Abstract
Background:
Metastatic breast cancer is a severe condition without curative treatment. How relative and absolute risk of distant metastasis varies over time since diagnosis, as a function of treatment, age and tumour characteristics, has not been studied in detail.
Methods:
A total of 9514 women under the age of 75 when diagnosed with breast cancer in Stockholm and Gotland regions during 1990–2006 were followed up for metastasis (mean follow-up=5.7 years). Time-dependent development of distant metastasis was analysed using flexible parametric survival models and presented as hazard ratio (HR) and cumulative risk.
Results:
A total of 995 (10.4%) patients developed distant metastasis; the most common sites were skeleton (32.5%) and multiple sites (28.3%). Women younger than 50 years at diagnosis, with lymph node-positive, oestrogen receptor (ER)-negative, >20 mm tumours and treated only locally, had the highest risk of distant metastasis (0–5 years' cumulative risk =0.55; 95% confidence interval (CI): 0.47–0.64). Women older than 50 years at diagnosis, with ER-positive, lymph node-negative and ⩽20-mm tumours, had the same and lowest cumulative risk of developing metastasis 0–5 and 5–10 years (cumulative risk=0.03; 95% CI: 0.02–0.04). In the period of 5–10 years after diagnosis, women with ER-positive, lymph node-positive and >20-mm tumours were at highest risk of distant recurrence. Women with ER-negative tumours showed a decline in risk during this period.
Conclusion:
Our data show no support for discontinuation at 5 years of clinical follow-up in breast cancer patients and suggest further investigation on differential clinical follow-up for different subgroups of patients.
Keywords: breast cancer, distant metastasis, risk, survival analysis, tumour characteristics, competing risk
The overall survival of breast cancer patients has increased quite remarkably in the past decades because of substantial improvements in diagnosis and treatment reaching 79% 5-year survival by the early 2000s (Verdecchia et al, 2007). In particular, new polychemotherapy regimens have contributed to about 25% decrease in annual death rates from 1980s and, in combination with 5-year tamoxifen treatment, have approximately halved death rates among middle-aged women with oestrogen receptor (ER)-positive tumours (Early Breast Cancer Trialists' Collaborative Group, 2005). However, the development of distant metastasis still means that the patient is beyond cure (Clarke et al, 2005; Early Breast Cancer Trialists' Collaborative Group, 2005; Verdecchia et al, 2007; Liu et al, 2010).
Whereas it is well known that molecular profiles of breast tumours influence prognosis and likelihood of distant recurrences (Grann et al, 2005; Dunnwald et al, 2007; Hsieh et al, 2009; Alford et al, 2012), it has only recently been shown that gene expression profiles of breast tumour samples could be used to predict relapses and metastatic pattern in breast cancer patients and to be potential candidate targets for new drugs (van de Vijver et al, 2002; Chambers et al, 2002; Brenton et al, 2005; Minn et al, 2005).
Several studies have already investigated the prognosis of breast cancer patients after developing distant metastasis, showing that age at initial diagnosis, hormonal receptor status and site of metastasis are the most relevant factors for predicting survival from occurrence of metastasis (Largillier et al, 2008; Foukakis et al, 2011; Schneider et al, 2011). Dent et al (2009a, 2009b) have shown that triple-negative tumours metastasise earlier and more frequently than other breast tumours; however, following 5 years from diagnosis the difference tends to disappear. Apart from the effect of ER-receptor status, not much is known about how time to first distant metastasis is influenced by age and tumour characteristics and how site of first distant metastasis changes by time since diagnosis (Biganzoli et al, 2003; Dent et al, 2007; Jensen et al, 2011; Frisk et al, 2012).
The risk of developing first distant metastasis may in fact vary over time since diagnosis across different subgroups of patients, and it is of importance to be able to more accurately predict the risk of tumour dissemination over time. We aimed to undertake a comprehensive analysis of factors affecting development, time and site of distant metastasis using a Swedish population-based cohort of breast cancer patients.
Materials and Methods
Data source
The Stockholm Breast Cancer Register (SBCR) is a population-based clinical register held by the Regional Cancer Centre of Stockholm–Gotland region, Sweden. The register contains data about all breast cancer diagnoses occurring in the Swedish counties of Stockholm and Gotland since 1976. The SBCR provides detailed clinical information, such as tumour characteristics and intention of treatment, for each patient.
Study cohort
A population-based cohort was selected from the SBCR, including all women diagnosed with first invasive breast cancer in the period of 1 January 1990 and 31 December 2006, younger than 75 years at diagnosis and without any previous occurrence of cancer. Patients were followed up for at most 10 years from the date of breast cancer diagnosis until the development of first distant metastasis (event), until death, diagnosis of second primary cancer or end of study period (31 December 2006). The records were linked to the Swedish Cancer Register (Barlow et al, 2009) for information on other invasive cancers through linkage by the personal identification number (unique for each Swedish resident and included in all Swedish population registers).
