Abstract
Nutrient over-enrichment is one of the classic triggering mechanisms for the occurrence of cyanobacteria blooms in aquatic ecosystems. In the Baltic Sea, cyanobacteria regularly occur in the late summer months and form nuisance accumulations in surface waters and their abundance has intensified significantly in the past 50 years attributed to human-induced eutrophication. However, the natural occurrence of cyanobacteria during the Holocene is debated. In this study, we present records of cyanobacteria pigments, water column redox proxies, and nitrogen isotopic signatures for the past ca. 8000 years from Baltic Sea sediment cores. Our results demonstrate that cyanobacteria abundance and nitrogen fixation are correlated with hypoxia occurring during three main intervals: (1) ca. 7000–4000 B.P. during the Littorina transgression, (2) ca. 1400–700 B.P. during the Medieval Climate Anomaly, and (3) from ca. 1950 A.D. to the present. Issues of preservation were investigated, and we show that organic matter and pigment profiles are not simply an artifact of preservation. These results suggest that cyanobacteria abundance is sustained during periods of hypoxia, most likely because of enhanced recycling of phosphorus in low oxygen conditions.
Introduction
The Baltic Sea, one of the largest brackish water bodies in the world, is vulnerable to hypoxia (dissolved oxygen < 2 mg/L) because of the limited bottom-water inflow of oxygenated waters from the adjacent Kattegat. Widespread hypoxia in the stratified water column1,2 maintains a ready resupply of phosphorus from the sediments.3,4 Together with enhanced denitrification, this leads to low surface water nitrogen/phosphorus (N/P) ratios following the spring bloom, favoring diazotrophic cyanobacteria blooms in the summer months. Unlike most phytoplankton, which require both high N and P conditions, diazotrophic cyanobacteria only require high P concentrations because they are “N-fixing” and can produce their own ammonia as a nutrient source from atmospheric N2 gas.5 Thus, diazotrophic cyanobacteria can flourish in the Baltic Sea during strongly N-limiting conditions and usually form blooms in the summer if the N/P ratio after the spring bloom is below the Redfield ratio of 16.1,6 In addition to low N/P ratios, the prevalence of cyanobacteria is also influenced by other environmental factors, such as light attenuation, water temperatures (which must be >15 °C for blooms to occur), and vertical mixing.7
Cyanobacteria have been shown to provide a positive feedback to eutrophication, by supplying new N to the system and enhancing the downward flux of degradable organic matter from surface waters, which elevates oxygen consumption and the regeneration of phosphate.2 Moreover, some species are toxic, and they are, therefore, problematic for recreation and fisheries.2 Because of these negative effects of cyanobacteria blooms, some scientists argue that efforts should be made to reduce their abundance and their contribution to the phytoplankton community.8 Others argue that cyanobacteria are a characteristic, natural feature of the Baltic Sea.9
Cyanobacteria have been shown to be present in the Baltic Sea since around 7000 years B.P.,9,10 but the triggers to past cyanobacteria blooms remain unclear. An improved understanding of the controls on cyanobacteria blooms will assist in developing solutions to reduce their occurrence in the future. In this study, we examine the presence of cyanobacteria pigments in sediment cores and compare these to proxies for past redox conditions to determine if there is a link between cyanobacteria and hypoxia in the Baltic Sea during the Holocene.
Materials and Methods
Sediment cores were taken with R/V Aranda in May/June 2009. Multi-cores (collecting the top 30 cm of the sediment) and gravity cores (collecting ∼4.5 m of sediment) were obtained at two sites in the Baltic Sea (Figure 1): LL19 in the Northern Gotland Basin (58.8807° N, 20.3108° E, and 169 m water depth) and F80 in the Fårö Deep (58.0000° N, 19.8968° E, and 191 m water depth). These sites were selected because we expected continuous accumulation of sediment in these deep basins over the Holocene. Multi-cores were sampled immediately in a nitrogen-filled glovebox. Gravity cores were cut into 1 m sections and stored in the dark at 4 °C. Gravity cores were subsampled in a nitrogen-filled glovebox in a dark lab for pigment analyses. Sample resolution varied between 1 and 5 cm, and the sample selection for each analysis varied slightly because of the availability of material. Subsamples were freeze-dried and homogenized with a mortar and pestle.
Figure 1.
