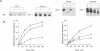Abstract
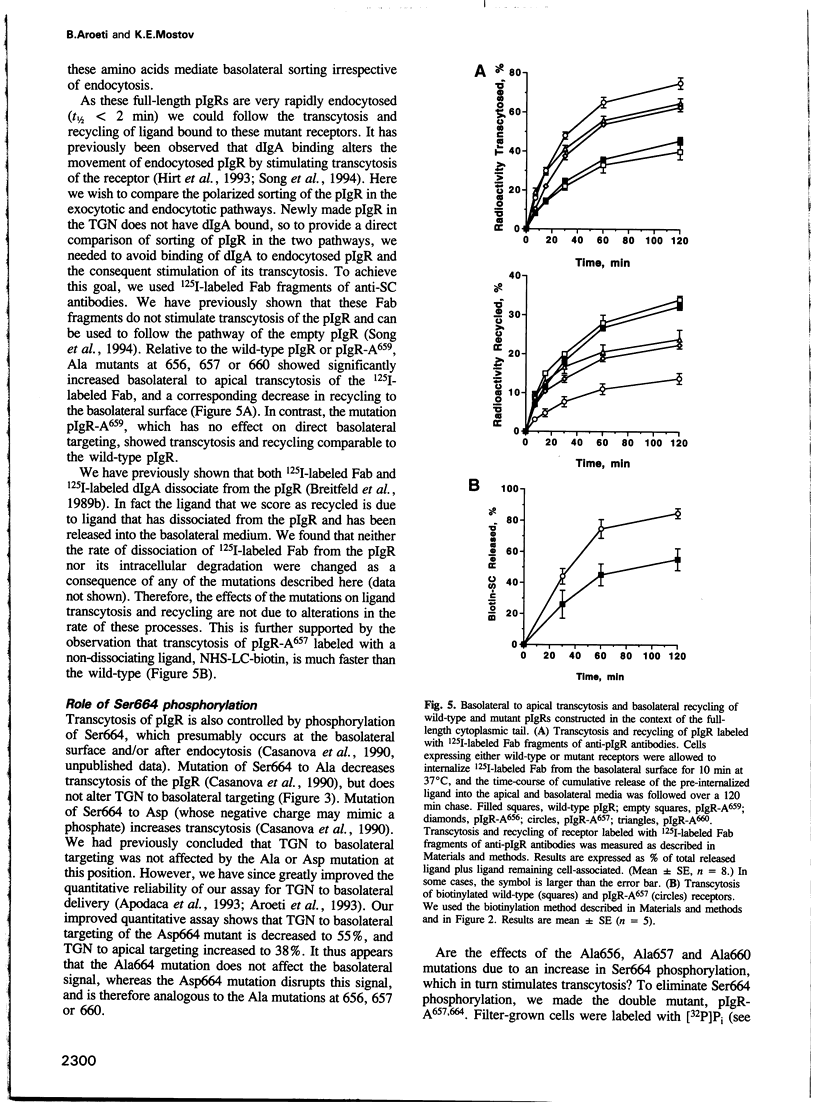
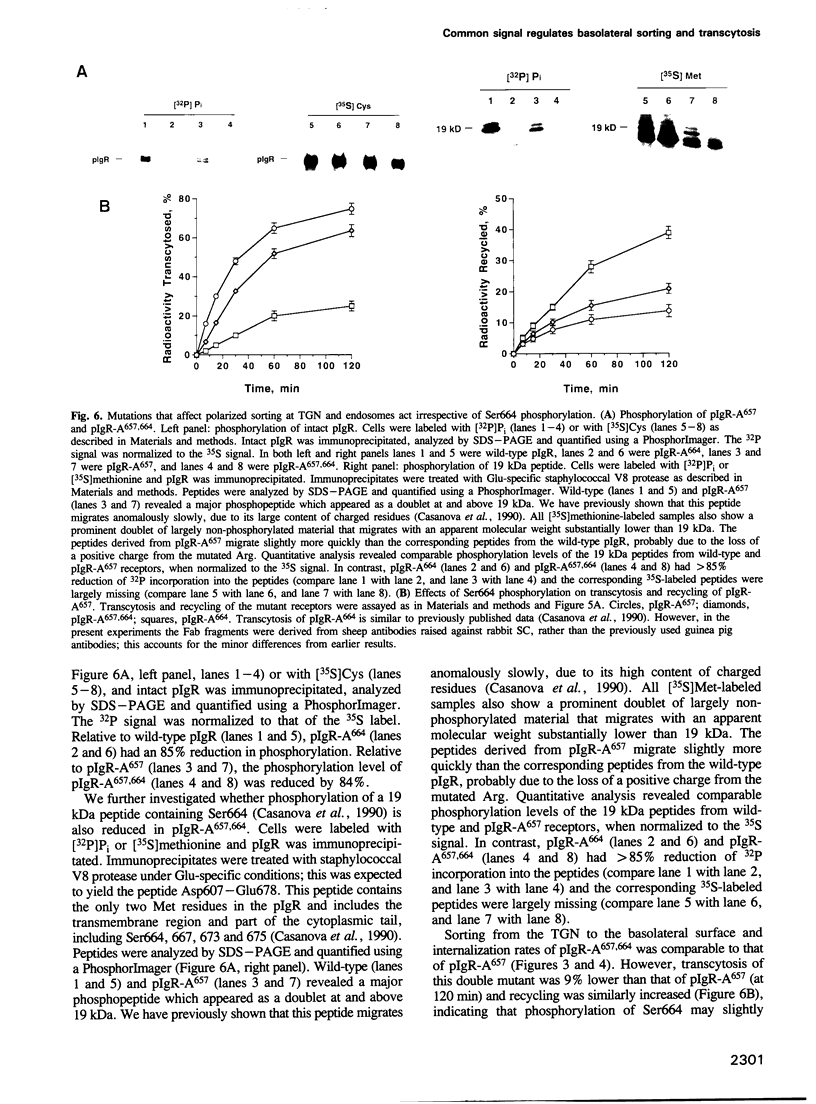
Polarized epithelial cells can sort plasma membrane proteins to the apical or basolateral domain either by direct targeting from the trans-Golgi network (TGN) or by targeting to one surface, followed by endocytosis and transcytosis to the opposite surface. In Madin-Darby canine kidney (MDCK) cells, targeting of the polymeric immunoglobulin receptor (pIgR) to the basolateral surface is controlled by a sorting signal residing in the membrane proximal 17 amino acids of the cytoplasmic domain of this receptor. We have recently found that individual mutations at any of three residues in this signal, His656, Arg657 and Val660, substantially decrease targeting from the TGN to the basolateral surface and correspondingly increase targeting from the TGN to the apical surface. Here we report that these mutations decrease the recycling of basolaterally endocytosed pIgR to that surface, and correspondingly increase its transcytosis to the apical surface. This effect occurred in mutant pIgRs that either contained the full-length cytoplasmic domain or were truncated to contain only the 17-residue basolateral targeting signal, and was independent of phosphorylation of pIgR at Ser664. Our results indicate that polarized sorting of the pIgR in the endocytotic and exocytotic pathways are controlled by the same amino acids.
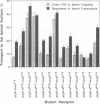
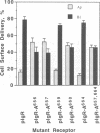
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apodaca G., Aroeti B., Tang K., Mostov K. E. Brefeldin-A inhibits the delivery of the polymeric immunoglobulin receptor to the basolateral surface of MDCK cells. J Biol Chem. 1993 Sep 25;268(27):20380–20385. [PubMed] [Google Scholar]
- Aroeti B., Kosen P. A., Kuntz I. D., Cohen F. E., Mostov K. E. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol. 1993 Dec;123(5):1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsel M., Mostov K. E. Possible role of both the alpha and beta gamma subunits of the heterotrimeric G protein, Gs, in transcytosis of the polymeric immunoglobulin receptor. J Biol Chem. 1993 Dec 5;268(34):25824–25835. [PubMed] [Google Scholar]
