Abstract
Background:
Thrombotic events are common in cancer patients and have been associated with an adverse prognosis in large registry-based studies.
Methods:
A retrospective cohort of 417 patients with ovarian cancer treated at a tertiary cancer centre between 2006 and 2009 was studied to identify the incidence and risk factors for thrombotic events and the prognostic impact of thrombosis. Patient outcomes were evaluated against a matched control group without thrombosis.
Results:
Ninety-nine thrombotic events occurred in 90 patients (21.6%) from 8 months before diagnosis to 56 months following diagnosis, peaking in the 4 months following diagnosis. Patients with thrombosis were older (mean 65 vs 61 years, P=0.007), had a worse performance status (PS ⩾2: 29.9% vs 9.5%, P<0.0001) and had a more advanced FIGO stage (FIGO III/IV 75.6% vs 56.9%, P<0.0001) than patients without thrombosis. Shorter overall survival was seen in patients with pulmonary embolism and pelvic/lower limb deep vein thrombosis than without thrombosis (P=0.001). When the control group was matched for stage and PS, no survival difference was seen (P=0.91).
Conclusion:
Ovarian cancer patients with thrombotic events had a shorter survival. However, when matched for prognostic factors (PS and FIGO stage), thrombosis did not impact upon prognosis.
Keywords: thromboembolism, deep venous thrombosis, pulmonary embolism, DVT, PE, ovarian cancer
Venous thromboembolism (VTE) is a major cause of morbidity and mortality in cancer patients. Up to 20% of patients with cancer will develop thromboembolic events (Lee et al, 2006) and up to 20% of patients with thromboembolism have an underlying diagnosis of malignancy (Caine et al, 2002). Venous thromboembolic events affects up to 4.1% of patients with cancer admitted to hospital (Khorana et al, 2007a) and thrombosis has been identified as a leading cause of death in patients with cancer (Chew et al, 2006; Khorana et al, 2007b). The true event burden is often underestimated as thrombotic events can be asymptomatic and venous thrombosis is detected in up to half of all cancer patients at post-mortem examinations (Gao and Escalante, 2004).
Among solid tumours, ovarian cancer is associated with one of the highest incidence rates of venous thrombosis (Khorana et al, 2007a). Patients with ovarian cancer have several disease and treatment-related features that are well recognised as risk factors. The tumour arises within the pelvis and the majority of patients have advanced disease at presentation. Patients undergo abdomino-pelvic surgery as a part of the definitive treatment of the disease and the mainstay of systemic treatment is platinum-based chemotherapy. D-dimer levels are often elevated in ovarian cancer patients (Satoh et al, 2007) and some histological subtypes such as clear cell carcinomas are more prone to thrombotic events than others (Duska et al, 2010). The molecular basis for increased risk of thrombotic events is unclear but overexpression of tissue factor associated with elevated D-dimer levels is believed to be a major factor in promoting a hypercoagulable state in ovarian cancer (Uno et al, 2007).
Much of the data on the prognostic role of thrombotic events in solid tumours are confounded by studies that comprise heterogeneous patient populations of multiple cancer types, with varying disease and treatment-associated risk factors. Studies also vary in their evaluation of patients for thrombosis, either by screening for thrombotic events or by investigation of patients based upon clinical suspicion of thrombosis. The latter approach better reflects the current established clinical practice. In this study, we therefore aimed to chart the natural history of thrombotic events in patients with ovarian cancer throughout the course of their treatment. We also sought to define the risk factors for thrombosis, identify periods of high risk during the patient's lifetime and assess the impact of thrombosis on long-term survival, and to identify the proportion of thrombotic events that are detected following clinical findings or incidentally on routine imaging.
Materials and methods
Patients with thrombosis (cases) were identified from a registry of patients with epithelial ovarian cancer referred to the gynaecological medical oncology department of a tertiary cancer centre between January 2006 and December 2009. During this period, hospital guidelines were to administer prophylactic anti-coagulation with low molecular weight heparin (LMWH) to all immobile patients during in-patient admissions. Electronic patient records of 417 patients were reviewed to identify patients with thrombosis.
A pilot was performed with a group of patients known to have thrombosis to identify the method with the greatest sensitivity and specificity of detection. The final method involved the use of the automatic search function of the text within the electronic record for the terms ‘thromb', ‘embol', ‘DVT' (deep vein thrombosis), and ‘PE' (for pulmonary embolism). The term ‘PE' was inserted as case sensitive to minimise the false positive rate. This method was used to search the records of patients referred from January 2006 to December 2009.
