Abstract
Low socioeconomic status (SES) influences disease incidence and contributes to poor health outcomes throughout an individual's life course across a wide range of populations. Low SES is associated with increased incidence of chronic kidney disease, progression to end-stage renal disease, inadequate dialysis treatment, reduced access to kidney transplantation, and poor health outcomes. Similarly, racial and ethnic disparities, which in the USA are strongly associated with lower SES, are independently associated with poor health outcomes. In this Review, we discuss individual-level and group-level SES factors, and the concomitant role of race and ethnicity that are associated with and mediate the development of chronic kidney disease, progression to end-stage renal disease and access to treatment.
Introduction
The burden of chronic kidney disease (CKD) in the USA is substantial and rising.1–3 The prevalence of CKD, defined by an estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73 m2, is estimated to be 15% of the US population, representing roughly 45 million individuals.2 Between two National Health and Nutrition Examination Surveys (NHANES) in 1988–1994 and 1999–2004, the prevalence of CKD increased by 30%.4 Although the incidence of end-stage renal disease (ESRD) is stable, at 255 per million population, an increased prevalence of ESRD is driven primarily by the aging population, and is currently 1,738 per million population in the USA.2
Inequities in CKD and ESRD incidence, risk factors and disease treatment have been repeatedly observed among various socioeconomic, racial and ethnic groups within the US population. Healthy People 2020—the US national blueprint for public health goals—explicitly aims for the elimination of socioeconomic-related health disparities among patients with kidney disease in the USA by 2020.5 Included in this goal is the reduction of disparities in the occurrence and outcomes of CKD, reflecting the marked and well-recognized inequities observed in the prevalence of CKD,6 progression of CKD to ESRD,7 and treatment of patients with ESRD8 among both adults9–11 and children.12,13
It is important to acknowledge the complexity of measuring socioeconomic status (SES) when discussing health disparities.14 Most of the evidence discussed below defines SES using the commonly available metrics of income, education and occupation. Although these proxy measures might not entirely capture an individual's SES, they remain the most accessible means of assessing SES.15 When addressing SES, it must be understood that in the USA, lower SES is strongly linked with race. The terms race and ethnicity are often used interchangeably in the medical literature; in this Review, we consider both race and ethnicity to be primarily social constructs assigned by an individual.16 The US population has an unusual association between race, ethnicity and poverty delineated in national data. For example, poverty is reported for 67% of black individuals compared with 12% of white individuals residing in urban US populations; among individuals aged ≤65 years, 31% of black individuals and 11% of white individuals have incomes below the poverty line.17 Efforts to understand the role of socioeconomic factors in the occurrence and outcomes of health disparities must account for both the effect of race and ethnicity as well as socioeconomic indicators.11
The mechanisms linking SES and race to the occurrence and outcomes of kidney disease are poorly understood. Norris and Nissenson recently described the associations between race, SES and CKD as being multilevel, ranging from individual to society, and multifactorial at each level of social organization.10 Understanding these complex relationships is essential if successful interventions to reduce these disparities are to be designed and conducted.9
In this Review, we summarize recent research that illustrates, and perhaps elucidates, this multilevel and multifactorial association between race, SES and kidney disease. We discuss the general framework for considering the multilevel role of poverty and kidney disease. We also briefly review the recent North American literature describing associations between SES, race, ethnicity and CKD throughout the life course of an individual, including genetic influences, the perinatal period, growth and development, differential exposure to risk factors for initiation and progression of CKD, and the course of treatment for advanced CKD. The degree to which studies from other populations parallel that of North America is also examined.
Framework for race, SES and CKD
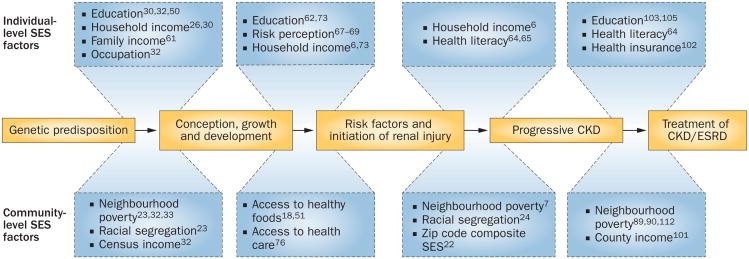
An important contribution of research to our understanding of the association between SES, race and disparities in CKD has been the recognition that attributes of place, independent of individual risk factors, can contribute to explanatory models. For example, the influence of individual SES attributes, such as income on dietary preferences, might be constrained by SES attributes of the community that limit the types of restaurants located in the community.18 Attributes of place that could modify individual risk are termed contextual risk factors, and models that include individual and contextual level of risk factors are called multilevel models (Figure 1). It is important to recognize that measures of individual SES and community SES, which aggregate information on all individuals within a community, may be discordant, and attributing group-level measures to individuals could result in misstating the nature of an association. This error is termed the ecological fallacy.19 The theoretical framework for examining the multilevel impact of SES on health is well established.20,21
Figure 1.
Multilevel framework of health disparities in patients with kidney disease. Racial and socioeconomic factors influence the development and treatment of CKD during the course of an individual's life. Genetic predisposition influences the development of CKD and may interact with individual-level socioeconomic factors such as education, income and health literacy. Environmental and community-level factors, such as neighbourhood poverty, diet, racial segregation and access to health care, influence the life course and treatment of CKD in addition to individual-level socioeconomic factors. Abbreviations: CKD, chronic kidney disease; ESRD, end-stage renal disease; SES, socioeconomic status.
One mechanism that could link individual SES and race to CKD risk in a multilevel context is access to health care. Several studies have documented that kidney disease is associated with area-based measures of access to care. Delayed referral to nephrology care is associated with increased morbidity, mortality, cost and incidence of ESRD and is more common among CKD patients with lower SES2,21–23 and patients of ethnic minority.24 In a population-based ecological study, Ward reported that the incidence of ESRD attributed to diabetes mellitus is associated with zip-code-level residence in areas with a shortage of primary care.22 The multilevel context of individual and neighbourhood SES factors on the progression of kidney disease is also modified by race and ethnicity. Residential segregation is a contextual, area-based measure that can modify the effect of an individual's SES, race and ethnicity on risk of CKD. For example, the association between race and the prevalence of hypertension varies substantially by residential racial segregation, where health disparities are more pronounced in more racially segregated areas.23 Volkova et al. reported that increasing neighbourhood poverty was associated with a greater disparity in incidence rates of ESRD between black and white individuals.7 Similarly, a 2010 study by Prakash et al. found that as the percentage of black individuals in a neighbourhood increases, the likelihood of access to pre-ESRD nephrology care decreases.24 Other community-level SES measures might also influence the racial disparities associated with the incidence of CKD and progression to ESRD, including residing in an urban area and treatment at an urban public health centre.6,25–27 The extension of this multilevel, contextual framework is a major area for contemporary CKD epidemiology and outcomes research. Below we describe recent research that provides additional evidence to clarify and extend this model.10,28
Perinatal factors, race and poverty
CKD occurs against a genetic background of variable susceptibility to kidney injury that must be accounted for in any comprehensive model of CKD incidence and progression. The recent identification of APOL1, a high-risk allele on chromosome 22 found largely among individuals of West African descent, is an example of the predisposing genetic background for CKD.29 The high but variable risk among individuals with two of these alleles is taken as evidence that genetic and environmental factors modify the resulting phenotype. Other genetic variants for CKD risk, including ELMO1, UMOD and ACTN4, illustrate the potential genetic complexity of risk of CKD. By contrast, the degree to which environmental and behavioural risk factors associated with lower SES from the prenatal period throughout adulthood might modify the phenotypic expression of these genetic variants is unclear.
