla (OR=1.02; 95% CI: 1.00–1.04; P=0.069). Only high PTEN expression approached a significant association with a poor pCR (OR=2.90; 95% CI: 0.95–8.97; P=0.063).
Table 4. Factors associated with pathological complete response in breast and axilla—Univariate analyses.
| P-valuea | OR (95% CI) | |
|---|---|---|
| Treatmentb (EC→DL as ref.) |
0.0381 |
2.783 (1.058–7.323) |
| ER-negative |
0.0144 |
3.373 (1.274–8.931) |
| PR-negative |
0.0191 |
3.375 (1.221–9.330) |
| Ki67 expression |
0.0690 |
1.019 (0.999–1.039) |
| Loss of PTEN expression |
0.0630 |
2.909 (0.944–8.967) |
| ERK expression |
0.5638 |
0.996 (0.984–1.009) |
| p-ERK expression |
0.2873 |
1.004 (0.997–1.011) |
| Ratio p-ERK/ERK |
0.2190 |
2.790 (0.543–14.331) |
| AKT expression |
0.1602 |
0.993 (0.982–1.003) |
| p-AKT expression |
0.5799 |
0.998 (0.993–1.004) |
| Ratio p-AKT/AKT | 0.8477 | 0.869 (0.207–3.650) |
Abbreviations: CI=confidence intervals; ER=estrogen receptors; OR=Odds ratio; PR=progesterone receptors.
P-values in bold are significant and P-values in italics approach significance.
Treatment is EC-DT, epirubicin plus cyclophosphamide x 4 cycles followed by docetaxel plus trastuzumab, and the treatment in the reference category is EC-DL, epirubicin plus cyclophosphamide x 4 cycles followed by docetaxel plus lapatinib.
Table 5. Factors associated with pathological complete response in breast and axilla—multivariate analyses.
| P-value | ORa | 95% CI | |
|---|---|---|---|
| Treatment EC→DH vs EC→DL |
0.0366 |
2.953 |
1.070–8.154 |
| ER central-negative vs positive | 0.0141 | 3.557 | 1.292–9.795 |
Abbreviations: CI=confidence interval; EC-DL=epirubicin plus cyclophosphamide × 4 cycles followed by docetaxel plus lapatinib; EC-DT=epirubicin plus cyclophosphamide × 4 cycles followed by docetaxel plus trastuzumab; ER=oestrogen receptor; OR=Odds ratio.
Significant variables (α=0.10) in univariate analysis were included: treatment, ER, PR, Ki67, PTEN. We used the stepwise method in SAS to identify variables associated to the dependent variable.
Multivariate analyses showed that only treatment (OR=2.95; 95% CI: 1.07–8.15; P=0.0366) and the absence of ER expression (OR=3.56; 95% CI: 1.29–9.80; P=0.014) were predictive of pCR in breast and axilla (Table 5).
Discussion
In this phase II trial we evaluated the efficacy and toxicity of trastuzumab and lapatinib when added to standard NAC (that is, epirubicin, cyclophosphamide, and docetaxel) for patients with HER2-positive early stage or inflammatory breast cancer. Our results showed that the EC-DT regimen was more efficacious and less toxic than the EC-DL schedule. Specifically, we observed statistically significant higher pCR rates in breast and axilla in patients receiving the EC-DT treatment than in those treated with the EC-DL therapy (P=0.0112). It is important to point out that pCR is increasingly accepted as a surrogate marker of disease response to therapy and it has been associated with a favourable long-term prognosis in patients with HER2+ tumours treated with NAC and trastuzumab, especially in the HER2+/ER− subgroup (Untch et al, 2010; Cortazar et al, 2012; von Minckwitz et al, 2012). With regards to toxicity, our data showed that, although reports of grade 3–4 neutropenia, leucopenia, and lymphopenia were similar between treatment arms, diarrhoea was significantly more frequent among the EC-DL patients and, in fact, more patients in this regimen discontinued treatment due to toxicity. It is worth noting that the cardiotoxicity observed in this trial—asymptomatic cardiotoxicity in <4% of patients—was lower both in severity and frequency than the levels found by Buzdar et al (2005) who reported that one out of 45 patients developed CHF, which they considered an exceptionally low rate.