Once patients with VTE were identified, the case notes were reviewed to collect demographic and clinical data including the details of thrombotic event, risk factors for thrombosis, details of the cancer diagnosis and treatment and clinical outcomes. A control cohort of patients without thrombosis was generated from the same database of patients with ovarian cancer managed through the gynaecological medical oncology department, stratified by stage and performance status (PS). These variables were chosen as they were found to have the greatest prognostic impact in the cohort of patients with thrombosis. The matching did not proceed for further known prognostic factors (e.g., age), nor was one-to-one matching performed.
Data were exported to Microsoft Excel and analysed using SPSS for Windows (Chicago, IL, USA) where P⩽0.05 was considered significant. Analyses were performed on all patients identified to have thrombotic events and separately on the subgroup of patients with PE and pelvic/lower limb DVT. The prognostic impact of clinical variables was tested by univariate survival analysis using the Kaplan–Meier method and the log-rank test. Multivariate survival analysis using forward stepwise Cox proportional hazards regression model was performed including thrombosis and prognostic variables which were identified as significant by univariate analysis. The multivariate modelling did not use interaction terms. In the multivariate analysis, cases with missing variables were excluded when that variable was considered as being part of the model. The association between thrombosis and clinical characteristics was compared using the Fisher's exact test. The difference between means was compared using the Student's t-test. As more than one thrombotic event occurred in several patients, all analyses were based on individual patients and not on thrombotic events. The study was carried out as a part of the clinical audit of anticoagulation of patients with ovarian cancer, and the project was prospectively approved by the institutional clinical audit committee.
Results
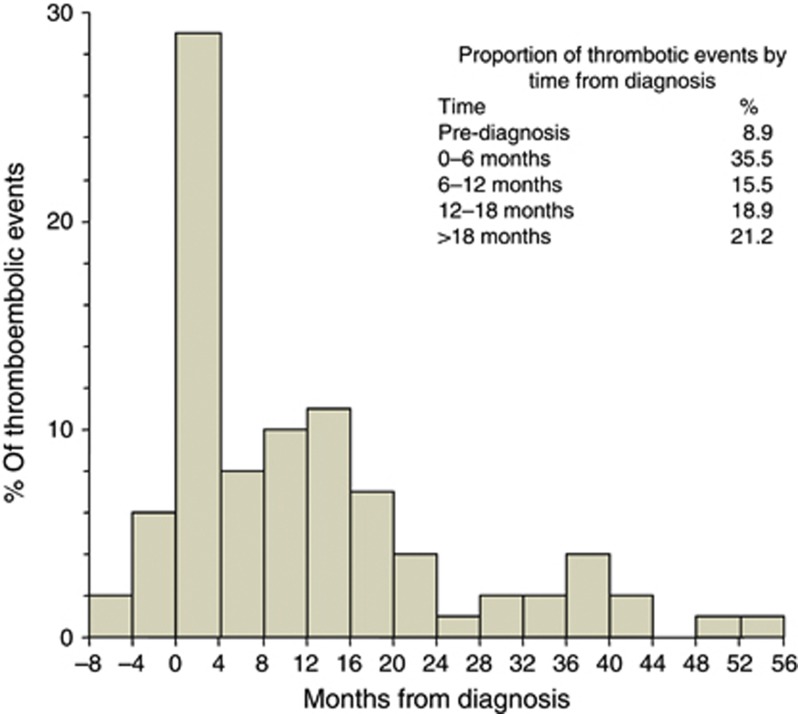
Four-hundred and seventeen patients with ovarian cancer were identified. Ninety-nine thrombotic events were observed in ninety patients. Nine patients had two separate events, giving a cumulative incidence rate of 21.6%. Of the patients with thrombosis, 93.3% were Caucasian and 2.2% Asian. Thrombotic events were most likely to occur within the first 4 months of diagnosis, although they occurred from 8 months before diagnosis until over 4 years following diagnosis (Figure 1). Further peaks in incidence albeit smaller were seen around 1 year after cancer diagnosis, and again after 3 years.
Figure 1.
Timing of thrombosis from diagnosis. The frequency of thrombotic events is shown (n=99) related to the number of months from diagnosis (time point 0). The negative figures on the x axis indicate months before diagnosis.
