Abstract
Interleukin 2 (IL-2) and Interleukin 15 (IL-15) are common γ-chain family cytokines involved in regulation of T cell differentiation and homeostasis. Despite signaling through the same receptors, IL-2 and IL-15 have non-redundant roles in T cell biology, both physiologically and at the cellular level. The mechanisms by which IL-2 and IL-15 trigger distinct phenotypes in T cells remain elusive. To elucidate these mechanisms, we performed a quantitative comparison of the phosphotyrosine signaling network and resulting phenotypes triggered by IL-2 and IL-15. This study revealed that the signaling networks activated by IL-2 or IL-15 are highly similar and that T cell proliferation and metabolism are controlled in a quantitatively distinct manner through IL-2/15 receptor signal strength independent of the cytokine identity. Distinct phenotypes associated with IL-2 or IL-15 stimulation therefore arise through differential regulation of IL-2/15R signal strength and duration due to differences in cytokine-receptor binding affinity, receptor expression levels, physiological cytokine levels, and cytokine-receptor intracellular trafficking kinetics. These results provide important insights into the function of other shared cytokine and growth factor receptors, quantitative regulation of cell proliferation and metabolism through signal transduction, and improved design of cytokine based clinical immunomodulatory therapies for cancer and infectious diseases.
Introduction
Interleukin-2 (IL-2) and Interleukin-15 (IL-15) are critically involved in the regulation of peripheral T lymphocyte homeostasis and differentiation. IL-2 and IL-15 were among the first cytokines shown to trigger proliferation of activated T cells in vitro and in vivo.1,2 Their ability to expand T cell numbers upon exogenous stimulation has made both cytokines extremely important in clinical settings as immunomodulatory and cancer immunotherapeutic agents.3–5 Both cytokines mediate their effects on T cells through a heterotrimeric receptor complex consisting of a cytokine specific α-chain (IL-2Rα or IL-15Rα), the common γ-chain (γc), and the IL-2/15 receptor β-chain (β).6–9 The α-chains function primarily as high affinity ligand capture receptors while signal transduction occurs exclusively through the β and γc chains, which are constitutively associated with the Janus kinases (JAK).10 As a consequence of sharing the β and the γc chains, stimulation of T cells with IL-2 and IL-15 results in the activation of similar signaling pathways which include the JAK-STAT, the Ras-Raf-MAPK, and the PI3K-Akt pathways.4,10,11 Additionally, stimulation of T cells with IL-2 or IL-15 has been shown to result in the induction of similar gene expression profiles.8,12,13 Despite signaling through the same receptors and sharing common effector pathways, multiple studies have reported unique and even antagonistic roles for IL-2 and IL-15 in the T cell immune response. IL-2 is critical for the clonal expansion of activated T cells, differentiation of effector and memory cytotoxic T lymphocytes (CTLs), and regulation of T cell peripheral tolerance.4,14,15 In contrast; IL-15 is critically involved in the maintenance and survival of memory CD8+ T cells, naïve CD8+ T cells, and natural killer cells. IL-2 and IL-15 stimulation of T cells can result in distinct phenotypic responses even at the cellular level.4,16,17 Antigen activated mouse CD8+ T cells cultured with IL-2 are metabolically more active and larger in size than cells cultured with IL-15 despite proliferating equivalently.18 Additionally, a transient pulse of IL-15, but not IL-2, triggers T cell proliferation in an in vitro assay.19,20
Multiple factors may contribute to functional differences triggered by IL-2 and IL-15 stimulation of T cells. IL-2 and IL-15 differ in their mode of presentation to T cells. IL-2 directly binds IL-2Rα chains expressed on T cells, whereas IL-15/IL-15Rα complexes on non-T cells are presented in trans to IL-2/15βγc complexes expressed on T cells in addition to directly binding IL-15Rα chains expressed on T cells.4,19,21 Binding affinity of cytokines for their respective α-chains may also play an important role in differentiating the response to IL-2 and IL-15, as the binding affinity of IL-15 for IL-15Rα chain is approximately 1000-fold higher compared to the affinity of IL-2 for IL-2Rα.19,20 In support of this, IL-2 mutants engineered with significantly higher binding affinity for IL-2Rα trigger equivalent proliferation compared to IL-15 upon pulse stimulation of T cells.20 Signaling kinetics have also been implicated in differential regulation of T cell phenotype, as differences in cell size and metabolic activity between antigen-activated mouse CD8+ T cells cultured with IL-2 and IL-15 were associated with different kinetics of PI3K/PDK1 signaling triggered by the two cytokines.18 Although these studies have unveiled myriad possibilities for the distinct phenotypes resulting from stimulation with these two cytokines, the molecular mechanisms leading to differential regulation of T cell proliferation and metabolism through IL-2 and IL-15 remain incompletely characterized.
To elucidate the molecular mechanisms underlying the distinct T cell phenotypes driven by IL-2 and IL-15, we compared phosphotyrosine signaling networks triggered by the two cytokines and determined that the signaling networks activated by IL-2 and IL-15 are virtually identical. Since the disparate phenotypic response was not encoded in the signaling network, we focused on the role of IL-2/15R signal strength and duration in regulating cell proliferation and metabolic activity in engineered and primary human T cells. Our results indicate that the strength of signal is directly proportional to cellular metabolic activity and increase in cell size, while cell proliferation requires a constant signal above a threshold. Intriguingly, phenotypic regulation is independent of cytokine identity when presentation and duration are held constant. These results provide key insights into the differential regulation of cell proliferation and metabolic activity through shared signaling receptors which ultimately informs improved cytokine based immunotherapies for the treatment of cancer, autoimmune disorders, and infectious disease.
Materials and Methods
Antibodies and Reagents
Recombinant human IL-2 and IL-15 were purchased from Peprotech (Rocky Hill, NJ). High affinity mutant IL-2 (mtIL-2) was a kind gift from K.D. Wittrup (MIT Koch Institute, Cambridge, MA). JAK Inhibitor I (JI) was purchased from EMD Millipore (Billerica, MA). Carboxyfluorescein succinimidyl ester (CFSE) and CellTrace Violet were purchased from Life Technologies (Grand Island, NY). Phycoerythrin conjugated anti-IL-2, anti-IL-15, and anti-IL-2Rβ, and Allophycocyanin conjugated anti-IL-2Rα and anti-IL-15Rα mAbs were purchased from R&D Systems (Minneapolis, MN). Alexa-fluor 647 conjugated anti-pSTAT5 (pY694) and anti-pS6 (pS235/pS236) antibodies were purchased from BD Biosciences (San Jose, CA). Human anti-CD3ε (clone UCHT1) and human anti-CD28 (clone 37407) mAbs were purchased from R&D Systems (Minneapolis, MN).
Cell Culture
F15R-Kit cell culture
F15R-Kit cells were a kind gift from the K.D. Wittrup (MIT, Cambridge, MA). F15R-Kit cells were maintained at 37 °C and 5% CO2 in RPMI 1640 supplemented with 10% FBS (heat inactivated), 2mM L-Glutamine, 1mM sodium pyruvate, 100U/ml penicillin-streptomycin, and 900μg/ml G418. Unless otherwise indicated, cells were cultured in 80pM IL-2 at a density of 2–3×105 cells/ml and passaged every 48h.
Primary human T cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) were isolated using ficoll-paque gradient centrifugation of unpurified human buffycoats (Research Blood Components, Boston, MA). CD4+ and CD8+ T cells were isolated from PBMCs using magnetic separation with EasySep CD4+ and CD8+ negative enrichment kits (STEMCELL Technologies) and maintained in RPMI 1640 supplemented with 10% FBS (heat inactivated), 100U/ml penicillin-streptomycin, 2mM L-Glutamine, and 1mM sodium pyruvate.
Mass spectrometry based phosphotyrosine profiling analysis
Lysate preparation for mass spectrometry based phosphotyrosine analysis
2 × 107 cytokine starved F15R-Kit cells were suspended in fresh culture medium at a density of 4 × 105 cell/ml and stimulated with 500pM IL-2 or 500pM IL-15 for 15min. Cells were washed twice with 50 ml phosphate buffered saline (PBS) and lysed in 8M urea supplemented with 1mM sodium orthovanadate. Cells cultured in medium alone were used as unstimulated controls. The lysates were incubated on ice for 20min then stored in −80°C.
Reduction, Alkylation and Tryptic Digestion
Proteins from cell lysates were quantified using the bicinchoninic assay (Pierce, Thermo Scientific, Rockford, IL), reduced using 10mM DL-Dithiothreitol (DTT) at 56°C for 45min, and alkylated using 50mM iodoacetamide at room temperature in the dark for 1 hour. Excess iodoacetamide was quenched with DTT to a final concentration of 25mM. Proteins were subsequently digested with trypsin (sequencing grade, Promega, Madison, WI), at an enzyme/substrate ratio of 1:100, at room temperature overnight in 100mM ammonium acetate (pH 8.9). Trypsin activity was quenched through addition of formic acid to a final concentration of 5%. Samples were desalted using a C18 cartridge (Waters, Milford, MA) and peptides were lyophilized and stored at −80°C.
iTRAQ labeling
Peptide labeling with iTRAQ 8plex (AB Sciex, Framingham, MA) was performed as previously described. Briefly, for each analysis, approximately 400μg (prior to desalting and processing) peptide for each condition was labeled with iTRAQ 8plex reagent. Lyophilized samples were dissolved in 30μl of 500mM triethylammonium bicarbonate, pH 8.5, and the iTRAQ reagent was dissolved in 70μl of isopropanol. The solution containing peptides and iTRAQ reagent was vortexed, incubated at room temperature for 2 hours and concentrated to 40μl. Samples labeled with eight different isotopic iTRAQ reagents were combined and concentrated. Peptides were then dissolved in 400μl of IP buffer (100mM Tris, 100mM NaCl, and 1% Nonidet P-40, pH 7.4) and the pH was adjusted to 7.4 prior to phosphotyrosine immunoprecipitation (IP). 3 biological replicates of F15R-Kit cells stimulated with 500pM IL-2 or IL-15 for 15 minutes, and two biological replicates of unstimulated cells (total 8 conditions) were labeled, combined and analyzed together.
