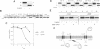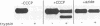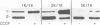Abstract
The membrane electrochemical potential is critical for the export of most periplasmic proteins in Escherichia coli. Its exact role during insertion of integral inner membrane proteins, however, remains obscure. Using derivatives of the inner membrane protein leader peptidase (Lep), we now show that the membrane potential appears to stimulate the membrane translocation of chain segments containing negatively charged residues, that positively charged regions appear to be more easily translocated in the absence of a potential, and that certain Lep constructs insert with different topologies in the presence and absence of a membrane potential, suggesting that the electrochemical potential introduces an asymmetry between the topological effects of positively and negatively charged amino acids during the process of membrane protein insertion in E. coli.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akita M., Sasaki S., Matsuyama S., Mizushima S. SecA interacts with secretory proteins by recognizing the positive charge at the amino terminus of the signal peptide in Escherichia coli. J Biol Chem. 1990 May 15;265(14):8164–8169. [PubMed] [Google Scholar]
- Andersson H., Bakker E., von Heijne G. Different positively charged amino acids have similar effects on the topology of a polytopic transmembrane protein in Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):1491–1495. [PubMed] [Google Scholar]
- Andersson H., von Heijne G. Position-specific Asp-Lys pairing can affect signal sequence function and membrane protein topology. J Biol Chem. 1993 Oct 5;268(28):21389–21393. [PubMed] [Google Scholar]
- Andersson H., von Heijne G. Sec dependent and sec independent assembly of E. coli inner membrane proteins: the topological rules depend on chain length. EMBO J. 1993 Feb;12(2):683–691. doi: 10.1002/j.1460-2075.1993.tb05702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Randall L. L. The requirement for energy during export of beta-lactamase in Escherichia coli is fulfilled by the total protonmotive force. EMBO J. 1984 Apr;3(4):895–900. doi: 10.1002/j.1460-2075.1984.tb01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Beckwith J. The role of charged amino acids in the localization of secreted and membrane proteins. Cell. 1990 Sep 21;62(6):1031–1033. doi: 10.1016/0092-8674(90)90378-r. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. Leader peptidase of Escherichia coli: critical role of a small domain in membrane assembly. Science. 1987 Feb 13;235(4790):783–787. doi: 10.1126/science.3544218. [DOI] [PubMed] [Google Scholar]
- Dalbey R. E., Wickner W. The role of the polar, carboxyl-terminal domain of Escherichia coli leader peptidase in its translocation across the plasma membrane. J Biol Chem. 1986 Oct 15;261(29):13844–13849. [PubMed] [Google Scholar]
- Daniels C. J., Bole D. G., Quay S. C., Oxender D. L. Role for membrane potential in the secretion of protein into the periplasm of Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5396–5400. doi: 10.1073/pnas.78.9.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date T., Zwizinski C., Ludmerer S., Wickner W. Mechanisms of membrane assembly: effects of energy poisons on the conversion of soluble M13 coliphage procoat to membrane-bound coat protein. Proc Natl Acad Sci U S A. 1980 Feb;77(2):827–831. doi: 10.1073/pnas.77.2.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewelling R. F., Hubbell W. L. The membrane dipole potential in a total membrane potential model. Applications to hydrophobic ion interactions with membranes. Biophys J. 1986 Feb;49(2):541–552. doi: 10.1016/S0006-3495(86)83664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallusser A., Kuhn A. Initial steps in protein membrane insertion. Bacteriophage M13 procoat protein binds to the membrane surface by electrostatic interaction. EMBO J. 1990 Sep;9(9):2723–2729. doi: 10.1002/j.1460-2075.1990.tb07459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavel Y., Steppuhn J., Herrmann R., von Heijne G. The 'positive-inside rule' applies to thylakoid membrane proteins. FEBS Lett. 1991 Apr 22;282(1):41–46. doi: 10.1016/0014-5793(91)80440-e. [DOI] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. The distribution of charged amino acids in mitochondrial inner-membrane proteins suggests different modes of membrane integration for nuclearly and mitochondrially encoded proteins. Eur J Biochem. 1992 May 1;205(3):1207–1215. doi: 10.1111/j.1432-1033.1992.tb16892.x. [DOI] [PubMed] [Google Scholar]
