Abstract
Racial and socioeconomic disparities exist in liver transplantation (LT) outcomes among adults, but little research exists for pediatric LT populations. We examined racial differences in graft survival and mortality within a retrospective cohort of pediatric and young adult LT recipients at a large children's transplant center in the Southeast between 1998 and 2011. The association between race/ethnicity and rates of graft failure and mortality was examined with Cox proportional hazards models that were adjusted for demographic and clinical factors as well as individual-level and census tract–level socioeconomic status (SES). Among the 208 LT recipients, 51.0% were white, 34.6% were black, and 14.4% were other race/ethnicity. Graft survival and patient survival were higher for whites versus minorities 1, 3, 5, and 10 years after transplantation. The 10-year graft survival rates were 84% [95% confidence interval (CI) = 76%-91%] for white patients, 60% (95% CI = 46%-74%) for black patients, and 49% (95% CI = 23%-77%) for other race/ethnicity patients. The 10-year patient survival rates were 92% (95% CI = 84%-96%), 65% (95% CI = 52%-79%), and 76% (95% CI = 54%-97%) for the white, black, and other race/ethnicity groups, respectively. In analyses adjusted for demographic, clinical, and socioeconomic characteristics, the rates of graft failure [black: hazard ratio (HR) = 2.59, 95% CI = 1.29-5.45; other: HR = 3.01, 95% CI = 1.23-7.35] and mortality (black: HR = 4.24, 95% CI = 1.54-11.69; other: HR=3.09, 95% CI= 0.78-12.19) were higher for minority groups versus whites. In conclusion, at a large pediatric transplant center in the Southeastern United States, racial/ethnic disparities exist in pediatric and young adult LT outcomes that are not fully explained by measured SES and clinical factors.
Liver transplantation (LT) is the definitive treatment for end-stage liver disease (ESLD) in pediatric populations. Patient survival has improved in recent decades: overall 1-year survival rates currently approach 90%, whereas rates were <70% before 1980.1,2 Research examining risk factors for patient and graft survival following LT have focused on recipient, donor, and intraoperative variables as well as posttransplant treatments and complications.3-11 Although many studies have included race/ethnicity in demographic risk stratification when the data have been available, health disparities in transplant outcomes have not been studied in depth in the pediatric LT recipient population. Health disparities, including racial, ethnic, and socioeconomic disparities, are increasingly appreciated as important factors in the health status of pediatric populations.12,13 Moreover, the effects of health disparities in childhood have profound effects on health in adulthood.14,15 As mortality due to childhood illnesses such as ESLD continues to decline, an improved understanding of predictors of long-term health is necessary for limiting morbidity and maintaining quality of life.
Several studies of the adult LT population have examined the role of race/ethnicity and socioeconomic status (SES) in transplant outcomes with variable results.16-22 Black race has been found to be an independent predictor of mortality among adult LT recipients, even after the introduction of the Model for End-Stage Liver Disease (MELD) score, which was intended to improve the equity of organ allocation.18-20 Several previous studies of adult LT recipients found no association between SES and LT outcomes, but national studies examining the effects of SES on LT outcomes are limited by inadequate measurements of SES factors.16,17,21 For example, one study of the adult LT population found that neighborhood-level income was not an independent predictor of poor outcomes, but the main exposure was measured on the zip code level (a less sensitive and robust measure of poverty) rather than the individual level.17,23
Research among adult LT recipients suggests that there may be racial and socioeconomic disparities in transplant outcomes, but little is known about the impact of race/ethnicity and SES on pediatric LT recipients. The purpose of this study was to examine the association of race/ethnicity with outcomes after LT, including patient and graft survival, in a single-center study with more robust SES information on both the individual level and the neighborhood level. We hypothesized that black and minority race/ethnicity was associated with poor LT outcomes at a large children's transplant center in the Southeastern United States. Furthermore, we hypothesized that SES may partially account for differential outcomes based on race.
Patients and Methods
Data Sources
All pediatric and young adult patients (age ≤ 22 years at our center) who underwent LT at Children's Hospital of Atlanta (CHOA) between January 1998 and December 2008 and were followed through November 2011 were included in this cohort study. Clinical, demographic, and outcome data were abstracted from patient charts with CHOA electronic medical systems (Epic and Organ Transplant Tracking Record), and they were used to determine exposure and outcome variables. Patients who are residents of Georgia and are in need of financial aid are typically referred to the Georgia Transplant Foundation (GTF); specific reasons for referral include assistance with the cost of medications, financial emergencies, lodging, and difficulty in accessing insurance. For this study, GTF provided individual SES and social support data for all CHOA patients referred to GTF over the time period of the study. The United Network for Organ Sharing (UNOS) provided data on donor characteristics, intraoperative characteristics, and posttransplant outcomes (graft failure and mortality).
Study Population
The study population consisted of 208 first LT recipients at CHOA during the study period. All patients were included, regardless of the geographic region (17% of the patients lived outside the state of Georgia, mostly in the Southeastern United States).
Outcome Variables
Outcome data for patient mortality and graft failure were examined through November 2011, and this allowed a minimum follow-up of 3 years. Dates for graft failure and mortality were derived from UNOS data as well as CHOA medical charts. There was 100% agreement between the mortality dates from the 2 data sources. Two graft failure dates reported in the CHOA medical charts were not reported by UNOS, and these were included in the analyses. Rejection episodes were tabulated from CHOA medical charts.
Primary Explanatory Variable
The primary explanatory variable for all analyses was race/ethnicity as reported in CHOA medical hospitalization records. We defined race/ethnicity as a social construct rather than a representation of genetic variability. Race/ethnicity data were obtained from CHOA medical records, but when data were missing, either UNOS or GTF data were used. If discrepancies in race/ethnicity were found between data sources, confirmation was sought from CHOA medical charts. Race/ethnicity was categorized as non-Hispanic white, black, or other race/ethnicity (Hispanic, Asian, Native Hawaiian or other Pacific Islander, multiracial, and unknown) for the analysis.
Patient-Level Covariates
Clinical and demographic variables were obtained from CHOA medical charts, and they included the age at transplant (which was further characterized as <1, 1-5, 6-10, 11-17, or 18-22 years), sex (male or female), and etiology of ESLD (which was categorized as acute hepatic necrosis, biliary atresia, cholestatic liver disease/cirrhosis, metabolic disease, benign and malignant neoplasms, noncholestatic liver disease/cirrhosis, or other). Transplant characteristics from UNOS data included the Pediatric End-Stage Liver Disease (PELD) era (before or after the introduction of the PELD score in 2002), graft type (deceased donor whole, deceased donor reduced, split, or living donor), donor age (0-18 or >18 years), donor sex, and cold ischemia time (hours).
The insurance status was considered the primary SES exposure of interest in the study. The insurance type was determined from CHOA medical charts and GTF records. Insurance was categorized as public [Medicaid (publicly and privately administered) and State Children's Health Insurance Program], private, or other (Consolidated Omnibus Budget Reconciliation Act, charity care, or other).24 Those categorized as having private insurance included participants with partial public coverage provided to disabled children (8.9% of the cohort).24 Noncompliance diagnoses (described as non-adherence in the Results and Discussion sections) were recorded from chart documentation. Noncompliance diagnoses were documented for tacrolimus levels outside the recommended ranges after transplantation. They also signified missed appointments and laboratory visits and other medication nonadherence. Although we were unable to obtain tacrolimus levels for all patients in the cohort throughout the follow-up period, we did obtain these values from the 2009-2011 follow-up for a subset of patients (n=30). A subanalysis showed 85% concordance between a diagnosis of noncompliance and tacrolimus levels outside this range.
Neighborhood-Level Covariates
Several neighborhood-level covariates were considered as key SES measures of interest for this study cohort. Patient addresses from CHOA medical charts were geocoded and linked with US Census data for all patients residing in the United States; census tracts are small geographic areas including approximately 4000 individuals. Thirteen participants (6.2%) had only zip code–level data, and in those cases, the closest geographical census tract was used. Neighborhood characteristics from the 2000 US Census were used to create a composite neighborhood deprivation index (NDI), which was considered the primary neighborhood-level SES measure of interest in this study (Neighborhood Change Database, GeoLytics, Inc., East Brunswick, NJ).
The NDI methodology is detailed elsewhere.25 It includes 8 variables within several domains related to health outcomes: income, poverty, employment, occupational status, family structure, and housing. Once all census data were pooled for 19 heterogeneous cities in the United States, Messer et al.25 used a principal components analysis to create the index. The mean was standardized at 0 with standard deviations at +1 and −1. A higher (positive) number represents more neighborhood-level deprivation, whereas a lower (negative) number represents less deprivation. Five of the 8 components of the index were also analyzed independently [household poverty rate (%), households seeking public assistance (%), female-headed households (%), households with male unemployment (%), and households with an annual income < $30,000 (%)] for the entire population and for the subset of individuals referred to GTF.
