Abstract
Background:
We aimed to define the incidence and risk of cardiovascular late effects (LEs) identified from inpatient hospital episode statistics (HES) among long-term survivors of cancer in young people by age at diagnosis (0–14 and 15–29 years).
Methods:
Records from the Yorkshire Specialist Register of Cancer in Children and Young People (1991–2006) were linked to inpatient HES data (1996–2011) to assess rates of cardiovascular LEs. Rates were compared with the general population in Yorkshire using age–sex-matched HES records for the entire region.
Results:
Of 3247 survivors of cancer, 3.6% had at least one cardiovascular LE. Overall, cardiovascular hospitalisations for the childhood cohort were threefold higher compared with the general population, but did not differ for young adults. For young adults, increased rates were limited to pericardial disease, cardiomyopathy and heart failure, pulmonary heart disease, hypertension and conduction disorders.
Conclusions:
Survivors of childhood and young adult cancer remain at increased risk of cardiovascular LEs compared with the general population.
Keywords: cardiovascular, childhood, teenage, survivors, late effects
In the United Kingdom, ∼80% of children and young people diagnosed with a malignancy can be expected to become a long-term survivor (Stiller, 2007; Cancer Research UK, 2012). However, this growing cohort are at increased risk of developing chronic health conditions, including malignant neoplasms, neurocognitive impairment and cardiotoxicity, with approximately two-thirds of survivors developing at least one such condition (Oeffinger et al, 2006). Late effects (LEs) associated with survival from childhood cancer are increasingly well described, although data reporting cardiovascular diseases identified from hospital episodes are limited (Neglia et al, 2001; Mulhern et al, 2004; Mulrooney et al, 2009; Tukenova et al, 2010; Blanco et al, 2012; Travis et al, 2012; van der Pal et al, 2012), whereas issues facing survivors diagnosed in young adulthood are even less clearly defined (Woodward et al, 2011). Furthermore, information has predominantly relied on self-reported outcomes in retrospective cohort studies (Oeffinger et al, 2006; Mulrooney et al, 2009), rather than more objective measures that may be achieved by the linkage of electronic health records (Hawkins, 2010; Zhang et al, 2013).
We have linked routinely collected population-based cancer registry data with administrative hospital admissions data in order to quantify the incidence and risk of cardiovascular LEs among survivors of childhood and young adult cancer.
Patients and methods
Case data
The Yorkshire Specialist Register of Cancer in Children and Young People (van Laar et al, 2010) was used to identify long-term survivors (at least 5 years post diagnosis) of childhood and young adult cancer (0–14 and 15–29 years inclusive) diagnosed between 1991 and 2006, providing at least 5 years follow-up data.
Hospital admissions data
Cases data were linked to inpatient HES data (1996–2011) using NHS number, date of birth, gender and postal code; cases were linked to outpatient HES in the same manner. Inpatient HES data were obtained for the general population resident within Yorkshire between 1996 and 2011, and were matched by age at hospital admission to the survivors' cohort in the following age–period groups: 5–34 years in 1996, 5–35 years in 1997 and up to 5–49 years in 2011.
Statistical methods
Cardiovascular LEs were grouped as follows: hypertension, cardiomyopathy and heart failure, coronary artery disease, pulmonary heart disease, pericardial disease, valvular heart disease, conduction disorders, cerebrovascular disease and operations and procedures requiring hospitalisation based on ICD–10 diagnosis codes and OPCS–4.5 procedure codes (Supplementary Table 1). Cases were divided into the following two groups: those with cardiovascular LEs (involving at least one cardiovascular hospitalisation occurring exclusively 5 or more years post diagnosis of cancer) and those without cardiovascular LEs (either no reported LEs or any cardiovascular episode before or within 5 years of cancer diagnosis (occurred in <1%)). Events were identified from all diagnosis and procedure fields within an episode; however, only the first occurrence of a particular event was included in the analysis so that ongoing conditions, which could be recorded multiple times, were not duplicated.