- Geller B., Zhu H. Y., Cheng S., Kuhn A., Dalbey R. E. Charged residues render pro-OmpA potential dependent for initiation of membrane translocation. J Biol Chem. 1993 May 5;268(13):9442–9447. [PubMed] [Google Scholar]
- Heijne G. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 1986 Nov;5(11):3021–3027. doi: 10.1002/j.1460-2075.1986.tb04601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S., Lee J. H., Ray D. S. High-level expression of M13 gene II protein from an inducible polycistronic messenger RNA. Gene. 1985;34(2-3):137–145. doi: 10.1016/0378-1119(85)90121-0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusters R., Dowhan W., de Kruijff B. Negatively charged phospholipids restore prePhoE translocation across phosphatidylglycerol-depleted Escherichia coli inner membranes. J Biol Chem. 1991 May 15;266(14):8659–8662. [PubMed] [Google Scholar]
- Lee J. I., Kuhn A., Dalbey R. E. Distinct domains of an oligotopic membrane protein are Sec-dependent and Sec-independent for membrane insertion. J Biol Chem. 1992 Jan 15;267(2):938–943. [PubMed] [Google Scholar]
- Nilsson I. M., Gafvelin G., von Heijne G. Different sec-requirements for signal peptide cleavage and protein translocation in a model E. coli protein. FEBS Lett. 1993 Feb 22;318(1):7–10. doi: 10.1016/0014-5793(93)81316-r. [DOI] [PubMed] [Google Scholar]
- Nilsson I., von Heijne G. Fine-tuning the topology of a polytopic membrane protein: role of positively and negatively charged amino acids. Cell. 1990 Sep 21;62(6):1135–1141. doi: 10.1016/0092-8674(90)90390-z. [DOI] [PubMed] [Google Scholar]
- Oliver D. B., Cabelli R. J., Dolan K. M., Jarosik G. P. Azide-resistant mutants of Escherichia coli alter the SecA protein, an azide-sensitive component of the protein export machinery. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8227–8231. doi: 10.1073/pnas.87.21.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puziss J. W., Strobel S. M., Bassford P. J., Jr Export of maltose-binding protein species with altered charge distribution surrounding the signal peptide hydrophobic core in Escherichia coli cells harboring prl suppressor mutations. J Bacteriol. 1992 Jan;174(1):92–101. doi: 10.1128/jb.174.1.92-101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J., Kuhn A. The function of a leader peptide in translocating charged amino acyl residues across a membrane. Science. 1990 Dec 7;250(4986):1418–1421. doi: 10.1126/science.2124001. [DOI] [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Rice M., Wickner W. Effects of two sec genes on protein assembly into the plasma membrane of Escherichia coli. J Biol Chem. 1985 Feb 10;260(3):1836–1841. [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W. Bacterial leader peptidase, a membrane protein without a leader peptide, uses the same export pathway as pre-secretory proteins. Cell. 1984 Apr;36(4):1067–1072. doi: 10.1016/0092-8674(84)90056-4. [DOI] [PubMed] [Google Scholar]
- Wolfe P. B., Wickner W., Goodman J. M. Sequence of the leader peptidase gene of Escherichia coli and the orientation of leader peptidase in the bacterial envelope. J Biol Chem. 1983 Oct 10;258(19):12073–12080. [PubMed] [Google Scholar]
- Zimmermann R., Watts C., Wickner W. The biosynthesis of membrane-bound M13 coat protein. Energetics and assembly intermediates. J Biol Chem. 1982 Jun 10;257(11):6529–6536. [PubMed] [Google Scholar]
- Zimmermann R., Wickner W. Energetics and intermediates of the assembly of Protein OmpA into the outer membrane of Escherichia coli. J Biol Chem. 1983 Mar 25;258(6):3920–3925. [PubMed] [Google Scholar]
- de Vrije T., de Swart R. L., Dowhan W., Tommassen J., de Kruijff B. Phosphatidylglycerol is involved in protein translocation across Escherichia coli inner membranes. Nature. 1988 Jul 14;334(6178):173–175. doi: 10.1038/334173a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Gavel Y. Topogenic signals in integral membrane proteins. Eur J Biochem. 1988 Jul 1;174(4):671–678. doi: 10.1111/j.1432-1033.1988.tb14150.x. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., Nielsen J., Hansen F. G. Promoters of the atp operon coding for the membrane-bound ATP synthase of Escherichia coli mapped by Tn10 insertion mutations. Mol Gen Genet. 1982;188(2):240–248. doi: 10.1007/BF00332682. [DOI] [PubMed] [Google Scholar]