Data Analysis
All demographic, clinical, and sociodemographic status variables were compared by race/ethnicity, graft status, and mortality. Differences were assessed by chi-square or analysis of variance tests (Fisher or Wilcoxon signedrank tests were used when data were nonparametric). Kaplan-Meier estimation methods were used to examine the time to graft failure and death, and the log-rank test was used to assess statistical significance.
Separate Cox proportional hazards models were created to examine the effects of black race and minority race/ethnicity on the rates of (1) graft failure and (2) mortality. Graft failure was defined to include first graft loss as well as patient death. Patients with multiple transplants were eliminated from the mortality analysis. The proportional hazards assumption was evaluated for all baseline variables determined to be influential a priori.26 Collinearity was assessed for all variables in both models. For purposes of modeling, variables were regrouped a priori into high-risk and low-risk groups. New variables were categorized as follows: age at transplant (< 1 versus ≥ 1 year), disease etiology (acute hepatic necrosis versus other etiologies), graft type (cadaveric, whole grafts versus technical variants), donor age (0-18 versus >18 years), and insurance type (public versus private). The NDI was used as a continuous variable.
Bivariate analyses of race/ethnicity and included variables were performed for outcomes of graft failure and mortality. Variables were determined a priori and were sequentially added to the model with race/ethnicity to adjust for clinical and demographic factors, and then SES variables were added to create the fully adjusted model. Interactions were assessed between SES variables (insurance type and NDI) and race/ethnicity with likelihood ratio tests. Likelihood ratio tests were also used to assess model significance and for the addition and removal of individual variables. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the effects of minority race/ethnicity were reported for each model.
Secondary Analyses
For residents of Georgia, we considered the effect of seeking GTF assistance as a potential SES indicator in the main analyses. However, the use of GTF assistance as an indicator variable in multivariate analyses did not meet the proportionality assumption in Cox models.
In addition, for the subset of patients who received assistance from GTF at the time of their initial evaluation, we sought to determine the agreement between these more detailed SES measures and the SES measures used for the entire study population (insurance status and NDI). These additional individual SES factors included net monthly income (used to calculate the net yearly income), highest parental employment (unemployed/disabled/retired, part-time employment, or full-time employment), highest parental education (some high school, high school or general equivalency diploma, some college or technical degree, traditional college, or graduate school), usage of public assistance [Supplemental Security Income (SSI), Temporary Assistance for Needy Families (TANF), or other governmental welfare], and family structure (single or widowed, divorced or separated, or married).
For all analyses, a 2-tailed significance level of P<0.05 was used. All statistical analyses were performed with SAS 9.3 (SAS, Cary, NC), and geocoding was performed with ArcGIS 10 (Environmental Systems Research Institute, Redlands, CA). This study protocol was approved by the institutional review board of Emory University.
Sensitivity Analyses
To examine the hypothesis that early outcomes reflect the primary disease, severity of illness at transplant, graft quality, and perioperative complications rather than SES, a further analysis of graft failure and mortality at different intervals after transplantation (3 months, 1 year, greater than one year). A separate analysis excluded those who died during the first year of follow-up. These crude models were then adjusted for SES indicators of the insurance type and NDI. An analysis of the patients in this study for whom MELD/PELD data were collected during the MELD/PELD era (2002-2008) was also performed in order to examine the contribution of health status to racial/ethnic differences in graft failure and mortality.
Results
Description of the Study Cohort
The patients in our cohort were categorized as white (51.0%), black (34.6%), Hispanic (7.2%), Asian (4.8%), Native Hawaiian or Pacific Islander (1.4%), or multiracial/unknown (1.0%). For the analysis, the participants were categorized as white (50.1%), black (34.9%), or other race/ethnicity (14.4%). The median follow-up time for the patients in this study was 8.3 years.
The median age at transplant for all participants was 3.5 years [interquartile range (IQR) = 1.0-13.0], with the majority of the patients (60.6%) receiving their transplants at ≤5 years of age. Black and other minority participants had higher frequencies of transplantation at an age < 1 year in comparison with white patients (Table 1). Biliary atresia was the most common reason for transplantation among all participants, with higher relative frequencies among minority patients.
Table 1. Clinical and Demographic Characteristics of the Study Population at the Time of Transplantation (1998-2008).
| Study Population (n=208) | Whites [n=106 (51.0%)] | Blacks [n=72 (34.6%)] | Other [n=30 (14.4%)] | P Value | |
|---|---|---|---|---|---|
| Clinical variables | |||||
| Age at transplant (years)* | 3.5 (1.0-13.0) | 4.2 (1.3-13.5) | 3.3 (0.9-13.5) | 1.2 (0.7-5.4) | 0.0140 |
| Age categories [n (%)] | 0.0234 | ||||
| <1 year | 55 (26.4) | 19 (17.9) | 23 (32.0) | 13 (43.3) | |
| 1-5 years | 71 (34.1) | 41 (38.7) | 20 (27.8) | 10 (33.3) | |
| 6-10 years | 25 (12.0) | 15 (14.2) | 7 (9.7) | 3 (10.0) | |
| 11-17 years | 36 (17.3) | 21 (19.8) | 12 (15.3) | 4 (13.3) | |
| 18-22 years | 21 (10.1) | 10 (9.4) | 11 (15.3) | 0 (0) | |
| Female sex [n (%)] | 117 (56.2) | 55 (51.9) | 46 (63.9) | 16 (53.3) | 0.2683 |
| Disease etiology [n (%)] | 0.0008 | ||||
| Acute hepatic necrosis | 32 (15.4) | 11 (10.4) | 12 (16.7) | 9 (30.0) | |
| Biliary atresia | 83 (39.9) | 30 (28.3) | 40 (55.6) | 13 (43.3) | |
| Cholestatic liver disease/cirrhosis | 10 (4.8) | 5 (4.7) | 3 (4.2) | 2 (6.7) | |
| Metabolic disease | 24 (11.5) | 18 (17.0) | 4 (5.6) | 2 (6.7) | |
| Neoplasms (benign and malignant) | 9 (4.3) | 7 (6.6) | 1 (1.4) | 1 (3.3) | |
| Noncholestatic liver disease/cirrhosis | 35 (16.8) | 23 (21.7) | 11 (15.3) | 1 (3.3) | |
| Other | 15 (7.2) | 12 (11.3) | 1 (1.4) | 2 (6.7) | |
| Pretransplant characteristics | |||||
| Era [n (%)]† | 0.5341 | ||||
| Pre-MELD/PELD era | 81 (38.9) | 42 (39.6) | 30 (41.7) | 9 (30.0) | |
| MELD/PELD era | 127 (61.1) | 64 (60.4) | 42 (58.3) | 21 (70.0) | |
| Initial MELD/PELD score at wait listing*‡ | 15 (6-23) | 12 (4-22) | 21 (11-26) | 10 (−1 to 22) | 0.0186 |
| MELD/PELD score at transplant* | 17 (6-25) | 12 (1-23) | 22 (15-27) | 15 (4-25) | 0.0040 |
| Wait-list time (days)*§ | 74 (6-182) | 70 (9-189) | 81 (3-182) | 54 (4-143) | 0.8412 |
| Medical condition at transplant [n (%)]§ | 0.0120 | ||||
| At home | 129 (63.5) | 74 (71.8) | 40 (56.3) | 15 (51.7) | |
| Hospitalized | 16 (7.9) | 3 (2.9) | 11 (15.5) | 2 (6.9) | |
| In intensive care unit | 58 (28.6) | 26 (25.2) | 20 (28.2) | 12 (41.4) | |
| Transplant characteristics (first transplant only) | |||||
| Transplant graft type [n (%)] | <0.0001 | ||||
| Deceased donor, whole | 117 (56.2) | 64 (60.4) | 39 (54.2) | 14 (46.7) | |
| Deceased donor, reduced | 63 (30.3) | 23 (21.7) | 25 (34.7) | 15 (50.0) | |
| Split | 8 (3.8) | 0 (0.0) | 7 (9.7) | 1 (3.3) | |
| Living donor | 20 (9.6) | 19 (17.9) | 1 (1.4) | 0 (0.0) | |
| Donor-recipient sex mismatch [n (%)]¶ | 107 (51.7) | 52 (49.5) | 37 (51.4) | 18 (60.0) | 0.5976 |
| Donor age [n (%)]¶ | 0.0300 | ||||
| 0-18 years | 122 (58.9) | 57 (54.3) | 51 (70.8) | 14 (46.7) | |
| >18 years | 85 (41.1) | 48 (45.7) | 21 (29.2) | 16 (53.3) | |
| Cold ischemia time (hours)#** | 8.5±3.8 | 8.6±4.6 | 8.5±2.4 | 7.9±2.8 | 0.7460 |
| Posttransplant characteristics | |||||
| Nonadherence [n (%)]†† | 87 (41.8) | 36 (34.0) | 41 (56.9) | 10 (33.3) | 0.0064 |
| 2 or more rejection episodes [n (%)]‡‡ | 85 (42.1) | 37 (36.3) | 38 (54.3) | 10 (33.3) | 0.0405 |
NOTE: P values in bold are statistically significant (P<0.05).
The data are presented as medians and IQRs.
The PELD severity score was introduced in 2002. It is used for patients less than 12 years old.