Map of the Baltic Proper showing the principle sub-basins, water depth, and locations of the cores collected in the Gotland Basin for this study: LL19 in the Northern Gotland Basin (58.8807° N, 20.3108° E, and 169 m water depth) and F80 in the Fårö Deep (58.0000° N, 19.8968° E, and 191 m water depth). Multi-cores (surface of ∼40 cm) and gravity cores (∼450 cm) were collected at both sites. Bathymetric and coastline data are presented in Miller cylindrical projection, taken from the General Bathymetric Chart of the Ocean (GEBCO) Digital Atlas.43
Multi-core and gravity core data were combined on the basis of overlaps in the geochemical profiles. The age models for both sites were constructed using a combination of 210Pb dating for multi-cores and tuning of the gravity core Corg profiles to the loss on ignition (LOI) profile of core 372740-3 from the Gotland Deep.11 Core 372740-3 was independently dated by identification of two Pb pollution isochrones12 and 10 paleomagnetic secular variation features (see the Supporting Information for more details on the construction of the age models for LL19 and F80 and errors in absolute age estimates).
To determine the ratio of molybdenum/aluminum (Mo/Al, %/%), sediment samples were dissolved in HF (40%) and a HClO4/HNO3 mixture in a closed Teflon bomb at 90 °C for 12 h. The acids were evaporated at 190 °C. The resulting gel was redissolved in HNO3 and analyzed by inductively coupled plasma–optical emission spectroscopy (ICP–OES) for Mo and Al (precision and accuracy < 5%). For total percent carbon and δ15N measurements, samples were analyzed using a Carlo Erba NC2500 analyzer connected to a Finnigan MAT Delta V mass spectrometer. The reproducibility was better than 0.15‰ for δ15N and <1% for total percent carbon.
For pigment analysis, sediment samples were mixed with cold high-performance liquid chromatography (HPLC)-grade acetone/methanol/Milli-Q water (80:15:5%), sonicated, and stored in a freezer (−20 °C) overnight. Extracts were centrifuged and filtered (0.45 μm) and then were quantitatively analyzed by HPLC on a Shimadzu Prominence HPLC equipped with an online photodiode array detector (SPD-M20A PDA) and an autosampler (Sil-10AF). The run program was programed as described by Reuss and Conley.13
Coefficients of determination (R2) were calculated between the complete data series of δ15N and each of the other sedimentary proxies (total percent carbon, echinenone, zeaxanthin, and Mo/Al) (n = 194). Because the sample selection varied per analysis, data series were interpolated between age 15 and 7700 years B.P. and points were extracted at every 50 years (n = 154) for calculation of the coefficients of determination and p values. Data were analyzed using the R statistical program (R 2.15.2).
Results and Discussion
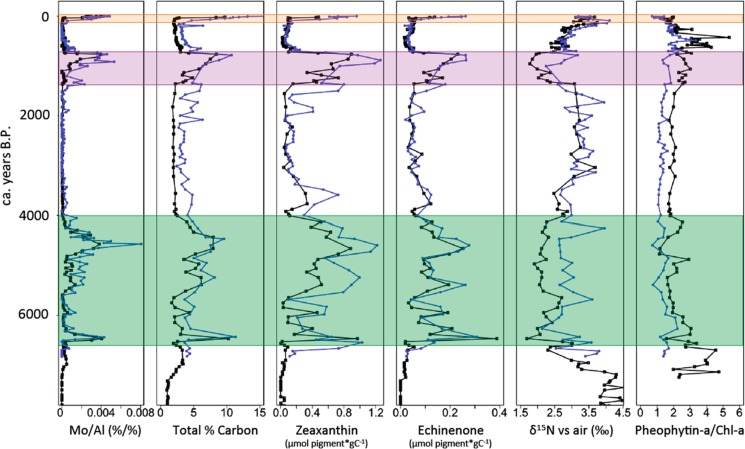
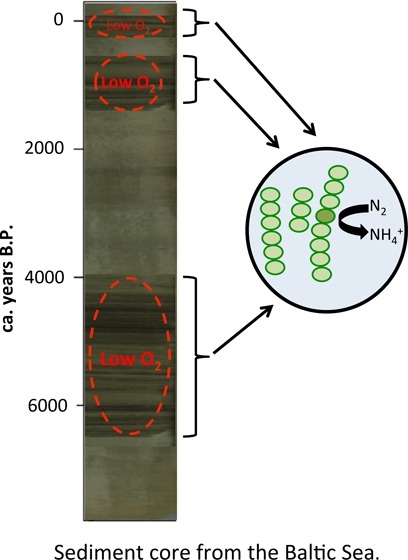