A possible contextual variable that modifies genetic predisposition is the family environment. A family history of ESRD in a first-degree relative is reported by over 10% of randomly selected, otherwise healthy, older adults (aged >45 years) in the USA.30 A family history of ESRD is twice as common among black individuals than among white individuals and, after adjusting for age and sex, significantly more likely among individuals with a family income below the poverty line or having less than a high-school education. Individuals with a family history of ESRD also have a decreased eGFR, increased albumin-to-creatinine ratio, and a substantially increased risk of progressing to ESRD. At present, the contribution of a shared genetic background to shared familial risk is not known, but the persistence of lower SES as a risk factor in multivariable models for a family history of ESRD suggests a possible gene–environment interaction that should be explored.
Some researchers have posited the low nephron hypothesis as one predisposing factor for progressive renal injury and strong evidence links measures of lower SES, including income, education and occupation, to low birth weight.31,32 In a systematic review by Blumenshine et al., most (88%) studies found an association between one or more measures of low SES and adverse birth outcomes.32 Many of these studies also reported racial or ethnic differences in the effects of SES on low birth weight. A review of studies that included a multilevel context consistently documented small to moderate effects of community SES measures.33 Independent of individual SES, community SES might explain as much as 10% of the variation in birth weight.33
A meta-analysis of the relationship between birth weight and CKD, defined as albuminuria, low eGFR or ESRD, found 31 studies that met the inclusion criteria with information from over 2 million individuals.34 Half of the studies reported a significant association between low birth weight and CKD, with a summary odds ratio (OR) of 1.73 (95% CI 1.44–2.08) for albuminuria, 1.81 (95% CI 1.19–2.77) for low eGFR, and 1.58 (95% CI 1.33–1.88) for ESRD.
Two studies on the influence of intrauterine environment further illustrate this concept. In a Danish cohort of patients with autosomal dominant polycystic kidney disease, low birth weight was associated with earlier onset of ESRD.35 Similarly, in a case report of two monozygotic twins with Alport syndrome who had variable progression of kidney disease, the twin with low birth weight had a faster rate of progression to CKD.36 The extent to which community SES might mediate the complex relationship between individual race, SES, low birth weight and CKD is unclear and a subject for further inquiry.
Mechanisms linking low birth weight with conditions predisposing to CKD, including hypertension, diabetes mellitus, dyslipidemia and obesity, as well as directly to microalbuminuria and decreased eGFR have recently been reviewed by Nistala and colleagues.37 Prenatal malnutrition and other adverse exposures during pregnancy, such as anaemia, iron deficiency, alcohol use, smoking, activation of the renin–angiotensin system and stress related to domestic violence,38 might contribute to the development of risk factors for future CKD.
Growth and development
Low birth weight might influence kidney function during subsequent growth and development. Racial differences in creatinine clearance,39 systolic and diastolic blood pressure,40 future risk of hypertension,41 and obesity42 have been extensively described in the Bogalusa Heart Study, a series of cross-sectional surveys conducted every 2–3 years from childhood (4–17 years) to adulthood (18–50 years). Black patients in the Bogalusa Heart Study had lower birth weight than did white patients before and after adjustment for gestational age, and low birth weight was associated with increased adult blood pressure among black and white individuals, an association that intensified equally with age.43 Although the number of incident cases of ESRD was low (n = 15), the occurrence of ESRD was higher than expected among young black males; these individuals were characterized by increased blood pressure and BMI during childhood.44
The role of community poverty has not been fully examined as a mediator of these relationships, and racial differences in CKD risk during growth and development might be modified further by contextual factors. It would be interesting to examine the hypothesis that individuals with a genetic predisposition to kidney injury, perhaps those born into a familial environment that further exposed them to risk factors for CKD and who may have encountered an adverse prenatal environment, are at increased risk, but not necessarily predestined, to develop CKD later in life. Furthermore, it is possible that these genetic, familial and prenatal risks might be modified by the communities in which these individuals are born and develop into adulthood. For example, in the US-based Moving to Opportunity Study, participants who were randomly assigned to receive a voucher to move from a high-poverty neighbourhood to a low-poverty neighbourhood had a reduced prevalence of extreme obesity and diabetes mellitus 10–15 years later compared with those who did not move from the high-poverty neighbourhood.45 This social experiment highlights the potential for community-based interventions to potentially impact the causal pathway between poverty and the development of risk factors for kidney disease.
Risk factors for CKD
Development of a CKD phenotype, and intermediate predisposing states in the causal pathway to kidney disease such as diabetes mellitus and hypertension, could also represent a continuing interaction with environmental influences throughout life that vary with race and SES.46–48 Described below are only some of the risk factors and mediators of disparities observed in CKD during the life course, including diet, obesity, diabetes mellitus, hypertension, educational attainment and health literacy. With each association, we highlight the potential multilevel nature of the exposure.
Dietary factors
Dietary risk factors vary with both SES and race. A study of diet using data from NHANES 1999–2002 found that among elderly adults, lower household income was associated with an inadequate diet.49 The Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) Study has reported that SES measures, including income, poverty–income ratio and education, are associated with diet quality among black patients, whereas a similar association was noted for education among white adults.50
Diet is known to influence the risk of CKD and related outcomes. A Cochrane Renal Group review of the association between low-protein diet and CKD reported by 10 studies found a lower risk of CKD among those with restricted protein intake (relative risk 0.68, 95% CI 0.55–0.84, P = 0.0002).51 Dietary salt consumption is associated with increased proteinuria and salt restriction contributes to delayed progression of CKD. Several studies have reported that black patients with hypertension had a higher GFR on a high versus low salt diet, but no differences in GFR were observed among white patients in response to changes in dietary sodium intake.52 Furthermore, Gutiérrez et al. analysed participants in the Chronic Renal Insufficiency Cohort Study for associations between race, SES and serum phosphate levels.53 Diets were comparable in caloric intake, but were lower in carbohydrate and higher in protein content among black individuals than in white individuals. Among black patients, phosphorus consumption was lower, but black patients had a higher serum phosphate level than did white patients, even after controlling for other covariates. Both crude and multivariable serum phosphate levels were inversely associated with annual income, and were higher among those who were unemployed and had lower education levels. Of interest, the racial differences in serum phosphate levels were noted in the higher but not the lowest income group, suggesting that SES is a potential mediator of this relationship.53
Neighbourhood poverty is known to influence diet independently of individual race, ethnicity and income. For example, Block and colleagues found associations between the density of fast-food restaurants and both neighbourhood race and income composition.18 A recent analysis of the Coronary Artery Risk Development in Young Adults (CARDIA) study illustrates how community availability of restaurants and grocery stores might influence eating patterns in low-income communities.54 An association between the density of neighbourhood fast-food restaurants and individual fast-food consumption was evident among low-income men, not significant among low-income women, and quite variable among other income groups.