Data were missing for 7.1% of the patients eligible for a MELD/PELD score (n = 127).
Data were missing for 3.4% of the patients.
Data were missing for 2.4% of the patients.
Data were missing for 0.4% of the patients.
Data were missing for 30.1% of the patients.
The data are presented as means and standard deviations.
Includes nonadherence to medications, office visits, and laboratory testing.
Data were missing for 2.9% of the patients.
For those who underwent transplantation during the MELD/PELD era (in 2002 or afterward), the MELD/PELD scores at wait-list entry and at transplant differed significantly across racial/ethnic groups (Table 1). Medical conditions at transplant were also significantly different, with higher percentages of black and other race/ethnicity participants undergoing transplantation while they were hospitalized or in intensive care (P = 0.01; Table 1). White patients received a higher percentage of whole grafts from deceased donors than black and other minority patients (60.4%, 54.2%, and 46.7%, respectively; P<0.001). The donor age differed significantly with race/ethnicity: black patients received more transplants from young donors (0-18 years old) in comparison with white and other minority patients (P=0.03). Sex, donor-recipient sex mismatch, and cold ischemia time did not vary significantly across the 3 racial categories (all P>0.05; Table 1).
SES of the Study Population
Data for the health insurance type were missing for 6 patients (2.9%), and 4 of these patients died after transplantation (2 black patients and 2 white patients). Although health insurance coverage was almost evenly split between public/other and private coverage (51.5% versus 48.5%), black and other minority race/ethnicity patients accounted for significantly higher percentages of public insurance utilization in comparison with white participants (64.3%, 75.9%, and 35.9%, respectively; P<0.0001). Neighborhood indicators (income, poverty, public assistance usage, female-headed households, and male unemployment) were all significantly different across racial groups, with the highest percentages of low SES indicators found for black participants (P<0.001; Table 2). Based on the aforementioned indicators, overall neighborhood deprivation was highest for black patients (P<0.0001).
Table 2. Socioeconomic and Social Status Variables by Race/Ethnicity for the Study Population From Transplantation Through the Follow-Up Period (1998-2011).
| Study Population (n=208) | Whites [n=106 (51.0%)] | Blacks [n=72 (34.6%)] | Other [n=30 (14.4%)] | P Value | |
|---|---|---|---|---|---|
| SES: health insurance coverage [n (%)]* | <0.0001 | ||||
| Public (or other) | 104 (51.5) | 37 (35.9) | 45 (64.3) | 22 (75.9) | |
| Private | 98 (48.5) | 66 (64.1) | 25 (35.7) | 7 (24.1) | |
| Neighborhood level indicators† | |||||
| Poverty (%)‡ | 9.0 (3.9-17.3) | 5.8 (3.3-13.3) | 14.4 (8.0-23.0) | 6.3 (2.6-13.1) | <0.0001 |
| Low parental education (%)ठ| 18.4 (10.6-30.8) | 16.6 (9.4-28.0) | 25.5 (11.7-35.5) | 13.6 (6.7-20.7) | 0.0023 |
| Sought public assistance: SSI, TANF, or welfare (%)‡ | 5.5 (2.2-10.5) | 4.7 (2.2-8.6) | 9.6 (4.5-16.3) | 3.1 (1.7-6.1) | <0.0001 |
| Yearly income < $30,000 (%)‡ | 24.4 (12.2-38.5) | 18.9 (11.0-32.1) | 32.0 (21.7-48.7) | 14.2 (7.6-28.7) | <0.0001 |
| Female-headed household (%)‡ | 20.8 (11.8-33.9) | 16.8 (10.0-26.4) | 31.7 (21.4-47.3) | 15.8 (10.0-29.4) | <0.0001 |
| Male unemployment (%)‡ | 4.0 (2.4-.4) | 3.0 (2.1-5.2) | 6.2 (3.5-8.9) | 3.4 (2.4-5.0) | <0.0001 |
| NDI‡¶ | −0.34±0.93 | −0.59±0.72 | 0.18±1.01 | −0.73±0.84 | <0.0001 |
NOTE: P values in bold are statistically significant (P<0.05).
Data were missing for 2.9% of the patients.
These indicators represent information for all households in a participant's census tract; data were missing for 0.9% of the patients because of addresses outside the United States.
The data are presented as medians and IQRs. §The parents did not complete high school.
The NDI represents the level of socioeconomic deprivation in a census tract and is based on an analysis of 19 heterogeneous US cities using 2000 US census data.25 If the NDI is 0, the deprivation for a given area is at the mean for this broad sample of US cities. An NDI of +1 or −1 represents a standard deviation of deprivation above or below the mean, respectively. Deprivation is based on the household poverty rate (%), households seeking public assistance (%), female-headed households (%), households with unemployed persons (%), households with an annual income<$30,000 (%), males employed in managerial and professional occupations (%), males and females with no high school education (%), and residents with crowded housing (%).
The data are presented as means and standard deviations.
Graft Failure and Mortality Outcomes
In all, 53 patients (25.5%) experienced graft failure during the median study follow-up of 8.3 years. When we compared baseline clinical, demographic, and SES indicators across graft statuses after transplantation, we found that only the age at transplant, race/ethnicity, MELD/PELD era, and graft type were significantly associated with the graft status after first transplant (Table 3). The MELD/PELD scores at wait-list entry and transplant were not associated with outcomes (P = 0.5676 and P = 0.7313; Table 3). Graft failure was most frequently due to primary nonfunction, chronic rejection, or hepatic artery thrombosis. Thirty-four patients (16.4%) died during the study period. Among all the indicators analyzed for mortality, only the age at transplant and race/ethnicity were significantly associated with mortality, with participants undergoing transplantation at <1 year showing the highest mortality rate (Table 4). The cause of death was most often related to an infection or a malignancy.
Table 3. Clinical, Demographic, and Socioeconomic Variables of the Study Population by Graft Status.
| Study Population (n=208) | Graft Failure [n=53 (25.5%)] | No Graft Failure [n=155 (74.5%)] | P Value | |
|---|---|---|---|---|
| Clinical and demographic variables | ||||
| Age at transplant (years)* | 3.5 (1.0-13.0) | 1.2 (0.8-13.9) | 4.0 (1.1-11.5) | 0.1545 |
| Age categories [n (%)] | <0.0001 | |||
| <1 year | 55 (26.4) | 21 (39.6) | 34 (21.9) | |
| 1-5 years | 71 (34.1) | 15 (28.3) | 56 (36.1) | |
| 6-10 years | 25 (12.0) | 0 (0.0) | 25 (16.1) | |
| 11-17 years | 36 (17.3) | 7 (13.2) | 29 (18.7) | |
| 18-22 years | 21 (10.1) | 10 (18.9) | 11 (7.1) | |
| Female sex [n (%)] | 117 (56.2) | 29 (54.7) | 88 (56.8) | 0.7944 |
| Disease etiology [n (%)] | 0.8603 | |||
| Acute hepatic necrosis | 32 (15.4) | 8 (15.1) | 24 (15.5) | |
| Biliary atresia | 83 (39.9) | 22 (41.5) | 61 (39.4) | |
| Cholestatic liver disease/cirrhosis | 10 (4.8) | 2 (3.8) | 8 (5.2) | |
| Metabolic disease | 24 (11.5) | 8 (15.1) | 16 (10.3) | |
| Neoplasms (benign and malignant) | 9 (4.3) | 3 (5.7) | 6 (3.9) | |
| Noncholestatic liver disease/cirrhosis | 35 (16.8) | 8 (15.1) | 27 (17.4) | |
| Other | 15 (7.2) | 2 (3.8) | 13 (8.4) | |
| Race/ethnicity [n (%)] | 0.0132 | |||
| White | 106 (51.0) | 18 (34.0) | 88 (56.8) | |
| Black | 72 (34.6) | 24 (45.3) | 48 (31.0) | |
| Other | 30 (14.4) | 11 (20.8) | 19 (12.3) | |
| Pretransplant characteristics | ||||
| Era [n (%)]† | 0.0163 | |||
| Pre-MELD/PELD era | 81 (38.9) | 28 (52.8) | 53 (34.2) | |
| MELD/PELD era | 127 (61.1) | 25 (47.2) | 102 (65.8) | |
| Initial MELD/PELD score at wait listing*‡ | 15 (6-23) | 12 (−1 to 24) | 15 (7-23) | 0.5676 |
| MELD/PELD score at transplant* | 17 (6-25) | 19 (4-27) | 17 (6-24) | 0.7313 |
| Wait-list time (days)*§ | 74 (6-182) | 80 (6-182) | 70 (6-188) | 0.8432 |
| Medical condition at wait listing [n (%)]† | 0.6181 | |||
| At home | 129 (63.5) | 30 (58.8) | 99 (65.1) | |
| Hospitalized | 16 (7.9) | 5 (9.8) | 11 (7.2) | |
| In intensive care unit | 58 (28.6) | 16 (31.4) | 42 (27.6) | |
| Transplant characteristics (first transplant only) | ||||
| Transplant graft type [n (%)] | <0.0001 | |||
| Deceased donor, whole | 117 (56.2) | 25 (47.2) | 92 (59.4) | |
| Deceased donor, reduced | 63 (30.3) | 17 (32.1) | 46 (29.7) | |
| Split | 8 (3.8) | 4 (7.5) | 4 (2.6) | |
| Living donor | 20 (9.6) | 7 (13.2) | 13 (8.4) | |
| Donor-recipient sex mismatch [n (%)]¶ | 107 (51.7) | 26 (49.1) | 81 (52.3) | 0.6564 |
| Donor age [n (%)]¶ | 0.1964 | |||
| 0-18 years | 122 (58.9) | 27 (50.9) | 95 (61.7) | |
| >18 years | 85 (41.1) | 26 (49.1) | 59 (38.3) | |
| Cold ischemia time (hours)#** | 8.5±3.8 | 8.5±3.0 | 8.5±4.0 | 0.9949 |
| Posttransplant characteristics | ||||
| Nonadherence [n (%)]†† | 87 (41.8) | 23 (43.4) | 64 (41.3) | 0.7885 |
| 2 or more rejection episodes [n (%)]‡‡ | 85 (42.1) | 24 (46.2) | 61 (40.7) | 0.4898 |
| SES: health insurance coverage [n (%)]§§ | 0.4131 | |||
| Public (or other) | 104 (51.5) | 28 (57.1) | 76 (49.7) | |
| Private | 98 (48.5) | 21 (42.9) | 77 (50.3) | |
| Neighborhood level indicator: NDI**†† | −0.34±0.93 | −0.30±0.99 | −0.35±0.91 | 0.6733 |
NOTE: P values in bold are statistically significant (P<0.05).