Phosphotyrosine Enrichment
Protein G agarose (80μl, EMD Millipore, Billerica, MA) beads were incubated with 3 phosphotyrosine antibodies; 12μg PT66 (Sigma-aldrich, St. Louis, MO), 12μg pY100 (CST, Beverly, MA) and 12μg 4G10 (EMD Millipore, Billerica, MA) and 200μlof IP buffer (100mM Tris, 1% NP-40, pH 7.4) was added and the mixture incubated for 8 hours at 4°C with rotation. iTRAQ 8plex labeled peptides re-suspended in IP buffer were added to antibody-conjugated protein G agarose beads and incubated overnight at 4°C with rotation. Peptide conjugated protein G agarose beads were rinsed with 400μl of IP buffer and 4 × 400μl of rinse buffer (100mM Tris, pH 7.4), and peptides were eluted into 70μl of 100mM glycine (pH 2.0). Phosphotyrosine peptides were further enriched using an offline immobilized metal affinity chromatography (IMAC) column. Peptides retained on the IMAC column were loaded onto a C18 pre-column and were subsequently separated by reverse phase HPLC (Agilent) over a 150 minute gradient prior to nanoelectrospray into an Orbitrap Elite mass spectrometer (Thermo scientific) for phosphotyrosine analyses. To correct for slight variations in the amount of sample in each of the iTRAQ channels, the mean iTRAQ ratios for all proteins identified in each biological replicate analysis was used to normalize the data. The mass spectrometer was operated in data-dependent mode with a full scan MS spectrum followed by MS/MS (CID was set at 35% energy for sequence information and HCD at 75% energy for iTRAQ quantification for Orbitrap Elite) for the top 10 precursor ions in each cycle. Ion trap injection time was set to 100 ms and FTMS injection time was set to 1000 ms with a resolution of 60000 across m/z 400–2000. For IT and FT-MS/MS scans, fragmentation was carried out on ions above a threshold of 500 counts and an FTMS resolution of 7500.
Phosphotyrosine Data analysis
Raw mass spectral data files (.RAW files) were converted into .mgf file format using DTAsupercharge 1.31 (http://msquant.sourceforge.net/). All resulting MS/MS peak lists were searched against a NCBI UniProt 2009 database containing Homo sapiens protein sequences (37, 743 entries) using Mascot version: 2.1.03 (Matrix Science). Trypsin enzyme specificity was applied with a maximum of 1 missed cleavage. Mass tolerance for precursor ions was set to 10 ppm and fragment ion mass tolerance was 0.8 Da. MS/MS spectra searches incorporated fixed modifications of carbamidomethylation of cysteine and iTRAQ 8plex modification of lysines and peptide N-termini. Variable modifications were oxidized methionine, and phosphorylation of serine, threonine, and tyrosine residues. Phosphotyrosine peptides were initially filtered using an arbitrary mascot score cut-off of 25. Precursor ions were manually evaluated and peptides with contaminating peaks present within the isolation window (ions with intensity > 25% of the base peak ± 1.5 m/z around the selected precursor ion m/z) were discarded as they may contribute to the relative iTRAQ intensities. iTRAQ intensity values were extracted from HCD scans using an in house python script which converted iTRAQ intensities into .txt format. We further imported this into Excel, and iTRAQ values were isotope corrected based on the iTRAQ8plex correction matrix (AB Sciex). Phosphotyrosine peptide iTRAQ ratios were normalized based on the mean relative protein quantification ratios obtained from the total protein.
Western Blotting
F15R-Kit cells were lysed on ice with radio immunoprecipitation assay (RIPA) buffer (50mM Tris-HCl, 150mM NaCl, 1% NP-40, 0.5% Sodium deoxycholate, and 0.1% sodium dodecyl sulfate (SDS)) supplemented with phosphatase and protease inhibitor (Thermo-Fisher Scientific, Rockford, IL). The lysates were cleared through centrifugation at 10,000Xg for 10min at 4°C, and boiled with laemmli sample buffer at 95°C for 5min. Sample protein concentrations were determined using the bicinchoninic acid (BCA) protein assay (Thermo-Fisher Scientific, Rockford, IL). The samples were separated via polyacrylamide gel electrophoresis using 10% polyacrylamide gels and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were probed overnight at 4°C with primary antibody, then washed and stained with HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibodies. The blots were developed using SuperSignal West Pico Chemiluminescent Substrate kit (Thermo-Fisher Scientific, Rockford, IL). RIPA buffer and laemmli sample buffers were purchased from Boston BioProducts (Ashland, MA). Pre-cast 10% Tris-HCl polyacrylamide gels were purchased from BioRad (Hercules, CA). Anti-pSTAT5 (pY694), anti-STAT5, anti-pERK, anti-ERK, and anti-β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA).
Flow Cytometry Analysis
Cell surface staining was performed according to the manufacturer’s recommended protocol for the particular antibody used. Phycoerythrin conjugated antibodies against human IL-2R α, IL-15Rα, IL-2Rβ, and IL-2Rγ proteins were purchased from R&D systems (Minneapolis, MN). Alexa-647 conjugated antibody against human 40S ribosomal protein S6 phosphorylated at Ser235 and Ser236 were purchased from BD Biosciences (San Jose, CA). For intracellular staining, cells were washed and fixed in a 1:1 dilution of PBS and BD Cytofix Buffer for 15min at room temperature. Fixed cells were permeabilized on ice for 30min in BD Phosflow Perm Buffer III and washed and stained with fluorochrome conjugated antibody (5 × 105 cells in 50μl) at 4°C for 60min. Samples were analyzed using either BD Accuri C6 cytometer or BD LSRFortessa cell analyzer (BD Biosciences) and data were analyzed using the FlowJo software (TreeStar Inc., Ashland, OR). Flow cytometry events were acquired ungated and live lymphocyte gates were created based on their forward and side light scatter profiles using FlowJo.
Proliferation assays
Cell counting using Trypan Blue exclusion
F15R-Kit cells cytokine starved for 48h were resuspended in fresh media at a density of 2 × 105 cells/ml and then cultured in the presence or absence of cytokines. For the pulse bioassay, cytokine starved F15R-Kit cells were stimulated with cytokine for 30min, washed three times and resuspended in cytokine-free culture media, and maintained at 37 °C and 5% CO2. Viable cell numbers were determined through Trypan blue exclusion using the Vi-CELL Cell Viability Analyzer (Beckman Coulter).
CFSE and CellTrace Violet staining
Cells were labeled with CFSE or CellTrace Violet dyes according to the manufacturer’s protocols at a final concentration of 0.5–2.5μM and 5μM, respectively. CFSE and CellTrace Violet fluorescence levels were measured using the BD Accuri C6 cytometer and the BD LSRFortessa analyzer respectively.
Cell size and proliferation experiments
F15R-Kit Cells
CFSE labeled cytokine-starved F15-Kit cells were seeded at a density of 2 × 105 cells/ml and pretreated with the indicated doses of JAK inhibitor (JI) for 30min at 37 °C. The cells were then cultured with 500pM of IL-2 or IL-15 at 37 °C and 5% CO2 for 6 days.
Primary human CD4+ and CD8+ T cells
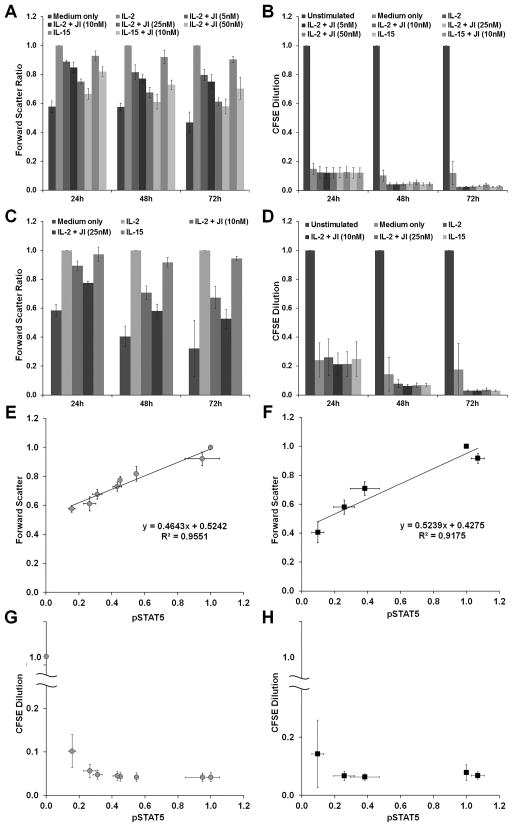
Purified CD4+ and CD8+ human primary T cells were labeled with CFSE (2.5μM) and added to 12-well tissue culture plates coated with 10μg/ml anti-CD3 mAb, supplemented with soluble anti-CD28 mAb (0.5μg/ml), and cultured at 37 °C and 5% CO2 for 72h. Cells cultured on uncoated plates in the absence of anti-CD28 served as unstimulated controls. Activated cells were washed, suspended in fresh medium, and cultured with the indicated dose of JI and 1nM IL-2, 1nM IL-15, or medium alone for 4 days.
Cells were passaged and cytokine and inhibitor levels were replenished every 48h. Forward scatter, CFSE dilution, and pSTAT5 levels were measured using the BD Accuri C6 cytometer at the indicated time-points after cytokine addition.