- Bomsel M., Mostov K. Role of heterotrimeric G proteins in membrane traffic. Mol Biol Cell. 1992 Dec;3(12):1317–1328. doi: 10.1091/mbc.3.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld P. P., Casanova J. E., Harris J. M., Simister N. E., Mostov K. E. Expression and analysis of the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells using retroviral vectors. Methods Cell Biol. 1989;32:329–337. doi: 10.1016/s0091-679x(08)61178-4. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., Harris J. M., Mostov K. E. Postendocytotic sorting of the ligand for the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells. J Cell Biol. 1989 Aug;109(2):475–486. doi: 10.1083/jcb.109.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C. B., Roth M. G. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991 Aug;114(3):413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E., Apodaca G., Mostov K. E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991 Jul 12;66(1):65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Breitfeld P. P., Ross S. A., Mostov K. E. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990 May 11;248(4956):742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Casanova J. E., Mishumi Y., Ikehara Y., Hubbard A. L., Mostov K. E. Direct apical sorting of rat liver dipeptidylpeptidase IV expressed in Madin-Darby canine kidney cells. J Biol Chem. 1991 Dec 25;266(36):24428–24432. [PubMed] [Google Scholar]
- Dargemont C., Le Bivic A., Rothenberger S., Iacopetta B., Kühn L. C. The internalization signal and the phosphorylation site of transferrin receptor are distinct from the main basolateral sorting information. EMBO J. 1993 Apr;12(4):1713–1721. doi: 10.1002/j.1460-2075.1993.tb05816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Peppard J., von Figura K., Hasilik A., Schwartz A. L. Intracellular receptor sorting during endocytosis: comparative immunoelectron microscopy of multiple receptors in rat liver. Cell. 1984 May;37(1):195–204. doi: 10.1016/0092-8674(84)90315-5. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Hirt R. P., Hughes G. J., Frutiger S., Michetti P., Perregaux C., Poulain-Godefroy O., Jeanguenat N., Neutra M. R., Kraehenbuhl J. P. Transcytosis of the polymeric Ig receptor requires phosphorylation of serine 664 in the absence but not the presence of dimeric IgA. Cell. 1993 Jul 30;74(2):245–255. doi: 10.1016/0092-8674(93)90416-n. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Harter C., Matter K., Mellman I. Basolateral sorting in MDCK cells requires a distinct cytoplasmic domain determinant. Cell. 1991 Sep 6;66(5):907–920. doi: 10.1016/0092-8674(91)90437-4. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Mostov K., Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990 May;110(5):1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low S. H., Wong S. H., Tang B. L., Subramaniam V. N., Hong W. J. Apical cell surface expression of rat dipeptidyl peptidase IV in transfected Madin-Darby canine kidney cells. J Biol Chem. 1991 Jul 15;266(20):13391–13396. [PubMed] [Google Scholar]