The data are presented as medians and IQRs.
The PELD severity score was introduced in 2002. It is used for patients less than 12 years old.
Data were missing for 7.1% of the patients eligible for a MELD/PELD score (n = 127).
Data were missing for 3.4% of the patients.
Data were missing for 2.4% of the patients.
Data were missing for 0.4% of the patients.
Data were missing for 30.1% of the patients.
The data are presented as means and standard deviations.
Includes nonadherence to medications, office visits, and laboratory testing.
Data were missing for 2.9% of the patients.
Data were missing for 2.9% of the patients.
This indicator represents information for all households in a participant's census tract; data were missing for 0.9% of the patients because of addresses outside the United States.
Table 4. Clinical, Demographic, and Socioeconomic Variables of the Study Population by Mortality Status.
| Study Population (n=208) | Deceased [n=34 (16.4%)] | Alive [n=174 (83.7%)] | P Value | |
|---|---|---|---|---|
| Clinical and demographic variables | ||||
| Age categories [n (%)] | 0.0025 | |||
| <1 year | 55 (26.4) | 13 (38.2) | 42 (24.1) | |
| 1-5 years | 71 (34.1) | 8 (23.5) | 63 (36.2) | |
| 6-10 years | 25 (12.0) | 0 (0.0) | 25 (14.4) | |
| 11-17 years | 36 (17.3) | 5 (14.7) | 31 (17.8) | |
| 18-22 years | 21 (10.1) | 8 (23.5) | 13 (7.5) | |
| Female sex [n (%)] | 117 (56.2) | 20 (58.8) | 97 (55.8) | 0.8506 |
| Disease etiology [n (%)] | 0.9065 | |||
| Acute hepatic necrosis | 32 (15.4) | 7 (20.6) | 25 (14.4) | |
| Biliary atresia | 83 (39.9) | 14 (41.2) | 69 (39.7) | |
| Cholestatic liver disease/cirrhosis | 10 (4.8) | 1 (2.9) | 9 (5.2) | |
| Metabolic disease | 24 (11.5) | 5 (14.7) | 19 (10.9) | |
| Neoplasms (benign and malignant) | 9 (4.3) | 1 (2.9) | 8 (4.6) | |
| Noncholestatic liver disease/cirrhosis | 35 (16.8) | 5 (14.7) | 30 (17.2) | |
| Other | 15 (7.2) | 1 (2.9) | 14 (8.0) | |
| Race/ethnicity [n (%)] | 0.0029 | |||
| White | 106 (51.0) | 9 (26.5) | 97 (55.7) | |
| 72 (34.6) | 20 (58.8) | 52 (29.9) | ||
| Other | 14 (14.4) | 5 (14.7) | 25 (14.4) | |
| Pretransplant characteristics | ||||
| Era [n (%)]* | 0.0672 | |||
| Pre-MELD/PELD era | 81 (38.9) | 18 (52.9) | 63 (36.2) | |
| MELD/PELD era | 127 (61.1) | 16 (47.1) | 111 (63.8) | |
| Initial MELD/PELD score at wait listing | 15 (6-23) 21 | (21 to 33) | 14 (6-23) | 0.5023 |
| MELD/PELD score at transplant† | 17 (6-25) | 21 (4-29) | 17 (6-24) | 0.4483 |
| Wait-list time (days)†§ | 74 (6-182) | 75 (5-165) | 74 (6-188) | 0.5820 |
| Medical condition at wait listing [n (%)]* | 0.5087 | |||
| At home | 129 (63.5) | 19 (59.4) | 110 (64.3) | |
| Hospitalized | 16 (7.9) | 4 (12.5) | 12 (7.0) | |
| In intensive care unit | 58 (28.6) | 9 (28.1) | 49 (28.7) | |
| Transplant characteristics (first transplant only) | ||||
| Transplant graft type [n (%)] | 0.7543 | |||
| Deceased donor, whole | 117 (56.2) | 19 (55.9) | 98 (56.3) | |
| Deceased donor, reduced | 63 (30.3) | 11 (32.4) | 52 (29.9) | |
| Split | 8 (3.8) | 2 (5.9) | 6 (3.4) | |
| donor | 20 (9.6) | 2 (5.9) | 18 (10.3) | |
| Donor-recipient sex mismatch [n (%)]¶ | 107 (51.7) | 16 (47.1) | 91 (52.6) | 0.5544 |
| Donor age [n (%)]¶ | 0.6921 | |||
| 0-18 years | 122 (58.9) | 19 (55.9) | 103 (59.5) | |
| >18 years | 85 (41.1) | 15 (44.1) | 70 (40.5) | |
| Cold ischemia time (hours)#** | 8.5±3.8 | 8.5±3.1 | 8.5±3.9 | 0.9492 |
| Posttransplant characteristics | ||||
| Nonadherence [n (%)]†† | 87 (41.8) | 13 (38.2) | 74 (42.5) | 0.6425 |
| 2 or more rejection episodes [n (%)]‡‡ | 85 (42.1) | 14 (41.2) | 71 (42.3) | 0.9069 |
| SES: health insurance coverage [n (%)]§§ | 0.3301 | |||
| Public (or other) | 104 (51.5) | 18 (60.0) | 86 (50.0) | |
| Private | 98 (48.5) | 12 (40.0) | 86 (50.0) | |
| Neighborhood level indicator: NDI**** | −0.34±0.93 | −0.13±1.04 | −0.38±0.91 | 0.1514 |
NOTE: P values in bold are statistically significant (P<0.05).
The PELD severity score was introduced in 2002. It is used for patients less than 12 years old.
The data are presented as medians and IQRs.
Data were missing for 7.1% of the patients eligible for a MELD/PELD score (n5127).
Data were missing for 3.4% of the patients.
Data were missing for 2.4% of the patients.
Data were missing for 0.4% of the patients.
Data were missing for 30.1% of the patients.
The data are presented as means and standard deviations.
Includes nonadherence to medications, office visits, and laboratory testing.
Data were missing for 2.9% of the patients.
Data were missing for 2.9% of the patients.
This indicator represents information for all households in a participant's census tract; data were missing for 0.9% of the patients because of addresses outside the United States.
Racial and Socioeconomic Differences in Patient Outcomes
The time to graft failure and mortality differed with race/ethnicity. Graft survival was lowest for patients classified as other race/ethnicity: the 10-year graft survival rates were 49.0% (95% CI = 21.5%-76.5%) for other race/ethnicity patients, 60.1% (95% CI = 46.2%-74.1%) for black patients, and 83.8% (95% CI576.4%-91.2%) for white patients (P = 0.0062; Fig. 1). Differences in graft survival between white and minority racial groups increased during the follow-up period (Fig. 1). Minority participants also had significantly lower survival rates throughout the study period: the 10-year survival rate was 65.5% (95% CI = 51.6%-79.3%) for black patients, 75.6% (95% CI = 54.2%-96.9%) for other race/ethnicity patients, and 91.9% (95% CI = 86.5%-97.3%) for white patients (P = 0.0020; Fig. 1).
Figure 1.