Glucose and Lactate measurements
F15R-Kit cells were cultured for 6 days with the indicated dose of JI and 500pM of IL-2 or IL-15. On day 6, cells were washed and seeded at a density of 2 × 105 cells/ml in fresh media, inhibitor, and cytokine. Culture media were collected 48h later and glucose and lactate levels were measured using the YSI 7100 Select Biochemistry Analyzer (YSI Incorporated, Yellow Springs, OH).
Statistical Analysis
Mean iTRAQ fold changes of individual phosphotyrosine sites generated through LC-MS/MS analysis were compared using unpaired student’s t-test. A p-value of less than 0.05 was considered statistically significant. For multiple comparison testing, one-way analysis of variance (ANOVA) was performed followed by unpaired student’s t-tests for comparison of individual populations. To account for the presence of type I error due to multiple hypotheses testing, p-values used for the determination of statistical significance were adjusted using the false discovery rate control method to correspond to a particular family-wise error rate (αfw). P-values less than the adjusted p-values corresponding to αfw of 0.05 were considered significant.
Results
Phosphotyrosine profiling of IL-2 or IL-15 stimulated T cells reveals the activation of highly similar signaling networks
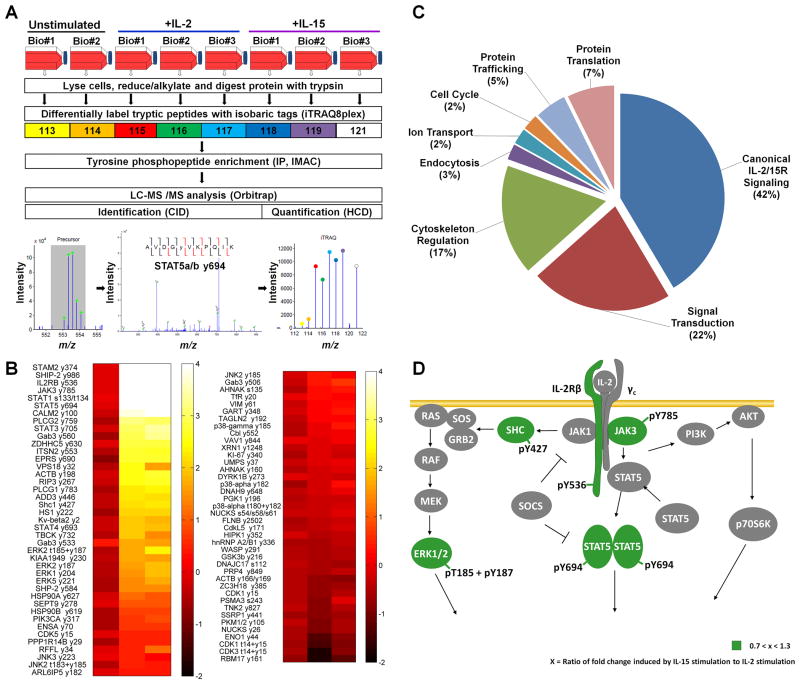
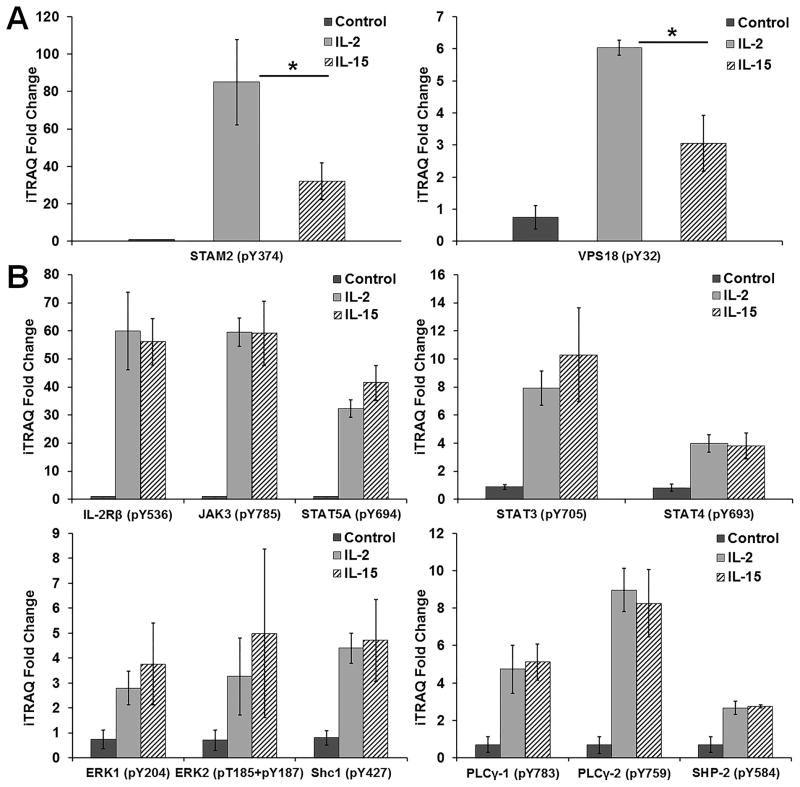
To quantitatively compare IL-2 and IL-15 mediated signal transduction in T cells at a network level, differentially iTRAQ8plex labeled tyrosine phosphorylated peptides from F15R-Kit cells stimulated for 15min with either no cytokine, IL-2 or IL-15 were immunoprecipitated with pan-specific anti-phosphotyrosine antibodies and analyzed using reverse phase LC-MS/MS (figure 1A).22–25 The choice of F15R-Kit cells, a human leukemia derived T cell line, as a model system was based on constitutive and stable expression of all four receptors of the IL-2/15R system in these cells (supplementary figure S1A–B).26 Phosphotyrosine profiling analysis led to the identification and quantification of 85 unique phosphorylation sites (76 pTyr, and 9 pSer/Thr) on 81 proteins measured across three biological replicates (figure 1B). To determine the minimum magnitude of difference detected through LC-MS/MS analysis in the present study, we analyzed the relative standard deviation (through calculation of the average coefficient of variation) across all conditions and biological replicates. Using twice the relative standard deviation as a cutoff, we defined a 1.54 fold-change in iTRAQ values as the minimum difference reliably detected through MS analysis in the current study. It is important to note that the relative standard deviation for the LC-MS/MS analysis is a combination of both the technical variations of LC-MS/MS and cell culture techniques and the inherent biological noise of our cell culture system. Consequently, the minimum difference threshold of 1.54 fold is specific to the present analysis and does not represent the sensitivity of the LC-MS/MS technique by itself. Out of 85 total phosphorylation sites, 41 sites (38 pTyr and 3 pSer/Thr) from 39 proteins showed a 1.54 fold or higher increase in phosphorylation level upon IL-2 or IL-15 stimulation. The IL-2 and IL-15 responsive phosphorylation sites belonged to proteins involved in the regulation of multiple cellular processes including cytoskeletal regulation, endocytosis, and protein translation, in addition to proteins known to be involved in signal transduction (figure 1C, supplementary table S1). Only 2 of the 39 IL-2 and IL-15 responsive proteins showed quantitatively distinct tyrosine phosphorylation responses, i.e. greater than 1.54 fold change, between IL-2 and IL-15 stimulation. IL-2 stimulation resulted in approximately 2.7-fold increase in phosphorylation of signal transducing adaptor molecule 2 (STAM2) on Tyr374 and a 2.0-fold increase in phosphorylation of vacuolar protein sorting 18 (VPS18) on Tyr32 relative to IL-15 stimulation (figure 2A). Both of these proteins have been shown to be involved in protein trafficking, endosomal sorting, and endocytosis (supplementary table S1).27–30
Figure 1. Mass spectrometry based phosphotyrosine profiling of F15R-Kit cells stimulated with IL-2 and IL-15.
(A) Schematic diagram of mass spectrometry based quantitative phosphotyrosine profiling experimental workflow. (B) Heat maps depicting 85 phosphorylation sites on 81 proteins quantified across F15R-Kit cell stimulated with IL-2 or IL-15 for 15 min. Heat maps represent log 2 transformed iTRAQ-8plex fold change values relative to one replicate of unstimulated F15R-Kit cells. The fold changes depicted for IL-2 and IL-15 are an average of 3 technical replicates. (C) Cellular processes regulated by the 41 proteins with greater than 1.5 fold increase in phosphorylation level upon IL-2 and IL-15 stimulation. (D) The relative increase in phosphorylation levels of canonical IL-2/15R signaling proteins triggered by IL-2 and IL-15 in F15R-Kit cells.
Figure 2. Quantification of phosphotyrosine sites regulated by IL-2 and IL-15 stimulation of F15R-Kit cells.
(A) Quantification of phosphotyrosine sites differentially regulated by IL-2 and IL-15 stimulation. iTRAQ fold changes in F15R-Kit cells stimulated with IL-2 and IL-15 normalized to unstimulated cells. Data represent an average of 3 biological replicates. * p-value <0.05. (B) Quantification of phosphotyrosine sites on canonical signaling proteins of the IL-2/15R signaling pathways. iTRAQ fold changes in F15R-Kit cells stimulated with IL-2 and IL-15 normalized to unstimulated cells. Data represent an average of 3 biological replicates.
Overall IL-2 or IL-15 stimulation resulted in an equivalent increase in phosphorylation levels of canonical IL-2/15R signaling pathway proteins, confirming previous reports (figure 1D, figure 2B). Beyond canonical signaling pathways, our analysis demonstrated that stimulation with IL-2 or IL-15 resulted in equivalent tyrosine phosphorylation of many proteins not known to be directly involved in IL-2/15R signaling, indicating that the signaling response to IL-2 and IL-15 is similar throughout the network.