These findings are consistent with a previous study evaluating the efficacy of trastuzumab and lapatinib in combination with EC-D chemotherapy in the neoadjuvant treatment of breast cancer (Untch et al, 2012). In contrast to our study, in this 2012 clinical trial, trastuzumab and lapatinib were added in both treatment arms from the first cycle of therapy. The pCR rates in patients treated with trastuzumab were superior to that of patients treated with lapaninib (30.3% vs 22.7%, P=0.04). However, not all evidence supports this finding. For instance, the NeoALTTO (Baselga et al, 2012) and the NSBAP B41 (Robidoux et al, 2012) trials, also in the neoadjuvant setting, randomised breast cancer patients to receive weekly paclitaxel in combination with either trastuzumab, lapatinib, or the double blockade with trastuzumab and lapatinib (as the only therapy in the NeoALTTO trial and following standard doxorubicin and cyclophosphamide therapy in the NSBAP B41 trial). These studies found no difference in pCR rates between the trastuzumab and the lapatinib arms, although there was a statistically significant benefit of the double blockade when compared with the trastuzumab-alone regimen.
To date, there are no clinically validated markers of resistance to HER2-targeted therapies. However, several mechanisms have been proposed such as intrinsic HER2 alterations, activation of compensatory signal transduction pathways, alterations in apoptosis and cell cycle control, or host factors that affect immunomodulatory function (Rexer and Arteaga, 2012).
In our trial, the hormonal status of patients was associated with the likelihood of CR, suggesting that oestrogen receptor may have a predictive role regarding a patient's response to a specific neoadjuvant anti-HER2 therapy. A crosstalk between the ER and HER2 pathways has been established clinically and in vitro as having a role in both intrinsic and acquired resistance to HER2-directed agents (Puglisi et al, 2012) as in sustained HER2 inhibition, ER acts as a survival pathway in ER-positive/HER2-positive cells. These data suggest the possibility that a subset of HER2-positive, ER-positive breast cancers are driven primarily by ER and biologically behave more like HER2-negative, ER-positive breast cancers (Nahta and O'Regan, 2012). Efforts to identify this subset of HER2-positive breast cancers might have a relevant role in predicting the clinical benefit of specific therapies. These efforts may also facilitate the design of new therapeutic approaches to improve treatment outcomes for patients with ER-positive, HER2-positive breast cancer.
Hyperactivation of PI3K signalling downstream of HER2, either through loss-of-function PTEN mutations or dominant activating mutations in the catalytic subunit of PI3K (PIK3CAα), seems to decrease T activity in breast cancer. Thus, it has been proposed as resistance mechanism to the anti-HER2 therapy (Dave et al, 2011). However, activation of the PI3K signalling pathway in the tumour measured by PTEN expression and the phosphorylated (that is, activated) form of AKT were not associated with response in our patients. Similar results have been reported recently by the North Central Cancer Treatment Group Trial N9831 in which the benefit of adjuvant trastuzumab for patients with HER2-positive breast cancer was independent of tumour PTEN status (Perez et al, 2013).
It has been described that HER2 signalling inhibition in breast cancer by specific therapies resulted in a compensatory activation of the ERK pathway mediated by activation of the HER family receptors, inducing receptor dimerisation and phosphorylation, HER3 overexpression and binding of adaptor molecules to receptors. In HER2-positive, ER-positive breast cancer, ERK activation in vitro has been involved as a compensatory signalling mechanism (Emde et al, 2011). However, the analysis of ERK activation in breast tumours in this study did not show an association with response to trastuzumab or lapatinib therapy.