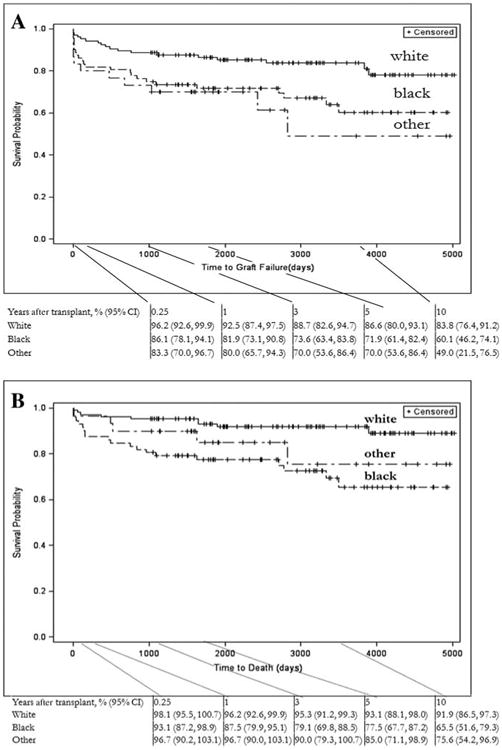
Kaplan-Meier estimates of posttransplant outcomes by race/ethnicity: (A) graft survival stratified by race/ethnicity and (B) patient survival stratified by race/ethnicity.
In crude Cox models of graft failure and mortality, black race and other minority race/ethnicity were associated with a significantly increased rate of graft failure across the follow-up period (black versus white: HR = 2.62, 95% CI = 1.34-5.13; other versus white: HR = 3.52, 95% CI = 1.54-8.02). When the models were adjusted for relevant clinical factors, this rate decreased for other minority race/ethnicity patients to 3.1 times the rate for white participants (HR = 3.2, 95% CI = 1.32-7.67) and for black patients to 2.5 times the rate for white patients (HR = 2.5, 95% CI =1.29-4.96). After SES variables were included, the rate of graft failure for other minority race/ethnicity participants decreased further to 3.0 times the rate for white participants (HR = 3.0, 95% CI = 1.23-7.35), and the rate for black participants increased to 2.6 times the rate for white participants (HR = 2.6, 95% CI = 1.29-5.45; Tables 5 and 6).
Table 5. Multivariate Cox Proportional Hazards Models for the Effect of Black Race on Graft Failure and Mortality With Adjustments for Clinical and Demographic Characteristics.
| Black: White [HR (95% CI)] | P Value | |
|---|---|---|
| Models for graft failure | ||
| Crude model: race/ethnicity alone | 2.62 (1.34-5.13) | 0.0048 |
| Race/ethnicity adjusted for clinical/demographic factors: race/ethnicity+-age at transplant+graft type | 2.53 (1.29-4.96) | 0.0136 |
| Fully adjusted model: race/ethnicity+age at transplant+graft type-+insurance type+NDI | 2.59 (1.29-5.45) | 0.0407 |
| Models for mortality | ||
| Crude model: race/ethnicity alone | 5.05 (2.00-12.73) | 0.0006 |
| Race/ethnicity adjusted for clinical/demographic factors: race/ethnicity+-age at transplant+medical condition at wait listing | 4.84 (1.91-12.26) | 0.0043 |
| Fully adjusted model: race/ethnicity+age at transplant+medical condition at wait listing+insurance type+NDI | 4.24 (1.54-11.69) | 0.0296 |
NOTE: P Values in bold are statistically significant (P<0.05).
Table 6. Multivariate Cox Proportional Hazards Models for the Effect of Other Minority Race/Ethnicity on Graft Failure and Mortality With Adjustments for Clinical and Demographic Characteristics.
| Other: White [HR (95% CI)] | P Value | |
|---|---|---|
| Models for graft failure | ||
| Crude model: race/ethnicity alone | 3.52 (1.54-8.02) | 0.0028 |
| Race/ethnicity adjusted for clinical/demographic factors: race/ethnicity+-graft type+PELD era+medical condition at wait listing | 3.18 (1.32-7.67) | 0.0132 |
| Fully adjusted model: race/ethnicity+graft type+PELD era+medical condition at wait listing+NDI | 3.01 (1.23-7.35) | 0.0214 |
| Models for mortality | ||
| Crude model: race/ethnicity alone | 2.88 (0.81-10.27) | 0.1035 |
| Race/ethnicity adjusted for clinical/demographic factors: race/ethnicity+-age at transplant+graft type+PELD era | 3.18 (0.82-12.38) | 0.3670 |
| Fully adjusted model: race/ethnicity+age at transplant+etiology of liver failure+NDI | 3.09 (0.78-12.19) | 0.5115 |
NOTE: P Values in bold are statistically significant (P<0.05).
In multivariate analyses, we observed an increased mortality rate when we examined the effect of minority race/ethnicity versus white patients (black versus white: HR=5.05, 95% CI = 2.00-12.73; other versus white: HR = 2.88, 95% CI = 0.81-10.27). After adjustments for relevant clinical factors, the mortality rate decreased for black patients (black versus white: HR = 4.84, 95% CI = 1.91-12.26), whereas the rate increased for other minority race/ethnicity patients (other versus white: HR = 3.18, 95% CI=0.82-12.38). When we included SES variables, the mortality rate for black participants and other minority race/ethnicity participants decreased (black versus white: HR = 4.24, 95% CI = 1.54-11.69; other versus white: HR = 3.09, 95% CI = 0.78-12.19; Tables 5 and 6). Rates of graft failure and mortality were significantly elevated for black patients versus white patients in all models, whereas rates for other minority race/ethnicity patients were significantly elevated only for graft failure. Notably, there was no significant interaction between SES variables (insurance type and NDI) and race/ethnicity.
With designations of mild, moderate, severe, and clinical, the number of rejections for a given individual ranged from 0 to 10 during the follow-up period. The total number of rejections did not significantly differ between racial and ethnic groups, with a median of 1 rejection for the white and other race/ethnicity groups and a median of 2 rejections for the black patients [white: median =1 (IQR = 0-2.0); black: median=2 (IQR = 0-3.0); other: median=1 (IQR=0-3.0); P = 0.1105]. However, a larger proportion of the black participants had 2 or more rejection episodes in comparison with the white and other race/ethnicity groups (P = 0.0405; Table 1). Rejection episodes also did not significantly differ for the transplant outcomes of graft failure and mortality (P for graft failure=0.4898 and P for mortality = 0.9069; Tables 3 and 4).
Secondary Analyses
GTF provided assistance to 114 patients (54.8%) in the study cohort, with black patients having the highest percentages for all individual indicators of low 7 SES and social support (Table 7). Among all patients who were referred for assistance to GTF, 56.9% received public assistance at the time of the initial evaluation, and 26.0% had “partial high school” listed as the highest level of parental education. The prevalence of low SES indicators among our minority patients was even more pronounced when the data were stratified by race. For example, in the population referred to GTF, 85.4% of the black participants and 73.3% of the other minority participants had net yearly incomes<$30,000, whereas 55.1% of the white participants did. When we examined net yearly incomes, we found that 55.1% of white GTF participants had household incomes<$30,000, whereas 19.8% by neighborhood level did for this group; among minority participants, this difference increased to 81.3% versus 27.1%. For all individual-level indicators, low SES variables were present at higher percentages among black patients. When neighborhood indicators were analyzed for the GTF subset (for which individual-level SES measures were available), neighborhood-level indicators underestimated all individual-level SES and social support indicators except for education level (data not shown).
Table 7. Socioeconomic and Social Status Variables by Race/Ethnicity for the Study Population From GTF From Transplantation Through the Follow-Up Period (1998-2011).
| Socioeconomic and Social Status | Study Population (n=208) | Whites [n=106 (51.0%)] | Blacks [n=72 (34.6%)] | Other [n=30 (14.4%)] | P Value |
|---|---|---|---|---|---|
| Sought GTF assistance [n (%)] | 114 (54.8) | 50 (47.2) | 49 (68.1) | 15 (50.0) | 0.0195 |
| Variables for patients who sought assistance | |||||
| Net yearly income<30,000 [n (%)]* | 79 (70.5) | 27 (55.1) | 41 (85.4) | 11 (73.3) | 0.0045 |
| Highest parental education [n (%)]† | 0.0012 | ||||
| Partial high school | 20 (26.0) | 6 (15.8) | 14 (46.7) | 0 | |
| High school graduate | 40 (52.0) | 21 (55.3) | 13 (43.3) | 6 (66.7) | |
| Partial college | 7 (9.1) | 2 (5.3) | 3 (10) | 2 (22.2) | |
| College or master's degree | 10 (13.0) | 9 (23.7) | 0 | 1 (11.1) | |
| Parental employment status [n (%)]‡ | 0.0018 | ||||
| Full-time employment or student | 44 (44.4) | 25 (56.8) | 10 (23.8) | 9 (69.2) | |
| Part-time employment | 14 (14.1) | 5 (11.4) | 6 (14.3) | 3 (23.1) | |
| Unemployed, disabled, or retired | 41 (41.4) | 14 (31.8) | 26 (61.9) | 1 (7.7) | |
| Public assistance as income source: SSI, TANF, or welfare [n (%)]§ | 58 (56.9) | 22 (47.8) | 31 (70.5) | 5 (41.7) | 0.0504 |
| Family structure [n (%)]║ | <0.0001 | ||||
| Married | 45 (42.1) | 25 (53.2) | 10 (21.7) | 10 (71.4) | |
| Divorced or separated | 12 (11.2) | 11 (23.4) | 0 | 1 (7.1) | |
| Single or widowed | 50 (46.7) | 11 (23.4) | 36 (78.3) | 3 (21.4) |
NOTE: P values in bold are statistically significant (P<0.05).