F15R-Kit cells proliferate equivalently in response to continuous stimulation with IL-2 and IL-15 but differ in their response to pulse stimulation
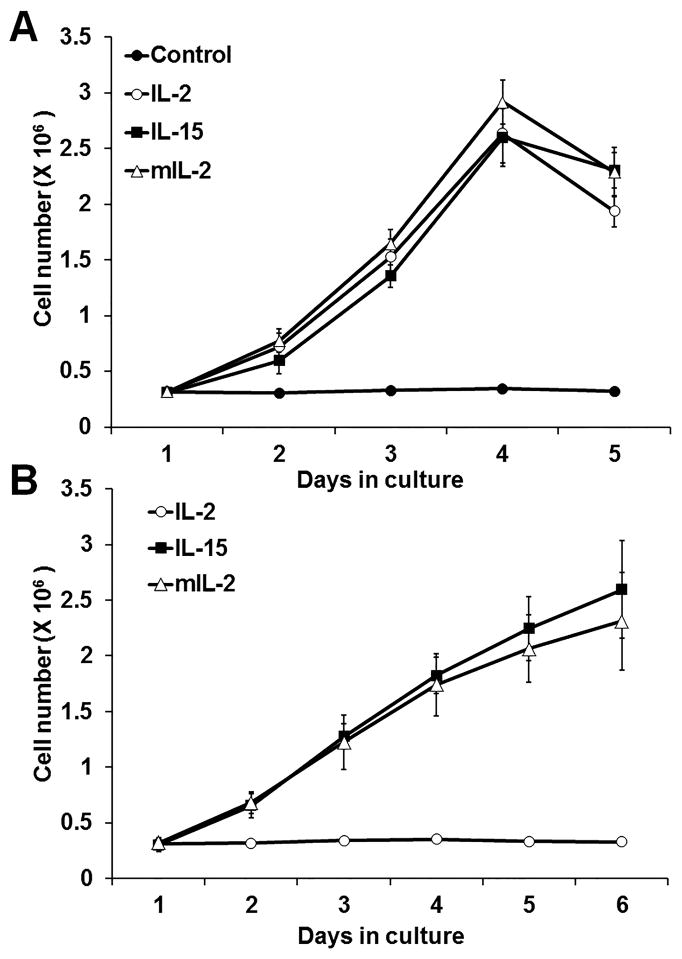
To understand how virtually identical signaling networks could give rise to qualitatively different phenotypic responses to IL-2 or IL-15 stimulation, we probed potentially revealing quantitative characteristics of receptor signaling and resultant cell phenotypic responses. To identify differential proliferation responses to the two cytokines, we counted viable F15R-Kit cell numbers following continuous stimulation with IL-2 or IL-15 over five days. F15R-Kit cells cultured in the continuous presence of IL-2 or IL-15 proliferated equivalently (figure 3A). To account for the effects of cytokine α-chain binding affinity on cell proliferation, F15R-Kit cells were cultured with an IL-2 mutant (mtIL-2) engineered to have a binding affinity for IL-2Rα approaching that of the IL-15-IL-15Rα complex. Proliferation of F15R-Kit cells cultured with mtIL-2 was quantitatively equivalent to cells cultured with either IL-2 or IL-15 (figure 3A). To compare the proliferative response of F15R-Kit cells pulse stimulated with IL-2, IL-15, or mtIL-2 for a defined 30 minutes, we used the pulse bioassay protocol described previously.20 Consistent with previous observations, F15R-Kit cells pulsed with IL-15 or mtIL-2 proliferated over a period of days without additional cytokine stimulation, while wild type IL-2 pulsed cells did not proliferate (figure 3B).
Figure 3. F15R-Kit cells proliferate equivalently in response to a continuous, but not pulsed, stimulation by IL-2, IL-15, and mtIL-2.
(A) Viable cell numbers over time for F15R-Kit cells cultured in the continuous presence of 500pM IL-2, IL-15, mtIL-2, or culture medium alone. (B) Viable cell numbers over time of F15R-Kit cells pulsed for 30min with 500pM IL-2, IL-15, and mtIL-2. Viable cell numbers were determined using Trypan Blue exclusion. Data represent Mean ± Standard Deviation (SD) for three independent experiments.
Increased initial receptor occupancy and surface persistence of cytokines after pulse stimulation mediates proliferation through increased duration of IL-2/15R signaling after cytokine withdrawal
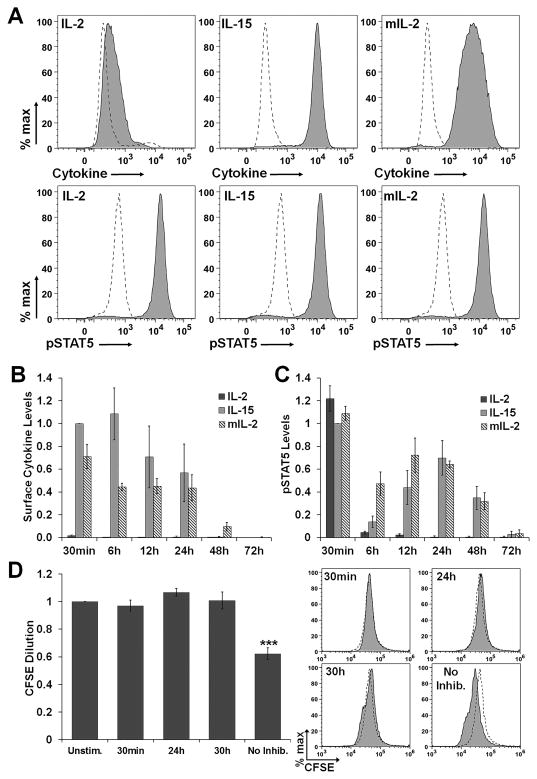
The proliferative response of F15R-Kit cells to cytokine pulse stimulation was previously found to be directly correlated to the initial cytokine surface receptor occupancy and surface persistence.20 We hypothesized that increased initial receptor occupancy and surface persistence may regulate proliferation through increased duration of IL-2/15R signal transduction after cytokine withdrawal. To test this hypothesis, the surface cytokine levels, IL-2/15R signal strength, and proliferation of F15R-Kit cells pulsed with IL-2, IL-15, or mtIL-2 were measured simultaneously at multiple time-points after cytokine withdrawal. As STAT5 proteins are tyrosine phosphorylated directly through association with the IL-2Rβ receptor, intracellular levels of phosphorylated STAT5 (pSTAT5) represent a proxy for IL-2/15R signal strength.10 F15R-Kit cells pulsed with IL-15 or mtIL-2 showed higher levels of surface-bound cytokine and surface persistence after cytokine withdrawal compared to wild-type IL-2 (figures 4A & 4B). Pulse stimulation of F15R-Kit cells with IL-2, IL-15, or mtIL-2 initially resulted in a quantitatively equivalent increase in pSTAT5 levels (figure 4A). Cells pulsed with IL-15 or mtIL-2 maintained high pSTAT5 levels for up to 72h after cytokine withdrawal, whereas the pSTAT5 levels of cells pulsed with wild-type IL-2 returned to basal levels by 6h (figure 4C, supplementary figure S2A–B).
Figure 4. F15R-Kit cell proliferation requires continuous activation of the IL-2/15R signaling.
(A) Representative flow cytometry histograms showing surface cytokine and intracellular pSTAT5 levels in F15R-Kit cells stimulated with 500pM of the indicated cytokine for 30min (solid histograms). Dotted histograms represent unstimulated cells. (B) Surface-bound cytokine levels and (C) intracellular pSTAT5 levels of F15R-Kit cells, pulse stimulated with 500pM cytokine measured at the indicated time points after cytokine withdrawal. Data represent median fluorescence intensity (MFI) of surface cytokine and pSTAT5 levels normalized to MFI of cells stimulated with IL-15 at the 30min time-point. (D) CFSE dilution values (left panel) and representative histograms of F15R-Kit CFSE levels (right panel) experiencing a defined duration of IL-2/15 receptor signaling, ***αFW < 0.001 compared to unstimulated cells. CFSE dilution values represent the ratio of CFSE MFI levels of cells cultured with IL-2 and JI to CFSE MFI of unstimulated cells 48h after cytokine addition. Solid histograms represent CFSE values for F15R-Kit cells cultured with IL-2 and JI and dashed histograms represent CFSE values of unstimulated cells 48h after cytokine addition. Data represent average ± SD for 3 independent experiments.
F15R-Kit cell proliferation requires a continuous signal input from the IL-2/15 receptor
F15R-kit cells pulsed with wild-type IL-2 maintained high pSTAT5 levels for up to 6–8h after cytokine withdrawal, yet did not proliferate. We hypothesized that a minimum duration of IL-2/15 receptor signaling might be required for T cell proliferation. To test this hypothesis, CFSE labeled F15R-Kit cells were cultured with a saturating IL-2 dose, and a saturating dose of Jak Inhibitor I (JI) was added at multiple time-points after cytokine addition. Saturating doses were defined as the cytokine dose that induced maximal STAT5 phosphorylation and the JI dose that resulted in maximum reduction in pSTAT5 levels after cytokine stimulation. The addition of JI at specified time-points after cytokine stimulation allowed experimental control over the duration of IL-2/15R signaling experienced by the F15R-Kit cells. The addition of JI at any time-point up to 30h after IL-2 addition prevented F15R-Kit cells from undergoing cell division (figure 4D). These results suggest that continuous signal transduction from the IL-2/15R is required for T cell proliferation.