Laminated sediments, indicative of hypoxic conditions, were observed during three intervals of the Holocene sedimentary record at the two sites: the Littorina Transgression, the Medieval Climate Anomaly (MCA), and the modern hypoxic interval. These intervals were also characterized by enhanced sedimentary Mo/Al (Figure 2). This proxy tracks the intensity of reducing conditions close to the sediment–water interface,14 because of the conversion of seawater MoO42– to particle-reactive thiomolybdates above a critical activity of hydrogen sulfide.15 Hence, our records indicate intermittent euxinic (i.e., sulfidic) conditions in the bottom waters of the deep basins during the Holocene (Figure 2). The hypoxic intervals are also characterized by enhanced organic carbon (Corg) contents, which we attribute to both enhanced preservation of organic matter under reducing conditions and enhanced primary productivity during the hypoxic intervals. Enhanced primary productivity was likely sustained by sedimentary phosphorus release under anoxic conditions, as shown by numerous studies.3,15−17 The centennial-scale oscillations in hypoxia during the Littorina Transgression and MCA were recently suggested to be related to shifts in the North Atlantic Oscillation (NAO) and amplified by internal feedbacks in the phosphorus cycle of the Baltic Sea.16 That study also showed that the replacement time of the Baltic Sea is sufficiently short for Mo/Al to be unaffected by reservoir effects and that Mo/Al varies in concert with organic carbon to total phosphorus (Corg/Ptot) ratios, confirming the role of phosphorus regeneration in sustaining hypoxia.
Figure 2.
Proxy profiles as a function of time in years B.P. for Northern Gotland Deep (LL19, black line) and Fårö Deep (F80, blue line). From left to right: molybdenum/aluminum (%/%) (euxinia proxy), total percent carbon (productivity proxy), zeaxanthin and echinenone (micromoles of pigment per gram of sediment normalized to total percent carbon) (cyanobacteria biomarkers), δ15N versus air (‰) (indicator of N fixation), and pheophytin a/chlorophyll a (mole ratio) (degradation proxy). The colored bars denote three intervals of hypoxia as determined by the occurrence of extensive laminated sediments: green, Littorina Transgression (7000–4000 years B.P.); purple, Medieval Climate Anomaly (1400–700 years B.P.); and orange, modern hypoxic period (∼60 years B.P. at F80 and ∼30 years B.P at LL19; present = A.D. 2010).
During the hypoxic intervals, we observe higher carbon-normalized concentrations of cyanobacteria pigments in the sediments (zeaxanthin and echinenone; Figure 2). The pigment concentrations also vary in concert with the centennial-scale oscillations in Mo/Al. Furthermore, δ15N signatures are inversely related to total carbon, pigment concentrations, and Mo/Al, indicating enhanced N fixation during hypoxic intervals.17 In combination, these results suggest that N-fixing cyanobacteria were more prevalent during intervals of hypoxic conditions in the Baltic Sea and less so during the intervening oxic intervals (see Table 1 for statistics).
Table 1. Correlation Statistics between the Biomarkers: Mo/Al, Total Percent Carbon, Zeaxanthin, Echinenone, and δ15N (n = 154)a.
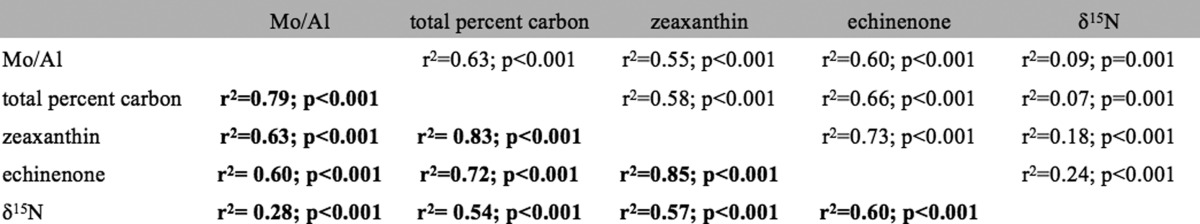
Significant inverse relationships (p < 0.001) between δ15N and other biomarkers were calculated for both sites: Northern Gotland Deep (LL19) (in bold) and Fårö Deep (F80) (not in bold).