Data were missing for 1.8% of the patients.
Data were missing for 32.5% of the patients.
Data were missing for 13.2% of the patients.
Data were missing for 10.5% of the patients.
Data were missing for 6.1% of the patients.
Sensitivity Analyses
Racial/ethnic differences in graft and patient survival increased throughout the follow-up (Fig. 1). When we examined graft survival and mortality at 90 days and 1 year, we found that the rates mostly remained unchanged after adjustments for SES when we compared black and white patients. There was a slight decline in the rates after an adjustment for SES when we examined the outcomes after 1 year of follow-up (crude rate of graft failure: HR = 1.95, 95% CI = 0.83-4.59; rate after adjustment: HR = 1.59, 95% CI = 0.57-4.45; crude mortality rate: HR = 4.01, 95% CI = 1.39-11.56; rate after adjustment: HR = 3.59, 95% CI =1.00-12.82). According to other/white race/ethnicity comparisons, rates of graft failure increased after adjustments for SES factors (crude rate of graft failure: HR = 2.77, 95% CI = 0.93-8.21; rate after adjustment: HR = 4.31, 95% CI = 1.22-15.20). When we examined those who survived for 1 year after transplantation, we found similar rates of graft failure and mortality for black participants after adjustments for SES (crude rate of graft failure: HR = 1.96, 95% CI = 0.80-4.83; rate after adjustment: HR = 1.56, 95% CI = 0.55-4.45; crude mortality rate: HR = 4.52, 95% CI = 1.42-14.43; rate after adjustment: HR = 3.48, 95% CI = 0.97-12.57). In other racial/ethnic groups, the rates of graft failure and mortality increased after adjustments for SES.
Although MELD/PELD scoring was not introduced until 2002 and thus could not be examined for the entire cohort, we compared patients with MELD/PELD scores to participants who underwent transplantation before 2002 when the MELD/PELD system was introduced. We found that adjusting for the MELD/PELD score attenuated the effect of minority race/ethnicity on graft failure and mortality (Table 8).
Table 8. Multivariate Cox Proportional Hazards Models for the Effect of Race/Ethnicity (Blacks and Other Minorities) on Graft Failure and Mortality With Adjustments for MELD/PELD Scores and SES Factors Among Patients Undergoing Transplantation After the Introduction of the MELD/PELD System (2002-2008).
| Black Versus White Race/Ethnicity | HR (95% CI) |
|---|---|
| Graft failure adjusted for SES | 2.01 (0.70-5.72) |
| Graft failure adjusted for SES and MELD/PELD | 1.62 (0.58-4.55) |
| Mortality adjusted for SES | 2.32 (0.63-8.63) |
| Mortality adjusted for SES and MELD/PELD | 1.98 (0.55-7.09) |
|
| |
| Other Versus White Race/Ethnicity | HR (95% CI) |
|
| |
| Graft failure adjusted for SES | 3.72 (1.05-13.26) |
| Graft failure adjusted for SES and MELD/PELD | 3.14 (1.01-9.78) |
| Mortality adjusted for SES | 3.81 (0.69-21.01) |
| Mortality adjusted for SES and MELD/PELD | 3.45 (0.70-16.93) |
Discussion
This study is the first to directly examine the effects of race/ethnicity on pediatric and young adult LT outcomes while adjusting for both individual-level and census tract–level SES characteristics in addition to baseline demographic and clinical characteristics. The degree of racial/ethnic and socioeconomic inequality found in the Southeastern United States is reflected in the findings of this study. Minorities had almost 4 times the mortality rate of whites and 2 to 3 times the graft failure rate of whites, even after adjustments for relevant clinical and SES factors. These results suggest that our measures of SES do not fully explain the effects of race/ethnicity on outcomes for pediatric and young adult LT recipients. We believe that other unknown or unmeasured factors may contribute to racial/ethnic disparities in outcomes. These factors most likely involve unmeasured differences in SES affecting pretransplant and posttransplant disease states. We found racial differences in nonadherence to medical care across racial groups, with black participants showing the highest proportion of nonadherence in our population.3 Given the strong correlation between adherence and SES, we suggest that unmeasured SES differences may explain many of the disparities found in our study.
We used race/ethnicity as a social construct in our analysis. We did not intend to describe genetic differences in disease outcomes, although admittedly we were unable to exclude a genetic contribution. Moreover, there are no well-described genetic differences in ESLD between racial groups. Although higher rates of biliary atresia have been reported for black populations, further studies have not consistently confirmed this difference.27 In general, the influence of race/ethnicity and SES on pediatric LT outcomes is not well understood. Few studies in the pediatric ESLD population have examined the impact of sociodemographic factors on LT outcomes. SES is often difficult to describe in epidemiological studies because of a lack of standardized reporting of many social variables related to health. Social support, family composition, parental education, and family income and wealth can all contribute to measures of SES in children and adolescents.15,28-32 These variables are often unmeasured in large, population-based studies. In any population, social inequality and social gradients are useful in describing the effects of SES.33,34 Along with individual SES factors, neighborhood measures of SES and inequality help to describe the impact of sociodemographic variables on a child's health sta-tus.31,32,35 A combination of neighborhood-level factors may be useful in describing environmental effects on SES; this is what Messer et al.25,36 used to describe perinatal outcomes in a diverse multistate US population.
Although prior studies have examined the potential influence of race/ethnicity on transplant outcomes,4-7,11,37,38 no other studies of which we are aware have documented an association between race/ethnicity and outcomes among pediatric LT recipients. For example, a study of national pediatric and adult data showed no significant differences in pediatric patient and graft survival (at 1, 3, 5, and 10 years) between racial/ethnic groups when national Scientific Registry of Transplant Recipients data from 1994 to 2006 were examined.37 However, these studies did not examine regional differences in racial/ethnic disparities. One potential explanation may be that there is an unexplored interaction between race/ethnicity and geographical region. In other solid organ transplantation, research has suggested an effect of race and SES on patient access and outcomes in pediatric populations.39,40 The Southeastern United States has been found to have higher levels of racial and socioeconomic inequalities, and health disparities have been described in adult access to transplantation.41,42 Thus, it is possible that racial/ethnic differences in transplant outcomes in the pediatric LT recipient population may also vary by region, and they could also be concentrated in the South or Southeastern United States
Additionally, there may be other unmeasured factors that could account for some of the observed racial/ethnic differences in outcomes. Differences in graft and patient survival increased between white and minority racial/ethnic groups during the follow-up period, and this could be partially explained by changes in operative characteristics and treatments after transplantation. Multivariate analyses only partially accounted for these. Sensitivity analyses showed that adjustments for clinical severity (MELD/PELD) partially explained differential outcomes by race/ethnicity. However, it is unclear why there are racial/ethnic differences in MELD/PELD scores, and future analyses should seek to examine why a racial/ethnic disparity in the health status of these pediatric patients exists. Phase of care, also explored in sensitivity analyses, revealed the potential importance of further adjustments for SES. Outcomes were universally worse for the minority groups studied, yet the exact mechanism of the differential outcomes between the minority groups remains unclear. Small sample sizes, which were most pronounced for the other race/ethnicity group, likely explain some of the paradoxical findings in the modeling. Thus, residual or unmeasured confounding could partially explain some of the racial/ethnic differences in patient and graft survival that we observed.
The strong effect of race/ethnicity and SES on health outcomes, however, is consistent with the existing literature on pediatric outcomes.14,43 Racial discrimination and social inequality in a child's perinatal life and childhood can affect long-term health and cause premature mortality.44 Moreover, black race is a predictor of poor health outcomes in all life stages, possibly because of an increased effect of social stressors and discrimination creating an allostatic load leading to chronic inflammation.14,43 National studies show black race as an independent predictor of acute and chronic graft rejection in pediatric LT, and this is consistent with a hypothesis of increased inflammation.6,45 Low SES may potentiate the effects of black and minority race/ethnicity on health outcomes; this can be seen in nonadherence to immunosuppressive medications among pediatric LT recipients and in the greater risk of graft rejection and graft failure.3,46 Public insurance, indicating a low SES, is associated with increased posttransplant mortality and wait-list times for children with biliary atresia.47 Thus, the results of our study are consistent with the existing literature on race/ethnicity and health in pediatric LT populations.
Although the thousands of children who have undergone LT account for only a small fraction of the 74.2 million children living in the United States, the public health impact of their illness is broad.48,49 Along with other survivors of childhood illness, these patients represent a new challenge to health care delivery and chronic care management, and they mirror models more frequently discussed for adults.5,50,51 Thus, a reliance on community resources and many other contextual factors likely become more important in maintaining health after transplantation.52 Sensitivity analyses in our study confirm a stronger effect of SES when it is examined later during follow-up.