The risk of cardiovascular LEs was modelled using Royston–Parmar relative survival, thereby adjusting for the risk of a cardiovascular event in the general population by attained age, year of event and sex (Royston and Lambert, 2006). Explanatory variables included gender, age and year at diagnosis, diagnostic group, deprivation (Index of Multiple Deprivation, Department for Communities and Local Government (2007)) and initial treatment type. Further models were fitted to the following subgroups:
Cases who received chemotherapy – to examine the effect of the number of different anthracycline drugs administered (in the absence of accurate dose information).
Cases who received radiotherapy (excluding cerebrovascular LEs) – to examine the effect of radiation to the chest.
Cumulative incidence for cardiovascular LEs was estimated, treating death without cardiovascular LE as a competing risk. Hospitalisation rate ratios (HRRs) comparing the survivor cohort with the general population were calculated overall and by age group using standardised mortality ratio techniques, and were standardised to the general population by single year of attained age, year of event and sex (Juul, 2006).
Results
Data linkage
A total of 3306 cases met the inclusion criteria, and of these cases 98% (n=3247) linked to at least one inpatient HES record. Outpatient records were available for 2412 (74%) cases; however, 99% of outpatient diagnosis codes were classified as ‘other and unknown causes of morbidity', and were therefore not used any further.
Cardiovascular LEs
One-hundred and nineteen (3.6%) individuals had at least one cardiovascular LE (n=40 and 79 for 0–14 and 15–29-year olds, respectively). The cumulative incidence was 7.5% (95% confidence interval (CI), 5.3%–10.3%) and 14.0% (95% CI, 9.9%–18.8%) for 0–14 and 15–29-year olds, respectively, at 20 years from diagnosis (Supplementary Figure 1). The majority of cases experienced one cardiovascular LE (70%), 15% experienced two and the remainder experienced between 3 and 12. The median time to cardiovascular LE was 10.2 years from diagnosis (IQR=6.8 to 13.4 years) which did not vary by type (Supplementary Figure 2).
Comparison of cancer cohort with the general population
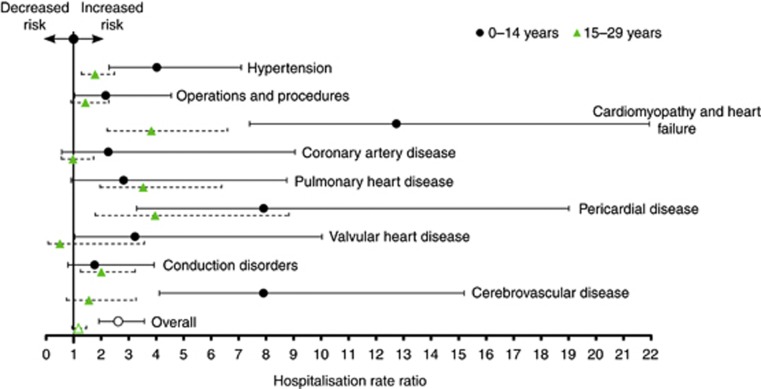
The crude incidence of cardiovascular LEs per 10 000 person-years was higher among cancer survivors than the general population (51.29 vs 35.19) (Table 1). Among childhood survivors, higher rates were observed for hypertension (7.8 vs 3.0), cardiomyopathy and heart failure (8.4 vs 0.9) and cerebrovascular disease (5.8 vs 0.9). For young adult survivors, higher incidence was observed for cardiomyopathy and heart failure (6.6 vs 2.3) and for pulmonary heart disease (6.06 vs 2.83). Overall, the rate of cardiovascular hospitalisations was higher for the childhood cohort compared with the general population (HRR=2.6, 95% CI 1.9–3.6), but not for the young adult cohort (HRR=1.2, 95% CI 0.9–1.5) (Figure 1). Among the younger cohort, there was a significant increased risk of cardiomyopathy and heart failure (HRR=12.7, 95% CI 7.4–21.9), cerebrovascular disease (HRR=7.9, 95% CI 4.1–15.2), pericardial disease (HRR=7.9, 95% CI 3.3–19.0), hypertension (HRR=4.0 95% CI 2.3–7.1), valvular heart disease (HRR=3.2, 95% CI 1.0–10.0) and operations and procedures (HRR=2.2, 95% CI 1.0–4.5). Despite no significant increased hospitalisation rate for young adults overall, there was a significant increase in the hospitalisation rate of pericardial disease (HRR=4.0, 95% CI 1.8–8.8), cardiomyopathy and heart failure (HRR=3.8, 95% CI 2.2–6.6), pulmonary heart disease (HRR=3.5, 95% CI 2.0–6.4), conduction disorders (HRR=2.0, 95% CI 1.2–3.2) and hypertension (HRR=1.8, 95% CI 1.3–2.5) in this age group. Results for both age groups combined are given in Supplementary Figure 3.