F15R-Kit cell proliferation, cell size, and glycolytic activity respond distinctly to IL-2/15R signal strength
To investigate the role of IL-2/15R signal strength in the regulation of T cell proliferation and cell size, CFSE labeled F15R-Kit cells were cultured with increasing doses of IL-2 or IL-15; for each condition, cell proliferation and cell size were measured over multiple days. F15R-Kit cell size increased with increasing cytokine dose (figure 5A & 5C) but proliferation remained equivalent for IL-2 or IL-15 stimulated cells (figure 5B & 5C). As the relationship between cytokine dose, cell size, and cell proliferation was identical for both IL-2 and IL-15, we hypothesized that T cell size and proliferation were regulated directly through the IL-2/15 receptor signal strength in a cytokine independent manner. To test this hypothesis, CFSE labeled F15R-Kit cells were cultured with a saturating dose of IL-2 or IL-15 along with a range of JI doses, allowing for direct control of the IL-2/15R signal strength upon IL-2 or IL-15 stimulation (figure 6A). For either IL-2 or IL-15 stimulation, F15R-Kit cell size decreased with increasing JI concentration (figure 6B), while proliferation remained unaffected (figure 6C). The relative differences in F15R-Kit cell size and the equivalent relative F15R-Kit proliferation levels were maintained for up to 6 days in culture (figure 6B–C).
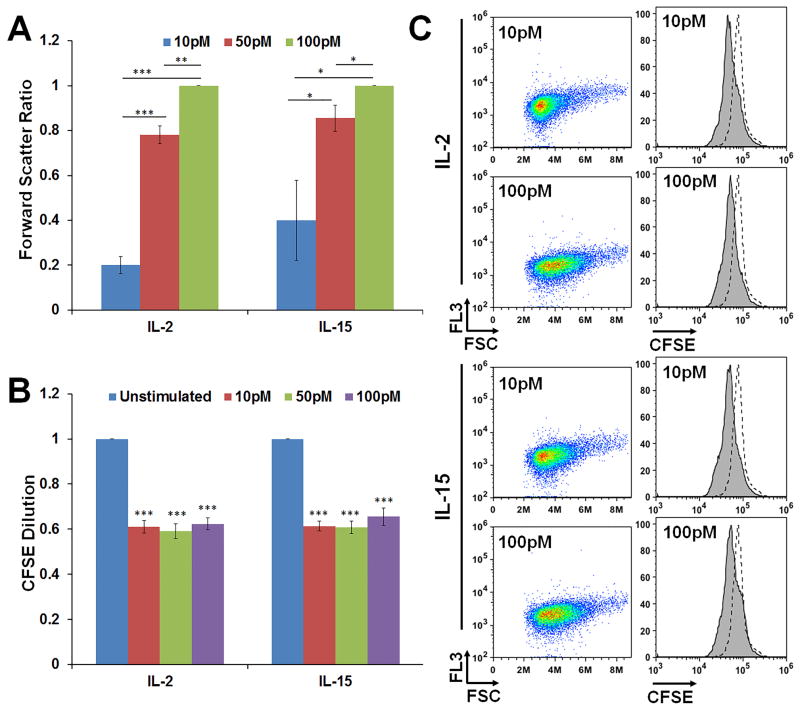
Figure 5. F15R-Kit cell size increases with cytokine dose but proliferation remains identical.
(A) Median FSC values for F15R-Kit cells cultured for 48h with the indicated doses of IL-2 or IL-15 normalized to the 100pM dose for each cytokine. *αfw < 0.05, **αfw < 0.01, ***αfw < 0.001. (B) Plot showing the ratios of the CFSE values of F15R-Kit cells cultured with the indicated dose of cytokine to the unstimulated cells at 48h. ***αfw < 0.001 compared to unstimulated cells. (C) Representative flow cytometry dot plots showing forward scatter and histograms showing CFSE levels of F15R-Kit cells 48h after treatment with the indicated dose of IL-2 or IL-15. Dotted histograms represent unstimulated cells. Data represent Mean ± SD for 3 independent replicates.
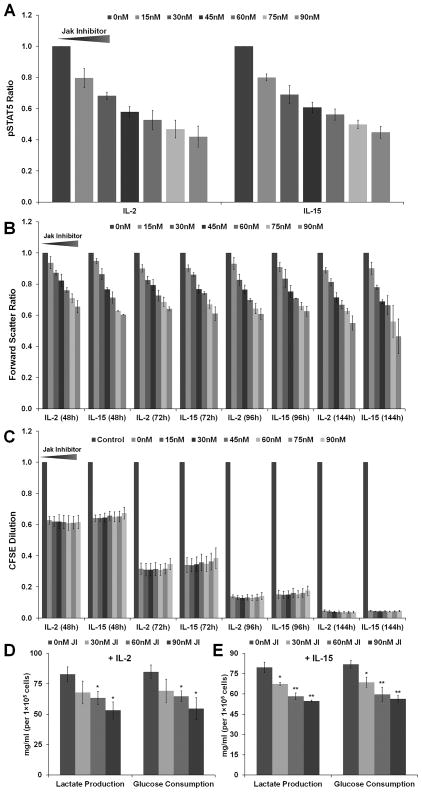
Figure 6. F15R-Kit cell size and glycolytic activity increases with increasing IL-2/15 receptor signal strength while proliferation remains identical.
Cytokine starved F15R-Kit cells were pretreated with the indicated dose of JAK Inhibitor I, stimulated with a 500pM dose of cytokine, and intracellular pSTAT5 levels, forward scatter, and CFSE levels measured at the indicated time-points using flow cytometry. (A) F15R-Kit intracellular pSTAT5 levels 15min after addition of IL-2 or IL-15. (B) F15R-Kit cells forward scatter values at the indicated time-periods after the addition of IL-2 or IL-15. (C) CFSE dilution levels compared to the unstimulated control at the indicated time points for cells cultured with IL-2 or IL-15. Glucose consumption and lactate production by F15R-Kit cells treated with the indicated dose of JAK Inhibitor I and stimulated with 500pM of (D) IL-2 or (E) IL-15, *αFW < 0.05, **αFW < 0.01 compared to 0nM JI condition. Data represent Mean ± SD for 3 independent replicates.
Increase in T cell size due to antigen or cytokine stimulation is related to increased cellular metabolic activity.31–34 To determine whether an increase in F15R-Kit cell size with IL-2/15R signal strength was also correlated with an increase in cell metabolism, we measured glucose consumption and lactate production by F15R-Kit cells maintained in culture with IL-2 or IL-15 in the presence of varying doses of JI. Glucose consumption and lactate production decreased with increasing JI dose for cells stimulated with either IL-2 or IL-15 (figure 6D–E), suggesting that metabolic activity is dependent on signal strength from the receptor, independent of cytokine identity.
Regulation of F15R-Kit cell size and proliferation through the IL-2/15 receptor signal strength is quantitatively distinct and cytokine independent
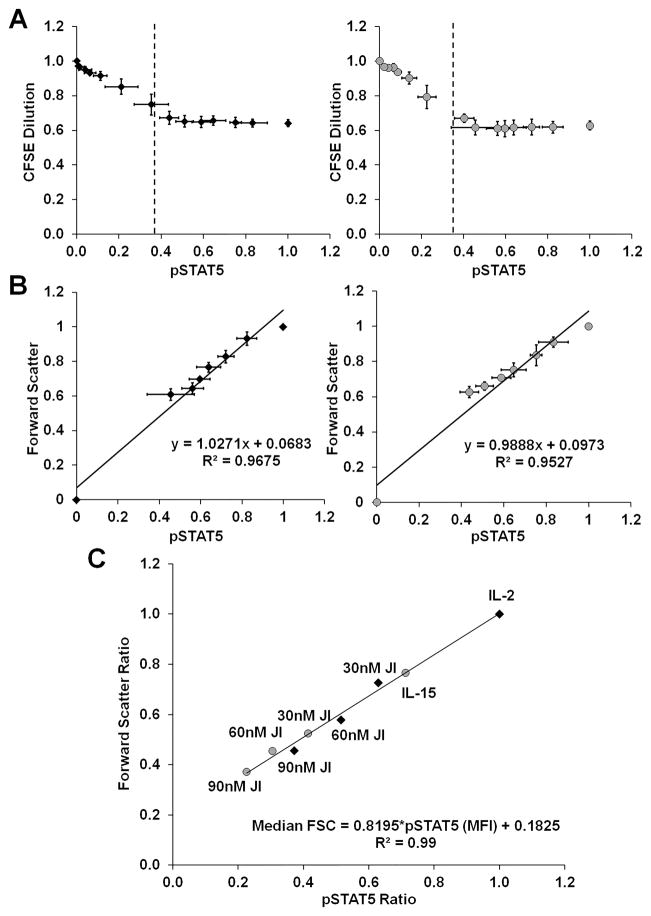
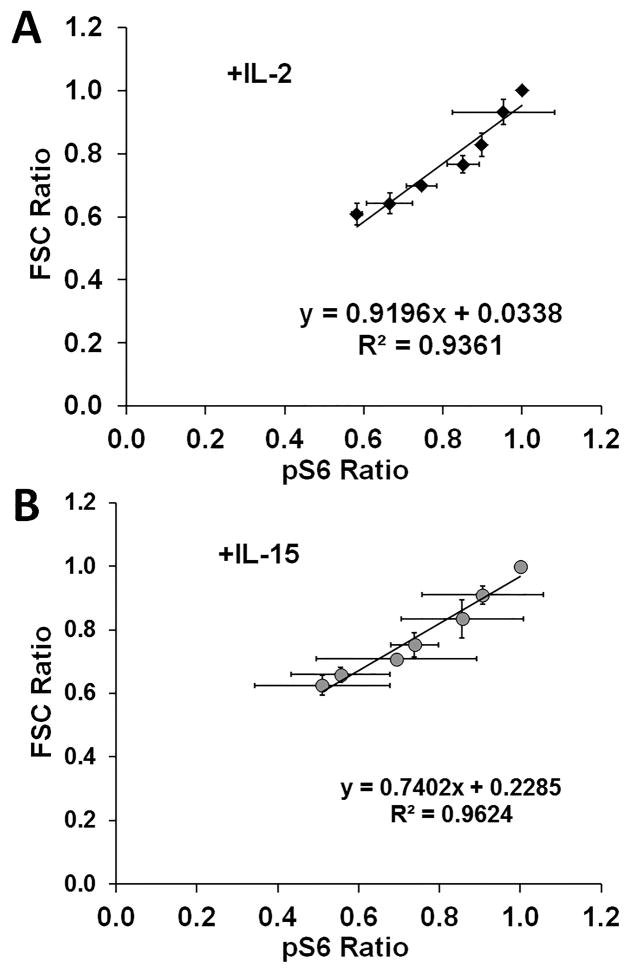
To directly establish the quantitative relationship between signal strength, cell size, and proliferation, CFSE labeled F15R-Kit cells were cultured with IL-2 or IL-15 along with a range of JI doses. FSC, CFSE dilution, and intracellular pSTAT5 levels were measured through flow cytometry after 48h in culture. CFSE dilution levels initially decreased with increasing pSTAT5 levels but plateaued independent of further increase in pSTAT5 levels beyond 40% of maximal pSTAT5 levels triggered by cytokine stimulation, suggesting a digital relationship between cell proliferation and IL-2/15R signal strength (figure 7A). In contrast to CFSE dilution, F15R-Kit FSC values increased linearly with respect to pSTAT5 levels, suggesting an analog relationship between cell size and signal strength (figure 7B). The PI3K/AKT/mTOR pathway has been shown to play a direct role in the regulation of T cell size and metabolic activity, and serine phosphorylation of 40S ribosomal protein S6 (pS6) is a reliable indicator of this pathway’s activation.18,35,36 Plotting F15R-Kit cell FSC values with respect to pS6 levels revealed an analog relationship between F15R-Kit cell size and mTOR pathway activation (figure 8), similar to our results for pSTAT5.