Survivors of pediatric LT continue to use high levels of health care resources and experience a great deal of morbidity from their disease because of posttransplant complications.49 These complications are the most important predictors of graft and patient survival, and their possible relationships with race/ethnicity and SES factors suggest avenues for more research and potential interventions in the posttransplant period.3,7,45 In our study, increasing disparities after transplantation imply the need for improved chronic care models.
Along with the posttransplant complications in our population, pretransplant factors such as waiting-list times and referral for transplantation also warrant further study. Racial/ethnic and SES variables may be important to these processes because they are related to access and health care utilization.14,43 A national multicenter study examining regional differences will help us to understand whether these disparities are present in other centers without the degree of racial/ethnic inequality present in the Southeastern United States. Although national cohorts and reported national statistics include reported race/ethnicity, there are no standardized measures of SES beyond insurance categories, and this makes the use of neighborhood-level indicators an important tool for additional research in this population.49
Several limitations exist in this study, and they are related to the small sample size, the limitations of the data sources, and the length of patient follow-up. Racial/ethnic categories were combined for the analysis, and this limited the conclusions that could be drawn for other races and ethnicities. Furthermore, the study was likely underpowered for detecting significant racial/ethnic differences in outcomes, as suggested by the wide confidence limits. This study was a single-center study in the Southeastern United States; the results may not be generalizable to other pediatric LT recipients outside this region. For example, our population included participants as old as 22 years at transplant: this is the standard for our transplant center but may not be the standard maximum age of transplantation for all pediatric LT centers. Residual or unmeasured confounding may be a possibility in this study. Although we did adjust for relevant pretransplant factors when they were available, we may not have fully captured disease severity before transplantation. For example, we were unable to use PELD scores in our analysis because of the high degree of missingness and exceptions in our population (they were available only for patients with chronic liver failure). We also had limited access to clinical data such as immunosuppressive regimens, medication adherence, and severity of illness before transplantation (eg, intensive care unit care, PELD scores, and pertinent laboratory findings).
Although we used numerous individual and neighborhood SES factors, our assessment of SES for each patient may have been incomplete. Multiple data sources provided different estimates of factors such as the insurance type, which required further comparisons in the transplant record, and the insurance type may have varied over time because of changes in coverage. Also, the insurance status provides incomplete information on the economic status of children because of additional programs and eligibilities for families for improving coverage.24 Direct individual SES and social support characteristics were limited to patients referred to GTF for financial need, so these more detailed SES measures were not available for the approximately 50% of our patients who did not seek assistance from GTF. However, because CHOA offers information about GTF services to all patients, we assume that those who did not seek GTF assistance had a higher SES than those who did seek assistance.
The use of data from the 2000 US Census for neighborhood characteristics may be another study limitation because many families in the Southeast experienced large economic fluctuations in the decade between 2000 and 2010, and some may have relocated during this period. Relocation alone, however, does not correlate with a significant change in SES.23 Furthermore, negative economic trends in the Southeastern United States imply that neighborhood covariates from 2000 underestimate SES for our cohort of patients. In our secondary analyses, we found that both insurance status and NDI data used in this study underestimated the SES of GTF patients. For many patients in our study who were referred to GTF, neighborhood census data used in the analysis severely underestimated the true level of deprivation, especially when they were examined by race/ethnicity. Thus, our estimates of the impact of minority race/ethnicity on poor outcomes after transplantation could potentially be underestimations of the racial/ethnic disparities that exist in mortality and graft failure after pediatric LT. Finally, we did not have access to data on tacrolimus levels for all of the patients in our cohort, and thus we may have underestimated nonadherence. However, an analysis of a subset of our cohort suggested 85% concordance between a diagnosis of noncompliance and out-of-range tacrolimus levels.
The major strength of our study is the examination of race/ethnicity with respect to transplant outcomes with the inclusion of social determinants of health. Our population included a higher percentage of black patients and lower percentages of other minority race/ethnicity patients in comparison with the national population, and this provided enough statistical power to examine racial/ethnic differences in patient outcomes for a relatively large pediatric cohort of LT recipients.49 We also provide the first use of individual SES (insurance status) in combination with neighborhood deprivation in the pediatric transplant literature. The index used to describe levels of deprivation in our population is based on census tract data, which are more sensitive and robust than data based on zip code–level information.35 Neighborhood indicators provide a community-level exposure of a participant's access to resources, and this is an important assessment of a patient's overall SES above and beyond individual-level SES data.
The difference in posttransplant outcomes between minority and white pediatric LT recipients implores further research in this area. Our study was unable to fully explain the factors that account for differential outcomes between racial groups. Pediatric populations demand special attention to reduce disparities because they are extremely vulnerable populations. Detailed measures of SES available on a national and reportable scale will significantly improve the analysis of pediatric LT outcomes in the future. National studies examining regional variations will also help us to understand whether disparities exist in regions with relatively less racial/ethnic and SES deprivation. The results of this study may also imply a need to risk-stratify for racial/ethnic and socioeconomic variables when we are examining pediatric LT outcomes. As survival after ESLD and pediatric LT continues to improve, an understanding of complex individual-and neighborhood-level SES factors is crucial for ensuring equality of outcomes across racial/ethnic groups. In the Southeast, more research is needed to determine the reasons for these racial/ethnic differences in outcomes in order to better identify interventions to reduce disparities in pediatric LT.
Acknowledgments
The authors are grateful for the support of Dr. Michael Kramer (Rollins School of Public Health) in providing the NDI for Georgia. They also thank Sandy McMath and GTF for their collaboration and data sharing.
Rachel E. Patzer was supported in part by grants from the National Center for Advancing Translational Sciences of the National Institutes of Health (awards ULl TR000454 and KL2TR000455). Patzer was also supported in part by the National Institute on Minority Health and Health Disparities (1R24MD008077-01).
Abbreviations
- CHOA
Children's Hospital of Atlanta
- CI
confidence interval
- ESLD
end-stage liver disease
- GTF
Georgia Transplant Foundation
- HR
hazard ratio
- IQR
interquartile range
- LT
liver transplantation
- MELD
Model for End-Stage Liver Disease
- NDI
neighborhood deprivation index
- PELD
Pediatric End-Stage Liver Disease
- SES
socioeconomic status
- SSI
Supplemental Security Income
- TANF
Temporary Assistance for Needy Families
- UNOS
United Network for Organ Sharing
Footnotes
The authors of this article have no relevant conflicts of interest to report.
The data reported here were supplied by the United Network for Organ Sharing as the contractor for the Organ Procurement and Transplantation Network. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the Organ Procurement and Transplantation Network or the US Government.
References
- 1.Ryckman FC, Bucuvalas JC, Nathan J, Alonso M, Tiao G, Balistreri WF. Outcomes following liver transplantation. Semin Pediatr Surg. 2008;17:123–130. doi: 10.1053/j.sempedsurg.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Andrews WS, Wanek E, Fyock B, Gray S, Benser M. Pediatric liver transplantation: a 3-year experience. J Pediatr Surg. 1989;24:77–82. doi: 10.1016/s0022-3468(89)80306-9. [DOI] [PubMed] [Google Scholar]
- 3.Berquist RK, Berquist WE, Esquivel CO, Cox KL, Wayman KI, Litt IF. Adolescent non-adherence: prevalence and consequences in liver transplant recipients. Pediatr Transplant. 2006;10:304–310. doi: 10.1111/j.1399-3046.2005.00451.x. [DOI] [PubMed] [Google Scholar]
- 4.Diamond IR, Fecteau A, Millis JM, Losanoff JE, Ng V, Anand R, Song C for SPLIT Research Group. Impact of graft type on outcome in pediatric liver transplantation: a report from Studies of Pediatric Liver Transplantation (SPLIT) Ann Surg. 2007;246:301–310. doi: 10.1097/SLA.0b013e3180caa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Limbers CA, Neighbors K, Martz K, Bucuvalas JC, Webb T, Varni JW, Alonso EM for Studies of Pediatric Liver Transplantation Functional Outcomes Group. Health-related quality of life in pediatric liver transplant recipients compared with other chronic disease groups. Pediatr Transplant. 2011;15:245–253. doi: 10.1111/j.1399-3046.2010.01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin SR, Atkison P, Anand R, Lindblad AS for SPLIT Research Group. Studies of Pediatric Liver Transplantation 2002: patient and graft survival and rejection in pediatric recipients of a first liver transplant in the United States and Canada. Pediatr Transplant. 2004;8:273–283. doi: 10.1111/j.1399-3046.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- 7.McDiarmid SV, Anand R, Martz K, Millis MJ, Mazariegos G. A multivariate analysis of pre-, peri-, and posttransplant factors affecting outcome after pediatric liver transplantation. Ann Surg. 2011;254:145–154. doi: 10.1097/SLA.0b013e31821ad86a. [DOI] [PubMed] [Google Scholar]
- 8.Rook M, Rand E. Predictors of long-term outcome after liver transplant. Curr Opin Organ Transplant. 2011;16:499–504. doi: 10.1097/MOT.0b013e32834a945d. [DOI] [PubMed] [Google Scholar]
- 9.Tiao G, Ryckman FC. Pediatric liver transplantation. Clin Liver Dis. 2006;10:169–197. doi: 10.1016/j.cld.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Abramson O, Rosenthal P. Current status of pediatric liver transplantation. Clin Liver Dis. 2000;4:533–552. doi: 10.1016/s1089-3261(05)70125-2. [DOI] [PubMed] [Google Scholar]
- 11.Rhee C, Narsinh K, Venick RS, Molina RA, Nga V, Engelhardt R, Martín MG. Predictors of clinical outcome in children undergoing orthotopic liver transplantation for acute and chronic liver disease. Liver Transpl. 2006;12:1347–1356. doi: 10.1002/lt.20806. [DOI] [PubMed] [Google Scholar]
- 12.HealthyPeople.gov. [Accessed October 2013]; http://www.healthypeople.gov/2020/default.aspx.