Hypertension and diabetes
Hypertension and diabetes mellitus, which account for over 60% of incident ESRD, are associated with both a higher prevalence of CKD and risk of progression to kidney failure.2 Both are more common among minorities and individuals with lower SES.48,55 The potential role that geographic factors might have in mediating these predisposing diseases has been suggested by two studies. Kershaw et al. used data from NHANES 1999– 2006 to describe racial disparities in the prevalence of hypertension by degree of racial residential segregation and neighbourhood poverty.56 After controlling for individual-level SES and other risk factors, black individuals were nearly three times more likely than white individuals to be hypertensive, and these disparities were attenuated by lesser degrees of residential segregation and increased among lower community SES. Egede et al. examined the association between haemoglobin A1c levels and race among military veterans with diabetes mellitus followed for 5 years.57 Glycaemic control was worse among black and Hispanic patients than among white patients, and lowest in Southern US states, a region characterized by an increased density of community poverty.
Obesity
Similarly, obesity is a recognized risk factor for CKD that varies with race and SES; obesity is also associated with an increased risk of proteinuria and progressive impairment of kidney function.58,59 Evidence indicates that weight reduction, including bariatric surgery, can retard or reverse progressive CKD.60 The well-described racial and ethnic differences in the prevalence of obesity begin in early childhood and persist into adulthood and are more pronounced among lower income individuals. For example, obese children and adolescents in NHANES I, NHANES II and NHANES 1999–2008 were more likely to be black and have a lower income than were wealthier NHANES participants.61
Education and health literacy
Educational attainment among individuals screened for high risk of CKD is inversely associated with an increased prevalence of hypertension, diabetes mellitus, decreased eGFR, and increased urinary albumin excretion.62 Similarly, impaired health literacy is more common among minorities and lower income individuals with CKD63 and is associated with impaired access to transplantation64 and increased risk of mortality65 among patients with ESRD. Health literacy might also influence the course of CKD. For example, lower health literacy might influence effective communication between the provider and patient.66 Greer and colleagues reported that discussions of CKD risk between patients and primary care physicians occurred less frequently among patients with lower educational attainment.66 Black individuals and those with low SES who are members of high-risk groups, including family members of patients with ESRD, do not necessarily perceive themselves as having an increased risk of CKD.67–69 It is possible that the misperception of the risk of CKD, in turn, might contribute to other risk-related behaviours, such as screening avoidance or medication noncompliance. This observation stands in contrast to knowledge about individual CKD status which, although generally very low, varies little among SES or racial/ethnic groups.70,71 Again, it is reasonable to hypothesize that the association between individual degrees of educational attainment, health literacy and perception of personal risk of CKD might be modified by attributes of community SES.
Identification and treatment of CKD
Multiple aspects of health care that differ among SES levels are associated with an increased risk of CKD and related outcomes. Minority race is associated with lack of health insurance and access to usual sources of care.72 In the Atherosclerosis Risk in Communities (ARIC) study, black individuals were more likely to lack health insurance and a usual source of health care. Racial disparities were reported in the incidence of CKD, where black patients had a 60% higher rate of CKD incidence than did white patients. Adjusting for SES factors, including annual household income and years of education, explained 64% of the excess CKD risk among black versus white patients. After adjusting for differences in access to health care this disparity was attenuated.73 Ward found that the incidence of diabetic ESRD was higher in California zip code areas characterized by higher proportions of uninsured and Medicaid insured hospitalizations.22 By contrast, areas characterized as under-served by primary-care clinics had lower rates of diabetic ESRD, the reasons for which are unknown. Black individuals were also less likely to have access to health care in the ARIC study, and limited access to health care was independently associated with an increased risk of CKD.73 These results suggest that the multilevel associations between race, SES and treatment for patients with CKD are complex and not yet fully understood.
Most individuals receive at least some primary care, although evidence exists that primary care for individuals with CKD is less than optimal. Evidence of provider awareness of impaired kidney function was examined among primary-care practices in a Kaiser Permanente-managed care population by assessing congruence between laboratory and claims data.74 Among >10,000 individuals with at least two eGFR measures of 10–59 ml/min/1.73 m2, only 14.4% had a CKD diagnosis at baseline. Similar variability in the quality of CKD care—defined as measures of kidney disease monitoring, cardiovascular disease management, metabolic bone disease and anaemia management, and drug safety—has been reported among primary-care clinics in the north-eastern USA, but care could be improved if primary-care physicians recognize the presence of CKD and refer patients to a nephrologist.75
Although the under-reporting of CKD might reflect administrative issues rather than physician awareness, it is also apparent that some primary-care physicians lack awareness of relevant guidelines for CKD care, may be uncertain with respect to CKD care, persist in relying on inaccurate serum creatinine values rather than on eGFR to identify CKD, and have uncertain relationships with referring nephrologists.76 The insecurity among primary-care physicians with respect to the identification and treatment of CKD may begin during physician training. A survey of internal medicine residents found multiple gaps in knowledge of CKD complications and management.77 Low patient awareness of CKD and ESRD suggests a need for health-care providers, including nephrologists, to improve patient education.78,79 Compared with white patients, black patients are less likely to have pre-ESRD nephrology care,24 receive adequate dialysis treatment,80,81 have an arteriovenous fistula placed for dialysis access,82 and have access to kidney transplantation.83
Management of stage 5 CKD
Similar to treatment for CKD, race and SES have an important role in access to kidney transplantation among patients with ESRD in the USA. Compared with white patients, black patients are reported as being less interested in transplantation,84,85 and have a lower rate of accessing multiple steps in the kidney transplant process, including referral for transplant evaluation,86 start of transplant evaluation process,87 completion of the kidney transplant evaluation,88 placement on the deceased donor waiting list,89,90 and receipt of a living or deceased donor kidney transplant.91–94 These disparities are observed among both paediatric13 and adult83 patients with ESRD, and the reasons are likely to be multifactorial.95–97
SES has been hypothesized to have a role, whereby poor or uninsured patients may have greater difficulty in navigating the multiple steps needed to receive a kidney transplant.85,98–102 For example, lower educational attainment is associated with a lower rate of early access to the waiting list,102 a lower rate of completing the transplantation evaluation,103,104 and lower access to a transplant105 than is advanced educational levels. Low provider awareness of kidney disease and poor communication may also have a role.106–108 Providers might be less likely to recommend transplantation to black patients based on the misconception that black patients do better on dialysis compared with receiving a transplant,84,106,107 and low SES may further contribute to this disparity.109 For example, providers might be less likely to refer patients who lack health insurance to cover the costs of transplantation.110 Other SES factors such as low health literacy are more frequently observed among minorities,111 which influences access to transplantation.64