Figure 7. Quantitatively distinct regulation of F15R-Kit cell size and proliferation through the IL-2/15 receptor signal strength.
CFSE dilution and FSC levels plotted against intracellular pSTAT5 levels of F15R-Kit cells cultured for 48h with multiple doses of JAK Inhibitor I and 500pM IL-2 (black diamonds) or 500pM IL-15 (gray circles) (A–B). (A) CFSE dilution vs. pSTAT5 levels. The black dotted lines represent the threshold signal strength above which proliferation is identical and independent of the specific pSTAT5 level. CFSE dilution values between 0.6 and 1 represent median fluorescence for populations where > 0% but <100% of cells complete one cell division. (B) Forward scatter vs. pSTAT5 levels for cells with signal strength above the threshold indicated in figure 5A. (C) Forward scatter vs. pSTAT5 levels of F15R-Kit cells cultured with the indicated doses of JAK Inhibitor I and 500pM IL-2 (black diamonds) or 500pM IL-15 (gray circles). IL-2/15 receptor signal strength is a superior predictor of cell size compared to JAK Inhibitor I dose, cytokine dose, or cytokine identity.
Figure 8. F15R-Kit cell size vs. mTOR signaling.
Forward scatter vs. 40S ribosomal protein S6 (pS6) levels of F15R-Kit cells cultured with various doses of JI. Forward scatter and pS6 values measured 96h after cytokine addition for cells stimulated with (A) 500pM IL-2 (black diamonds) or (B) 500pM IL-15 (gray circles).
To determine whether IL-2/15R signal strength measurements alone were sufficient for predicting F15R-Kit cell size and proliferation in response to cytokine stimulation, CFSE labeled F15R-Kit cells were cultured with IL-2 or IL-15 along with three different doses of JI. The stimulation, processing, and analysis of the varying cytokine and JI treatments were performed simultaneously to minimize variations introduced through the experimental techniques. Consistent with earlier results, the FSC of F15R-Kit cell size increased linearly with receptor signal strength (figure 7C & supplementary figure S2C) but proliferation remained identical (supplementary figure S2C). Data in figure 7C demonstrate that IL-2/15R signal strength was not only sufficient but a superior indicator of F15R-Kit cell size compared to cytokine identity, cytokine dose, or JI concentration.
The quantitative relationship between cell size, proliferation, and IL-2/15R signal strength is conserved in antigen-activated primary human T lymphocytes
To determine the physiological relevance of the quantitative relationship between IL-2/15R signal strength, cell size, and proliferation discovered using F15R-Kit cells, we replicated the experiments with primary human CD4+ and CD8+ T lymphocytes. Primary T cells were activated in vitro using anti-CD3 and anti-CD28 stimulation, which led to an up-regulation of all four IL-2/15 receptor chain surface levels (supplementary figure S1C). Similar to F15R-Kit cells, IL-2 and IL-15 stimulated CD4+ and CD8+ T cells decreased in size with increasing JI dose (figure 9A & 9C) while proliferation levels remained unaffected (figure 9B & 9D). Differences in cell size were maintained for multiple days in cell culture, during which time the cell proliferation rates remained constant (figure 9A–D). Similar to F15R-Kit cells, plotting FSC and CFSE dilution levels of the primary human T cells with respect to intracellular pSTAT5 levels as a proxy for IL-2/15R signal strength revealed an analog relationship with cell size and a digital relationship with cell proliferation for both CD4+ and CD8+ T lymphocytes (figures 9E–H).
Figure 9. Quantitatively distinct regulation of cell size and proliferation through the IL-2/15 receptor signal strength in primary human T lymphocytes.
Primary human CD4+ and CD8+ T cells were activated with anti-CD3 and anti-CD28 stimulation, treated with the indicated JAK Inhibitor I dose, and stimulated with 1nM IL-2 or IL-15. Forward scatter of (A) CD4+ T cells and (C) CD8+ T cells at the indicated time-points after cytokine addition. CFSE dilution values for (B) CD4+ T cells and (D) CD8+ T cells at the indicated time-points after cytokine addition. Non-activated T cells were used as the negative control for measuring CFSE dilution. Forward scatter vs. intracellular pSTAT5 levels at 48h after cytokine addition for (E) CD4+ T cells (gray circles) and (F) CD8+ T cells (black squares). CFSE dilution vs. intracellular pSTAT5 levels at 48h after cytokine addition for (G) CD4+ T (gray circles) cells and (H) CD8+ T (black squares) cells.
Discussion
IL-2 and IL-15 signal through the same receptors on the surface of T cells, yet play distinct roles in the T cell immune response physiologically and at the cellular level. Results from the present study provide new insights into mechanisms by which IL-2 and IL-15 trigger distinct phenotypes in T cells.
To generate an unbiased, global, and quantitative view of the signal transduction triggered by IL-2 and IL-15 stimulation in T cells, we quantified changes in the phosphotyrosine proteome triggered in F15R-Kit cells upon IL-2 and IL-15 stimulation.22–25 With the exception of a quantitative difference in STAM2 and VPS18 phosphorylation, IL-2 and IL-15 stimulation resulted in qualitatively and quantitatively identical signal transduction through the IL-2/15R in F15R-Kit cells. These results suggest that phenotypic differences induced by IL-2 and IL-15 in T cells are likely not a result of distinct signal transduction from the shared IL-2/15R. Interestingly, both STAM2 and VPS18 proteins are known to be involved in the regulation of cytokine and growth factor receptor endocytosis and intracellular trafficking.27–30 The effects of potential differences in receptor intracellular trafficking on cellular phenotypes remain to be characterized.
Results from our investigation into the distinct proliferative responses of T cells to pulsed stimulation with IL-2 or IL-15 revealed a critical role of IL-2/15R signal duration in regulating T cell proliferation. We found that the increased receptor occupancy and surface persistence of IL-15 and mtIL-2, a result of their higher α-chain binding affinity, drove proliferation through prolonged duration of signaling from the receptor after cytokine withdrawal compared to cells pulsed with wild-type IL-2. A closer examination of the inability of F15R-Kit cells pulse stimulated with wild-type IL-2 to proliferate despite signaling for ~6h revealed that T cell proliferation required a constant signal input from the IL-2/15R. This result may be related to the role of IL-2 signaling in T cell peripheral tolerance, where a requirement for constant IL-2/15R signaling for cell division might ensure proliferation of activated T cells only during the early immune response, when high levels of IL-2 are produced and secreted by antigen activated T lymphocytes.3,4,15,37
Investigation into the distinct metabolic activity triggered by IL-2 and IL-15 in T cells revealed the critical role played by IL-2/15R signal strength in the regulation of T cell proliferation and metabolism. We found that F15R-Kit cell size increased with IL-2/15R signal strength while proliferation remained identical for both IL-2 and IL-15 stimulated cells. As the relative differences in cell size were maintained over several days while proliferation rates remained constant, the cell size differences were not a result of differential rates of cell cycle progression. This result suggests that cell proliferation and size were both directly regulated through IL-2/15R signal strength, but in a quantitatively distinct manner. F15R-Kit cell proliferation changed digitally with IL-2/15R signal strength, whereas F15R-Kit cell size showed an analog linear dependence on IL-2/15R signal strength.
