Abstract
Objective
Interleukin-19 (IL-19) is putative Th2, anti-inflammatory interleukin. Its expression in, and potential role in atherogenesis is unknown. IL-19 is not detected in normal artery, and is expressed to a greater degree in plaque from symptomatic vs. asymptomatic patients, suggesting a compensatory-counter regulatory function. We tested if IL-19 could reduce atherosclerosis in susceptible mice, and identified plausible mechanisms.
Approach and Results
LDLR−/− mice fed an atherogenic diet and injected with either 1.0ng/g/day or 10.0ng/g/day rmIL-19 had significantly less plaque area in the aortic arch compared with controls (p<0.0001). Weight gain, cholesterol and triglyceride levels were not significantly different. Gene expression in splenocytes from IL-19 treated mice demonstrated immune cell Th2 polarization, with decreased expression of T-bet, IFNγ, IL-1β and IL-12β, and increased expression of GATA3 and FoxP3 mRNA. A greater percentage of lymphocytes were Th2 polarized in IL-19 treated mice. Cellular characterization of plaque by immunohistochemistry demonstrated IL-19 treated mice have significantly less macrophage infiltrate compared with controls (p<0.001). Intravital microscopy revealed significantly less leukocyte adhesion in wild-type mice injected with IL-19 and fed an atherogenic diet compared with controls. Treatment of cultured endothelial cells (EC), vascular smooth muscle cells (VSMC), and bone marrow-derived macrophages (BMDM) with IL-19 resulted in a significant decrease in chemokine mRNA, and in the mRNA-stability protein HuR.
Conclusions
These data suggest IL-19 is a potent inhibitor of experimental atherosclerosis, with diverse mechanisms including immune cell polarization, decrease in macrophage adhesion, and decrease in gene expression. This may identify IL-19 as a novel therapeutic to limit vascular inflammation.
Keywords: atherosclerosis, Interleukin-19, chemokines, macrophage
Introduction
Atherosclerosis is a chronic vascular inflammatory disease (1). A series of cytokine-mediated interactions between lymphocytes, macrophage, endothelial, and vascular smooth muscle cells result in local inflammation of the arterial wall (1,2). While a multitude of potential mechanisms have been investigated for the initiation and propagation of atherosclerosis, the role of excess low density lipoprotein in inducing vascular inflammation is a widely acknowledged mechanism in atherogenesis. The Th1 arm of adaptive immunity is characterized by secretion of pro-inflammatory cytokines, and atherosclerosis in particular has been described as a Th1 inflammatory disease (3). Not surprisingly, a large number of studies advocate the importance of Th1 interleukins in the atherosclerotic disease process based on the predominance of Th1 lymphocytes and their cytokines in both human and mouse atherosclerotic lesions (4-6). By comparison, a much smaller number of studies focus on the role of endogenous counter-regulatory mechanisms in atherogenesis. The Th2 arm of immunity elaborate anti-inflammatory interleukins which tend to limit the magnitude of the inflammatory response. Despite their potential for anti-inflammatory effects, uncertainty remains concerning the potential for direct protective effects of Th2 positive cells and interleukins in atherogenesis. The majority of these important studies focus on the archetypical anti-inflammatory cytokine, IL-10. These principal studies suggest that reduction of atherosclerosis attributed to IL-10 is mediated by modulation of immune function by polarizing the T lymphocyte Th2/Th1 ratio toward a more anti-inflammatory phenotype (6-11). However, injection of IL-4 into ApoE−/− mice does not reduce development of atherosclerotic lesions, and lesions were in fact reduced in IL-4/ApoE double knock out mice (12,13). Moreover, very little has been published regarding potential protective effects of Th2 interleukins on resident vascular cells (EC and VSMC) in addition to inflammatory cells. Recognition of anti-atherosclerotic interleukins, particularly those which may have direct anti-inflammatory activity on resident vascular cells in addition to immune polarization are needed and would have considerable therapeutic potential.
IL-19 was discovered in 2001, and is a member of an IL-10 sub-family which also includes IL-20, IL-22, and IL-24 (14,15). IL-19 is considered to be anti-inflammatory because in T-lymphocytes it promotes the Th2, rather than the Th1 response (16,17). IL-19 signals through the IL-20 heterodimeric receptor complex, a class II cytokine receptor (16,18). Like typical class II receptors, IL-20Rs signal through the JAK-STAT family of signal transducers, and IL-19 activates STAT3 in VSMC, and STAT3 and p44/42 in EC (19,20). Expression of these receptors is tissue-restricted and cytokine inducible (15). Aside from its anti-inflammatory effects, IL-19 is unique among interleukins, including members of its own family. For example, neither IL-10, IL-22, IL-24, nor IL-4 are expressed by EC or VSMC, precluding any autocrine effects of these interleukins on the vasculature (21). IL-19 expression has been associated with some immune-linked diseases, such as rheumatoid arthritis and psoriasis (22). Interestingly, psoriasis has recently been linked with atherosclerosis (23). We have reported a unique role for IL-19 in vascular disease (19,20,24). IL-19 is expressed in EC and VSMC in injured, but not naive arteries, and in stimulated, but not unstimulated cultured EC and VSMC (19,24). This was novel and unexpected because IL-19 expression was previously thought to be restricted to immune cells (14,15,18,21). Expression of IL-19 in atherosclerotic plaque has not been reported, and a role for IL-19 in development of atherosclerosis is currently unknown.