Table 1. Number of casesa and crude incidence per 10 000 person-years by cardiovascular category and age group.
|
Age at diagnosisb |
||||||
|---|---|---|---|---|---|---|
| |
0–14 years |
15–29 years |
Total |
|||
|
Category |
Cancer survivors |
General population |
Cancer survivors |
General population |
Cancer survivors |
General population |
| N (incidence) | N (incidence) | N (incidence) | ||||
| Hypertension |
12 (7.75) |
4009 (2.57) |
35 (17.68) |
27 293 (16.80) |
47 (13.32) |
31 580 (9.91) |
| Cardiomyopathy and heart failure |
13 (8.39) |
1343 (0.86) |
13 (6.57) |
3681 (2.27) |
26 (7.37) |
5786 (1.82) |
| Operations and procedures |
7 (4.52) |
3413 (2.19) |
18 (9.09) |
11 689 (7.19) |
25 (7.08) |
21 497 (6.75) |
| Conduction disorders |
6 (3.87) |
4740 (3.04) |
17 (8.59) |
10 664 (6.56) |
23 (6.52) |
15 790 (4.96) |
| Cerebrovascular disease |
9 (5.81) |
1479 (0.95) |
7 (3.54) |
5252 (3.23) |
16 (4.53) |
7816 (2.45) |
| Pulmonary heart disease |
3 (1.94) |
1606 (1.03) |
12 (6.06) |
4599 (2.83) |
15 (4.25) |
5622 (1.76) |
| Coronary artery disease |
2 (1.29) |
847 (0.54) |
12 (6.06) |
10 080 (6.20) |
14 (3.97) |
17 328 (5.44) |
| Pericardial disease |
5 (3.23) |
1386 (0.89) |
6 (3.03) |
2592 (1.60) |
11 (3.12) |
2661 (0.84) |
| Valvular heart disease |
3 (1.94) |
1032 (0.66) |
1 (0.51) |
1986 (1.22) |
4 (1.13) |
4038 (1.27) |
| Totalc |
60 (38.73) |
19 855 (12.71) |
121 (61.12) |
77 836 (47.90) |
181 (51.29) |
112 118 (35.19) |
| Total person-years | 15 492.43 | 15 613 297 | 19 796.53 | 16 246 855 | 35 288.96 | 31 860 152 |
For each case, multiple occurrences of the same diagnosis were not counted.
Age at admission for general population corresponds to age of survivor cohort at admission dependent on their age at diagnosis.
Total number of events does not equal to the total number of cases, as 30% of cases experienced multiple cardiovascular diagnoses.
Figure 1.
HRRs and 95% confidence intervals comparing cardiovascular LEs among cancer survivors with the general population by age at diagnosis.
Predictors of cardiovascular LEs
There was significant evidence of an increased risk of cardiovascular LEs for those diagnosed aged 15–29 years compared with those diagnosed aged 0–14 years in the unadjusted analysis (HR=1.69, P-value=0.007); no further differences in demographic and clinical variables were observed (Supplementary Table 2). The excess hazard ratio of age at diagnosis in the adjusted relative survival model was 1.35; however, this effect was not significant (Table 2).