Organic matter is often better preserved in anoxic sediments because of the comparatively slow rate of microbial degradation in the absence of oxygen.18 To examine if our cyanobacteria abundance trends are influenced by preservation artifacts, we calculated the molar ratio of pheophytin a/chlorophyll a (pheophytin a/chl a).19 Pheophytin a is a degradation product of chlorophyll a; hence, low values of this ratio indicate good preservation of the initial material, and high values indicate poor preservation of the initial material. As shown in Figure 2, pheophytin a/chl a molar ratios show no systematic relationship (F80, r2 = 0.026 and p value = 0.031; LL19, r2 = 0.026 and p value = 0.029) with redox conditions, as represented by Mo/Al. These observations suggest that changes in the pigment concentration in the sediments are dominantly controlled by changes in the initial flux of the pigments to the sediments, i.e., by cyanobacteria abundance in the surface waters, and less by preferential preservation effects after sedimentation.
Three prominent intervals of frequent hypoxia and high cyanobacteria abundance occurred in the past ca. 7500 years. The first and longest of these, the Littorina Transgression (ca. 7000–4000 B.P.), followed the seawater intrusion through the Danish straits, which transformed the freshwater Ancylus Lake to the brackish Littorina Sea.20 This intrusion of seawater increased the stratification of the water column of the Baltic Sea and has been hypothesized to be the primary cause of deep-water hypoxia during this interval.21 It is likely that widespread hypoxia and euxinia in bottom waters stimulated sediment-bound P to be released into the water column,9,22,23 and the low N/P conditions created an ideal environment for diazotrophic cyanobacteria to thrive.9 The highest ratio between zeaxanthin and β-carotene (unpublished data), which is an indicator of the proportion of cyanobacteria in the phytoplankton community, was observed following seawater intrusion, as seen in previous studies (e.g., see refs (4 and 9)). Additionally, this period coincides with the Holocene Thermal Maximum (HTM) when warmer surface waters may have favored cyanobacteria blooms. Around ca. 4000 B.P., the Littorina Sea stabilized and salinity decreased because of the reduction in the depth of the Danish straits, increasing vertical mixing and replenishing oxygen to the deep basins.24 This shift reduced surface water phosphate concentrations and increased the N/P ratio, making conditions less favorable to diazotrophic cyanobacteria after ca. 4000 B.P.8
Hypoxia and high productivity was again observed ca. 1400–700 B.P. during the MCA (Figure 2). Several important factors may have contributed to hypoxia during this interval. First, northern Europe experienced milder winters because of a persistently positive phase of the NAO climate mode.25 This increase in winter temperatures may have been sufficient to increase thermal stratification, hence decreasing bottom-water oxygen concentrations and stimulating the release of P from the sediments. As during the HTM, the warmer temperature of the MCA may also have favored cyanobacteria.26 Second, the population for many of the countries in the Baltic Sea watershed (i.e., Denmark, Germany, Poland, and Sweden) nearly doubled within 300 years,27 leading to a change in land use28 and increased terrestrial nutrient runoff.29 The consequent spread of hypoxia in the Baltic Sea re-established the conditions required for diazotrophic cyanobacteria to thrive.
During the Little Ice Age (LIA), which followed the MCA, the NAO shifted to a more persistently negative phase.25 This may have led to an increase in storm frequency30 and cooler sea surface temperatures in the Baltic Sea.26,31 In addition, the population decreased during the 14th century when the bubonic plague (Black Death) and famine hit Europe. For example, the Swedish population is estimated to have decreased approximately by one-third during this time,32 leading to a 30–50% farm abandonment in some parts of Sweden.33 This could potentially have caused a decrease in nutrient runoff into the Baltic Sea. In combination, these conditions were less favorable for hypoxia and cyanobacteria blooms.