Many investigators have posited that atherosclerosis development is influenced by a balance between pro and anti-inflammatory cytokines, implying that IL-19 may regulate the development of atherosclerosis (3,5-7). The goals of this study were to determine if IL-19 was expressed in atherosclerotic plaque, to test the hypothesis that IL-19 could reduce atherosclerosis in susceptible mice, and to identify potential mechanisms for these effects.
Materials and Methods
Detailed description of Mice, study design, lesion analysis, immunohistochemistry, adhesion assay, intravital microscopy, cell culture, quantitative RT-PCR are presented in the Detailed materials and methods section.
Results
IL-19 is expressed in human atherosclerotic plaque
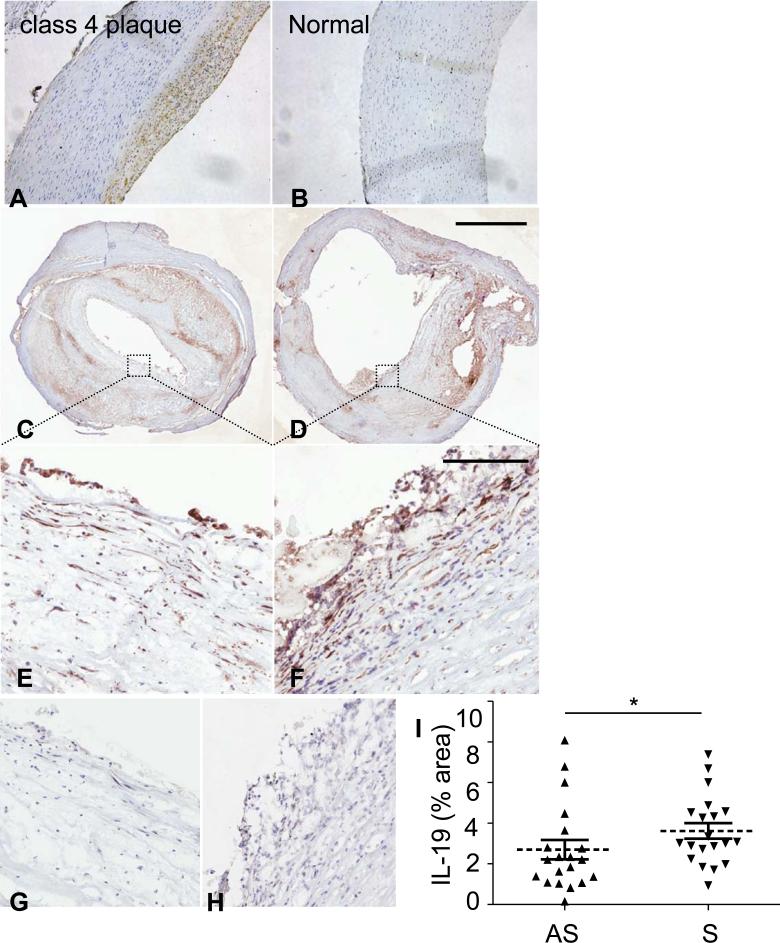
The atherosclerotic plaque microenvironment is biased to Th1 activation in humans and hypercholesterolemic mice (25). IL-19 content in atherosclerotic plaque has never been reported. Several human coronary arteries obtained post-mortem were immunostained with IL-19 antibody to characterize the cellular distribution in atherosclerosis. Overall, very little IL-19 immunoreactivity was detected in normal arteries, but surprisingly, we did observe consistent immunodetection in leukocyte, EC, and VSMC in Stary plaque types 4 and 5 (26). In the representative photomicrograph shown in Figure 1A, B, abundant IL-19 immunoreactivity localized within plaque from a human patient with type 4 plaque. Very little to no IL-19 immunoreactivity was detected in medial VSMC in this artery. We did not observe IL-19 expression in Stary plaque classification types 1 or 2. IL-19 cell-specific expression in a representative plaque was established in EC, VSMC, and inflammatory cells by immunoreactive co-localization with the EC marker Von Willebrand, smooth muscle cell α actin, and leukocyte common antigen CD45 (Supplemental Data IA).
Figure 1.
IL-19 expression in human atherosclerotic plaque. A. IL-19 is expressed in atherosclerotic, but not in normal human artery, Representative immunohistochemical analysis of serial sections from vessels obtained from a normal, and a human atherosclerotic coronary (Stary Class 4) artery immunostained with anti-IL-19 antibody. Carotid endarterectomy sections. C. asymptomatic. D. symptomatic E. high power asymptomatic, F. High power symptomatic. Red-brown staining indicates antibody recognition, sections were counterstained with hematoxylin. G., H., serial sections of E. and F. stained with isotype specific control antibody. Magnification 200X (A., B.), scale bar for C., D.=2mm, EH=200um). I. Significantly higher IL-19 levels are observed in plaques from symptomatic patients compared with asymptomatic patients (P<0.05, n=20 patients each group).
Expression of IL-19 was also demonstrated in human carotid endarterectomy sections by immunohistochemistry (Fig. 1C-G). Consistent with a compensatory-counter regulatory role for this cytokine in regulation of atherosclerosis, significantly higher IL-19 levels were found in plaques from patients with symptoms (stroke, transient ischemic attacks, amaurosis fugax) when compared to those from patients without symptoms (P=0.03, n=20 in each group; Fig. 5I). Clinical characteristics of the individuals included in this study are described in Supplemental Data Table 1. Interestingly, a significant negative correlation was observed between the levels of IL-19 (expressed as % positive IL-19 area of the plaque) and levels of soluble CD40 ligand (sCD40L) measured in plasma (r= −0.368; p=0.042). CD40L is a key mediator of cell communication in the immune system and has the ability to trigger the production of inflammatory cytokines such as IL-1 to enhance the density of cell adhesion molecules in vascular endothelial cells (27). As in the atherosclerotic coronary arteries, IL-19 was clearly detected in multiple cell types in these samples (Fig 1E-F).