The regulation of T cell size by IL-2/15R signal strength appears to be mediated through its effects on T cell metabolic activity, as glycolytic activity of F15R-Kit cells correlated directly with IL-2/15R signal strength and cell size. Proliferation remained identical for cells with glycolytic activity above a minimum threshold, suggesting that cytokine stimulation triggers metabolic activity in excess of proliferative demand. Although not directly measured in this study, the increase in glycolytic activity of F15R-Kit cells upon IL-2 or IL-15 stimulation is likely a result of increased expression and activity of glucose transporters (GLUTs) and metabolic enzymes.31–35,38 The analog relationship between glycolytic activity and consequently cell size may be a result of the direct dependence of GLUT and glycolytic enzyme expression levels on IL-2/15R signal strength.35,36,38,39 Our results showing a linear relationship between cell size and mTOR pathway activation for both IL-2 and IL-15 stimulated F15R-Kit cells lend support to this idea.36,38,39
Our experiments with primary human CD8+ and CD4+ T cells showed that the quantitatively distinct and cytokine independent regulation of T cell size and proliferation is a physiologically conserved mechanism for IL-2 and IL-15 mediated regulation of distinct T cell phenotypes. To our knowledge, this is the first time a stable analog relationship between IL-2/15R signal strength and cell size has been demonstrated for primary human CD8+ and CD4+ T lymphocytes. Interestingly, the significant difference in cell size upon IL-2 or IL-15 stimulation previously reported in mouse CD8+ T cells was not observed for either CD8+ or CD4+ human T lymphocytes.18 It is possible that activated human T cells express a higher amount of IL-15Rα on their surface compared to activated murine T cells either due to inherent species specific differences or distinct stimulation protocols used (anti-CD3/CD28 for humans vs. antigenic peptides for mice). The higher IL-15Rα expression of human T cells would result in similar IL-2/15R signal strength and consequently cell size for cells stimulated with IL-2 and IL-15.
Recent studies in mouse models have revealed that IL-2 and IL-15 can differ in their mode of presentation in vivo. Even though both IL-2 and IL-15 stimulate T cells as soluble ligands,4,9 IL-15 is thought to be mainly presented through a process known as trans-presentation.4,8,19,21 This mode of presentation involves the interaction of IL-15-IL-15Rα complexes expressed on the surface of dendritic cells and macrophages with the IL-2/15βγc complexes expressed on T cells.4,8,19,21 Although trans-presentation was not present in our system, results from the current study can provide insights into its effects on IL-15 signaling. As IL-15-IL-15Rα complexes presented in trans can directly stimulate the IL-2Rβγc complexes independent of the T cell surface expression of IL-15Rα, and no known evidence exists of their ability to be internalized by the stimulated cells, trans presentation can potentially increase the strength and duration of IL-15 signal transduction relative to stimulation by soluble IL-15. 4,18,19,40 Our current results suggest that T cells stimulated with IL-15 in trans would be metabolically more active, larger in size, and more proliferative compared to cells stimulated with soluble IL-15. In support of this idea, stimulation of T cells with soluble complexes of IL-15-IL-15Rα results in stronger and more persistent signaling and a more potent phenotypic response compared to stimulation with soluble IL-15.40,41 It is possible that soluble and trans-presented IL-15 play distinct roles physiologically. Soluble IL-15 may primarily function to provide viability and proliferation signals for T cell populations that express IL-15Rα chains on their surfaces, for instance in the context of processes such as maintenance and self-renewal of memory T cells. Alternatively, trans-presented IL-15 may primarily function to provide a spatially localized “growth factor” like signal stimulating both metabolic activity and proliferation of T cells independent of IL-15Rα expression, which may be critical for the generation and maintenance of tissue localized effector T cell populations.
In summary, the present study investigated mechanisms allowing IL-2 and IL-15 to mediate distinct phenotypes in T cells despite signaling through the same receptors. We found that the strength and duration of a signaling pathway can directly affect cellular phenotypes, and regulation of distinct phenotypes may not require activation of qualitatively distinct signaling pathways. These results may explain the ability of cytokines to regulate different phenotypes in T cells while signaling through shared receptors. In support of this, a recent study explained differences in IL-7-elicited viability and proliferation behavior among various mouse strain naive CD8+ T cells in terms of analogous quantitative dependences of IL-7 receptor signaling strength and duration.42 As T cell effector functions and differentiation are intimately linked to their metabolic activity, the quantitative regulation of T cell metabolism and proliferation described in the present study may provide important design principles for improved cytokine mediated immunotherapies.3–5,18,32 Engineering mutant versions of IL-2 or IL-15 with defined effects on IL-2/15R signal strength and/or duration can allow for a more precise control over the nature of T cell immune response required for the treatment of a particular disease. Thus, our results may provide important insights into the design of cytokine based immunotherapies for cancer, autoimmune disorders, and infectious diseases.
Supplementary Material
Acknowledgments
Funding for this research was provided in part by NIH U54CA11927 and R01 AI065824. This work was partially supported by the Institute for Collaborative Biotechnologies through grant W911NF-09-0001 from the U.S. Army Research Office. The content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
The authors would like to thank Amanda Del Rosario for critical reading of the manuscript, members of the White lab for technical assistance and helpful discussions, Maria Foley and Talitha Forcier for technical assistance with isolation and culture of human T lymphocytes, Glen Pardis and the flow cytometry core facility at the Koch Institute for assistance in flow cytometry experiments, Annie Gai (Wittrup Lab, MIT) for kindly providing F15R-Kit cells and human IL-2 mutants, and Matthew Vander Heiden for helpful discussions.
Abbreviations
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- pTyr
phosphotyrosine
- FSC
forward scatter
- SSC
side scatter
- JI
Jak Inhibitor I
Footnotes
Authorship and Conflict of Interest
A.A., H.J, and L.G. performed experiments and data analysis, A.A., D.A.L, and F.M.W. conceived the study and wrote the manuscript. All authors reviewed and edited drafts of the manuscript.
References
- 1.Burton JD, Bamford RN, Peters C, Grant AJ, Kurys G, Goldman CK, Brennan J, Roessler E, Waldmann TA. A lymphokine, provisionally designated interleukin T and produced by a human adult T-cell leukemia line, stimulates T-cell proliferation and the induction of lymphokine-activated killer cells. Proc Natl Acad Sci U S A. 1994;91:4935–4939. doi: 10.1073/pnas.91.11.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 3.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 4.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann TA, Conlon KC, Stewart DM, Worthy TA, Janik JE, Fleisher TA, Albert PS, Figg WD, Spencer SD, Raffeld M, Decker JR, Goldman CK, Bryant BR, Petrus MN, Creekmore SP, Morris JC. Phase 1 trial of IL-15 trans presentation blockade using humanized Mikβ1 mAb in patients with T-cell large granular lymphocytic leukemia. Blood. 2013;121:476–484. doi: 10.1182/blood-2012-08-450585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong JL, Farner NL, Widmer MB, Giri JG, Sondel PM. Interaction of IL-15 with the shared IL-2 receptor beta and gamma c subunits. The IL-15/beta/gamma c receptor-ligand complex is less stable than the IL-2/beta/gamma c receptor-ligand complex. J Immunol. 1996;156:1339–1348. [PubMed] [Google Scholar]
- 7.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. Embo j. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ring AM, Lin JX, Feng D, Mitra S, Rickert M, Bowman GR, Pande VS, Li P, Moraga I, Spolski R, Ozkan E, Leonard WJ, Garcia KC. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat Immunol. 2012;13:1187–1195. doi: 10.1038/ni.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stauber DJ, Debler EW, Horton PA, Smith KA, Wilson IA. Crystal structure of the IL-2 signaling complex: paradigm for a heterotrimeric cytokine receptor. Proc Natl Acad Sci U S A. 2006;103:2788–2793. doi: 10.1073/pnas.0511161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaffen SL. Signaling domains of the interleukin 2 receptor. Cytokine. 2001;14:63–77. doi: 10.1006/cyto.2001.0862. [DOI] [PubMed] [Google Scholar]
- 11.Delespine-Carmagnat M, Bouvier G, Bertoglio J. Association of STAT1, STAT3 and STAT5 proteins with the IL-2 receptor involves different subdomains of the IL-2 receptor β chain. European Journal of Immunology. 2000;30:59–68. doi: 10.1002/1521-4141(200001)30:1<59::AID-IMMU59>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.Kovanen PE, Rosenwald A, Fu J, Hurt EM, Lam LT, Giltnane JM, Wright G, Staudt LM, Leonard WJ. Analysis of gamma c-family cytokine target genes. Identification of dual-specificity phosphatase 5 (DUSP5) as a regulator of mitogen-activated protein kinase activity in interleukin-2 signaling. J Biol Chem. 2003;278:5205–5213. doi: 10.1074/jbc.M209015200. [DOI] [PubMed] [Google Scholar]