The onset of modern hypoxic conditions in the late 20th century at both sites is directly linked to excess nutrient loading from agricultural activities and urban development in the past century.34,35 From 1850 to 1980, N and P loads in the Baltic Sea increased on average 4.5-fold.36 In the spring, algal blooms thrive in these highly nutrient-enriched waters. Sedimentation of the spring bloom, in addition to organic matter runoff, increases microbial respiration, resulting in hypoxia and creating an ideal environment for cyanobacteria blooms to form in the summer months.37 Current climate change likely intensifies hypoxia26 because of the reduction in vertical mixing of the water column, therefore favoring cyanobacteria blooms.38
Perspectives
By reconstructing long-term trends, we conclude that multiple stressors, including climate variability, stratification, and anthropogenic activity, have influenced the occurrence of hypoxia in the Baltic Sea at different times during the Holocene. However, each hypoxic interval has been characterized by abundant cyanobacteria blooms, implying a close coupling between the two phenomena. Because of the limitations of sampling resolution, it remains difficult to determine the exact sequence of events at the onset of each hypoxic interval, i.e., the potential lead lag between hypoxia and cyanobacteria blooms. Theoretically, an external input of P could trigger cyanobacteria blooms by lowering surface water N/P ratios, leading to increased oxygen demand and hypoxia. Alternatively, hypoxia could be triggered by a change in stratification, leading to sedimentary P release and favoring cyanobacteria blooms. However, it is clear that once hypoxia is established, efficient phosphorus regeneration from sediments and cyanobacteria are closely coupled,39,40 sustaining a “vicious” circle of eutrophication.2
Recent observations and models suggest that hypoxic and suboxic regions of many marine and freshwater systems are likely to expand and become shallower with warmer temperatures.41 Hence, lakes,38 marine waters above oxygen minimum zones,42 and the Baltic Sea as reported here may all experience expanded hypoxia in the future. Coupled to the stress of anthropogenic nutrient loading and climate change, this global expansion of hypoxia may be expected to drive an increase in the global prevalence of cyanobacteria blooms.
Acknowledgments
The authors thank the captain, crew, and scientific party of the R/V Aranda cruise in 2009 for their assistance with the fieldwork. The authors also thank H. Siegmund for helping us use the mass spectrometer. This research was funded by a FORMAS Strong Research Environment (Multistressors), the K. & A. Wallenberg Foundation, the Netherlands Organization for Scientific Research (NWO Vidi), the European Union (EU) BONUS Project HYPER, the European Research Council (ERC) under the European Community’s Seventh Framework Programme for ERC Starting Grant 278364, and the Danish Council for Independent Research, Natural Sciences.
Supporting Information Available
Information on how the age models were constructed for the cores on the basis of two lead (Pb) isochrones identified from neighboring cores. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Supplementary Material
References
- Stal L. J.; Albertano P.; Bergman B.; von Brockel K.; Gallon J. R.; Hayes P. K.; Sivonen K.; Walsby A. E. BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea—Responses to a changing environment. Cont. Shelf Res. 2003, 2317–191695–1714. [Google Scholar]
- Vahtera E.; Conley D. J.; Gustafsson B. G.; Kuosa H.; Pitkanen H.; Savchuk O. P.; Tamminen T.; Viitasalo M.; Voss M.; Wasmund N.; Wulff F. Internal ecosystem feedbacks enhance nitrogen-fixing cyanobacteria blooms and complicate management in the Baltic Sea. Ambio 2007, 362–3186–194. [DOI] [PubMed] [Google Scholar]
- Jilbert T.; Slomp C. P.; Gustafsson B. G.; Boer W. Beyond the Fe–P-redox connection: Preferential regeneration of phosphorus from organic matter as a key control on Baltic Sea nutrient cycles. Biogeosciences 2011, 861699–1720. [Google Scholar]