- Ludwig T., Griffiths G., Hoflack B. Distribution of newly synthesized lysosomal enzymes in the endocytic pathway of normal rat kidney cells. J Cell Biol. 1991 Dec;115(6):1561–1572. doi: 10.1083/jcb.115.6.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992 Nov 27;71(5):741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Matter K., Whitney J. A., Yamamoto E. M., Mellman I. Common signals control low density lipoprotein receptor sorting in endosomes and the Golgi complex of MDCK cells. Cell. 1993 Sep 24;74(6):1053–1064. doi: 10.1016/0092-8674(93)90727-8. [DOI] [PubMed] [Google Scholar]
- Mostov K., Apodaca G., Aroeti B., Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992 Feb;116(3):577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J. Regulation of cell surface polarity from bacteria to mammals. Science. 1992 Nov 6;258(5084):948–955. doi: 10.1126/science.1439806. [DOI] [PubMed] [Google Scholar]
- Okamoto C. T., Shia S. P., Bird C., Mostov K. E., Roth M. G. The cytoplasmic domain of the polymeric immunoglobulin receptor contains two internalization signals that are distinct from its basolateral sorting signal. J Biol Chem. 1992 May 15;267(14):9925–9932. [PubMed] [Google Scholar]
- Pimplikar S. W., Simons K. Regulation of apical transport in epithelial cells by a Gs class of heterotrimeric G protein. Nature. 1993 Apr 1;362(6419):456–458. doi: 10.1038/362456a0. [DOI] [PubMed] [Google Scholar]
- Prill V., Lehmann L., von Figura K., Peters C. The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J. 1993 May;12(5):2181–2193. doi: 10.1002/j.1460-2075.1993.tb05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Powell S. K. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Song W., Bomsel M., Casanova J., Vaerman J. P., Mostov K. Stimulation of transcytosis of the polymeric immunoglobulin receptor by dimeric IgA. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):163–166. doi: 10.1073/pnas.91.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Brewer C. B., Roth M. G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993 Feb 15;268(5):3313–3320. [PubMed] [Google Scholar]
- Vaux D. The structure of an endocytosis signal. Trends Cell Biol. 1992 Jul;2(7):189–192. doi: 10.1016/0962-8924(92)90232-c. [DOI] [PubMed] [Google Scholar]
- Wessels H. P., Hansen G. H., Fuhrer C., Look A. T., Sjöström H., Norén O., Spiess M. Aminopeptidase N is directly sorted to the apical domain in MDCK cells. J Cell Biol. 1990 Dec;111(6 Pt 2):2923–2930. doi: 10.1083/jcb.111.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]