Table 2. Excess HRs and 95% CI obtained from a Royston–Parmar relative survival model, modelling the risk of a cardiovascular late event for long-term survivors of cancer diagnosed between 1991 and 2006 aged 0–14 and 15–29 years inclusive.
| |
|
95% CI |
|
|
|---|---|---|---|---|
| Variable | HR | Lower | Upper | P-value |
|
Sex | ||||
| Male | 1 | |||
| Female |
1.43 |
0.76 |
2.68 |
0.265 |
|
Age at diagnosis | ||||
| 0–14 | 1 | |||
| 15–29 |
1.35 |
0.67 |
2.71 |
0.391 |
|
Year of diagnosis | ||||
| 1991–2006 |
1.01 |
0.89 |
1.14 |
0.887 |
|
Diagnostic group | ||||
| Other solid tumours | 1 | |||
| Leukaemia | 1.97 | 0.81 | 4.76 | 0.132 |
| Lymphoma | 1.49 | 0.60 | 3.68 | 0.389 |
| CNS tumours |
1.55 |
0.56 |
4.28 |
0.394 |
|
Treatment group | ||||
| Sx alone/No treatment recorded | 1 | |||
| Cx (+/− Sx) | 1.23 | 0.58 | 2.63 | 0.592 |
| Rt (+/− Sx) | 0.39 | 0.04 | 3.66 | 0.407 |
| Cx+RT (+/− Sx) |
0.92 |
0.34 |
2.54 |
0.880 |
|
Deprivationa | ||||
| Most deprived (5) | 1 | |||
| 4 | 0.58 | 0.23 | 1.47 | 0.253 |
| 3 | 0.81 | 0.33 | 1.97 | 0.645 |
| 2 | 1.07 | 0.46 | 2.47 | 0.882 |
| Least deprived (1) | 0.35 | 0.05 | 2.31 | 0.274 |
Abbreviations: CI=confidence interval; HR=hazard ratio.
Index of Multiple Deprivation 2007.
There was no significant difference in the risk of cardiovascular LEs according to the number of anthracycline drugs administered (HR=0.56; 95% CI 0.2–1.4; P-value=0.204). We observed borderline significant evidence of an increased risk of cardiovascular LEs (excluding cerebrovascular disease) for those who received radiotherapy to the chest (HR=7.36; 95% CI 0.97–55.7; P-value=0.053) (Supplementary Tables 3 and 4).
Discussion
We report the first population-based study, extending over 15 years, of cardiovascular LEs in survivors of childhood and young adults with cancer using person-linked electronic health records of specialist cancer registry and administrative hospital admission data. Notably, there was evidence of a significant increase in cardiovascular morbidity in survivors of childhood cancer compared with the general population, whereas within individuals diagnosed in young adulthood the increased incidence was limited to pericardial disease, cardiomyopathy and heart failure, pulmonary heart disease, conduction disorders and hypertension compared with the general population.
Treatment-specific analysis showed an increase in the risk of cardiovascular LEs for those who received chest radiation which is consistent with previous studies (Mulrooney et al, 2009; Tukenova et al, 2010). However, increases in the risk of cardiovascular LEs according to the use and number of anthracyclines were not observed. We did not have access to chemotherapy and radiotherapy dose information within this study; this has recently been addressed by others for survivors of childhood cancer (Mulrooney et al, 2009; Tukenova et al, 2010; Blanco et al, 2012; van der Pal et al, 2012); however, more comprehensive studies using accurate treatment data are still required. Furthermore, this line of enquiry should be extended to young adult cancer survivors in order to facilitate evidence-based risk stratification of follow-up and aftercare (Jefford et al, 2013).
We undertook a bespoke patient-level linkage of clinical and administrative databases to study the effect of clinical exposures on long-term cardiovascular outcomes over many thousands of patient-years. However, the study depends on the quality of data coding which is likely to vary across England, however, by focusing on one region we may have mitigated some bias. The inability to adequately interrogate outpatient data highlights a potential underestimation of the true size of the problem; therefore, further work should concentrate on these data.
It is important that those at risk of developing cardiovascular LEs are supported with strategies to maximise cardiovascular health and given access to appropriate health surveillance. Furthermore, all of their current and potential future health carers should be made aware of the specific risks for these individuals.