Racial disparities in access to kidney transplantation are particularly pronounced in poor neighbourhoods.89,90,112 Saunders et al. reported that black patients who live in poor neighbourhoods are less likely to access the transplant waiting list than are poor white patients residing in the same neighbourhood poverty level (hazard ratio 0.79, 95% CI 0.70–0.89), and this disparity is particularly pronounced in the southeastern USA (hazard ratio 0.43, 95% CI 0.22–0.64).90 Other community factors, such as living in a rural area113 or living farther from a transplant centre may have less of a role in the observed disparities,89 but this effect could be mixed owing to increased regional travel among those living farther from a transplant centre.100
The degree to which either individual or community SES explains the observed racial disparities in access to kidney transplantation is unclear. Schold et al. reported that racial disparities observed in a population of patients referred for renal transplantation in Florida, USA, are largely explained by SES, as measured by health insurance and county income.101 Other studies, however, have found that even after accounting for SES, racial disparities still persist. Hall et al. reported that disparities in access to waitlisting were somewhat attenuated after adjusting for individual and neighbourhood SES, where health insurance coverage and zip code poverty explained 21% of the waitlisting disparity.83 In the south-eastern USA, where there is a higher concentration of poverty and a higher burden of ESRD, individual and community poverty explained 30% of the racial disparity in access to kidney transplantation, but even after adjustment for SES, black patients had a 59% lower rate of transplantation than did white individuals.87 These results suggest that there is considerable variability in the degree to which SES explains observed racial differences in treatment among patients with ESRD.
Evidence from other countries
Our focus on North American research regarding the influence of SES and race on the occurrence and outcomes of CKD reflects, in part, unique societal and health-care issues in the USA. It is important to note that the association between SES and health disparities has also been reported extensively for international populations.114,115 It is unclear whether our model describing the relationship between SES, race and CKD has limited applicability to other countries or whether it may be compatible with evidence from other populations. We highlight several recent examples of these relationships in other countries.
Genetic predisposition, growth and development
The genesis of the idea that growth and development in utero and throughout childhood can influence the development of disease later in life originated with studies by Barker and colleagues in the UK116 and was initially applied to CKD by Luyckx and Brenner.31 The Uppsala Family Study reported by Nitsch et al. examined Swedish pairs of consecutive healthy full siblings to identify determinants of kidney function as measured by levels of cystatin C.117 Lower maternal education attainment was associated with a higher level of cystatin C, and after adjusting for gestational age, maternal parity, sex, and age of the child, there was a nonsignificant decrease in cystatin C levels as birth weight increased. Associations between higher blood pressure and albumin excretion and lower birth weight have been reported from Brazil118 and Croatia119 and for microalbuminuria in children born during the Dutch famine in World War II.120
Progressive CKD
The early interest in the association between SES and chronic disease originated in Europe, including associations with hypertension, diabetes mellitus and cardiovascular disease, which are major risk factors for and correlates of kidney injury.121,122
Lower SES, measured by self-reported occupational grade and/or salary range, was associated with decreased eGFR among white participants in the Whitehall II cohort in the UK.123 The age-adjusted prevalence of severe CKD was inversely correlated with lower levels of an ecologic measure of SES, the British Index of Multiple Deprivation, in a study from Sheffield, UK.124 Hossain et al. found that area measures of lower SES were independently associated with an increased prevalence of heavy proteinuria, rapid progression of CKD and increased risk of ESRD among a clinic population in the UK.125 Maheswaran et al. found lower area SES was associated with increased prevalence rates for renal replacement therapy in the Trent Region, UK.126
A moderately strong association was reported between higher incident ESRD rates in Australian capital cities and greater socioeconomic disadvantage as measured by an index of SES disadvantage derived from Australian census data.127 Lower SES, measured by an index derived from education, highest professional position achieved and household net income, was associated with the severity of CKD among German patients with type 2 diabetes mellitus.128 The prevalence of CKD was increased among Swedish families with unskilled workers and individuals with ≤9 years of education.129 The incidence of ESRD among Danish individuals was significantly higher among individuals with lower income, lower educational attainment and those of non-Western origin.130 Maori and Pacific Islanders living in New Zealand, who are characterized by higher poverty rates, had a substantially higher prevalence of microalbuminuria and incidence of renal replacement therapy and lower kidney transplant and graft survival rates than did European descendents in New Zealand.131
Care of patients with CKD and ESRD
A report from India found that patients who were referred late for renal replacement therapy were more likely to have lower SES and educational attainment and to report financial barriers to care.132 In a separate study, patients with lower SES were more likely to die and less likely to receive a transplant once on hemodialysis.133
Conclusions
The association between CKD and economically disadvantaged groups throughout the world suggests that circumstances intrinsic to SES that are shared by these individuals are fundamental to the occurrence and outcomes of their disease. Some of the environmental and community-level factors that might account for this common risk, although not yet fully explored in a multilevel context, have been incorporated into Figure 1. One potentially interesting concept that has been revealed by this Review is that additional cross-population, multilevel studies of SES and CKD similar to those discussed above are needed.
What are the implications of the research discussed above? Growing evidence indicates that community-level and individual-level SES factors independently influence risk factors for the occurrence and progression of CKD throughout an individual's life course through mechanisms that are, as yet, not well understood. Elucidation of these mechanisms might identify targets for therapeutic and public health intervention. If the complex interaction between genetic predisposition to CKD, birth weight and family environment, and individual-level and contextual-level risk factors during adulthood on kidney disease is a valid model, then public health efforts to prevent CKD might well begin antepartum among populations at high risk.
Key points.
Socioeconomic status (SES) contributes to the variability in incidence, prevalence and treatment of chronic kidney disease and end-stage renal disease
The interplay between race and SES is complex, and efforts to understand health disparities in patients with kidney disease must account for these effects
The mechanisms linking poverty to kidney disease outcomes are multifactorial and multilevel, where contextual-level SES factors in addition to individual-level SES factors contribute to kidney disease outcomes
The mechanisms by which SES influences the development of risk factors for chronic kidney disease throughout the course of an individual's life are not entirely clear
Review criteria.
We searched PubMed for English-language, full-text manuscripts published between 1995 and 2011, with search terms that included “socioeconomic status”, “poverty”, “multilevel”, “education”, “health insurance”, “kidney disease”, “chronic kidney disease” and “end-stage renal disease”. We also manually searched the reference lists of reviewed articles and used Web of Science® to identify articles that cited a paper selected for review.