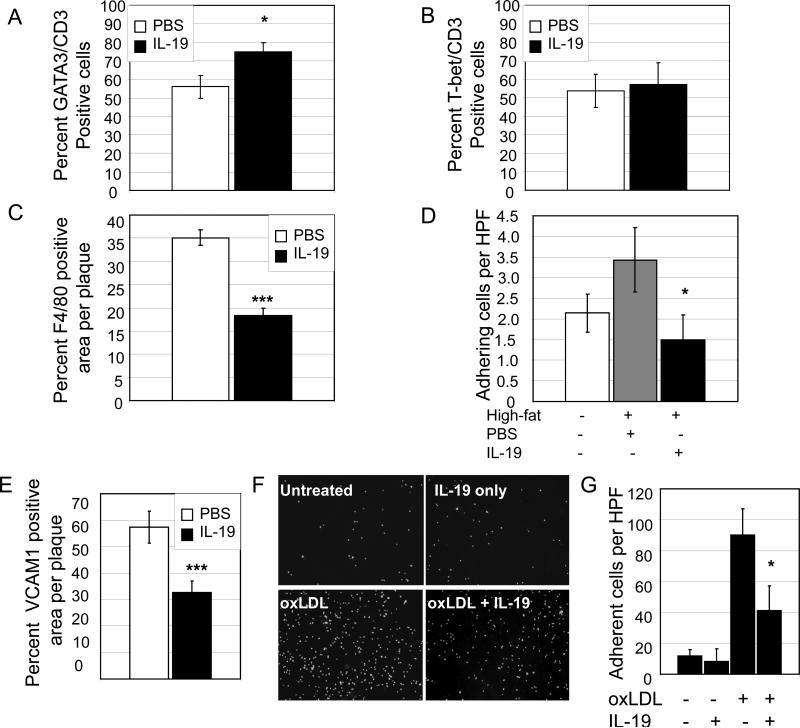
Figure 5.
T lymphocyte and macrophage infiltrate into atherosclerotic lesions. A. Multiple serial sections of the aortic root from the aortic sinus to disappearance of valve cusps per root of 10ng/g/day IL-19 treated and PBS control mice were sectioned and immunostained using CD3 and GATA3 antibody. The number of CD3 positive cells that also stained positive for GATA3 were quantitated by counting individual cells. p<0.001, n=8 mice, using at least three sections per aortic root. B. The number of CD3 positive cells that stained positive for T-bet were quantitated by counting individual cells. There was no significant difference between groups. C. IL-19 reduces macrophage infiltrate in atherosclerotic lesions. Representative fluorescent photomicrographs of aortic root immunostained with F4/80 antibody. Multiple serial sections of the aortic root from the aortic sinus to disappearance of valve cusps per root of 10ng/g/day IL-19 treated and PBS control mice were sectioned and immunostained using F4/80 antibody. Positively stained areas were quantitated as a percentage of total lesion area by quantitative morphometry. p<0.001, n=8 mice, using at least three sections per aortic root. D. IL-19 reduces leukocyte-endothelial interactions in vivo. Wild-type C57B6 mice were fed an atherogenic diet for 12 weeks, during which time they were injected with 10ng/g/day IL-19 or PBS. Leukocyte adhesion was quantitated by intravital microscopy. IL-19 treatment significantly reduces leukocyte adhesion (p<0.05, n=5 mice per group). E. Percent of VCAM1 positive cells in lesions. Positively stained areas were quantitated as a percentage of total lesion area by quantitative morphometry as described for F4/80. F. IL-19 reduces monocyte adhesion to endothelial cell monolayers. ECs grown on glass coverslips were pre-treated with IL-19 for 16 hours, then 50mg/ml oxLDL for 6 hours, prior to addition of THP1 monocytes. Representative photomicrograph shown. G. Results represent means P<0.05 for five high power fields from triplicate experiments.
This is the first description of IL-19 expression in atherosclerotic plaque. IL-19 detection in plaque, but not medial VSMC suggested a compensatory-counter regulatory role for this cytokine in regulation of atherosclerosis.