The cohort comprised 14 188 women. Those who had a metastatic disease at diagnosis (stage IV, n=264), were diagnosed with first distant metastasis occurring within 3 months from breast cancer diagnosis (n=44), had tumour size less than 1 mm (n=52), received neoadjuvant treatment (n=798) and did not undergo surgery for breast cancer (n=217) were excluded. Women who were diagnosed with second primary cancer at the time of breast cancer diagnosis (n=226) were also excluded because of impossibility to infer origin of metastasis. Finally, women who were referred as dying from breast cancer without any record of distant metastasis were also excluded (n=240), as it was not possible to assess whether this was due to missing information about metastatic status or due to the inaccuracy in the reported underlying cause of death. Of the remaining patients (n=12 322), 1189 (9.7%) had subsequent distant metastasis within 10 years of initial diagnosis.
Information on date of breast cancer diagnosis, planned adjuvant treatment and site of first distant metastasis was complete for all patients. Information on number of positive lymph nodes and tumour size was available for 94.6% and 98.4% of patients, respectively. Information on ER status was available for 80.3% of patients. For this reason, in the analysis we eventually included only patients with information available for each covariate used in the models (n=9514), of which 995 (10.4%) developed distant metastasis within 10 years of initial diagnosis.
Information on the cause of death was obtained from the Swedish Cause of Death Register (Rutqvist, 1985). Patients with underlying cause of death other than breast cancer (International Classification of Diseases (ICD) version 8=174, ICD9=174, ICD10=C50) were censored. Information on the site of first metastasis was obtained from the SBCR and was divided into eight groups according to the ICD code: skeleton, lung, pleura, liver, central nervous system (CNS), skin, other sites and multiple sites of first distant metastasis (defined as distant metastasis diagnosed within 2 months from the first distant metastasis diagnosis).
Ethics
This study has an ethical permission from the regional ethics committee at the Karolinska Institutet.
Statistical analysis
This cohort study was analysed using survival methodology with adjustment for competing risks. All women were followed up and they contributed to risk–time from the date of diagnosis until the date of first distant metastasis (event) or diagnosis of second primary cancer, date of death, 10 years of follow-up or end of study, 31 December 2006 (censoring).
Rates of first distant metastasis were calculated as number of events (development of first distant metastasis) divided by total risk–time. These rates were modelled using flexible parametric survival models (FPMs) that use a restricted cubic spline function for the cumulative baseline hazard rate (Royston and Parmar, 2002; Lambert and Royston, 2009). The models estimate hazard ratios (HRs) with 95% confidence intervals (CIs) as measure of association between exposures and outcome. For the baseline rate, we used a spline with five degrees of freedom (two knots at each boundary and four intermediate knots placed at quintiles of the distribution of events). The underlying timescale used in the analysis was time since diagnosis of breast cancer.
From the FPM it is also possible to post estimate the cumulative risk of developing metastasis in the presence of competing risks (Hinchliffe and Lambert, 2012). We estimated the cumulative risk for first distant metastasis within 5 years from diagnosis for various covariate patterns in the presence of competing event death due to causes other than breast cancer. We further estimated the cumulative risk of metastasis during the period of 5–10 years after diagnosis, conditional upon surviving and being metastasis-free at 5 years (that is, conditional probabilities). Variables were categorised as follows: age at breast cancer diagnosis (⩽50, 51–60, 61–74 years), calendar period at breast cancer diagnosis (1990–1994, 1995–1999, 2000–2006), tumour size (⩽20 mm, >20 mm), lymph node status (positive/negative), ER status (ER-positive/negative) and treatment. Treatment was categorised as local treatment (surgery without adjuvant treatment; surgery with radiotherapy only (RT)) and systemic treatment (any combination with either chemotherapy (CT) or hormone therapy (HT)).
Firstly, we estimated HRs by age and tumour characteristics using non-proportional hazards models, which allow HR to vary over follow-up (that is, time-dependent effects).
Secondly, we estimated 5-year cumulative risks of developing a first distant metastasis in the presence of competing event death due to other causes. As cumulative risk is an absolute measure, results are shown for a given set of characteristics (covariate patterns). We also report cumulative risks during 5–10 years after diagnosis conditional on surviving 5 years answering the question: what is the probability that a patient who has survived 5 years without metastasis will develop first distant metastasis in the coming years? The data were analysed using Stata Intercooled 12.0 (StataCorp. 2009, Stata Statistical Software: Release 12. College Station, TX, USA: StataCorp LP) and R package version 2.15.1 for calculating CIFs 5–10 years.
Results
The mean age of the patients was 56.4 years at diagnosis. The tumour characteristics were distributed as follows: 63.5% of patients had lymph node-negative tumours, 69% had tumours of size 20 mm or less and 82.3% had ER-positive tumours (Table 1). In our cohort, 87.9% of women underwent systemic adjuvant treatment. The overall rate of first distant metastasis was 19.4 per 1000 person–years (95% CI: 18.2–20.6).