Footnotes
Competing interests: The authors declare no competing interests.
Author contributions: R. E. Patzer and W. M. McClellan contributed equally to discussion of content for the article, researching data to include in the manuscript, writing, reviewing and editing of the manuscript before submission.
Contributor Information
Rachel E. Patzer, Emory University School of Medicine, Department of Surgery, Emory Transplant Center, 101 Woodruff Circle, 5125 WMB, Atlanta, GA 30322, USA
William M. McClellan, Emory University, Rollins School of Public Health, Department of Epidemiology, 1518 Clifton Avenue N.E., Atlanta, GA 30312, USA
References
- 1.Grassmann A, Gioberge S, Moeller S, Brown G. ESRD patients in 2004: global overview of patient numbers, treatment modalities and associated trends. Nephrol Dial Transplant. 2005;20:2587–2593. doi: 10.1093/ndt/gfi159. [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System. US Renal Data System 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States [online] 2010 http://www.usrds.org/atlas10.aspx.
- 3.Castro AF, Coresh J. CKD surveillance using laboratory data from the population-based National Health and Nutrition Examination Survey (NHANES) Am J Kidney Dis. 2009;53(Suppl. 3):S46–S55. doi: 10.1053/j.ajkd.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coresh J, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services. National Healthcare Quality Report [online] 2010 http://www.ahrq.gov/qual/nhqr10/nhqr10.pdf.
- 6.McClellan WM, et al. Poverty and racial disparities in kidney disease: the REGARDS study. Am J Nephrol. 2010;32:38–46. doi: 10.1159/000313883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkova N, et al. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19:356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClellan WM, et al. Geographic concentration of poverty and arteriovenous fistula use among ESRD patients. J Am Soc Nephrol. 2010;21:1776–1782. doi: 10.1681/ASN.2009121235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powe NR. Let's get serious about racial and ethnic disparities. J Am Soc Nephrol. 2008;19:1271–1275. doi: 10.1681/ASN.2008040358. [DOI] [PubMed] [Google Scholar]
- 10.Norris K, Nissenson AR. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol. 2008;19:1261–1270. doi: 10.1681/ASN.2008030276. [DOI] [PubMed] [Google Scholar]
- 11.Young BA. The interaction of race, poverty, and CKD. Am J Kidney Dis. 2010;55:977–980. doi: 10.1053/j.ajkd.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minnick ML, Boynton S, Ndirangu J, Furth S. Sex, race, and socioeconomic disparities in kidney disease in children. Semin Nephrol. 2010;30:26–32. doi: 10.1016/j.semnephrol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Patzer RE, et al. Racial disparities in pediatric access to kidney transplantation: does socioeconomic status play a role? Am J Transplant. 2012;12:369–378. doi: 10.1111/j.1600-6143.2011.03888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56:769–784. doi: 10.1016/s0277-9536(02)00073-4. [DOI] [PubMed] [Google Scholar]
- 15.Galobardes B, Shaw M, Lawlor DA, Davey Smith G, Lynch J. In: Methods in Social Epidemiology. 1st. Oakes JM, Kaufman JS, editors. Jossey-Bass; San Francisco: 2006. pp. 47–85. [Google Scholar]
- 16.Winker MA. Measuring race and ethnicity: why and how? JAMA. 2004;292:1612–1614. doi: 10.1001/jama.292.13.1612. [DOI] [PubMed] [Google Scholar]
- 17.Tareen N, et al. Chronic kidney disease in African American and Mexican American populations. Kidney Int Suppl. 2005;97:S137–S140. doi: 10.1111/j.1523-1755.2005.09723.x. [DOI] [PubMed] [Google Scholar]
- 18.Block JP, Scribner RA, DeSalvo KB. Fast food, race/ethnicity, and income: a geographic analysis. Am J Prev Med. 2004;27:211–217. doi: 10.1016/j.amepre.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Blakely TA, Woodward AJ. Ecological effects in multi-level studies. J Epidemiol Commun Health. 2000;54:367–374. doi: 10.1136/jech.54.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diez Roux AV. Next steps in understanding the multilevel determinants of health. J Epidemiol Commun Health. 2008;62:957–959. doi: 10.1136/jech.2007.064311. [DOI] [PubMed] [Google Scholar]
- 21.Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. 1998;88:216–222. doi: 10.2105/ajph.88.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward MM. Access to care and the incidence of end-stage renal disease due to diabetes. Diabetes Care. 2009;32:1032–1036. doi: 10.2337/dc09-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kershaw KN, et al. Metropolitan-level racial residential segregation and black-white disparities in hypertension. Am J Epidemiol. 2011;174:537–545. doi: 10.1093/aje/kwr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prakash S, et al. Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol. 2010;21:1192–1199. doi: 10.1681/ASN.2009101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez RA, et al. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med. 2007;146:493–501. doi: 10.7326/0003-4819-146-7-200704030-00005. [DOI] [PubMed] [Google Scholar]
- 26.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55:992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall YN, Choi AI, Chertow GM, Bindman AB. Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol. 2010;5:828–835. doi: 10.2215/CJN.09011209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diez Roux AV. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91:1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman DJ, Pollak MR. Genetics of kidney failure and the evolving story of APOL1. J Clin Investig. 2011;121:3367–3374. doi: 10.1172/JCI46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClellan WM, et al. Association of family history of ESRD, prevalent albuminuria, and reduced GFR with incident ESRD. Am J Kidney Dis. 2012;59:25–31. doi: 10.1053/j.ajkd.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luyckx VA, Brenner BM. The clinical importance of nephron mass. J Am Soc Nephrol. 2010;21:898–910. doi: 10.1681/ASN.2009121248. [DOI] [PubMed] [Google Scholar]
- 32.Blumenshine P, Egerter S, Barclay CJ, Cubbin C, Braveman PA. Socioeconomic disparities in adverse birth outcomes: a systematic review. Am J Prev Med. 2010;39:263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 33.Sellström E, Bremberg S. The significance of neighbourhood context to child and adolescent health and well-being: a systematic review of multilevel studies. Scand J Public Health. 2006;34:544–554. doi: 10.1080/14034940600551251. [DOI] [PubMed] [Google Scholar]
- 34.White SL, et al. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis. 2009;54:248–261. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 35.Orskov B, Christensen KB, Feldt-Rasmussen B, Strandgaard S. Low birth weight is associated with earlier onset of end-stage renal disease in Danish patients with autosomal dominant polycystic kidney disease. Kidney Int. 2012;81:919–924. doi: 10.1038/ki.2011.459. [DOI] [PubMed] [Google Scholar]