The distribution of age, FIGO stage and PS in the 417 patients with and without thrombotic events is shown in Table 1. As expected, 75% of patients had FIGO stage III/IV disease. In all, 10.1% of patients with stage I/II disease and 26.8% with stage III/IV disease were diagnosed with a thrombotic event (P=0.0008). Patients with thrombosis were older (mean 65 vs 61 years, P=0.007), had a worse PS (PS ⩾2: 29.9% vs 9.5%, P<0.0001) and had a more advanced FIGO stage (FIGO III/IV 75.6% vs 56.9%, P<0.0001) than patients without thrombosis. The majority of tumours were adenocarcinoma not otherwise specified (34%), followed by serous carcinoma (34.4%), endometrioid carcinoma (8.9%), clear cell carcinoma (6.7%) and carcinosarcoma (1.1%).
Table 1. Characteristics of patients with and without thrombosis.
| Characteristic | Thrombosis (n=90) | No thrombosis (n=327) |
|---|---|---|
|
Age at baseline (years) | ||
| Mean | 65 | 61 |
| Range |
37–90 |
18–91 |
|
FIGO stage
n
(%) | ||
| I | 9 (10.0) | 101 (30.6) |
| II | 6 (6.7) | 32 (9.8) |
| III | 42 (46.7) | 149 (45.6) |
| IV | 26 (28.9) | 37 (11.3) |
| Unknown |
7 (7.8) |
8 (2.4) |
|
WHO performance status | ||
| 0 | 12 (13.3) | 192 (58.7) |
| 1 | 45 (50.0) | 86 (26.3) |
| 2 | 22 (24.4) | 15 (4.6) |
| 3 | 4 (4.4) | 16 (4.9) |
| 4 | 1 (1.1) | 0 (0.0) |
| Unknown | 6 (6.7) | 18 (5.5) |
Abbreviations: FIGO=International Federation of Gynecology and Obstetrics; WHO=World Health Organisation.
Data on the location of the thrombotic event are shown in Table 2. Forty-one (45.6%) of the thrombotic events were pulmonary emboli (PE), of which 25 (60.1%) were classified as a central PE and 16 (29.9%) as a peripheral PE. Thirty (33.3%) patients sustained a DVT. The most common locations for DVT included femoral (20; 48.8%) and pelvic (8; 19.5%) veins. Venous thrombotic events were most often symptomatic (67.8%), with the remainder discovered incidentally on routine imaging. Eight (8.8%) patients had an arterial event.
Table 2. Details of thrombotic event (n=99).
| Characteristic | Incidence n (%) |
|---|---|
|
Location of event | |
|
PE | |
| Central | 25 (27.8) |
| Peripheral |
16 (17.8) |
|
Pelvic/lower extremity DVT | |
| Calf | 2 (2.2) |
| Femoral | 20 (22.2) |
| Pelvic |
8 (8.9) |
|
Other venous | |
| Visceral | 2 (2.2) |
| Upper limb | 2 (2.2) |
| Subclavian |
1 (1.1) |
|
Arterial | |
| Arterial NOS | 3 (3.3) |
| Limb | 2 (2.2) |
| Brain | 2 (2.2) |
| Intra-cardiac | 1 (1.1) |
|
Unknown |
6 (6.7) |
|
Detection of venous events | |
| Incidental | 29 (32.2) |
| Symptomatic | 61 (67.8) |
Abbreviations: DVT=deep vein thrombosis; NOS=not otherwise specified; PE=pulmonary embolism;
The frequencies of risk factors in patients with thrombotic events are shown in Table 3. Nearly three quarters of patients (72.2%) had at least one co-morbidity, with hypertension (25.6%) and diabetes mellitus (10%) being the most common. In addition, 5 patients (5.6%) underwent surgery up to 4 weeks before the thrombosis and 38 patients (42.2%) received chemotherapy (16 first-line chemotherapy, 15 second-line, 7 third-line or greater) up to 4 weeks before the thrombosis. Exclusion of patients with arterial, visceral and central venous catheter (CVC) related thromboses from the analysis showed very similar distribution of risk factors.