Table 1. Frequency distributions and rates of first distant metastasis in the study cohort of breast cancer patients in the Stockholm and Gotland Swedish counties, 1990–2006, followed for up to 10 years since diagnosis.
| Total N (%) | First distant metastasis N (%) | Rate of metastasis per 1000 person–years (95% CI) | |
|---|---|---|---|
|
Age at diagnosis | |||
| ⩽50 Years | 2913 (30.6) | 400 (40.2) | 25.0 (22.6–27.6) |
| 51–60 Years | 3039 (31.9) | 271 (27.2) | 16.6 (14.7–18.7) |
| 61–74 Years |
3562 (37.4) |
324 (32.6) |
17.1 (15.3–19.1) |
|
Period of breast cancer diagnosis | |||
| 1990–1994 | 2622 (27.6) | 496 (49.8) | 24.2 (22.2–26.4) |
| 1995–1999 | 2242 (23.6) | 324 (32.6) | 18.9 (16.9–21.0) |
| 2000–2006 |
4650 (48.9) |
175 (17.6) |
12.8 (11.1–14.9) |
|
ER status | |||
| Positive | 7830 (82.3) | 688 (69.1) | 16.1 (14.9–17.4) |
| Negative |
1684 (17.7) |
307 (30.9) |
35.7 (32.0–40.0) |
|
Lymph nodes status | |||
| Negative | 6043 (63.5) | 352 (35.4) | 10.4 (9.4–11.5) |
| Positive |
3471 (36.5) |
643 (64.6) |
36.9 (34.1–39.9) |
|
Tumour size | |||
| ⩽20 mm | 6567 (69.0) | 457 (45.9) | 12.5 (11.4–13.7) |
| >20 mm |
2947 (31.0) |
538 (54.1) |
36.2 (33.3–39.4) |
|
Treatment | |||
| Local (surgery +/− RT only) | 1147 (12.1) | 193 (19.4) | 24.5 (21.3–28.2) |
| Systemic (with CT and/or HT) |
8367 (87.9) |
802 (80.6) |
18.5 (17.2–19.8) |
| Total | 9514 (100.0) | 995 (100.0) | 19.4 (18.2–20.6) |
Abbreviations: CI=confidence interval; CT=chemotherapy; ER=oestrogen receptor; HT=hormone therapy; RT=radiation therapy.
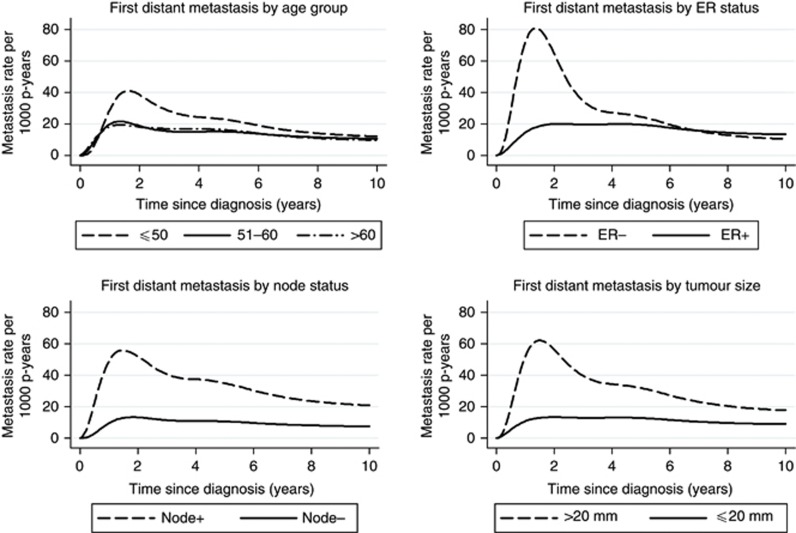
Figure 1 shows rates of first distant metastasis in relation to time since breast cancer diagnosis for different age groups of patients and tumour characteristics. Women younger than 50 years at breast cancer diagnosis, women with ER-negative tumours, positive lymph nodes and tumours larger than 20 mm, all showed a peak in the rate of first distant metastasis at about 2 years, in comparison with fairly stable rates in other subgroups of patients. In particular, women with ER-negative tumours showed a sharp decrease in the rate of first distant metastasis after 2 years since breast cancer diagnosis, whereas women with ER-positive tumours showed rather stable rates of first distant metastasis over time from about 1 year up until 10 years since breast cancer diagnosis.
Figure 1.
Estimated rates of first distant metastasis within 10 years of diagnosis of first invasive breast cancer in women diagnosed between 1990 and 2006 in Stockholm–Gotland Swedish counties, according to age and tumour characteristics.