- Westman P.; Borgendahl J.; Bianchi T. S.; Chen N. Probable causes of cyanobacteria expansion in the Baltic Sea: Role of anoxia and phosphorus retention. Estuaries 2003, 26, 680–689. [Google Scholar]
- Graneli E.; Wallstrom K.; Larsson U.; Graneli W.; Elmgren R. Nutrient limitation of primary production in the Baltic Sea area. Ambio 1990, 193142–151. [Google Scholar]
- Niemi Å. Blue-green algal blooms and N/P ratio in the Baltic Sea. Acta Bot. Fenn. 1979, 110, 57–61. [Google Scholar]
- Paerl H.Nutrient and other enviornmental controls of harmful cyanobacterial blooms laong the freshwater-marine continuum. In Cyanobacterial Harmful Algal Blooms: State of the Sceince and Research Needs; Hudnell H., Ed.; Springer: Berlin, Germany, 2008; Vol. 619, pp 217–237. [DOI] [PubMed] [Google Scholar]
- Zillen L.; Conley D. J. Hypoxia and cyanobacteria blooms—Are they really natural features of the late Holocene history of the Baltic Sea?. Biogeosciences 2010, 782567–2580. [Google Scholar]
- Bianchi T. S.; Engelhaupt E.; Westman P.; Andren T.; Rolff C.; Elmgren R. Cyanobacterial blooms in the Baltic Sea: Natural or human-induced?. Limnol. Oceanogr. 2000, 453716–726. [Google Scholar]
- Poutanen E. L.; Nikkila K. Carotenoid pigments as tracers of cyanobacterial blooms in recent and post-glacial sediments of the Baltic Sea. Ambio 2001, 304–5179–183. [PubMed] [Google Scholar]
- Lougheed B. C.; Snowball I.; M. M.; Kabel K.; Muscheler R.; Virtasalo J. J.; Wacker L. Using an independent geochronology based on palaeomagnetic secular variation (PSV) and atmospheric Pb deposition to date Baltic Sea sediments and infer 14C reservoir age. Quat. Sci. Rev. 2012, 42, 43–58. [Google Scholar]
- Zillen L.; Lenz C.; Jilbert T. Stable lead (Pb) isotopes and concentrations—A useful independent dating tool for Baltic Sea sediments. Quat. Geochronol. 2012, 8, 41–45. [Google Scholar]
- Reuss N.; Conley D. J. Effects of sediment storage conditions on pigment analysis. Limnol. Oceanogr. 2005, 3, 477–487. [Google Scholar]
- Alelson J. M.; Helz G. R.; Miller C. V. Reconstucting the rise of recent coastal anoxia; molybdenum in Chesapeake Bay sediments. Geochim. Cosmochim. Acta 2001, 65, 237–252. [Google Scholar]
- Helz G. R.; Miller C. V.; Charnock J. M.; Mosselmans J. F. W.; Pattrick A. D.; Garner C. D.; Vaughan D. J. Mechanism of molybdenum removal from the sea and its concentration in black shales: EXAFS evidence. Geochim. Cosmochim. Acta 1996, 60193631–3642. [Google Scholar]
- Jilbert T.; Slomp C. P. Rapid high amplitude variability in Baltic Sea hypoxia during the Holocene. Geology 2013, 10.1130/G34804.1. [DOI] [Google Scholar]
- Michener R.; Schell D., Stable isotopes ratio as tracers in marine aquatic foodwebs. In Stable Isotopes in Ecology and Enviornmental Science; Lajtha K., Michener R. H., Eds.; Blackwell Scientific: Oxford, U.K., 1994; pp 138–186. [Google Scholar]
- Bianchi T. S.; Dibb J. E.; Findlay S. Early diagenesis of plant pigments in Hudson River sediments. Estuarine, Coastal Shelf Sci. 1993, 366517–527. [Google Scholar]
- Reuss N.; Conley D. J.; Bianchi T. S. Preservation conditions and the use of sediment pigments as a tool for recent ecological reconstruction in four Northern European estuaries. Mar. Chem. 2005, 953–4283–302. [Google Scholar]
- Bjorck S. A review of the history of the Baltic Sea, 13.0–8.0 ka BP. Quat. Int. 1995, 27, 19–40. [Google Scholar]
- Zillen L.; Conley D. J.; Andren T.; Andren E.; Bjorck S. Past occurrences of hypoxia in the Baltic Sea and the role of climate variability, environmental change and human impact. Earth-Sci. Rev. 2008, 911–477–92. [Google Scholar]
- Conley D. J.; Humborg C.; Rahm L.; Savchuk O. P.; Wulff F. Hypoxia in the Baltic Sea and basin-scale changes in phosphorus biogeochemistry. Environ. Sci. Technol. 2002, 36245315–5320. [DOI] [PubMed] [Google Scholar]
- Wulff F.; Rahm L.; Larsson P.. A Systems Analysis of the Baltic Sea; Springer: Berlin, Germany, 2001; Vol. 148. [Google Scholar]