Table 3. Thrombotic risk factors in patients with thrombosis.
| |
All thrombotic events (n=90) |
PE and pelvic/lower limb DVT (n=77) |
|---|---|---|
| Risk factor | Incidence n (%) | Incidence n (%) |
|
Number of co-morbidities | ||
| 0 | 24 (26.7) | 18 (23.4) |
| 1 | 31 (34.4) | 29 (37.7) |
| 2 | 20 (22.2) | 16 (20.8) |
| 3 | 11 (12.2) | 10 (13.0) |
| 4 | 2 (2.2) | 1 (1.3) |
| 5 | 1 (1.1) | 1 (1.3) |
| Unknown |
1 (1.1) |
1 (1.3) |
|
Type of co-morbidity | ||
| Diabetes mellitus | 9 (10) | 9 (11.7) |
| Hypertension | 23 (25.6) | 20 (26.0) |
| History of cancer | 9 (10) | 7 (9.1) |
| Ischaemic heart disease | 6 (6.7) | 5 (6.5) |
| Cerebral-vascular disease | 3 (3.3) | 2 (2.6) |
| COPD |
1 (1.2) |
1 (1.3) |
|
Smoking status | ||
| Current smoker | 12 (13.3) | 7 (9.1) |
| Ex-smoker | 19 (21.1) | 17 (22.1) |
| Non-smoker | 43 (47.8) | 41 (53.2) |
| Unknown |
16 (17.8) |
12 (15.6) |
|
Family history of VTE | ||
| Yes | 2 (2.2) | 2 (2.6) |
| No | 73 (81.1) | 62 (80.5) |
| Unknown |
15 (16.7) |
13 (16.9) |
|
Use of prothrombotic drugs | ||
| Diuretics | 12 (13.3) | 11 (14.3) |
| Other | 2 (2.2) | 1 (1.3) |
| Mean BMI (kg m−2) | 27.7±6.7 | 27.9±6.9 |
Abbreviations: BMI=body mass index; COPD=chronic obstructive pulmonary disease; DVT=deep vein thrombosis; PE=pulmonary embolism; VTE=venous thromboembolism.
Univariate analysis (summarised in Table 4) identified a significant association between survival and advanced stage (P=0.002), increased age (P=0.047) and WHO PS of 2 or more (P=0.0001) for patients with thrombosis (n=90). There was no difference in survival between patients in whom thrombotic events were symptomatic and asymptomatic (P=0.52). A non-statistically significant association between the number of co-morbidities and shorter survival was found in patients with thrombosis (P=0.39). Patients on first-line treatment, who had experienced VTE, showed a trend towards a worse prognosis than patients on second, third or fourth line of chemotherapy; however, this was not statistically significant (P=0.28). In all, 22.2% had an in-patient admission within 4 weeks before the diagnosis of thrombosis and this was significantly associated with worse survival (P=<0.001). No difference in survival was seen between patients with DVT and PE (P=0.38). After exclusion of patients with arterial, visceral and CVC related thrombosis from the analysis, there remained a significant association between survival and performance score (P=0.005) and FIGO stage (P=<0.001). A Cox regression model was performed on 80 patients with complete data identified to have a thrombotic event, incorporating the variables that were identified as significant by univariate analysis. Two independent variables that emerged as significant in the final model were PS ⩾2 (P=0.000003) and FIGO stage III/IV (P=0.0003) (Table 5). These two variables remain significant (PS⩾2 P=0.00015 and FIGO stage III/IV P=0.001) when the multivariate model was performed following the exclusion of patients with visceral, arterial and CVC related thromboses.
Table 4. Univariate survival analysis (all cause mortality) for patients with thrombosis.
| |
All thrombotic events (n=90) |