Table 2 shows frequency distribution of sites of first distant metastasis by time-to-first-distant metastasis subdivided into 0–2, 2–5 and 5–10 years since diagnosis. Overall, metastasis to the skeleton (32.5%) and multiple sites of metastasis (28.3%) were the most frequent presentations of distant metastasis within 10 years. No particular combination pattern of multiple sites of first distant metastasis was found in the cohort. The site distribution of first distant metastasis changed significantly (P<0.05) over time for the following sites: skeleton, CNS and liver. The proportion of first distant metastasis to the skeleton increased from 29.9% of all first distant metastasis in the first 2 years to 36.5% in the period of 5–10 years since breast cancer diagnosis. The proportion of CNS and liver metastasis instead decreased from 6.8% and 15.4%, respectively, in the first 2 years to 1.8% and 8.0%, respectively, in 5–10 years since breast cancer diagnosis. Between 5 and 10 years since breast cancer diagnosis, 274 (27.5%) of all first distant metastasis were diagnosed.
Table 2. Frequencies and proportion of first distant metastases, by time since diagnosis and site, in the study cohort of breast cancer patients in the Stockholm and Gotland Swedish counties, 1990–2006, followed for up to 10 years since diagnosis.
| Site of first distant metastasis | 0–2 Years, n (%) | 2–5 Years, n (%) | 5–10 Years, n (%) | 0–10 Years, n (%) |
|---|---|---|---|---|
| Skeleton |
97 (29.9) |
126 (31.7) |
100 (36.5) |
323 (32.5) |
| Lung |
24 (7.4) |
41 (10.3) |
22 (8.0) |
87 (8.7) |
| Liver |
50 (15.4) |
36 (9.1) |
22 (8.0) |
108 (10.9) |
| Pleura |
12 (3.7) |
17 (4.3) |
16 (5.8) |
45 (4.5) |
| CNS |
22 (6.8) |
17 (4.3) |
5 (1.8) |
44 (4.4) |
| Other |
31 (10.0) |
32 (8.1) |
43 (15.7) |
106 (10.7) |
| Multiple |
88 (27.2) |
128 (32.2) |
66 (24.1) |
282 (28.3) |
| Total (any site) | 324 (100.0) | 397 (100.0) | 274 (100.0) | 995 (100.0) |
Abbreviation: CNS=central nervous system.
Table 3 shows time-dependent HRs of developing first distant metastasis by age and tumour characteristics at 2, 5 and 10 years from breast cancer diagnosis. For women with positive lymph nodes, the hazard of developing metastasis was still significantly increased at 10 years after diagnosis (HR=2.6; 95% CI: 1.9–3.5) compared with women with negative lymph nodes. Women with ER-negative tumours had an increased hazard at 2 (HR=2.7; 95% CI: 2.2–3.3) and 5 (HR=1.4; 95% CI: 1.1–1.7) years from breast cancer diagnosis compared with women with ER-positive tumours; at 10 years from diagnosis, the same HR was not significantly increased anymore (HR=0.9; 95% CI: 0.6–1.4). Having a tumour larger than 20 mm at breast cancer diagnosis was still causing an increased hazard of developing first distant metastasis at 10 years (HR=1.5; 95% CI: 1.1–2.0).
Table 3. Adjusted HRa and 95% CIs of developing a first distant metastasis by patient and tumour characteristics in the study cohort of breast cancer patients in the Stockholm and Gotland Swedish counties, 1990–2006, followed for up to 10 years since diagnosis.
| At 2 years, HR (95% CI) | At 5 years, HR (95% CI) | At 10 years, HR (95% CI) | |
|---|---|---|---|
|
Age | |||
| ⩽50 Years | 1.5 (1.2–1.9) | 1.1 (0.9–1.3) | 1.1 (0.8–1.5) |
| 51–60 Years | 1.0 (0.8–1.3) | 0.9 (0.8–1.1) | 1.0 (0.7–1.4) |
| 61–74 Yearsb |
1.0 |
1.0 |
1.0 |
|
Lymph nodal status | |||
| Negative lymph nodesb | 1.0 | 1.0 | 1.0 |
| Positive lymph nodes |
3.2 (2.6–4) |
2.9 (2.5–3.4) |
2.6 (1.9–3.5) |
|
ER status | |||
| ER+b | 1.0 | 1.0 | 1.0 |
| ER− |
2.7 (2.2–3.3) |
1.4 (1.1–1.7) |
0.9 (0.6–1.4) |
|
Tumour size | |||
| 1–20 mmb | 1.0 | 1.0 | 1.0 |
| >20 mm | 2.7 (2.2–3.3) | 1.8 (1.6–2.1) | 1.5 (1.1–2) |
Abbreviations: CI=confidence intervals; ER=oestrogen receptor; HR=hazard ratios.
HRs adjusted for all variables in the table, treatment and calendar period. Time-varying effects for age, lymph node status, ER status and tumour size.
Reference group.