IL-19 decreases atherosclerotic plaque area in LDLR−/− mice
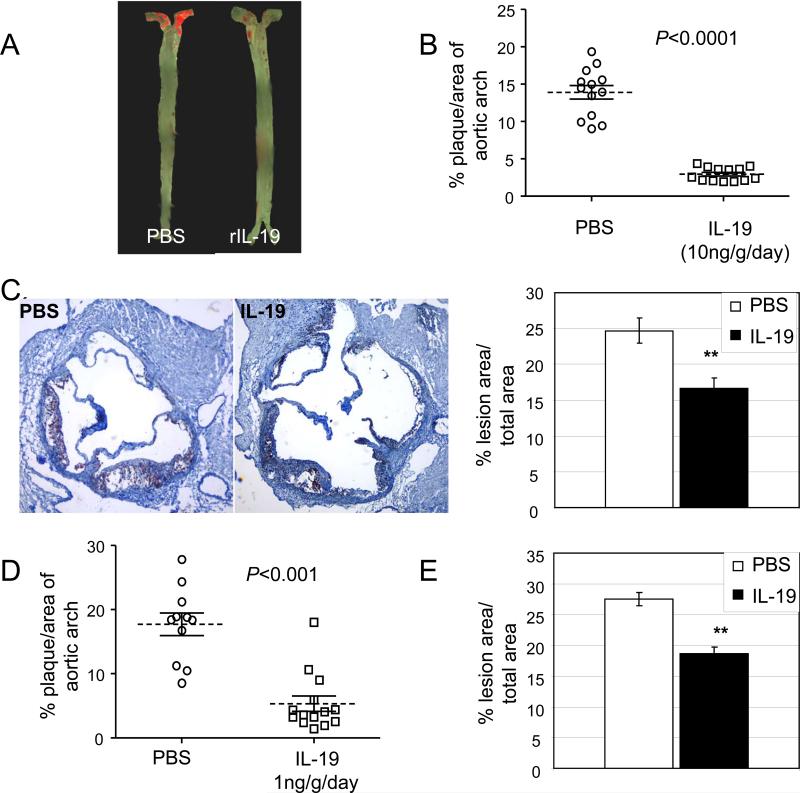
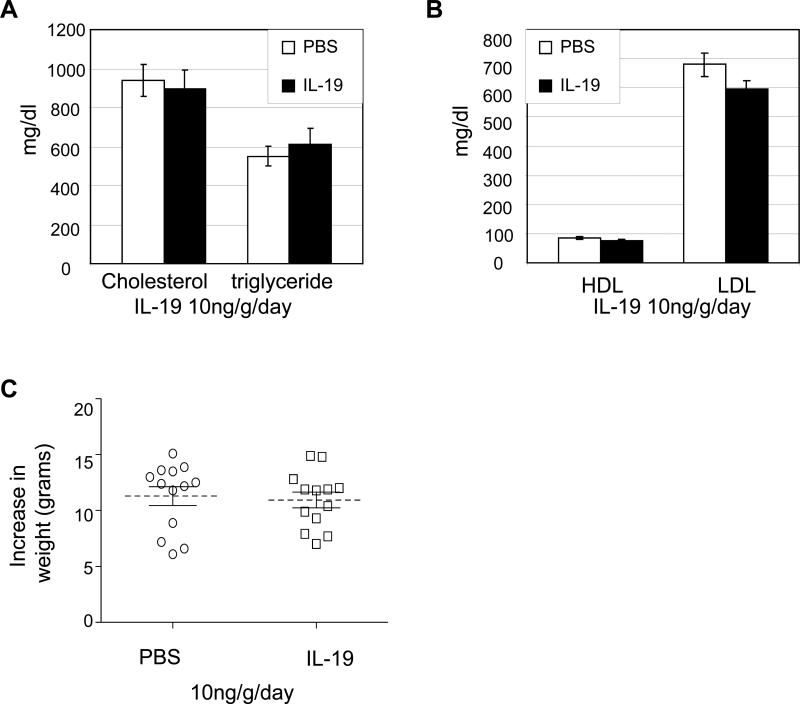
We hypothesized that systemic administration of IL-19 would be protective and decrease atherosclerosis. Similar to human, little to no IL-19 is detected in normal mouse aorta, but abundant IL-19 is detected in lesions from LDLR−/− mice (Supplemental Data Figure 1B). LDLR−/− mice were utilized for inhibition experiments because they do not develop atherosclerotic lesions until fed a high fat diet, allowing synchronization of initiation of atherosclerosis with IL-19 administration. Mice were injected i.p. with 10ng/g/day murine recombinant IL-19, or an equal volume of PBS for 5 consecutive days per week for 13 weeks. Surface lesion area determined by en face staining of aortic arch and quantitative morphometry show a significant reduction in lesion area between the PBS control and IL-19-treated mice (Figure 2A) (13.9±0.9% vs. 2.9±0.25%, respectively; p<0.0001, n=13 in each group). There was no significant difference in lesion area between sexes in either group. Similar results were obtained using ApoE−/− mice fed an atherogenic diet for 12 weeks (26.78±3.41% vs. 10.36±0.94%, p<0.0001, for PBS and IL-19-treated mice, respectively) (Supplemental data Figure 1C). Lesion area assessed by quantitative morphometry in multiple serial transverse sections of Oil Red O stained aortic root was significantly reduced in IL-19 injected mice compared with PBS controls (24.7±1.8% vs. 16.6±1.5% for PBS and IL-19 treated, respectively, p<0.01) (Figure 2C). In a second cohort we determined that systemic administration of as little as 1ng/g/day IL-19 could significantly reduce lesion area (17.7±1.7% vs. 5.3±1.2% for PBS and IL-19 treated mice p<0.0001, n=11 and 13, respectively) (Figure 2D). Interquartile range values for all studies are presented in Table 1. Lesion area in Oil Red O stained aortic root was significantly lower in 1ng/g/day IL-19-injected mice compared with PBS controls (27.4±1.1% vs. 18.6±1.1% for PBS and IL-19 treated, respectively, p<0.01) (Figure 2E.). There was no significant difference in serum lipid profiles (Figure 3A and B). There was no significant difference in weight gain (11.29±0.8 vs. 10.95±0.7 grams for PBS and IL-19 in the 10ng/g/day, or 11.99±1.4 vs. 8.9±0.9 grams for PBS and IL-19 in the 1ng/g/day study, respectively) (Figure 3C) during the course of either dose study. This is the first report demonstrating that systemic administration of IL-19 is anti-atherogenic, and the remainder of the study was directed toward elucidating mechanisms for this effect.
Figure 2.