PE and pelvic/lower limb DVT (n=77) |
||||||
|---|---|---|---|---|---|---|---|---|
| Co-variate | n | 3-Year survival (%) | CI | P-value | n | 3-Year survival (%) | CI | P-value |
|
Performance score | ||||||||
| 0 | 12 | 60.6 | 25.1–83.4 | <0.001 | 9 | 50.0 | 13.7–78.5 | 0.005 |
| 1 | 45 | 50.7 | 34.1–65.2 | 41 | 47.8 | 31.0–62.8 | ||
| 2+ |
27 |
23.1 |
9.3–40.4 |
|
22 |
27.3 |
11.1–46.4 |
|
|
FIGO stage | ||||||||
| I/II | 15 | 86.7 | 56.3–96.5 | 0.002 | 14 | 92.9 | 58.8–99.0 | <0.001 |
| III/IV |
68 |
30.1 |
18.6–42.4 |
|
57 |
25.7 |
14.4–38.5 |
|
|
Age | ||||||||
| <60 | 29 | 56.6 | 34.7–73.6 | 0.047 | 25 | 59.8 | 36.6–77.0 | 0.242 |
| 60<70 | 26 | 41.8 | 21.7–60.7 | 22 | 44.6 | 21.9–65.1 | ||
| ⩾70 |
35 |
30.1 |
15.5–46.1 |
|
30 |
35.5 |
18.4–53.0 |
|
|
Chemotherapy <4 weeks | ||||||||
| Chemotherapy | 38 | 37.1 | 23.2–51.0 | 0.263 | 30 | 35.3 | 21.1–49.8 | 0.335 |
| No chemotherapy |
52 |
47.9 |
30.3–63.5 |
|
47 |
47.0 |
27.5–64.3 |
|
|
Line of treatment | ||||||||
| First | 16 | 40.2 | 16.6–63.0 | 0.283 | 11 | 45.5 | 16.7–70.7 | 0.314 |
| Second | 15 | 39.0 | 13.7–64.0 | 13 | 35.2 | 11.2–60.8 | ||
| Third | 4 | NR | NR | 3 | NR | NR | ||
| Fourth |
3 |
66.7 |
5.4–94.5 |
|
3 |
66.7 |
5.4–94.5 |
|
|
Number of co-morbidities | ||||||||
| 0 | 24 | 58.1 | 34.3–75.9 | 0.393 | 18 | 66.3 | 36.2–84.7 | 0.350 |
| 1 | 31 | 41.8 | 23.7–58.9 | 29 | 41.0 | 22.3–58.9 | ||
| 2 | 20 | 26.7 | 7.8–50.4 | 16 | 36.9 | 11.8–62.8 | ||
| 3–5 |
14 |
35.7 |
13.0–59.4 |
|
13 |
38.5 |
14.1–62.8 |
|
|
Smoking status | ||||||||
| Current smoker | 12 | 18.7 | 1.27–52.5 | 0.582 | 7 | 42.9 | 9.8–73.5 | 0.454 |
| Ex-smoker | 19 | 44.7 | 21.8–55.3 | 17 | 37.6 | 14.4–61.0 | ||
| Never smoker | 43 | 51.4 | 34.5–66.0 | 41 | 49.4 | 32.9–63.9 | ||
| Unknown |
16 |
25.0 |
6.9–48.7 |
|
12 |
22.2 |
4.1–49.2 |
|
|
Prothrombotic medications | ||||||||
| Yes | 14 | 35.7 | 13.0–59.4 | 0.478 | 12 | 25.0 | 6.0–50.5 | 0.437 |
| No |
76 |
43.1 |
30.8–54.8 |
|
65 |
44.0 |
31.1–56.2 |
|
|
Mode of detection of thrombosis | ||||||||
| Incidental | 29 | 29.2 | 13.0–47.6 | 0.522 | 26 | 31.7 | 14.4–50.6 | 0.778 |
| Symptomatic | 61 | 48.3 | 34.5–60.8 | 51 | 44.6 | 30.1–58.1 | ||
Abbreviations: CI=confidence interval; DVT=deep vein thrombosis; FIGO=International Federation of Gynecology and Obstetrics; NR=not reached.
Table 5. Multivariate survival analysis final model for patients with thrombosis.
| Co-variate | n | β | Standard error | Hazard ratio | 95% CI | P-value |
|---|---|---|---|---|---|---|
|
All patients with thrombosis (n=80) | ||||||
|
PS | ||||||
| 0 | 12 | 0 | 0.000003 | |||
| 1 | 43 | 0.403 | 0.456 | 1.5 | 0.61–3.66 | |
| ⩾2 |
25 |
1.891 |
0.491 |
6.63 |
2.53–17.35 |
|
|
Stage | ||||||
| I/II | 14 | 0 | 0.0003 | |||
| III/IV |
66 |
1.811 |
0.498 |
6.11 |
2.30–16.23 |
|
|
PE and pelvic/lower limb DVT (n=68) | ||||||
|
PS | ||||||
| 0 | 9 | 0 | 0.00015 | |||
| 1 | 39 | 0.052 | 0.500 | 1.054 | 0.40–2.81 | |
| ⩾2 |
20 |
1.429 |
0.519 |
4.173 |
1.51–11.53 |
|
|
Stage | ||||||
| I/II | 13 | 0 | 0.001 | |||
| III/IV | 55 | 1.722 | 0.53 | 5.597 | 1.98–15.82 | |
Abbreviations: CI=confidence interval; DVT=deep vein thrombosis; PE=pulmonary embolism; PS=performance status.