Acknowledgments
This paper presents independent research commissioned by the Candlelighters Trust (grant reference number RG.EPID.474842 to RGF). AG's time was funded by Macmillan Cancer Support, Candlelighters Trust and the Yorkshire Cancer Network; the latter additionally funded the purchase of HES data. We are grateful to Paula Feltbower for data collection and the co-operation of all oncologists, pathologists, GPs and medical records staff in Yorkshire. We acknowledge the work of Rebecca Birch and colleagues at NYCRIS for their advice and support in the use of HES data, and we also acknowledge the work by the NHS Information Centre for performing the HES data linkage.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Blanco JG, Sun CL, Landier W, Chen L, Esparza-Duran D, Leisenring W, Mays A, Friedman DL, Ginsberg JP, Hudson MM, Neglia JP, Oeffinger KC, Ritchey AK, Villaluna D, Relling MV, Bhatia S. Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes—a report from the Children's Oncology Group. J Clin Oncol. 2012;30:1415–1421. doi: 10.1200/JCO.2011.34.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Research UK 2012CancerStats: Teenage and Young Adult Cancer—SurvivalAvailable at http://www.cancerresearchuk.org/cancer-info/cancerstats/keyfacts/teenage-and-young-adult-cancer/#survive (accessed: 23/01/2013).
- Department for Communities and Local Government 2007The English Indices of Deprivation. London.
- Hawkins MM. Survivorship outcomes research based on record linkage. Pediatr Blood Cancer. 2010;55:224–225. doi: 10.1002/pbc.22580. [DOI] [PubMed] [Google Scholar]
- Jefford M, Rowland J, Grunfeld E, Richards M, Maher J, Glaser A. Implementing improved post-treatment care for cancer survivors in England, with reflections from Australia, Canada and the USA. Br J Cancer. 2013;108:14–20. doi: 10.1038/bjc.2012.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul S. An Introduction to Stata for Health Researchers. Stata Press: Texas, USA; 2006. [Google Scholar]
- Mulhern R, Merchant T, Gajjar A, Reddick W, Kun L. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5:399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. Br Med J. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neglia J, Friedman D, Yasui Y, Mertens A, Hammond S, Stovall M, Donaldson S, Meadows A, Robison L. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93:618. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- Oeffinger K, Mertens A, Sklar C, Kawashima T, Hudson M, Meadows A, Friedman D, Marina N, Hobbie W, Kadan-Lottick N. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- Royston P, Lambert PC. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model. Stata Press books: Texas, USA; 2006. [Google Scholar]
- Stiller C. Childhood Cancer in Britain: Incidence, Survival, Mortality. Oxford University Press: Oxford, UK; 2007. [Google Scholar]
- Travis LB, Ng A, Allan JM, Pui C, Kennedy AR, Xu X, Purdy JA, Applegate K, Yahalom J, Constine LS, Gilbert ES, Boice JB., Jr Second malignant neoplasms and cardiovascular disease following radiotherapy. J Natl Cancer Inst. 2012;104:1–14. doi: 10.1093/jnci/djr533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, Guerin S, Pacquement H, Aouba A, Hawkins M. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- van der Pal HJH, van Dalen EC, van Delden E, van Dijk IW, Kok WE, Geskus RB, Sieswerda E, Oldenburger F, Koning CC, van Leeuwen FE, Caron HN, Kremer LC. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- van Laar M, McKinney PA, Parslow RC, Glaser A, Kinsey SE, Lewis IJ, Picton SV, Richards M, Shenton G, Stark DP, Norman P, Feltbower RG. Cancer incidence among the south Asian and non-south Asian population under 30 years of age in Yorkshire, UK. Br J Cancer. 2010;103:1448–1452. doi: 10.1038/sj.bjc.6605903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward E, Jessop M, Glaser A, Stark D. Late effects in survivors of teenage and young adult cancer: does age matter. Ann Oncol. 2011;12:2561–2568. doi: 10.1093/annonc/mdr044. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lorenzi MF, Goddard K, Spinelli JJ, Gotay C, McBride ML. Late morbidity leading to hospitalization among 5-year survivors of young adult cancer: A report of the childhood, adolescent, and young adult cancer survivors (CAYACS) research program. Int J Cancer. 2013;134 (5:1174–1182. doi: 10.1002/ijc.28453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.