IL-19 reduces atherosclerosis. A. Representative photomicrograph of aortic arch from LDLR−/− mice after consuming atherogenic diet for 13 weeks, injected with either PBS or 10ng/g/day. Surface lesion en face stained with Sudan IV. B. Graphic depiction of atherosclerotic lesion size quantitated from en face stained aortic arches as depicted in “A”, (n=13 each). C. Representative photomicrographs of aortic root stained with Oil Red O from mice treated with 10ng/g/day IL-19 or PBS. Quantitation of lesion area from four transverse serial sections from the aortic sinus to disappearance of valve cusps per aortic root from mice were stained with Oil Red O, and positive stained areas quantitated. D. Graphic depiction of atherosclerotic lesion size quantitated from aortic arches from LDLR−/− mice after consuming atherogenic diet for 13 weeks, injected with either PBS or 1ng/g/day IL-19 (n=11, n=13, respectively). E. Quantitation of cross-sectional lesion area from aortic root from mice either PBS or 1ng/g/day IL-19 5 days per week were stained with Oil Red O, and positive stained areas quantitated.
Table 1.
Interquartile range (IQR) values for all studies.
| Study | mouse | condition | median (%) | IQR (%) |
|---|---|---|---|---|
| 10ng/g/day | (LDLR−/−) | PBS | 16.83 | 14.73-21.72 |
| IL-19 | 2.00 | 1.32- 2.75 | ||
| Ing/g/day | (LDLR−/−) | PBS | 18.41 | 11.27-27.81 |
| IL-19 | 3.81 | 2.50- 6.72 | ||
| 10ng/g/day | (ApoE−/−) | PBS | 25.45 | 18.06-43.92 |
| IL-19 | 10.24 | 8.05-12.06 |
Figure 3.
IL-19 does not modify serum lipids or weight. A. Cholesterol and triglycerides, B. HDL and LDL in mice fed atherogenic diet for 13 weeks receiving either PBS or 10ng/g/day IL-19, at time of euthanasia do not statistically differ between control and IL-19 groups (n=9 each). C. Weight gain does not statistically differ between control and IL-19 groups (n=13 each).
IL-19 polarizes leukocytes to a Th2-like profile
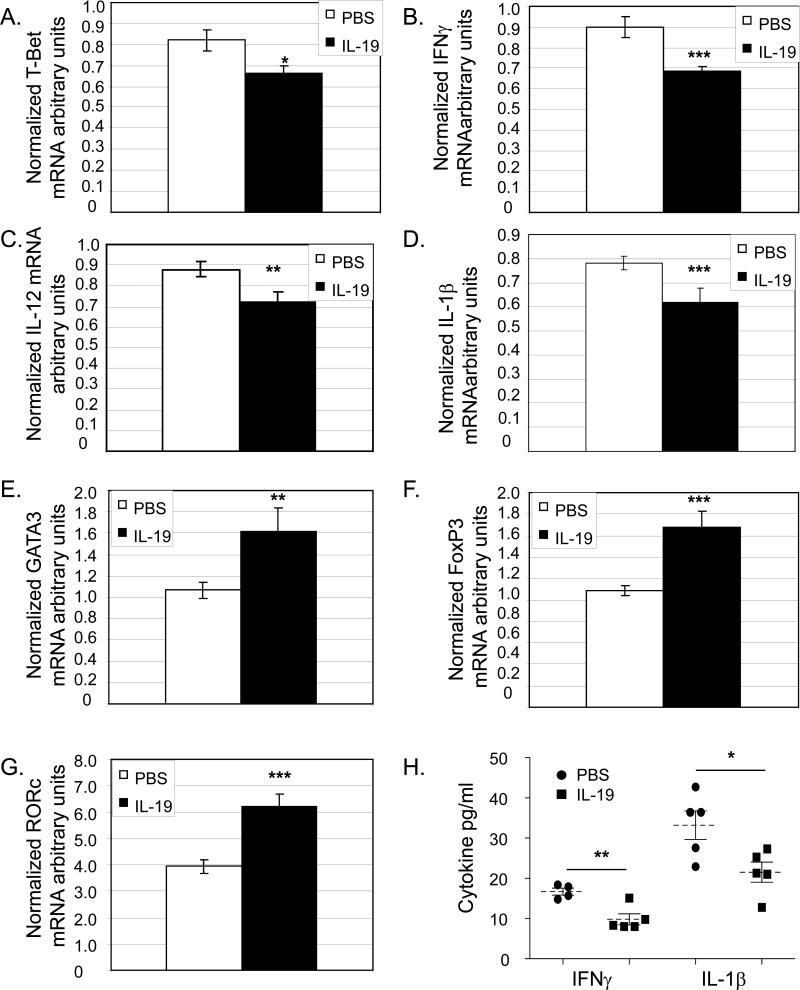
Atherosclerosis is highly influenced by the Th1/Th2 balance (28,29). The global immune status of these mice was determined by quantitation of Th1 and Th2 marker expression in splenocytes immediately removed from mice at the termination of the study. Quantitative RT-PCR demonstrates that splenocytes from IL-19 injected mice had significantly lower mRNA levels of the Th1 markers T-bet (0.82±0.05 vs. 0.66± 0.04 for PBS and IL-19 treated, respectively, p<0.05), and IFNγ (0.89±0.05 vs. 0.68±0.02 for PBS and IL-19 treated, respectively, p<0.05) (Figure 4). Concordantly, splenocytes from IL-19 injected mice had significantly higher levels of Th2 markers GATA3 (1.06±0.08 vs. 1.62±0.22 for PBS and IL-19 respectively p<0.001), and FoxP3 (1.08±0.05 vs. 1.68±0.15 for PBS and IL-19, respectively, p<0.001) mRNA. IL-19 treated mice had significantly higher RORγ mRNA compared with controls (3.93±0.27 vs. 6.23±0.46 for PBS and IL-19, respectively, p<0.01). Quantitative RT-PCR also shows mRNA for IL-1β and IL-12p40, both potent pro-inflammatory cytokines, are significantly decreased in IL-19 injected mice, (0.78±0.28 vs.0.61±0.5 for IL-1β, p<0.001, and 0.88±0.30 vs. 0.72±0.04 p<0.01, for PBS and IL-19, respectively). Cytokine protein was assayed by quantitative Luminex analysis and determined significantly less IFNγ and IL-1β protein in IL-19-treated mice compared with controls (16.70±0.8 vs. 9.84±1.3 pg/ml IFNγ for PBS and IL-19, and 33.20±5.5 vs. 21/54±2.4 pg/ml IL-1γ for PBS and IL-19, respectively, p<0.05). GATA3 protein is also increased in spleen from IL-19 injected mice (Supplemental data IID).