Table 4 shows cumulative risks of developing first distant metastasis within 5 years of diagnosis, and within 10 years surviving 5 years without metastasis, according to all different covariate patterns. Among those with an adjuvant treatment combination including CT and/or HT, the highest risk of first distant metastasis in the period of 0–5 years was found in patients of 50 years of age or less, with tumour size >20 mm, positive lymph nodes and ER-negative tumours at breast cancer diagnosis (cumulative risk =0.45; 95% CI: 0.38–0.53); whereas the lowest risk was among patients aged 51–74 years with negative lymph nodes, tumour size ⩽20 mm and with ER-positive tumours at breast cancer diagnosis (cumulative risk =0.03; 95% CI: 0.02–0.04). For the same two groups of patients in the period of 5–10 years following diagnosis (that is, among those who survived metastasis-free until 5 years), the risk dropped (cumulative risk=0.09; 95% CI: 0.05–0.17) for the group originally at the highest risk, whereas it remained the same (cumulative risk=0.03; 95% CI: 0.02–0.04) for the group at the lowest risk. The risk at 5–10 years following diagnosis was similar to the risk for the period of 0–5 years in all women with ER-positive tumours and negative lymph nodes, regardless of treatment, tumour size and age. This was also true for women with ER-positive tumours and positive lymph nodes, if the tumour size at diagnosis was ⩽20 mm. For all patients with ER-negative tumours instead, the risk at 5–10 years following diagnosis was significantly lower compared with the risk for the period of 0–5 years regardless of age, tumour size and nodes. Women treated with systemic adjuvant treatment (that is, CT and/or HT) always had lower risks compared to women treated with surgery only, with or without RT.
Table 4. Cumulative risk of first distant metastasis within 0–5 years and 5–10 years of diagnosis for women with breast cancer in Stockholm and Gotland Countries, 1995–99a.
| |
|
Local treatment (surgery ±RT only)b |
Systemic treatment (CT and/or HT)b |
||
|---|---|---|---|---|---|
| Age group | Tumour characteristics | 0–5 Years | 5–10 Years | 0–5 Years | 5–10 Years |
|
⩽50 Years | |||||
|
Positive nodes | |||||
| ER−, size >20 mm | 0.55 (0.47–0.64) | 0.12 (0.07–0.23) | 0.45 (0.38–0.53) | 0.09 (0.05–0.17) | |
| ER−, size <20 mm | 0.30 (0.24–0.37) | 0.12 (0.08–0.18) | 0.23 (0.19–0.28) | 0.09 (0.06–0.13) | |
| ER+, size >20 mm | 0.30 (0.25–0.37) | 0.19 (0.14–0.25) | 0.24 (0.20–0.27) | 0.14 (0.11–0.18) | |
| |
ER+, size <20 mm |
0.15 (0.12–0.19) |
0.12 (0.09–0.16) |
0.11 (0.09–0.13) |
0.09 (0.07–0.12) |
|
Negative nodes | |||||
| ER−, size >20 mm | 0.22 (0.17–0.27) | 0.06 (0.04–0.11) | 0.17 (0.13–0.20) | 0.05 (0.03–0.08) | |
| ER−, size <20 mm | 0.10 (0.08–0.13) | 0.05 (0.03–0.07) | 0.08 (0.06–0.10) | 0.04 (0.03–0.05) | |
| ER+, size >20 mm | 0.10 (0.08–0.13) | 0.07 (0.05–0.10) | 0.08 (0.06–0.10) | 0.06 (0.04–0.08) | |
| |
ER+, size <20 mm |
0.05 (0.04–0.06) |
0.05 (0.04–0.06) |
0.04 (0.03–0.04) |
0.04 (0.03–0.04) |
|
51–60 Years | |||||
|
Positive nodes | |||||
| ER−, size >20 mm | 0.46 (0.39–0.55) | 0.13 (0.07–0.24) | 0.37 (0.31–0.43) | 0.10 (0.05–0.18) | |
| ER−, size <20 mm | 0.24 (0.19–0.30) | 0.11 (0.07–0.17) | 0.18 (0.15–0.23) | 0.08 (0.05–0.13) | |
| ER+, size >20 mm | 0.24 (0.20–0.30) | 0.17 (0.12–0.23) | 0.19 (0.16–0.22) | 0.13 (0.10–0.17) | |
| |
ER+, size <20 mm |
0.12 (0.09–0.15) |
0.11 (0.08–0.15) |
0.09 (0.07–0.11) |
0.08 (0.06–0.11) |
|
Negative nodes | |||||
| ER−, size >20 mm | 0.17 (0.14–0.22) | 0.06 (0.04–0.10) | 0.13 (0.10–0.16) | 0.05 (0.03–0.08) | |
| ER−, size <20 mm | 0.08 (0.06–0.10) | 0.04 (0.03–0.07) | 0.06 (0.05–0.08) | 0.03 (0.02–0.05) | |
| ER+, size >20 mm | 0.08 (0.06–0.10) | 0.07 (0.05–0.09) | 0.06 (0.05–0.08) | 0.05 (0.04–0.07) | |
| |
ER+, size <20 mm |
0.04 (0.03–0.05) |
0.04 (0.03–0.05) |
0.03 (0.02–0.03) |
0.03 (0.02–0.04) |
|
61–74 Years | |||||
|
Positive nodes | |||||
| ER−, size >20 mm | 0.47 (0.40–0.55) | 0.13 (0.07–0.24) | 0.38 (0.32–0.44) | 0.10 (0.05–0.19) | |
| ER−, size <20 mm | 0.25 (0.20–0.31) | 0.11 (0.07–0.18) | 0.19 (0.16–0.24) | 0.08 (0.05–0.13) | |