- Gustafsson B. G.; Westman P. On the causes for salinity variations in the Baltic Sea during the last 8500 years. Paleoceanography 2002, 17312-1–12-14. [Google Scholar]
- Trouet V.; Esper J.; Graham N. E.; Baker A.; Scourse J. D.; Frank D. C. Persistent positive North Atlantic Oscillation mode dominated the Medieval Climate Anomaly. Science 2009, 324592378–80. [DOI] [PubMed] [Google Scholar]
- Kabel K.; Moros M.; Porsche C.; Neumann T.; Adolphi F.; Andersen T. J.; Siegel H.; Gerth M.; Leipe T.; Jansen E.; Sinninghe Damste J. S. Impact of climate change on the Baltic Sea ecosystem over the past 1,000 years. Nat. Clim. Change 2012, 212871–874. [Google Scholar]
- McEvedy C.; Jones R.. Atlas or World Population History; Penguin Books: London, U.K., 1978; pp 19–119. [Google Scholar]
- Mydral J.The agricultural transformation of Sweden, 1000–1300. In Medieval Farming and Technology. The Impact of Agricultural Change in Northwest Europe; Astil G., Langdon J., Eds. Brill: Leiden, Netherlands, 1997; pp 147–171. [Google Scholar]
- Bradshaw E. G.; Rasmussen P.; Nielsen H.; Andersen N. J. Mid- to Late-Holocene land change and lake development at Dallund Sø, Denmark: Trend in lake primary production as reflected by algal and macrophyte remains. Holocene 2005, 15, 1130–1142. [Google Scholar]
- Andersson Palm L.Livet, Kärleken och Döden (Live, Love and Death); Historiska Institutionen: Gothenburg, Sweden, 2011; p 203. [Google Scholar]
- Clarke M. L.; Rendell H. M. The impact of North Atlantic storminess on western European coasts: A review. Quat. Int. 2009, 1951–231–41. [Google Scholar]
- Skog L.; Hauska H. Spatial modeling of the Black Death in Sweden. Trans. GIS 2013, 174589–611. [Google Scholar]
- Antonson H. The extent of farm desertion in central Sweden during the late medieval agrarian crisis: Landscape as a source. J. Hist. Geogr. 2009, 354619–641. [Google Scholar]
- Eriksson H.; Pastuszak M.; Lofgren S.; Morth C. M.; Humborg C. Nitrogen budgets of the Polish agriculture 1960–2000: Implications for riverine nitrogen loads to the Baltic Sea from transitional countries. Biogeochemistry 2007, 852153–168. [Google Scholar]
- Gren I. M.; Soderqvist T.; Wulff F. Nutrient reductions to the Baltic Sea: Ecology, costs and benefits. J. Environ. Manage. 1997, 512123–143. [Google Scholar]
- Gustafsson B. G.; Schenk F.; Blenckner T.; Eilola K.; Markus Meier H. E.; Müller-Karulis B.; Neumann T.; Ruoho-Airola T.; Savchuk O. P.; Zorita E. Reconstructing the development of Baltic Sea eutrophication 1850–2006. Ambio 2012, 41, 534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. L. M.; Holland D. P.; Longmore A. R. Effect of a flood event on the dynamics of phytoplankton and biogeochemistry in a large temperate Australian lagoon. Limnol. Oceanogr. 2010, 5531123–1133. [Google Scholar]
- Paerl H. W.; Huisman J. Climate—Blooms like it hot. Science 2008, 320587257–58. [DOI] [PubMed] [Google Scholar]
- Conley D. J.; Carstensen J.; Vaquer-Sunyer R.; Duarte C. M. Ecosystem thresholds with hypoxia. Hydrobiologia 2009, 629, 21–29. [Google Scholar]
- Kemp W. M.; Testa J.; Conley D. J.; Gilbert D.; Hagy J. D. Temporal responses of coastal hypoxia to nutrient loading and physical controls. Biogeosciences 2009, 6, 2985–3008. [Google Scholar]
- Stramma L.; Johnson G. C.; Sprintall V.; Mohrholz V. Expanding oxygen-minimum zones in the tropical oceans. Science 2008, 320, 655–658. [DOI] [PubMed] [Google Scholar]
- Uloa O.; Canfield D. E.; DeLong E. F.; Letelierd R. M.; Stewart F. J. Microbial oceanography of anoxic oxygen minimum zones. Proc. Natl. Acad. Sci. U. S. A. 2012, 1094015996–16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British Oceanographic Data Centre. Centenary Edition of the GEBCO Digital Atlas, published on CD-ROM on behalf of the Intergovernmental Oceanographic Commission and the International Hydrographic Organization as part of the General Bathymetric Chart of the Oceans; British Oceanographic Data Centre: Liverpool, U.K., 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.