Figure 4.
IL-19 polarizes the adaptive immune response to Th2 and T reg. A.–G., quantitative RT-PCR on RNA extracted from spleen from mice receiving either PBS or 10ng/g/day IL-19. Spleens were removed at the time of euthanasia, RNA extracted and reverse transcribed, and amplified using the primer pairs shown. p<0.05, 0.01, or 0.001, as shown. n=9 spleen each group. H. Cytokine protein concentration from spleen determined by Luminex analysis. P<0.01 or 0.5, n=4 or 5 spleen per group.
Local lymphocyte polarization in plaque was characterized by immunohistochemistry (Supplemental Data IIA,B and Figure 5). A significant increase in the percentage of GATA3 positive T lymphocytes was noted in IL-19 treated mice, compared with PBS injected controls (75.10±4.70% vs. 56.15±6.15 for IL-19 and PBS, respectively, p<0.05 ) (Figure 5A). No difference in T-bet positive T lymphocytes was noted between groups. Collectively, these data suggest one potential mechanism for the IL-19 anti-atherogenic effect is polarization of the immune response to the Th2 and possibly, T regulatory phenotypes.
IL-19 decreases macrophage accumulation in atherosclerotic lesions
To further characterize the cellular content of atherosclerotic lesions, macrophage infiltrate was assessed by immunohistochemistry. Multiple serial sections throughout the aortic root from IL-19 treated and control mice were immunostained using F4/80 antibody to detect macrophage (Supplemental Data IIC). Positively stained areas were quantitated as a percentage of total area by quantitative morphometry. Significantly less macrophage infiltrate in plaque from IL-19 injected mice was observed compared with PBS control mice (20.0±2.7% vs. 36.5±2.2%, respectively, p<0.001) (Figure 5C). These data suggest that at least in this experimental model of atherosclerosis, IL-19 can reduce macrophage accumulation.
IL-19 decreases leukocyte-endothelial adhesion in mice fed an atherogenic diet
Decreased macrophage infiltrate in atherosclerotic lesions suggested that IL-19 could reduce leukocyte-EC interaction induced by a chronic atherogenic diet in vivo. For these experiments, wild-type C57BL/6 mice were fed an atherogenic diet for 12 weeks, and injected 5 consecutive days per week with 10ng/g/day IL-19 or PBS. Leukocyte-EC interaction was assessed in vivo by quantitative intravital microscopy. Figure 5D shows that IL-19 significantly reduced leukocyte adhesion induced by an atherogenic diet (2.1±0.4 for no atherogenic diet, and 3.5±.78 vs. 1.5±0.5 cells/100μm for PBS and IL-19 treated mice, respectively, p<0.05). There was no statistical difference in rolling between PBS and IL-19 treated mice. Three additional experiments were performed to characterize a cellular mechanism. First, immunohistochemistry of VCAM1 abundance in plaque was performed. Positively stained areas were quantitated as a percentage of total area and determined significantly less VCAM1 immunoreactivity in IL-19-treated compared with PBS control mice (32.52±4.4% vs. 57.43±6.08% for IL-19 and PBS, respectively, p<0.01) (Figure 5E and Supplemental Data III). Second, we used an endothelial cell monolayer adhesion assay and determined that pretreatment of cultured EC with IL-19 could significantly reduce monocyte adhesion to EC oxLDL stimulated EC monolayers (89.9±17.3 vs. 41.1±16.3 monocytes/HPF, P<0.05) (Figures 5F and G.) . Third, Human ECs were pre-treated with IL-19 prior to stimulation with oxLDL, and lysates blotted with anti-VCAM1 antibody. Supplemental Data IIIB,C shows that IL-19 pre-treatment significantly decreases VCAM-1 protein abundance. Collectively, these data suggest that a second mechanism for IL-19 anti-atherosclerotic effects is a reduction in leukocyte-EC interactions.
IL-19 decreases expression of chemokines in cultured EC, VSMC, and macrophage
Leukocyte homing to the atherosclerotic lesion is mediated by the local chemokine gradient. We investigated if IL-19 could decrease expression of chemokines in EC, VSMC, and macrophage. Cultured human coronary artery EC, VSMC, and mouse bone marrow-derived macrophages (BMDM) were pre-treated with IL-19 for varying times, then challenged with TNFα. Cytokine mRNA was determined by quantitative RT-PCR. Figures 6A-C demonstrate that in all cell types tested, IL-19 has a potent inhibitory effect on IL-1β, IL-8, and MCP-1 mRNA accumulation, each of which is a potent leukocyte chemoattractant. Interestingly, efficacy of IL-19 inhibition of mRNA varied for different cell types. Differential sensitivity to IL-19 implies complex and perhaps cell type-specific regulatory mechanisms for IL-19 inhibitory effects. Together, this demonstrates that IL-19 can have direct anti-atherogenic effects on resident vascular cells as well as immune cells, suggesting a third mechanism for IL-19 anti-atherosclerotic effects.