The main limitation of our study is that we report findings from a randomised phase II trial when results from phase III trials using the same therapy are already published. In fact, our findings support those of the GeparQuinto study (Untch et al, 2012), a phase III trial, showing that combining anthracyclines and taxanes sequentially plus trastuzumab is more effective than the same therapy plus lapatinib. However, the relevance of our results is derived from the key difference between the two studies—that is, the timing of the anti-HER2 therapy (trastuzumab or lapatinib). In the GeparQuinto trial, patients receive trastuzumab concurrently with anthracyclines, whereas in our study the anti-HER2 therapy is only given with docetaxel. This difference is important for two reasons. First, since questions regarding the long-term cardiac toxicity associated with the combination of anthracyclines and trastuzumab still remain, our design avoids this potential cause of toxicity. Second, sequential drug delivery, as designed in our study, does not appear to compromise treatment effectiveness, given the similar pCRs found in both studies (and consistently greater for chemotherapy+trastuzumab than for chemotherapy+lapatinib). Our data have been confirmed by recently published results from the study Z1041, which show that the concurrent use of trastuzumab with anthracyclines, vs sequential use, does not increase pCR rates (Buzdar et al, 2013). It is also worth noting that, although there are no direct comparisons, pCR rates from anthracyclines and taxanes+trastuzumab (30–65% Alba et al, 2011; Untch et al, 2012; Buzdar et al, 2013) are equal or greater than the pCR from taxanes plus double-modulation with lapatinib or pertuzumab (25–51%) (Baselga et al, 2012; Gianni et al, 2012; Guarnieri et al, 2012). These data may generate hypotheses regarding the potential for anthracyclines plus double modulation to further increase pCR rates in this population. Thus, the main contribution of our study to the existing literature and clinical practice is that it confirms that trastuzumab is more effective than lapatinib, and with lower acute toxicity, when delivered with chemotherapy in the neoadjuvant treatment of patients with tumours HER2+. Our results further confirm that it is possible to attain a pCR similar to double modulation or to protocols using anthracyclines concurrently with trastuzumab, while reducing potential late cardiotoxicity.
In conclusion, in the neoadjuvant treatment of HER2-positive breast cancer patients, anthracycline- and taxane-based standard chemotherapy with concurrent (only with taxane) trastuzumab has shown to be more efficacious and less toxic than the standard chemotherapy with lapatinib. Furthermore, our study confirms that ER expression has a predictive role in the efficacy of standard NAC combined with a HER2-targeted agent. It is essential for future research to focus on the following: (a) the biology and implications of the relationship between biomarkers and HER2-related signalling pathways; and (b) the role of said relationships in the efficacy of the anti-HER2 therapy.
Acknowledgments
We thank Dr JI Chacón from H. Virgen de la Salud (Toledo), Dra M Muñoz from Clínic i Provincial (Barcelona), Dra M Margelí from H Germans Trias i Pujol (Barcelona), Dr A Plazaola from Onkologikoa (San Sebastián), Dr N Batista from H Univ de Canarias (Las Palmas), Dr MA Seguí from Consorci Sanitari Parc Taulí (Sabadell), Dra A Santaballa from H Univ La Fe (Valencia), and Dra A Miguel from H Althaia-Manresa (Barcelona) and the respective pathology departments for their valuable support in the recruitment of patients for this study. We also thank all the participating patients, clinicians, GEICAM, and the local research staff. We also thank María Isabel Casas (GEICAM) for her contribution in the statistical analysis of this study. We thank Hosanna Soler-Vila, PhD for her contribution to this manuscript as medical writer. This work was supported by GlaxoSmithkline SA (GSK).
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Members of Spanish Breast Cancer Research Group (GEICAM), Spain. Previously presented at San Antonio Breast Cancer Symposium in 2011 (poster discussion).