- 36.Rajan T, Barbour SJ, White CT, Levin A. Low birth weight and nephron mass and their role in the progression of chronic kidney disease: a case report on identical twins with Alport disease. Nephrol Dial Transplant. 2011;26:4136–4139. doi: 10.1093/ndt/gfr252. [DOI] [PubMed] [Google Scholar]
- 37.Nistala R, et al. Prenatal programming and epigenetics in the genesis of the cardiorenal syndrome. Cardiorenal Med. 2011;1:243–254. doi: 10.1159/000332756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silverman JG, Decker MR, Reed E, Raj A. Intimate partner violence victimization prior to and during pregnancy among women residing in 26 U.S. states: associations with maternal and neonatal health. Am J Obstet Gynecol. 2006;195:140–148. doi: 10.1016/j.ajog.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 39.Berenson GS, et al. Creatinine clearance, electrolytes, and plasma renin activity related to the blood pressure of white and black children—the Bogalusa Heart Study. J Lab Clin Med. 1979;93:535–548. [PubMed] [Google Scholar]
- 40.Cruickshank JK, et al. Origins of the “black/white” difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa Heart study. Circulation. 2005;111:1932–1937. doi: 10.1161/01.CIR.0000161960.78745.33. [DOI] [PubMed] [Google Scholar]
- 41.Chen W, Srinivasan SR, Ruan L, Mei H, Berenson GS. Adult hypertension is associated with blood pressure variability in childhood in blacks and whites: the Bogalusa Heart Study. Am J Hypertens. 2011;24:77–82. doi: 10.1038/ajh.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deshmukh-Taskar P, et al. Tracking of overweight status from childhood to young adulthood: the Bogalusa Heart Study. Eur J Clin Nutr. 2006;60:48–57. doi: 10.1038/sj.ejcn.1602266. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Srinivasan SR, Berenson GS. Amplification of the association between birthweight and blood pressure with age: the Bogalusa Heart Study. J Hypertens. 2010;28:2046–2052. doi: 10.1097/HJH.0b013e32833cd31f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muntner P, et al. End-stage renal disease in young black males in a black-white population: longitudinal analysis of the Bogalusa Heart Study. BMC Nephrol. 2009;10:40. doi: 10.1186/1471-2369-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludwig J, et al. Neighborhoods, obesity, and diabetes—a randomized social experiment. N Engl J Med. 2011;365:1509–1519. doi: 10.1056/NEJMsa1103216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Langenberg C, et al. Social circumstances and education: life course origins of social inequalities in metabolic risk in a prospective national birth cohort. Am J Public Health. 2006;96:2216–2221. doi: 10.2105/AJPH.2004.049429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wills AK, et al. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8:e1000440. doi: 10.1371/journal.pmed.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoham DA, Vupputuri S, Kshirsagar AV. Chronic kidney disease and life course socioeconomic status: a review. Adv Chronic Kidney Dis. 2005;12:56–63. doi: 10.1053/j.ackd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Bowman S. Low economic status is associated with suboptimal intakes of nutritious foods by adults in the National Health and Nutrition Examination Survey 1999–2002. Nutr Res. 2007;27:515–523. [Google Scholar]
- 50.Raffensperger S, et al. Effect of race and predictors of socioeconomic status on diet quality in the HANDLS Study sample. J Natl Med Assoc. 2010;102:923–930. doi: 10.1016/s0027-9684(15)30711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fouque D, Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database of Systematic Reviews. (3):Art. No.: CD001892. doi: 10.1002/14651858.CD001892.pub3. http://dx.doi.org/10.1002/14651858.CD001892.pub3. [DOI] [PubMed]
- 52.Jones-Burton C, et al. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol. 2006;26:268–275. doi: 10.1159/000093833. [DOI] [PubMed] [Google Scholar]
- 53.Gutiérrez OM, et al. Low socioeconomic status associates with higher serum phosphate irrespective of race. J Am Soc Nephrol. 2010;21:1953–1960. doi: 10.1681/ASN.2010020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boone-Heinonen J, et al. Fast food restaurants and food stores: longitudinal associations with diet in young to middle-aged adults: the CARDIA study. Arch Intern Med. 2011;171:1162–1170. doi: 10.1001/archinternmed.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 56.Kershaw KN, et al. Metropolitan-level racial residential segregation and black-white disparities in hypertension. Am J Epidemiol. 2011;174:537–545. doi: 10.1093/aje/kwr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egede LE, et al. Regional, geographic, and racial/ethnic variation in glycemic control in a national sample of veterans with diabetes. Diabetes Care. 2011;34:938–943. doi: 10.2337/dc10-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ejerblad E, et al. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 59.Kalaitzidis RG, Siamopoulos KC. The role of obesity in kidney disease: recent findings and potential mechanisms. Int Urol Nephrol. 2011;43:771–784. doi: 10.1007/s11255-011-9974-1. [DOI] [PubMed] [Google Scholar]
- 60.Afshinnia F, Wilt TJ, Duval S, Esmaeili A, Ibrahim HN. Weight loss and proteinuria: systematic review of clinical trials and comparative cohorts. Nephrol Dial Transplant. 2010;25:1173–1183. doi: 10.1093/ndt/gfp640. [DOI] [PubMed] [Google Scholar]
- 61.Murasko JE. Trends in the associations between family income, height and body mass index in US children and adolescents: 1971– 1980 and 1999–2008. Ann Hum Biol. 2011;38:290–306. doi: 10.3109/03014460.2010.537698. [DOI] [PubMed] [Google Scholar]
- 62.Choi AI, et al. Association of educational attainment with chronic disease and mortality: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;58:228–234. doi: 10.1053/j.ajkd.2011.02.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green JA, et al. Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2011;6:1354–1360. doi: 10.2215/CJN.09761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grubbs V, Gregorich SE, Perez-Stable EJ, Hsu CY. Health literacy and access to kidney transplantation. Clin J Am Soc Nephrol. 2009;4:195–200. doi: 10.2215/CJN.03290708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cavanaugh KL, et al. Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol. 2010;21:1979–1985. doi: 10.1681/ASN.2009111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Greer RC, Cooper LA, Crews DC, Powe NR, Boulware LE. Quality of patient-physician discussions about CKD in primary care: a cross-sectional study. Am J Kidney Dis. 2011;57:583–591. doi: 10.1053/j.ajkd.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waterman AD, Browne T, Waterman BM, Gladstone EH, Hostetter T. Attitudes and behaviors of African Americans regarding early detection of kidney disease. Am J Kidney Dis. 2008;51:554–562. doi: 10.1053/j.ajkd.