Figure 6.
IL-19 decreases mRNA abundance of chemotactic cytokines in resident vascular and immune cells. A.-C. Cultured VSMC or EC were serum-starved 24 hours. BMDM were isolated from C57B6 mice. All cells were pre-treated with IL-19 for the times shown, then stimulated with 10ng/ml TNFa for four hours. Total RNA was reverse transcribed and target mRNA quantitated by qRT-PCR. mRNA was normalized to GAPDH. Differences in IL-19 pretreated vs. untreated treated controls are significant where indicated (p<0.05 or 0.01, n=3 experiments), asterisk are for all targets at that time, unless otherwise noted. D. Chronic treatment with IL-19 reduces HuR protein. Cultured VSMC or EC were serum-starved 24 hours. BMDM were isolated from C57B6 mice. Cells were stimulated with IL-19 continuously for 4 days, changing the media and adding fresh IL-19 every 24 hours. At the indicated times lysates were immunoblotted with HuR and GAPDH antibody. Western blots shown are representative of at least 3 independent experiments, with identical results.
We previously reported that IL-19 does not inhibit NF-κB activity, but transiently decreases activation of the mRNA stability factor HuR (20,30). Each of the chemokines tested contains an ARE (AU-rich stability element) in its 3’UTR, which is recognized by HuR. To determine potential molecular mechanisms for IL-19 anti-inflammatory effects, we chronically treated cultured EC, VSMC, and BMDM with IL-19 continuously for 4 days, changing the media and adding fresh IL-19 every 24 hours. Figure 6D shows that chronic stimulation of each of these cell types with IL-19 decreases protein abundance of HuR. This is significant as HuR preferentially stabilizes inflammatory transcripts.
Discussion
The major finding from this manuscript is that systemic administration of IL-19 attenuates atherosclerosis in LDLR−/− mice. Adaptive immune polarization, reduction in macrophage infiltration, inflammatory cytokine gene expression in immune and resident vascular cells are likely mechanisms. This is the first description of IL-19 inhibition of atherosclerosis, and is conceptually novel in that IL-19 can have direct atheroprotective effects on non-immune cells. It was unexpected that we were able to detect IL-19 protein in multiple cell types in atherosclerotic plaque from human patients in both coronary and carotid arteries, as current understanding indicates that Th1 cytokine expression dominates, in contrast to Th2 cytokines which are far less prevalent in human atherosclerotic lesions (6,29,30). It was particularly interesting that IL-19 immunoreactivity localized primarily to the plaque, and little to none was detected in medial VSMC, which is similar to expression of IL-20R1 and R2 which was detected in EC and mononuclear cells in human atherosclerotic lesions, but at much lower levels in normal aorta sections (31). IL-19 expression in plaque but not normal media suggested a compensatory, counter regulatory function for this cytokine. Similarly, elevated levels of IL-19 in plaque from symptomatic patients may occur in an attempt to limit local inflammation. Longitudinal, prospective studies will be required to test this conjecture. IL-19 expression in resident vascular cells (EC and VSMC) also implied a potential novel function for IL-19 independent of lymphocyte Th2 polarization.
Recently, serum IL-19 levels in humans have been reported (32). In patients selected for coronary artery bypass graft surgery (CABG), IL-19 levels were 34.4±17.6 ng/ml prior to surgery. IL-19 rose to 541.3±110.4 ng/ml 24 hours post-surgery, and declined to 77.2±24.9 ng/ml 96 hours after surgery. In our study, systemic administration of 10ng/g/day IL-19 almost completely inhibited plaque formation in the aortic arch, and as little as 1ng/g/day IL-19 decreased plaque area by 70%, suggesting potent anti-atherosclerotic effects of IL-19. This is a straight-forward study design that does not rely on any genetic modification of IL-19, and suggests therapeutic potential for IL-19. IL-19 reduction of plaque size is likely not due to reduction in lipid as there is no significant difference in total serum cholesterol, triglycerides, HDL or LDL in IL-19 treated mice compared with controls, nor is there any difference in weight gain between these two populations. In contrast to IL-19, IL-10 has lipid-lowering effects, as serum cholesterol is significantly reduced in mice receiving IL-10, which also may have contributed to decreased plaque in that study (10).
Some Th2 interleukins have been shown to decrease atherosclerosis in mice; albeit with less potency than IL-19. Systemic IL-10 gene expression mediated by adenovirus injection reduced atherosclerosis by immune cell modulation and reduction of plaque inflammatory cell infiltrate (10). Transfer of bone marrow from IL-10−/− into LDLR−/− mice resulted in increased atherosclerotic plaque formation compared with controls, which was attributed to a decrease in macrophage foam cell apoptosis and monocyte activation (11). Similarly, LDLR−/− mice receiving BM from IL-10 transgenic mice showed a significant decrease in plaque area (8). Together, these studies suggest IL-10 anti-atherogenic effects are mediated by modulation of the adaptive immune response. Intimal area of cross sections of atherosclerotic plaque was reduced in ApoE−/− mice receiving serial injections of 1μg of IL-33; however, lesion area was not measured in this study (33,34). Counter intuitively, IL-4−/− mice do not have increased atherosclerosis, and subcutaneous injection of 1.1/g/day recombinant IL-4 into ApoE−/− mice does not reduce development of atherosclerotic lesions (12,35). Further, lesions were actually reduced in area in IL-4−/−/ApoE−/− double knockout mice, and reconstitution of LDLR−/− mice with IL-4−/− bone marrow also reduces lesion formation.