- 13.Institute of Medicine's Committee on Quality of Health Care in America. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 14.Braveman P, Barclay C. Health disparities beginning in childhood: a life-course perspective. Pediatrics. 2009;124(suppl 3):S163–S175. doi: 10.1542/peds.2009-1100D. [DOI] [PubMed] [Google Scholar]
- 15.Goodman E, Slap GB, Huang B. The public health impact of socioeconomic status on adolescent depression and obesity. Am J Public Health. 2003;93:1844–1850. doi: 10.2105/ajph.93.11.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathur AK, Sonnenday CJ, Merion RM. Race and ethnicity in access to and outcomes of liver transplantation: a critical literature review. Am J Transplant. 2009;9:2662–2668. doi: 10.1111/j.1600-6143.2009.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo HY, Thuluvath PJ. Outcome of liver transplantation in adult recipients: influence of neighborhood income, education, and insurance. Liver Transpl. 2004;10:235–243. doi: 10.1002/lt.20069. [DOI] [PubMed] [Google Scholar]
- 18.Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. 2002;359:287–293. doi: 10.1016/S0140-6736(02)07494-9. [DOI] [PubMed] [Google Scholar]
- 19.Lee TH, Shah N, Pedersen RA, Kremers WK, Rosen CB, Klintmalm GB, Kim WR. Survival after liver transplantation: is racial disparity inevitable? Hepatology. 2007;46:1491–1497. doi: 10.1002/hep.21830. [DOI] [PubMed] [Google Scholar]
- 20.Ananthakrishnan AN, Saeian K. Racial differences in liver transplantation outcomes in the MELD era. Am J Gastroenterol. 2008;103:901–910. doi: 10.1111/j.1572-0241.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoo HY, Galabova V, Edwin D, Thuluvath PJ. Socioeconomic status does not affect the outcome of liver transplantation. Liver Transpl. 2002;8:1133–1137. doi: 10.1053/jlts.2002.37000. [DOI] [PubMed] [Google Scholar]
- 22.Mathur AK, Schaubel DE, Gong Q, Guidinger MK, Merion RM. Racial and ethnic disparities in access to liver transplantation. Liver Transpl. 2010;16:1033–1040. doi: 10.1002/lt.22108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the Public Health Disparities Geocoding Project. Am J Public Health. 2003;93:1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Georgia Department of Community Health. [Accessed October 2013];Eligibility criteria chart: eligibility for major coverage groups within Medicaid. http://dch.georgia.gov/eligibility-criteria-chart.
- 25.Messer LC, Laraia BA, Kaufman JS, Eyster J, Holzman C, Culhane J, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83:1041–1062. doi: 10.1007/s11524-006-9094-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. 2nd. New York, NY: Springer; 2005. [Google Scholar]
- 27.Yoon PW, Bresee JS, Olney RS, James LM, Khoury MJ. Epidemiology of biliary atresia: a population-based study. Pediatrics. 1997;99:376–382. doi: 10.1542/peds.99.3.376. [DOI] [PubMed] [Google Scholar]
- 28.Entwislea DR, Astone NM. Some practical guidelines for measuring youth's race/ethnicity and socioeconomic status. Child Dev. 1994;65:1521–1540. [Google Scholar]
- 29.Goodman E. The role of socioeconomic status gradients in explaining differences in US adolescents' health. Am J Public Health. 1999;89:1522–1528. doi: 10.2105/ajph.89.10.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman E, Adler NE, Kawachi I, Frazier AL, Huang B, Colditz GA. Adolescents' perceptions of social status: development and evaluation of a new indicator. Pediatrics. 2001;108:E31. doi: 10.1542/peds.108.2.e31. [DOI] [PubMed] [Google Scholar]
- 31.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18:341–378. doi: 10.1146/annurev.publhealth.18.1.341. [DOI] [PubMed] [Google Scholar]
- 32.Bradley RH, Corwyn RF. Socioeconomic status and child development. Annu Rev Psychol. 2002;53:371–399. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 33.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995:80–94. [PubMed] [Google Scholar]
- 34.Marmot M. Social determinants of health inequalities. Lancet. 2005;365:1099–1104. doi: 10.1016/S0140-6736(05)71146-6. [DOI] [PubMed] [Google Scholar]
- 35.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Campo P, Burke JG, Culhane J, Elo IT, Eyster J, Holzman C, et al. Neighborhood deprivation and preterm birth among non-Hispanic black and white women in eight geographic areas in the United States. Am J Epidemiol. 2008;167:155–163. doi: 10.1093/aje/kwm277. [DOI] [PubMed] [Google Scholar]
- 37.Neff GW, Kemmer N, Kaiser T, Zacharias V, Majoras N, Safdar K. Outcomes in adult and pediatric liver transplantation among various ethnic groups. Transplant Proc. 2007;39:3204–3206. doi: 10.1016/j.transproceed.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 38.McDiarmid SV, Anand R Lindblad AS; for Principal Investigators and Institutions of the Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Development of a pediatric end-stage liver disease score to predict poor outcome in children awaiting liver transplantation. Transplantation. 2002;74:173–181. doi: 10.1097/00007890-200207270-00006. [DOI] [PubMed] [Google Scholar]
- 39.Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr. 2005;147:739–743. doi: 10.1016/j.jpeds.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Patzer RE, Amaral S, Klein M, Kutner N, Perryman JP, Gazmararian JA, McClellan WM. Racial disparities in pediatric access to kidney transplantation: does socioeconomic status play a role? Am J Transplant. 2012;12:369–378. doi: 10.1111/j.1600-6143.2011.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt JB. The topography of poverty in the United States: a spatial analysis using county-level data from the Community Health Status Indicators project. Prev Chronic Dis. 2007;4:A111. [PMC free article] [PubMed] [Google Scholar]
- 42.Patzer RE, Perryman JP, Schrager JD, Pastan S, Amaral S, Gazmararian JA, et al. The role of race and poverty on steps to kidney transplantation in the Southeastern United States. Am J Transplant. 2012;12:358–368. doi: 10.1111/j.1600-6143.2011.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanders-Phillips K, Settles-Reaves B, Walker D, Brownlow J. Social inequality and racial discrimination: risk factors for health disparities in children of color. Pediatrics. 2009;124(suppl 3):S176–S186. doi: 10.1542/peds.2009-1100E. [DOI] [PubMed] [Google Scholar]
- 44.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 45.Gupta P, Hart J, Cronin D, Kelly S, Millis JM, Brady L. Risk factors for chronic rejection after pediatric liver transplantation. Transplantation. 2001;72:1098–1102. doi: 10.1097/00007890-200109270-00020. [DOI] [PubMed] [Google Scholar]
- 46.Kaufman JS, Cooper RS. Commentary: considerations for use of racial/ethnic classification in etiologic research. Am J Epidemiol. 2001;154:291–298. doi: 10.1093/aje/154.4.291. [DOI] [PubMed] [Google Scholar]
- 47.Arnon R, Annunziato RA, Willis A, Parbhakar M, Chu J, Kerkar N, Shneider BL. Liver transplantation for children with biliary atresia in the Pediatric End-Stage Liver Disease era: the role of insurance status. Liver Transpl. 2013;19:543–550. doi: 10.1002/lt.23607. [DOI] [PubMed] [Google Scholar]
- 48.Federal Interagency Forum on Child and Family Statistics. America's Children: Key National Indicators of Well-Being, 2011. Washington, DC: US Government Printing Office; 2011. [Google Scholar]
- 49.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR) OPTN/SRTR 2010 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2011. [Google Scholar]
- 50.Alonso EM. Quality of life for pediatric liver recipients. Liver Transpl. 2009;15(suppl 2):S57–S62. doi: 10.1002/lt.21904. [DOI] [PubMed] [Google Scholar]
- 51.Ng VL, Alonso EM, Bucuvalas JC, Cohen G, Limbers CA, Varni JW, et al. for Studies of Pediatric Liver Transplantation (SPLIT) Research Group. transplantation performed in the US and Canada: report of the Studies of Pediatric Liver Transplantation experience. J Pediatr. 2012;160:820–826.e3. doi: 10.1016/j.jpeds.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288:1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]