References
- Alba E, Albanell J, De la Haba J, Barnadas A, Calvo L, Sánchez P, Ramos M, Rojo F, Burgués O, Porras I, Tibau A, Carrasco E, Cámara MC, Lluch A.2011Lapatinib vs Trastuzumab in combination with standard EC-D chemotherapy in the neaodjuvant treatment of HER2+ patients. Results from the GEICAM 2006–14 Phase II Randomized Trial Cancer Res 71(24 SupplAbstract PD07-04. [Google Scholar]
- Alba E, Chacon JI, Lluch A, Anton A, Estevez L, Cirauqui B, Carrasco E, Calvo L, Segui MA, Ribelles N, Alvarez R, Sanchez-Muñoz A, Sanchez R, Garcia-Asenjo JA, Rodriguez-Martin C, Escudero MJ, Albanell J. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat. 2012;136:487–493. doi: 10.1007/s10549-012-2100-y. [DOI] [PubMed] [Google Scholar]
- Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G, Goldhirsch A, Chang TW, Horváth Z, Coccia-Portugal M, Domont J, Tseng LM, Kunz G, Sohn JH, Semiglazov V, Lerzo G, Palacova M, Probachai V, Pusztai L, Untch M, Gelber RD, Piccart-Gebhart M, NeoALTTO Study Team NeoALTTO Study Team. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertucci F, Birnbaum D. Reasons for breast cancer heterogeneity. J Biol. 2008;7:6. doi: 10.1186/jbiol67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, Pusztai L, Green MC, Arun BK, Giordano SH, Cristofanilli M, Frye DK, Smith TL, Hunt KK, Singletary SE, Sahin AA, Ewer MS, Buchholz TA, Berry D, Hortobagyi GN. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2–positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Buzdar AU, Suman VJ, Meric-Bernstam F, Leitch AM, Ellis MJ, Boughey JC, Unzeitig G, Royce M, McCall LM, Ewer MS, Hunt KK, American College of Surgeons Oncology Group investigators Fluorouracil, epirubicin, and cyclophosphamide (FEC-75) followed by paclitaxel plus trastuzumab versus paclitaxel plus trastuzumab followed by FEC-75 plus trastuzumab as neoadjuvant treatment for patients with HER2-positive breast cancer (Z1041): a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1317–1325. doi: 10.1016/S1470-2045(13)70502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, Pusztai L, Green MC, Singletary SE, Hunt KK, Sahin AA, Esteva F, Symmans WF, Ewer MS, Buchholz TA, Hortobagyi GN. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. doi: 10.1158/1078-0432.CCR-06-1345. [DOI] [PubMed] [Google Scholar]
- Cameron D, Casey M, Press M, Lindquist D, Pienkowski T, Romieu CG, Chan S, Jagiello-Gruszfeld A, Kaufman B, Crown J, Chan A, Campone M, Viens P, Davidson N, Gorbounova V, Raats JI, Skarlos D, Newstat B, Roychowdhury D, Paoletti P, Oliva C, Rubin S, Stein S, Geyer CE. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analysis. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- Cancer Therapy Evaluation Program (CTEP) 2006. Common Terminology Criteria for Adverse Events (CTCAE). Version 3.0, DCTD, NCI, NIH, DHHS. August 9, Available at http://ctep.cancer.gov/forms/CTCAEv3.pdf Accessed on 20 August 20 2013.
- Cheang MCU, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortazar P, Zhang L, Untch M, Mehta K, Costantino J, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Zujewski JA, Justice R, Loibl S, Wickerham L, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas E, Bergh J, Semiglazov V, Prowell T, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching P, Swain SM, Slaets L, Tang S, Gerber B, Geyer C, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Mincwitz G.2012Meta-analysis results from the collaborative trials in neoadjuvant breast cancer (CTNeoBC) Cancer Res 72(24 SupplAbstract nr S1–S11. [Google Scholar]
- Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H, Narasanna A, Chakrabarty A, Hilsenbeck SG, Huang J, Rimawi M, Schiff R, Arteaga C, Osborne CK, Chang JC. Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol. 2011;29:166–173. doi: 10.1200/JCO.2009.27.7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leo A, Gomez HL, Aziz Z, Zvirbule Z, Bines J, Arbushites MC, Guerrera SF, Koehler M, Oliva C, Stein SH, Williams LS, Dering J, Finn RS, Press MF. Phase III, double blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emde A, Mahlknecht G, Maslak K, Ribba B, Sela M, Possinger K, Yarden Y. Simultaneous inhibition of estrogen receptor and the HER2 pathway in breast cancer: effects of HER2 abundance. Transl Oncol. 2011;4:293–300. doi: 10.1593/tlo.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB, Jr, Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NV. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15:2483–2493. doi: 10.1200/JCO.1997.15.7.2483. [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, Vazquez F, Byakhow M, Lichinitser M, Climent MA, Ciruelos E, Ojeda B, Mansutti M, Bozhok A, Baronio R, Feyereislova A, Barton C, Valagussa P, Baselga J. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi G, Szado T, Ratnayake J, Ross G, Valagussa P. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, Ng C, Franco SX, Chow LW, Arbushites MC, Casey MA, Berger MS, Stein SH, Sledge GW. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- Guarneri V, Broglio K, Kau SW, Cristofanilli M, Buzdar AU, Valero V, Buchholz T, Meric F, Middleton L, Hortobagyi GN, Gonzalez-Angulo AM. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- Guarneri V, Frassoldati A, Bottini A, Cagossi K, Bisagni G, Sarti S, Ravaioli A, Cavanna L, Giardina G, Musolino A, Untch M, Orlando L, Artioli F, Boni C, Generali DG, Serra P, Bagnalasta M, Marini L, Piacentini F, D'Amico R, Conte P. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol. 2012;30:1989–1995. doi: 10.1200/JCO.2011.39.0823. [DOI] [PubMed] [Google Scholar]
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Hortobagyi GN, Rouzier R, Kuerer H, Sneige N, Buzdar AU, Kau SW, Fornage B, Sahin A, Broglio K, Singletary SE, Valero V. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–9311. doi: 10.1200/JCO.2005.02.5023. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Joensuu H, Kellokumpu-Lehtinen PL, Bono P, Alanko T, Kataja V, Asola R, Utriainen T, Kokko R, Hemminki A, Tarkkanen M, Turpeenniemi-Hujanen T, Jyrkkiö S, Flander M, Helle L, Ingalsuo S, Johansson K, Jääskeläinen AS, Pajunen M, Rauhala M, Kaleva-Kerola J, Salminen T, Leinonen M, Elomaa I, Isola J, FinHer Study Investigators Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, Colleoni M, Denkert C, Eiermann W, Jackesz R, Makris A, Miller W, Pierga JY, Semiglazov V, Schneeweiss A, Souchon R, Stearns V, Untch M, Loibl S. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18:1927–1934. doi: 10.1093/annonc/mdm201. [DOI] [PubMed] [Google Scholar]
- Konecny GE, Pegram MD, Venkatesan N, Finn R, Yang G, Rahmeh M, Untch M, Rusnak DW, Spehar G, Mullin RJ, Keith BR, Gilmer TM, Berger M, Podratz KC, Slamon DJ. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE. Clinical course of breast cancer patients with complete pathological tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- Luporsi E, André F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, Tubiana-Mathieu N, Sigal-Zafrani B, Arnould L, Gompel A, Egele C, Poulet B, Clough KB, Crouet H, Fourquet A, Lefranc JP, Mathelin C, Rouyer N, Serin D, Spielmann M, Haugh M, Chenard MP, Brain E, de Cremoux P, Bellocq JP. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty M, Cognetti F, Maraninchi D, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O'Byrne K, Conte PF, Green M, Ward C, Mayne K, Extra JM. Randomised phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, Klos KS, Li P, Monia BP, Nguyen NT, Hortobagyi GN, Hung MC, Yu D. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Nahta R, O'Regan RM. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat. 2012;135:39–48. doi: 10.1007/s10549-012-2067-8. [DOI] [PubMed] [Google Scholar]
- Ogston KN, Miller ID, Payne S, Hutcheon AW, Sarkar TK, Smith I, Schofield A, Heys SD. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003;12:320–327. doi: 10.1016/s0960-9776(03)00106-1. [DOI] [PubMed] [Google Scholar]
- Okano J, Gaslightwala I, Birnbaum MJ, Rustgi AK, Nakagawa H. Akt/protein kinase B isoforms are differentially regulated by epidermal growth factor stimulation. J Biol Chem. 2000;275:30934–30942. doi: 10.1074/jbc.M004112200. [DOI] [PubMed] [Google Scholar]