| ER+, size >20 mm | 0.25 (0.20–0.30) | 0.17 (0.13–0.22) | 0.19 (0.16–0.23) | 0.13 (0.10–0.17) | |
| |
ER+, size <20 mm |
0.12 (0.10–0.15) |
0.11 (0.08–0.15) |
0.09 (0.08–0.11) |
0.08 (0.07–0.11) |
|
Negative nodes | |||||
| ER−, size >20 mm | 0.18 (0.14–0.22) | 0.06 (0.04–0.10) | 0.13 (0.11–0.17) | 0.05 (0.03–0.08) | |
| ER−, size <20 mm | 0.08 (0.07–0.11) | 0.05 (0.03–0.07) | 0.06 (0.05–0.08) | 0.03 (0.02–0.05) | |
| ER+, size >20 mm | 0.08 (0.07–0.11) | 0.07 (0.05–0.09) | 0.06 (0.05–0.08) | 0.05 (0.04–0.07) | |
| ER+, size <20 mm | 0.04 (0.03–0.05) | 0.04 (0.03–0.05) | 0.03 (0.02–0.04) | 0.03 (0.02–0.04) | |
Abbreviations: CT=Chemotherapy; ER=oestrogen receptor; HT=hormone therapy; RT=radiation therapy.
Models are based on the full data set, but only estimates for women diagnosed at 1995–1999 are shown, as they have chance of being followed up to 10 years.
Local treatment: patients undergoing surgery with or without RT; systemic treatment: patients undergoing any treatment combination including CT and/or HT.
Discussion
We found that the site distribution of first distant metastasis over time changed significantly for metastasis to the skeleton, CNS and liver. The risk of developing distant metastasis within 10 years of breast cancer diagnosis significantly varied in different subgroups of patients. The risk remained non-negligible up to 10 years since diagnosis particularly among women with positive lymph nodes. The risk was high in particular among patients with ER-negative tumours within the first 5 years of diagnosis, whereas it significantly decreased after 5 years since diagnosis. The risk of developing distant metastasis remained instead rather stable for most subgroups of patients with ER-positive tumours independent of age and other tumour characteristics.
In our cohort, one-third of first distant metastasis was diagnosed in the skeleton. This proportion significantly increased over time since diagnosis, whereas the proportion of metastasis to the liver and CNS significantly decreased. This seems to reflect the natural history of distant recurrences as women with ER-positive tumours more often develop metastasis to the skeleton later during follow-up, whereas women with ER-negative tumours more often develop early liver and CNS metastasis (Kennecke et al, 2010).
Interestingly, the risk for developing distant metastasis over time since diagnosis mirrors the pattern observed for breast cancer-specific mortality that has been previously studied in this same cohort: lymph node status and tumour size at diagnosis are significant prognosticators of distant recurrence, as well as of breast cancer death, at 10 years since breast cancer diagnosis, whereas ER status is not. These findings confirm that distant metastasis still indicate a very poor prognosis in breast cancer patients (Hilsenbeck et al, 1998; Louwman et al, 2008; Colzani et al, 2011).
Cumulative risk estimates of developing distant metastasis were obtained for specific subgroups of patients while taking into account the competing risk of dying from other causes and by allowing time-dependent effects (interaction with time since diagnosis) for age and tumour characteristics. The use of cumulative risk as a function of time is of relevant clinical value as it allows a quantitative estimation of what is the actual probability of developing distant metastasis for any given subgroup of breast cancer patients at different time points. This measurement may help clinicians to better estimate the individual risk of developing first distant metastasis during the first 5 years as well as 5–10 years after diagnosis. One of the main messages from this study stems from the fact that the risk of developing distant metastasis carries over significantly to the second 5 years of follow-up for most metastasis-free patients, particularly those patients with lymph node-positive tumours where the risk at 5–10 years after diagnosis is always higher or equal to 8%. In addition, for some subgroups of patients with ER-positive tumours, the cumulative risk of developing distant metastasis in the period of 0–5 and 5–10 years is essentially the same.