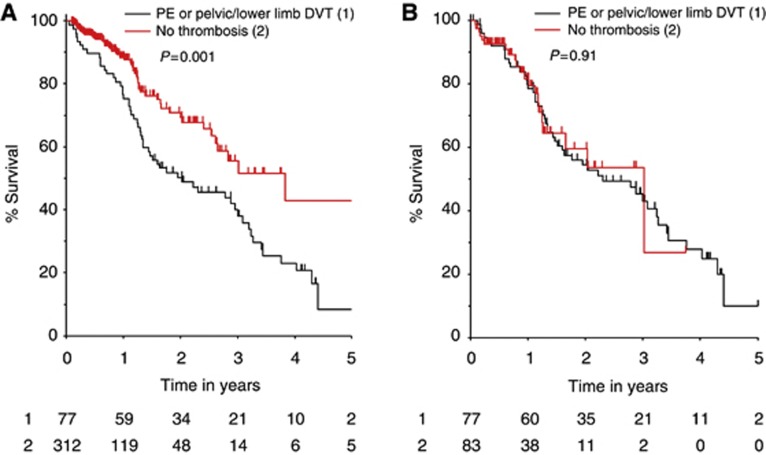
Survival analysis was performed on the patients with thrombosis after exclusion of the arterial, visceral and CVC related events (n=77). Survival analysis of all 417 patients demonstrated a significantly worse survival in the patients with PE or pelvic/lower limb DVT compared with all remaining patients without a diagnosis of a thrombotic event (Figure 2A). The median survival for the patients with thrombosis is 24.0 months, compared with 36.8 months for the patients without thrombosis (P=0.001). However, no survival difference was observed between the same cases and the control group matched for stage and PS (P=0.91; Figure 2B).
Figure 2.
(A) Kaplan–Meier curve for overall survival of patients with ovarian cancer and pulmonary embolism (PE) or pelvic/lower limb deep vein thrombosis (DVT) (black) and of all remaining patients with ovarian cancer and no thrombosis (grey). (B) Kaplan–Meier curve for overall survival of patients with ovarian cancer and PE or pelvic/lower limb DVT (black) and a cohort of patients with ovarian cancer and no thrombosis, matched for FIGO stage and performance status (grey).
After the thrombotic event, all patients were anti-coagulated with LMWH. Forty-five patients (50%) were on anti-coagulation for <6 months. The main reason for early termination was patient death from disease (38; 84.4%). Only one patient (2.2%) had a significant major bleeding complication of haematemesis as a result of the anti-coagulation treatment. Thirty-eight patients (42.2%) had treatment lasting for longer than 6 months, and three patients (3.3%) were currently still receiving treatment for their thrombosis at the time of analysis.
Discussion
This study charts the natural history of thrombotic events associated with ovarian cancer. One in five patients developed a thrombosis during their cancer-associated lifetime. The Californian cancer registry data reported incidence rates of venous thrombosis in ovarian cancer of 1.6% for early stage and 6.7% for advanced stage of disease (Rodriguez et al, 2007), and Khorana et al (2007a) reported incidence rates in ovarian cancer of 5.6%. The incidence rate in our study is higher than reported in the literature and reflects a poor prognostic group. Patients with good prognosis early-stage disease not requiring adjuvant chemotherapy and borderline ovarian tumours who were not referred to our institution were excluded. The incidence rate in patients undergoing surgery was much lower and reflects surgical practice wherein peri-operative thromboprophylaxis is routine. The peak in incidence in the immediate months following diagnosis seen in our study confirms previously published findings in ovarian cancer (Rodriguez et al, 2007). This peak may be due in part to the detection of incidental thrombotic events during staging investigations.
The crude survival rates in the current study corroborate findings from previous studies. The median survival in ovarian cancer patients with thrombotic events was 2 years compared with 3 years in counterparts without thrombosis. There was no difference in survival between patients whose thrombotic event was detected due to symptoms and those that were detected asymptomatically. However, there were significant differences in risk factors for thrombosis between the cohorts of patients with and without thrombosis. Patients with thrombosis were older at diagnosis, had more advanced disease and poorer PS than patients without thrombosis. When the outcomes of patients with thrombosis were compared with a control group matched by stage and PS no survival difference was observed.