- Perez EA, Dueck AC, McCullough AE, Chen B, Geiger XJ, Jenkins RB, Lingle WL, Davidson NE, Martino S, Kaufman PA, Kutteh LA, Sledge GW, Harris LN, Gralow JR, Reinholz MM. Impact of PTEN protein expression on benefit from adjuvant Trastuzumab in early-stage human epidermal growth factor receptor 2-positive breast cancer in the North Central Cancer Treatment Group N9831 Trial. J Clin Oncol. 2013;31:2115–2122. doi: 10.1200/JCO.2012.42.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Láng I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Rüschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD, Herceptin Adjuvant (HERA) Trial Study Team Trastuzumab after adjuvant chemotherapy in HER-2 positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Puglisi F, Minisini AM, De Angelis C, Arpino G. Overcoming treatment resistance in HER2-positive breast cancer: potential strategies. Drugs. 2012;72:1175–1193. doi: 10.2165/11634000-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- Rexer BN, Arteaga CL. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncol. 2012;17:1–16. doi: 10.1615/critrevoncog.v17.i1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robidoux A, Tang g, Rastogi P, Geyer CE, Azar CA, Atkins JN, Fehrenbacher L, Bear HD, Baez-Diaz L, Kuebler JP, Margolese RG, Farrar WB, Brufsky A, Shibata HR, Bandos H, Paik S, Costantino JP, Swain SM, Mamounas EP, Wolmark N. Evaluation of lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer: NSABP protocol B-41. J Clin Oncol. 2012;30 (18 suppl:LBA506. doi: 10.1016/S1470-2045(13)70411-X. [DOI] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER-2 positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Rouzier R, Extra JM, Klijanienko J, Falcou MC, Asselain B, Vincent-Salomon A, Vielh P, Bourstyn E. Incidence and prognostic significance of complete axillary downstaging after primary chemotherapy in breast cancer patients with T1 to T3 tumors and cytologically proven axillary metastatic lymph nodes. J Clin Oncol. 2002;20:1304–1310. doi: 10.1200/JCO.2002.20.5.1304. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D, CONSORT Group CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J, Breast Cancer International Research Group Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpress HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector NL, Xia W, Burris H, 3rd, Hurwitz H, Dees EC, Dowlati A, O'Neil B, Overmoyer B, Marcom PK, Blackwell KL, Smith DA, Koch KM, Stead A, Mangum S, Ellis MJ, Liu L, Man AK, Bremer TM, Harris J, Bacus S. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–2512. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- Untch M, Loibl S, Bischoff J, Eidtmann H, Kaufmann M, Blohmer JU, Hilfrich J, Strumberg D, Fasching PA, Kreienberg R, Tesch H, Hanusch C, Gerber B, Rezai M, Jackisch C, Huober J, Kühn T, Nekljudova V, von Minckwitz G, German Breast Group (GBG) Arbeitsgemeinschaft Gynäkologische Onkologie-Breast (AGO-B) Study Group Lapatinib versus trastuzumab in combination with neoadjuvant anthracycline-taxane-based chemotherapy (GeparQuinto, GBG 44): a randomised phase 3 trial. Lancet Oncol. 2012;13:135–144. doi: 10.1016/S1470-2045(11)70397-7. [DOI] [PubMed] [Google Scholar]
- Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, Bauerfeind I, Hilfrich J, Eidtmann H, Gerber B, Hanusch C, Kühn T, du Bois A, Blohmer JU, Thomssen C, Dan Costa S, Jackisch C, Kaufmann M, Mehta K, von Minckwitz G. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28:2024–2031. doi: 10.1200/JCO.2009.23.8451. [DOI] [PubMed] [Google Scholar]
- von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, Jackisch C, Kaufmann M, Konecny GE, Denkert C, Nekljudova V, Mehta K, Loibl S. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF, American Society of Clinical Oncology. College of American Pathologists American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002;21:6255–6263. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]