- 13.Kovanen PE, Young L, Al-Shami A, Rovella V, Pise-Masison CA, Radonovich MF, Powell J, Fu J, Brady JN, Munson PJ, Leonard WJ. Global analysis of IL-2 target genes: identification of chromosomal clusters of expressed genes. Int Immunol. 2005;17:1009–1021. doi: 10.1093/intimm/dxh283. [DOI] [PubMed] [Google Scholar]
- 14.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 15.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 16.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 17.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 18.Cornish GH, Sinclair LV, Cantrell DA. Differential regulation of T-cell growth by IL-2 and IL-15. Blood. 2006;108:600–608. doi: 10.1182/blood-2005-12-4827. [DOI] [PubMed] [Google Scholar]
- 19.Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- 20.Rao BM, Driver I, Lauffenburger DA, Wittrup KD. High-affinity CD25-binding IL-2 mutants potently stimulate persistent T cell growth. Biochemistry. 2005;44:10696–10701. doi: 10.1021/bi050436x. [DOI] [PubMed] [Google Scholar]
- 21.Burkett PR, Koka R, Chien M, Chai S, Chan F, Ma A, Boone DL. IL-15R alpha expression on CD8+ T cells is dispensable for T cell memory. Proc Natl Acad Sci U S A. 2003;100:4724–4729. doi: 10.1073/pnas.0737048100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotechnol. 2002;20:301–305. doi: 10.1038/nbt0302-301. [DOI] [PubMed] [Google Scholar]
- 23.Johnson H, Del Rosario AM, Bryson BD, Schroeder MA, Sarkaria JN, White FM. Molecular characterization of EGFR and EGFRvIII signaling networks in human glioblastoma tumor xenografts. Mol Cell Proteomics. 2012;11:1724–1740. doi: 10.1074/mcp.M112.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JE, White FM. Quantitative analysis of phosphotyrosine signaling networks triggered by CD3 and CD28 costimulation in Jurkat cells. J Immunol. 2006;176:2833–2843. doi: 10.4049/jimmunol.176.5.2833. [DOI] [PubMed] [Google Scholar]
- 25.Iwai LK, Benoist C, Mathis D, White FM. Quantitative phosphoproteomic analysis of T cell receptor signaling in diabetes prone and resistant mice. J Proteome Res. 2010;9:3135–3145. doi: 10.1021/pr100035b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori T, Uchiyama T, Tsudo M, Umadome H, Ohno H, Fukuhara S, Kita K, Uchino H. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987;70:1069–1072. [PubMed] [Google Scholar]
- 27.Endo K, Takeshita T, Kasai H, Sasaki Y, Tanaka N, Asao H, Kikuchi K, Yamada M, Chenb M, O’Shea JJ, Sugamura K. STAM2, a new member of the STAM family, binding to the Janus kinases. FEBS Lett. 2000;477:55–61. doi: 10.1016/s0014-5793(00)01760-9. [DOI] [PubMed] [Google Scholar]
- 28.Stuible M, Abella JV, Feldhammer M, Nossov M, Sangwan V, Blagoev B, Park M, Tremblay ML. PTP1B targets the endosomal sorting machinery: dephosphorylation of regulatory sites on the endosomal sorting complex required for transport component STAM2. J Biol Chem. 2010;285:23899–23907. doi: 10.1074/jbc.M110.115295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huizing M, Didier A, Walenta J, Anikster Y, Gahl WA, Kramer H. Molecular cloning and characterization of human VPS18, VPS 11, VPS16, and VPS33. Gene. 2001;264:241–247. doi: 10.1016/s0378-1119(01)00333-x. [DOI] [PubMed] [Google Scholar]
- 30.Peng C, Ye J, Yan S, Kong S, Shen Y, Li C, Li Q, Zheng Y, Deng K, Xu T, Tao W. Ablation of vacuole protein sorting 18 (Vps18) gene leads to neurodegeneration and impaired neuronal migration by disrupting multiple vesicle transport pathways to lysosomes. J Biol Chem. 2012;287:32861–32873. doi: 10.1074/jbc.M112.384305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauer DE, Harris MH, Plas DR, Lum JJ, Hammerman PS, Rathmell JC, Riley JL, Thompson CB. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. Faseb j. 2004;18:1303–1305. doi: 10.1096/fj.03-1001fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 33.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 34.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long M, Adler AJ. Cutting edge: Paracrine, but not autocrine, IL-2 signaling is sustained during early antiviral CD4 T cell response. J Immunol. 2006;177:4257–4261. doi: 10.4049/jimmunol.177.7.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato N, Patel HJ, Waldmann TA, Tagaya Y. The IL-15/IL-15Ralpha on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc Natl Acad Sci U S A. 2007;104:588–593. doi: 10.1073/pnas.0610115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer MJ, V, Mahajan S, Chen J, Irvine DJ, Lauffenburger DA. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naive CD8(+) T cells. Immunol Cell Biol. 2011;89:581–594. doi: 10.1038/icb.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damjanovich S, Bene L, Matko J, Alileche A, Goldman CK, Sharrow S, Waldmann TA. Preassembly of interleukin 2 (IL-2) receptor subunits on resting Kit 225 K6 T. Proc Natl Acad Sci U S A. 1997;94:13134–13139. doi: 10.1073/pnas.94.24.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bakker BI, Bodnár A, van Dijk EM, Vámosi G, Damjanovich S, Waldmann TA, van Hulst NF, Jenei A, Garcia-Parajo MF. Nanometer-scale organization of the alpha subunits of the receptors for IL2 and IL15 in human T lymphoma cells. J Cell Sci. 2008;121:627–633. doi: 10.1242/jcs.019513. [DOI] [PubMed] [Google Scholar]
- 45.Vamosi G, Bodnar A, Vereb G, Jenei A, Goldman CK, Langowski J, Toth K, Matyus L, Szollosi J, Waldmann TA, Damjanovich S. IL-2 and IL-15 receptor alpha-subunits are coexpressed in a supramolecular. Proc Natl Acad Sci U S A. 2004;101:11082–11087. doi: 10.1073/pnas.0403916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liparoto SF, Myszka DG, Wu Z, Goldstein B, Laue TM, Ciardelli TL. Analysis of the role of the interleukin-2 receptor gamma chain in ligand binding. Biochemistry. 2002;41:2543–2551. doi: 10.1021/bi011692m. [DOI] [PubMed] [Google Scholar]
- 47.Pesesse X, Dewaste V, De Smedt F, Laffargue M, Giuriato S, Moreau C, Payrastre B, Erneux C. The Src homology 2 domain containing inositol 5-phosphatase SHIP2 is recruited to the epidermal growth factor (EGF) receptor and dephosphorylates phosphatidylinositol 3,4,5-trisphosphate in EGF-stimulated COS-7 cells. J Biol Chem. 2001;276:28348–28355. doi: 10.1074/jbc.M103537200. [DOI] [PubMed] [Google Scholar]
- 48.Prasad N, Topping RS, Decker SJ. Src family tyrosine kinases regulate adhesion-dependent tyrosine phosphorylation of 5′-inositol phosphatase SHIP2 during cell attachment and spreading on collagen I. J Cell Sci. 2002;115:3807–3815. doi: 10.1242/jcs.00070. [DOI] [PubMed] [Google Scholar]
- 49.Kurzer JH, Argetsinger LS, Zhou YJ, Kouadio JL, O’Shea JJ, Carter-Su C. Tyrosine 813 is a site of JAK2 autophosphorylation critical for activation of JAK2 by SH2-B beta. Mol Cell Biol. 2004;24:4557–4570. doi: 10.1128/MCB.24.10.4557-4570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benitah SA, Valerón PF, Rui H, Lacal JC. STAT5a activation mediates the epithelial to mesenchymal transition induced by oncogenic RhoA. Mol Biol Cell. 2003;14:40–53. doi: 10.1091/mbc.E02-08-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez R, Matsuda M, Storey A, Katan M. Requirements for distinct steps of phospholipase Cgamma2 regulation, membrane-raft-dependent targeting and subsequent enzyme activation in B-cell signalling. Biochem J. 2003;374:269–280. doi: 10.1042/BJ20021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu G, Chen Y, Schuman J, Wang D, Wen R. Phospholipase Cgamma2 plays a role in TCR signal transduction and T cell selection. J Immunol. 2012;189:2326–2332. doi: 10.4049/jimmunol.1103458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng H, Ross JA, Frost JA, Kirken RA. Phosphorylation of human Jak3 at tyrosines 904 and 939 positively regulates its. Mol Cell Biol. 2008;28:2271–2282. doi: 10.1128/MCB.01789-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim MJ, Kim E, Ryu SH, Suh PG. The mechanism of phospholipase C- gamma1 regulation. Exp Mol Med. 2000;32:101–109. doi: 10.1038/emm.2000.18. [DOI] [PubMed] [Google Scholar]
- 55.Brunati AM, Donella-Deana A, James P, Quadroni M, Contri A, Marin O, Pinna LA. Molecular features underlying the sequential phosphorylation of HS1 protein and its association with c-Fgr protein-tyrosine kinase. J Biol Chem. 1999;274:7557–7564. doi: 10.1074/jbc.274.11.7557. [DOI] [PubMed] [Google Scholar]
- 56.Cavnar PJ, Mogen K, Berthier E, Beebe DJ, Huttenlocher A. The actin regulatory protein HS1 interacts with Arp2/3 and mediates efficient neutrophil chemotaxis. J Biol Chem. 2012;287:25466–25477. doi: 10.1074/jbc.M112.364562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gotoh N, Toyoda M, Shibuya M. Tyrosine phosphorylation sites at amino acids 239 and 240 of Shc are involved in epidermal growth factor-induced mitogenic signaling that is distinct from Ras/mitogen-activated protein kinase activation. Mol Cell Biol. 1997;17:1824–1831. doi: 10.1128/mcb.17.4.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakahira M, Tanaka T, Robson BE, Mizgerd JP, Grusby MJ. Regulation of signal transducer and activator of transcription signaling by the tyrosine phosphatase PTP-BL. Immunity. 2007;26:163–176. doi: 10.1016/j.immuni.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 59.Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Lennartsson J, Burovic F, Witek B, Jurek A, Heldin CH. Erk 5 is necessary for sustained PDGF-induced Akt phosphorylation and inhibition of apoptosis. Cell Signal. 2010;22:955–960. doi: 10.1016/j.cellsig.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 61.Yang X, Dutta U, Shaw LM. SHP2 mediates the localized activation of Fyn downstream of the α6β4 integrin to promote carcinoma invasion. Mol Cell Biol. 2010;30:5306–5317. doi: 10.1128/MCB.00326-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu W, Gong D, Bar-Sagi D, Cole PA. Site-specific incorporation of a phosphotyrosine mimetic reveals a role for tyrosine phosphorylation of SHP-2 in cell signaling. Mol Cell. 2001;8:759–769. doi: 10.1016/s1097-2765(01)00369-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.