This study challenges prevailing opinion that thrombotic events are always associated with a worse prognosis in ovarian cancer. A Danish cancer registry study identified venous thrombosis within 4 months before the diagnosis of ovarian cancer and showed a worse prognosis in the group with thrombosis compared to without (1-year survival 44% and 63%, respectively). Although there was an increase in the proportion of patients in the group with thrombosis with stage 4 disease (35% vs 31%) there was no difference in distribution of PS between the groups (Tetsche et al, 2006). A study of venous thrombosis during first-line chemotherapy in ovarian cancer identified no difference in stage of disease or PS between the groups with and without thrombosis. Once again the prognosis was worse in the group with thrombosis (median survival 29.8 months (thrombosis) and 36.2 months (no thrombosis)) (Fotopoulou et al, 2008). Our study differs from these studies in that we included events occurring up to a year before diagnosis and throughout all subsequent follow-up visits up to 4 years following diagnosis. The differences observed may reflect a temporal association between diagnosis of thrombotic events and patient outcomes. For example, a thrombotic event before diagnosis may reflect underlying aggressive disease biology, whereas that occurring during chemotherapy may reflect a temporary hypercoagulable state. Peaks of incidence appear to be associated with disease activity, that is, diagnosis or relapse and its treatment. Our study included thrombosis during the later stages of disease that may simply reflect natural progression of disease (characterised by advanced FIGO stage and poor PS) and this may be responsible for the absence of a prognostic impact of thrombotic events in this patient cohort. In support of this is the observation that venous thrombosis did not affect prognosis in a cohort of patients with relapsed ovarian cancer (Fotopoulou et al, 2009). Thrombotic events may therefore not be an independent predictor of poor outcomes in all circumstances.
The highest risk of thrombotic events was in the immediate 0–4 months after diagnosis. Thrombosis pre-dated the diagnosis of cancer in 13% (12 out of 90) of cases. Further smaller spikes in incidence were noted a year later likely related to new disease relapse and re-initiation of chemotherapy. This finding validates the current approach of thromboprophylaxis trials with chemotherapy targeting patients with new cancer diagnoses and also confirms that treatment duration of 6 months is appropriate given that the incidence falls sharply after the first few months. This decrease could be the result of response to treatment and disease remission. Over the next few years the falling incidence reflects a declining patient population as the majority succumb to their disease.
The study findings raise some pertinent questions. Is a thrombotic event an independent predictor of poor outcomes in ovarian cancer and is preventive therapy likely to be beneficial, that is, reduce incidence of thrombosis and improve survival with low risk of bleeding? The current clinical practice guidelines suggest that prophylactic anticoagulation should be considered in selected high-risk outpatients as well as in hospitalised patients with cancer (Lyman et al, 2013). Studies to date that also included small subsets of ovarian cancer patients have not answered this question satisfactorily as the additional benefit of venous thromboembolism prophylaxis with LMWH in these trials was too small to be clinically meaningful (Agnelli et al, 2009, 2012). The problem in part may be due to failure to recruit sufficient numbers of patients with poor PS who are the group at highest risk of thrombotic complications.
Although warfarin remains the mainstay of anticoagulant therapy for treatment of thromboembolic disease outside of the setting of cancer, LMWH has been studied extensively and has been adopted as the anticoagulant of choice in the oncology setting (Lyman et al, 2013). Although the novel oral anticoagulants, direct thrombin inhibitors and factor-Xa inhibitors, are gaining acceptance as alternative oral anticoagulants to warfarin (den Exter et al, 2013), they have not yet been sufficiently studied to be recommended for the treatment or prophylaxis of thrombosis in patients with cancer. An advantage to their use is that they do not require routine laboratory monitoring and they would be more convenient than LMWH which requires daily subcutaneous administration. However, a noteworthy limitation is the absence of an intervention to reverse their action in the event of significant bleeding. They do however represent an exciting area of future research.
Although the findings of the current study have generated new hypotheses, any conclusions drawn are limited by the exploratory nature of the study, and these hypotheses now warrant further investigation to confirm their possible significance. Bonferroni correction for multiple tests has not been applied in this exploratory analysis. Although all patients in the case and control cohorts received standard multimodality therapy according to local guidance, treated through the same outpatient department, as no clinical data were collected on the control cohort beyond the data on stage and PS, we cannot exclude differences in clinical management which may impact upon risk of thrombosis.
In summary, thrombotic events in ovarian cancer are associated with poor survival; however, the outcomes are no worse than in patients with equivalent stage of disease and matched for PS. Women with advanced disease and poor PS constitute a high-risk sub-population and future trials of thromboprophylaxis in patients undergoing chemotherapy must focus on this patient group to maximise the potential patient benefit.