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 68.Jurkovitz C, Hylton TN, McClellan WM. Pathogenesis and treatment of kidney disease and hypertension prevalence of family history of kidney disease and perception of risk for kidney disease: a population-based study. Am J Kidney Dis. 2005;46:11–17. doi: 10.1053/j.ajkd.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 69.Boulware LE, Carson KA, Troll MU, Powe NR, Cooper LA. Perceived susceptibility to chronic kidney disease among high-risk patients seen in primary care practices. J Gen Intern Med. 2009;24:1123–1129. doi: 10.1007/s11606-009-1086-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Plantinga LC, Tuot DS, Powe NR. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17:225–236. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuot DS, et al. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6:1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaskin DJ, et al. Examining racial and ethnic disparities in site of usual source of care. J Natl Med Assoc. 2007;99:22–30. [PMC free article] [PubMed] [Google Scholar]
- 73.Evans K, et al. Race differences in access to health care and disparities in incident chronic kidney disease in the US. Nephrol Dial Transplant. 2011;26:899–908. doi: 10.1093/ndt/gfq473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guessous I, McClellan W, Vupputuri S, Wasse H. Low documentation of chronic kidney disease among high-risk patients in a managed care population: a retrospective cohort study. BMC Nephrol. 2009;10:25. doi: 10.1186/1471-2369-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allen AS, et al. Primary care management of chronic kidney disease. J Gen Intern Med. 2011;26:386–392. doi: 10.1007/s11606-010-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fox CH, Brooks A, Zayas LE, McClellan W, Murray B. Primary care physicians' knowledge and practice patterns in the treatment of chronic kidney disease: an Upstate New York Practice-based Research Network (UNYNET) study. J Am Board Fam Med. 2006;19:54–61. doi: 10.3122/jabfm.19.1.54. [DOI] [PubMed] [Google Scholar]
- 77.Agrawal V, Agarwal M, Ghosh AK, Barnes MA, McCullough PA. Identification and management of chronic kidney disease complications by internal medicine residents: a national survey. Am J Ther. 2011;18:e40–e47. doi: 10.1097/MJT.0b013e3181bbf6fc. [DOI] [PubMed] [Google Scholar]
- 78.Finkelstein FO, et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int. 2008;74:1178–1184. doi: 10.1038/ki.2008.376. [DOI] [PubMed] [Google Scholar]
- 79.Flessner MF, et al. Prevalence and awareness of CKD among African Americans: the Jackson Heart Study. Am J Kidney Dis. 2009;53:238–247. doi: 10.1053/j.ajkd.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Owen WF, Jr, Chertow GM, Lazarus JM, Lowrie EG. Dose of hemodialysis and survival: differences by race and sex. JAMA. 1998;280:1764–1768. doi: 10.1001/jama.280.20.1764. [DOI] [PubMed] [Google Scholar]
- 81.Leonard MB, Stablein DM, Ho M, Jabs K, Feldman HI. Racial and center differences in hemodialysis adequacy in children treated at pediatric centers: a North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) report. J Am Soc Nephrol. 2004;15:2923–2932. doi: 10.1097/01.ASN.0000143475.39388.DE. [DOI] [PubMed] [Google Scholar]
- 82.Wasse H, Hopson SD, McClellan W. Racial and gender differences in arteriovenous fistula use among incident hemodialysis patients. Am J Nephrol. 2010;32:234–241. doi: 10.1159/000318152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hall YN, Choi AI, Xu P, O'Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol. 2011;22:743–751. doi: 10.1681/ASN.2010080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients' preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–1669. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 85.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280:1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 86.Garg PP, Frick KD, Diener-West M, Powe NR. Effect of the ownership of dialysis facilities on patients' survival and referral for transplantation. N Engl J Med. 1999;341:1653–1660. doi: 10.1056/NEJM199911253412205. [DOI] [PubMed] [Google Scholar]
- 87.Patzer RE, et al. The role of race and poverty on steps to kidney transplantation in the southeastern United States. Am J Transplant. 2012;12:358–368. doi: 10.1111/j.1600-6143.2011.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weng FL, Joffe MM, Feldman HI, Mange KC. Rates of completion of the medical evaluation for renal transplantation. Am J Kidney Dis. 2005;46:734–745. doi: 10.1053/j.ajkd.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 89.Patzer RE, et al. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20:1333–1340. doi: 10.1681/ASN.2008030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Saunders MR, Cagney KA, Ross LF, Alexander GC. Neighborhood poverty, racial composition and renal transplant waitlist. Am J Transplant. 2010;10:1912–1917. doi: 10.1111/j.1600-6143.2010.03206.x. [DOI] [PubMed] [Google Scholar]
- 91.Ashby VB, et al. Geographic variability in access to primary kidney transplantation in the United States, 1996–2005. Am J Transplant. 2007;7:1412–1423. doi: 10.1111/j.1600-6143.2007.01785.x. [DOI] [PubMed] [Google Scholar]
- 92.Stolzmann KL, et al. Trends in kidney transplantation rates and disparities. J Natl Med Assoc. 2007;99:923–932. [PMC free article] [PubMed] [Google Scholar]
- 93.Waterman AD, Rodrigue JR, Purnell TS, Ladin K, Boulware LE. Addressing racial and ethnic disparities in live donor kidney transplantation: priorities for research and intervention. Semin Nephrol. 2010;30:90–98. doi: 10.1016/j.semnephrol.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weng FL, Reese PP, Mulgaonkar S, Patel AM. Barriers to living donor kidney transplantation among black or older transplant candidates. Clin J Am Soc Nephrol. 2010;5:2338–2347. doi: 10.2215/CJN.03040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Powe NR. To have and have not: health and health care disparities in chronic kidney disease. Kidney Int. 2003;64:763–772. doi: 10.1046/j.1523-1755.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- 96.Wolfe RA, et al. Differences in access to cadaveric renal transplantation in the United States. Am J Kidney Dis. 2000;36:1025–1033. doi: 10.1053/ajkd.2000.19106. [DOI] [PubMed] [Google Scholar]
- 97.Navaneethan SD, Singh S. A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant. 2006;20:769–775. doi: 10.1111/j.1399-0012.2006.00568.x. [DOI] [PubMed] [Google Scholar]
- 98.Soucie JM, Neylan JF, McClellan W. Race and sex differences in the identification of candidates for renal transplantation. Am J Kidney Dis. 1992;19:414–419. doi: 10.1016/s0272-6386(12)80947-4. [DOI] [PubMed] [Google Scholar]
- 99.Gaylin DS, et al. The impact of comorbid and sociodemographic factors on access to renal transplantation. JAMA. 1993;269:603–608. [PubMed] [Google Scholar]