Patients with ER-positive and lymph node-negative tumours show no change and very low risk over time. This could be explained by a very good effect of treatment, or alternatively even by overtreatment since patients undergoing local treatment show a similar low risk. More clinical attention should, however, be given to other subgroups in which follow-up could be intensified and treatment could be improved. In particular, women with ER-positive, lymph node-positive, >20 mm tumours still have a risk higher than 10% to develop first distant metastasis in the period of 5–10 years after diagnosis. Of note, all subgroups of patients with ER-negative tumours have a sharp significant decrease in risk of developing distant metastasis in the period of 5–10 years following diagnosis compared with the period of 0–5 years, independent of age and other tumour characteristics. The American Society of Clinical Oncology (ASCO) has recently concluded in a review of the clinical practice guidelines of primary breast cancer follow-up that there is no present need for updating the current guidelines (Khatcheressian et al, 2013). Although evidence supporting the change of current practice is rather weak (Robertson et al, 2011), following future improvements in prevention and treatment of metastatic breast cancer, a differential follow-up of patients with ER-positive and ER-negative tumours could be considered, given their remarkably different risks of spreading.
This study has some relevant strengths. We used a large cohort of women followed up to 10 years with accurate and complete information, enabling us to apply a comprehensive design and methodology. We analysed the risk of developing distant metastasis from many different perspectives, providing a thorough picture of the topic by analysing the proportion of first distant metastasis in different sites, the relative (HR) and absolute risk (cumulative risk) of developing metastasis at different follow-up times according to different patient and tumour characteristics by taking into account competing risks, and allowing the main effects to vary over time.
This paper has some limitations as well. The date of diagnosis of distant metastasis might be subject to timing of clinical work-ups and type of follow-up. In addition, site of first distant metastasis could be affected by detection bias. As in all studies requiring a long follow-up, the estimated cumulative risk of first distant metastasis might not reflect current risk as it was observed in women diagnosed between 1995 and 1999. In particular, adjuvant treatment has changed and we do not know whether the same risk patterns are observed in recently diagnosed patients: for instance, aromatase inhibitors have been widely used instead of tamoxifen from early 2000s, and high risk patients with ER-positive tumours are today offered extended endocrine therapy up to 10 years (Burstein et al, 2010).
In conclusion, there is still a clinically relevant risk of developing first distant metastasis from 5 to 10 years after breast cancer diagnosis in several groups of patients, especially those with positive lymph nodes at diagnosis. Patients with negative lymph nodes and ER-positive tumours, unlike those with ER-negative tumours, have a very similar low risk of developing first distant metastasis in the first 5 years and in the second 5 years of follow-up, independent of age, other tumour characteristics and competing risks of dying due to other causes. Upcoming improvements in metastasis prevention and treatment should elicit further research aimed at identifying specific clinical follow-ups for different subgroups of breast cancer patients. Five-year metastasis-free survival may not any longer be an appropriate outcome indicator measurement tool for breast cancer patients, particularly for those with ER-positive tumours.
Acknowledgments
We thank the Stockholm Breast Cancer Registry and the Stockholm Breast Cancer Group for the data made available and for the help in the data cleaning and study design phase. This research was funded by the King Gustaf V Jubilee Fund, Swedish Research Council (grant no: 521-2008-2728); Swedish Cancer Society (grant no: CAN 2010/807); and the Swedish Cancer Society (grant no: 5128-B07-01PAF to KC). This study was supported by the Cancer Risk Prediction Center (CRisP; www.crispcenter.org), a Linneus Centre (Contract ID 70867902) financed by the Swedish Research Council. We also thank Dr Paul Lambert from the University of Leicester (UK) for the methodological support in the analysis.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Alford SH, Toy K, Merajver SD, Kleer CG. Increased risk for distant metastasis in patients with familial early-stage breast cancer and high EZH2 expression. Breast Cancer Res Treat. 2012;132 (2:429–437. doi: 10.1007/s10549-011-1591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow L, Westergren K, Holmberg L, Talback M. The completeness of the Swedish Cancer Register: a sample survey for year 1998. Acta Oncol. 2009;48 (1:27–33. doi: 10.1080/02841860802247664. [DOI] [PubMed] [Google Scholar]
- Biganzoli E, Boracchi P, Coradini D, Grazia Daidone M, Marubini E. Prognosis in node-negative primary breast cancer: a neural network analysis of risk profiles using routinely assessed factors. Ann Oncol. 2003;14 (10:1484–1493. doi: 10.1093/annonc/mdg422. [DOI] [PubMed] [Google Scholar]
- Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application. J Clin Oncol. 2005;23 (29:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ. ASCO clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28 (23:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366 (9503:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- Colzani E, Liljegren A, Johansson AL, Adolfsson J, Hellborg H, Hall PF, Czene K. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29 (30:4014–4021. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Time to disease recurrence in basal-type breast cancers: effects of tumor size and lymph node status. Cancer. 2009;115 (21:4917–4923. doi: 10.1002/cncr.24573. [DOI] [PubMed] [Google Scholar]
- Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115 (2:423–428. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13 (15 Pt 1:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9 (1:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early Breast Cancer Trialists' Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365 (9472:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- Foukakis T, Fornander T, Lekberg T, Hellborg H, Adolfsson J, Bergh J. Age-specific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res Treat. 2011;130 (2:553–560. doi: 10.1007/s10549-011-1594-z. [DOI] [PubMed] [Google Scholar]
- Frisk G, Svensson T, Backlund LM, Lidbrink E, Blomqvist P, Smedby KE. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer. 2012;106 (11:1850–1853. doi: 10.1038/bjc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grann VR, Troxel AB, Zojvalla NJ, Jacobson JS, Hershman D, Neugut AI. Hormone receptor status and survival in a population-based cohort of patients with breast carcinoma. Cancer. 2005;103 (11:2241–2251. doi: 10.1002/cncr.21030. [DOI] [PubMed] [Google Scholar]
- Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. Breast Cancer Res Treat. 1998;52 (1-3:227–237. doi: 10.1023/a:1006133418245. [DOI] [PubMed] [Google Scholar]
- Hinchliffe SR, Lambert PC. Extending the flexible parametric survival model for competing risks. Stata J. 2012;12:674–687. [Google Scholar]
- Hsieh SM, Look MP, Sieuwerts AM, Foekens JA, Hunter KW. Distinct inherited metastasis susceptibility exists for different breast cancer subtypes: a prognosis study. Breast Cancer Res. 2009;11 (5:R75. doi: 10.1186/bcr2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AO, Jacobsen JB, Norgaard M, Yong M, Fryzek JP, Sorensen HT. Incidence of bone metastases and skeletal-related events in breast cancer patients: a population-based cohort study in Denmark. BMC Cancer. 2011;11:29. doi: 10.1186/1471-2407-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, Nielsen TO, Gelmon K. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28 (20:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, Hantel A, Henry NL, Muss HB, Smith TJ, Vogel VG, Wolff AC, Somerfield MR, Davidson NE. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2013;31 (7:961–965. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- Lambert PC, Royston P. Further developments of flexible parametric models for survival analysis. Stata J. 2009;9:265–290. [Google Scholar]
- Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, Courdi A, Hannoun-Levi JM, Ettore F, Birtwisle-Peyrottes I, Balu-Maestro C, Marcy PY, Raoust I, Lallement M, Chamorey E. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19 (12:2012–2019. doi: 10.1093/annonc/mdn424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MT, Huang WT, Wang AY, Huang CC, Huang CY, Chang TH, Pi CP, Yang HH. Prediction of outcome of patients with metastatic breast cancer: evaluation with prognostic factors and Nottingham prognostic index. Support Care Cancer. 2010;18 (12:1553–1564. doi: 10.1007/s00520-009-0778-0. [DOI] [PubMed] [Google Scholar]
- Louwman WJ, Voogd AC, van Dijck JA, Nieuwenhuijzen GA, Ribot J, Pruijt JF, Coebergh JW. On the rising trends of incidence and prognosis for breast cancer patients diagnosed 1975-2004: a long-term population-based study in southeastern Netherlands. Cancer Causes Control. 2008;19 (1:97–106. doi: 10.1007/s10552-007-9075-8. [DOI] [PubMed] [Google Scholar]
- Minn AJ, Kang Y, Serganova I, Gupta GP, Giri DD, Doubrovin M, Ponomarev V, Gerald WL, Blasberg R, Massague J. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J Clin Invest. 2005;115 (1:44–55. doi: 10.1172/JCI22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson C, Arcot Ragupathy SK, Boachie C, Dixon JM, Fraser C, Hernandez R, Heys S, Jack W, Kerr GR, Lawrence G, MacLennan G, Maxwell A, McGregor J, Mowatt G, Pinder S, Ternent L, Thomas RE, Vale L, Wilson R, Zhu S, Gilbert FJ. The clinical effectiveness and cost-effectiveness of different surveillance mammography regimens after the treatment for primary breast cancer: systematic reviews registry database analyses and economic evaluation. Health Technol Assess. 2011;15 (34:1–322. doi: 10.3310/hta15340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- Rutqvist LE. Validity of certified causes of death in breast carcinoma patients. Acta Radiol Oncol. 1985;24 (5:385–390. doi: 10.3109/02841868509134405. [DOI] [PubMed] [Google Scholar]
- Schneider M, Zuckerman IH, Onukwugha E, Pandya N, Seal B, Gardner J, Mullins CD. Chemotherapy treatment and survival in older women with estrogen receptor-negative metastatic breast cancer: a population-based analysis. J Am Geriatr Soc. 2011;59 (4:637–646. doi: 10.1111/j.1532-5415.2011.03351.x. [DOI] [PubMed] [Google Scholar]
- van de Vijver M, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernard R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347 (25:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8 (9:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]