Acknowledgments
We thank Dilly Goonetilleke and Kieran Crabtree of the Research and Development department at The Christie NHS Foundation Trust for compiling and maintaining the ovarian cancer clinical database.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Agnelli G, George DJ, Kakkar AK, Fisher W, Lassen MR, Mismetti P, Mouret P, Chaudhari U, Lawson F, Turpie AG. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366 (7:601–609. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- Agnelli G, Gussoni G, Bianchini C, Verso M, Mandala M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G, Passalacqua R, Ricci S, Gasparini G, Lorusso V, Bonizzoni E, Tonato M. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomised, placebo-controlled, double-blind study. Lancet Oncol. 2009;10 (10:943–949. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4 (6:465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166 (4:458–464. doi: 10.1001/archinte.166.4.458. [DOI] [PubMed] [Google Scholar]
- den Exter PL, Kooiman J, van der Hulle T, Huisman MV. New anticoagulants in the treatment of patients with cancer-associated venous thromboembolism. Best Pract Res Clin Haematol. 2013;26 (2:163–169. doi: 10.1016/j.beha.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Duska LR, Garrett L, Henretta M, Ferriss JS, Lee L, Horowitz N. When ‘never-events' occur despite adherence to clinical guidelines: the case of venous thromboembolism in clear cell cancer of the ovary compared with other epithelial histologic subtypes. Gynecol Oncol. 2010;116 (3:374–377. doi: 10.1016/j.ygyno.2009.10.069. [DOI] [PubMed] [Google Scholar]
- Fotopoulou C, duBois A, Karavas AN, Trappe R, Aminossadati B, Schmalfeldt B, Pfisterer J, Sehouli J. Incidence of venous thromboembolism in patients with ovarian cancer undergoing platinum/paclitaxel-containing first-line chemotherapy: an exploratory analysis by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group. J Clin Oncol. 2008;26 (16:2683–2689. doi: 10.1200/JCO.2008.16.1109. [DOI] [PubMed] [Google Scholar]
- Fotopoulou C, Karavas A, Trappe R, Chekerov R, Lichtenegger W, Sehouli J. Venous thromboembolism in recurrent ovarian cancer-patients: a systematic evaluation of the North-Eastern German Society of Gynaecologic Oncology Ovarian Cancer Study Group (NOGGO) Thromb Res. 2009;124 (5:531–535. doi: 10.1016/j.thromres.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Gao S, Escalante C. Venous thromboembolism and malignancy. Expert Rev Anticancer Ther. 2004;4 (2:303–320. doi: 10.1586/14737140.4.2.303. [DOI] [PubMed] [Google Scholar]
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110 (10:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5 (3:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- Lee AY, Levine MN, Butler G, Webb C, Costantini L, Gu C, Julian JA. Incidence, risk factors, and outcomes of catheter-related thrombosis in adult patients with cancer. J Clin Oncol. 2006;24 (9:1404–1408. doi: 10.1200/JCO.2005.03.5600. [DOI] [PubMed] [Google Scholar]
- Lyman GH, Khorana AA, Kuderer NM, Lee AY, Arcelus JI, Balaban EP, Clarke JM, Flowers CR, Francis CW, Gates LE, Kakkar AK, Key NS, Levine MN, Liebman HA, Tempero MA, Wong SL, Prestrud AA, Falanga A. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31 (17:2189–2204. doi: 10.1200/JCO.2013.49.1118. [DOI] [PubMed] [Google Scholar]
- Rodriguez AO, Wun T, Chew H, Zhou H, Harvey D, White RH. Venous thromboembolism in ovarian cancer. Gynecol Oncol. 2007;105 (3:784–790. doi: 10.1016/j.ygyno.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Satoh T, Oki A, Uno K, Sakurai M, Ochi H, Okada S, Minami R, Matsumoto K, Tanaka YO, Tsunoda H, Homma S, Yoshikawa H. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer. 2007;97 (8:1053–1057. doi: 10.1038/sj.bjc.6603989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsche MS, Norgaard M, Pedersen L, Lash TL, Sorensen HT. Prognosis of ovarian cancer subsequent to venous thromboembolism: a nationwide Danish cohort study. BMC Cancer. 2006;6:189. doi: 10.1186/1471-2407-6-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno K, Homma S, Satoh T, Nakanishi K, Abe D, Matsumoto K, Oki A, Tsunoda H, Yamaguchi I, Nagasawa T, Yoshikawa H, Aonuma K. Tissue factor expression as a possible determinant of thromboembolism in ovarian cancer. Br J Cancer. 2007;96 (2:290–295. doi: 10.1038/sj.bjc.6603552. [DOI] [PMC free article] [PubMed] [Google Scholar]