- 100.Axelrod DA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5:2276–2288. doi: 10.2215/CJN.04940610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schold JD, et al. Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol. 2011;6:1760–1767. doi: 10.2215/CJN.08620910. [DOI] [PubMed] [Google Scholar]
- 102.Keith D, Ashby VB, Port FK, Leichtman AB. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clin J Am Soc Nephrol. 2008;3:463–470. doi: 10.2215/CJN.02220507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Neyhart CD. Education of patients pre and post-transplant: improving outcomes by overcoming the barriers. Nephrol Nurs J. 2008;35:409–410. [PubMed] [Google Scholar]
- 104.Patzer RE, et al. Impact of a patient education program on disparities in kidney transplant evaluation. Clin J Am Soc Nephrol. 2012;7:648–655. doi: 10.2215/CJN.10071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goldfarb-Rumyantzev AS, et al. Effect of education on racial disparities in access to kidney transplantation. Clin Transplant. 2010;26:74–81. doi: 10.1111/j.1399-0012.2010.01390.x. [DOI] [PubMed] [Google Scholar]
- 106.Reddan DN, Szczech LA, Klassen PS, Owen WF., Jr Racial inequity in America's ESRD program. Semin Dial. 2000;13:399–403. doi: 10.1046/j.1525-139x.2000.00109.x. [DOI] [PubMed] [Google Scholar]
- 107.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343:1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 108.Kucirka LG, Grams ME, Balhara KS, Jaar BG, Segev DL. Disparities in provision of transplant information affect access to kidney transplantation. Am J Transplant. 2012;12:351–357. doi: 10.1111/j.1600-6143.2011.03865.x. [DOI] [PubMed] [Google Scholar]
- 109.Gordon EJ, Sehgal AR. Patient-nephrologist discussions about kidney transplantation as a treatment option. Adv Ren Replace Ther. 2000;7:177–183. doi: 10.1053/rr.2000.5268. [DOI] [PubMed] [Google Scholar]
- 110.Pradel FG, Jain R, Mullins CD, Vassalotti JA, Bartlett ST. A survey of nephrologists' views on preemptive transplantation. Clin J Am Soc Nephrol. 2008;3:1837–1845. doi: 10.2215/CJN.00150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Green JA, et al. Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2011;6:1354–1360. doi: 10.2215/CJN.09761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hall YN, O'Hare AM, Young BA, Boyko EJ, Chertow GM. Neighborhood poverty and kidney transplantation among US Asians and Pacific Islanders with end-stage renal disease. Am J Transplant. 2008;8:2402–2409. doi: 10.1111/j.1600-6143.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 113.Tonelli M, Klarenbach S, Rose C, Wiebe N, Gill J. Access to kidney transplantation among remote- and rural-dwelling patients with kidney failure in the United States. JAMA. 2009;301:1681–1690. doi: 10.1001/jama.2009.545. [DOI] [PubMed] [Google Scholar]
- 114.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 115.Mackenbach JP, et al. Socioeconomic inequalities in health in 22 European countries. N Engl J Med. 2008;358:2468–2481. doi: 10.1056/NEJMsa0707519. [DOI] [PubMed] [Google Scholar]
- 116.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 117.Nitsch D, et al. Fetal, developmental, and parental influences on cystatin C in childhood: the Uppsala Family Study. Am J Kidney Dis. 2011;57:863–872. doi: 10.1053/j.ajkd.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 118.Salgado CM, Jardim PC, Teles FB, Nunes MC. Influence of low birth weight on microalbuminuria and blood pressure of school children. Clin Nephrol. 2009;71:367–374. doi: 10.5414/cnp71367. [DOI] [PubMed] [Google Scholar]
- 119.Laganovic M, et al. Kidney volume and albuminuria as markers of birth weight-blood pressure relationship in essential hypertension. Kidney Blood Press Res. 2009;32:399–404. doi: 10.1159/000260041. [DOI] [PubMed] [Google Scholar]
- 120.Painter RC, et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J Am Soc Nephrol. 2005;16:189–194. doi: 10.1681/ASN.2004060474. [DOI] [PubMed] [Google Scholar]
- 121.Banks J, Marmot M, Oldfield Z, Smith JP. Disease and disadvantage in the United States and in England. JAMA. 2006;295:2037–2045. doi: 10.1001/jama.295.17.2037. [DOI] [PubMed] [Google Scholar]
- 122.Banks JW, Marmot M, Oldfield Z, Smith JP. The SES health gradient on both sides of the Atlantic: IZA Discussion Paper No 2539 [online] 2007 http://ssrn.com/abstract=957287.
- 123.Al-Qaoud TM, Nitsch D, Wells J, Witte DR, Brunner EJ. Socioeconomic status and reduced kidney function in the Whitehall II Study: role of obesity and metabolic syndrome. Am J Kidney Dis. 2011;58:389–397. doi: 10.1053/j.ajkd.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bello AK, Peters J, Rigby J, Rahman AA, El-Nahas M. Socioeconomic status and chronic kidney disease at presentation to a renal service in the United Kingdom. Clin J Am Soc Nephrol. 2008;3:1316–1323. doi: 10.2215/CJN.00680208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hossain MP, Palmer D, Goyder E, El Nahas AM. Association of deprivation with worse outcomes in chronic kidney disease: findings from a hospital-based cohort in the United Kingdom. Nephron Clin Pract. 2012;120:c59–c70. doi: 10.1159/000334998. [DOI] [PubMed] [Google Scholar]
- 126.Maheswaran R, et al. Socioeconomic deprivation, travel distance, and renal replacement therapy in the Trent Region, United Kingdom 2000: an ecological study. J Epidemiol Commun Health. 2003;57:523–524. doi: 10.1136/jech.57.7.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cass A, Cunningham J, Wang Z, Hoy W. Regional variation in the incidence of end-stage renal disease in Indigenous Australians. Med J Aust. 2001;175:24–27. doi: 10.5694/j.1326-5377.2001.tb143507.x. [DOI] [PubMed] [Google Scholar]
- 128.Wolf G, Busch M, Müller N, Müller UA. Association between socioeconomic status and renal function in a population of German patients with diabetic nephropathy treated at a tertiary centre. Nephrol Dial Transplant. 2011;26:4017–4023. doi: 10.1093/ndt/gfr185. [DOI] [PubMed] [Google Scholar]
- 129.Fored CM, et al. Socio-economic status and chronic renal failure: a population-based case-control study in Sweden. Nephrol Dial Transplant. 2003;18:82–88. doi: 10.1093/ndt/18.1.82. [DOI] [PubMed] [Google Scholar]
- 130.Hommel K, Rasmussen S, Kamper AL, Madsen M. Regional and social inequalities in chronic renal replacement therapy in Denmark. Nephrol Dial Transplant. 2010;25:2624–2632. doi: 10.1093/ndt/gfq110. [DOI] [PubMed] [Google Scholar]
- 131.Collins JF. Kidney disease in Maori and Pacific people in New Zealand. Clin Nephrol. 2010;74(Suppl. 1):S61–S65. [PubMed] [Google Scholar]
- 132.Parameswaran S, et al. Referral pattern of patients with end-stage renal disease at a public sector hospital and its impact on outcome. Natl Med J India. 2011;24:208–213. [PubMed] [Google Scholar]
- 133.Abraham G, et al. Resource settings have a major influence on the outcome of maintenance hemodialysis patients in South India. Hemodial Int. 2010;14:211–217. doi: 10.1111/j.1542-4758.2010.00441.x. [DOI] [PubMed] [Google Scholar]