A tip in the Th1/Th2 balance of these seemingly opposing forces toward the Th2 profile has been proposed to have anti-atherosclerotic effects (3-5). Gene expression in splenocytes from IL-19 injected mice indicated that IL-19 appeared to induce Th2 polarization, as Th1 markers T-bet and IFNγ, as well as the pro-inflammatory cytokines IL-1β and IL-12p40 were decreased, and Th2 and T reg markers such as GATA3 and FoxP3 were increased in IL-19-injected mice. This shift in immune system polarization may provide at least one mechanism for IL-19's anti-atherosclerotic effects. Th17 cell differentiation requires the Retinoid-related Orphan Receptor (RORγt), which participates in IL-17 expression. At present, the role of Th17 cells and the IL-17 family of interleukins in atherosclerosis is unclear, and appear to be contextual and circumstantial of the approach used, as some studies indicate pro-atherogenic effects and others suggest IL-17 is atheroprotective (36,37). Pertinant to our study, IL-17 athero-protection was associated with a reduction in VCAM-1 expression, similar to what we observed for IL-19 (37). Consequently, IL-19 induction of RORc mRNA may corroborate an atheroprotective role for Th17 cells, or on the other hand, contradict the apparent overall anti-inflammatory effects of IL-19. The precise role of IL-19 in regulation of this complex T cell subset and IL-17 family of interleukins are beyond the scope of the present study.
T regulatory cells express the FoxP3 (forkhead/winged helix) transcription factor, and a protective role for this T cell subset has been proposed (38). IL-19 increased FoxP3 mRNA abundance in spleen, but we were not able to detect FoxP3 positive cells in plaque, which could suggest that adaptive immunity may be shifted, but not the local response in the plaque. Alternatively, it has been shown that FoxP3 positive cells are present in very low numbers in plaque, and qRT-PCR from spleen is more sensitive than immunohistochemistry from sections of aortic root (39). Like most studies which utilize animal models, one limitation of this study is that the definitive distinctions in immune polarization observed in mice are often much less clear in humans. Future studies are needed to characterize IL-19 effect on global and local adaptive immunity in the context of atherosclerosis.
Monocyte adhesion constitutes a key cellular event in initiation of atherosclerosis (40). Cellular characterization of plaque determined significantly less macrophage infiltrate in IL-19 injected mice compared with PBS controls. Extending these data, quantitative intravital microscopy determined that leukocyte adhesion is decreased in wild-type mice fed an atherogenic diet One limitation of the intravital study is that endothelium of mesenteric post-capillary venules may not faithfully mirror conditions of the developing atherosclerotic plaque. However, it does allow for direct quantification of leukocyte trafficking in vivo and supports our immunohistochemical determination that IL-19 can reduce leukocyte/EC interactions in an inflammatory environment. The present study also showed that IL-19 can reduce oxLDL-driven VCAM-1 expression in cultured EC, and also reduce monocyte adhesion to oxLDL-stimulated EC monolayers. This is in contrast to IL-4, which has been shown to increase VCAM-1 expression and leukocyte adhesiveness (41). Together, these approaches identify a second mechanism whereby IL-19 may attenuate plaque formation. IL-19 expression in EC, VSMC, and macrophage in atherosclerotic plaque led us to hypothesize that IL-19 would have anti-inflammatory effects on these cells. Treatment of each these cells with IL-19 prior to TNFα stimulation lead to a significant decrease in mRNA for MCP-1, IL-8, IL-1β, all potent chemoattractants. This may account for the observed decreased macrophage accumulation in plaque, and also decreased adhesion assayed by intravital microscopy. IL-10 is acknowledged to reduce NF-κB activity in VSMC, but did not reduce TNFα or IL-1β-induced expression inflammatory genes (42). In the present study, IL-19 inhibition of mRNA varied for different cell types suggesting complex, cell specific regulatory mechanisms for IL-19 inhibitory effects. Taken in total, our present data are particularly intriguing in their demonstration that a presumed Th2 interleukin can have direct anti-inflammatory effects on cells outside of the T cell lineage, particularly EC and VSMC.
A plausible mechanism for IL-19 induced decrease in these transcripts is its effect on HuR, an inflammation-specific mRNA stability factor (43). We previously reported that in cultured, primary human VSMC, IL-19 inhibitory effects are NF-κB-independent, but did decrease the mRNA stability of inflammatory transcripts by decreasing nuclear to cytoplasmic translocation of HuR (20,28). The present study extends that report, showing that a single addition of IL-19 can rapidly and transiently decrease mRNA abundance of chemokine transcripts in multiple cell types. We then attempted to mirror the in vivo scenario in which IL-19 is injected into mice on a daily basis by multiple additions of IL-19 to these cells and demonstrated HuR protein abundance was reduced. Thus, both reduction in HuR cytoplasmic translocation as well as protein abundance is induced by IL-19. Decreased HuR protein abundance, with subsequent reduction in inflammatory gene mRNA provides a third mechanism for IL-19 attenuation of atherosclerosis.
In summary, IL-19 is expressed in multiple cell types in human atherosclerotic plaque, demonstrated efficacy of IL-19 to reduce atherosclerotic lesion size in mice. The probable mechanisms are a polarization of adaptive immunity to the Th2 phenotype, a decrease in macrophage infiltrate into the plaque, and a decrease in inflammatory gene expression in EC, VSMC, and macrophage. What is particularly important about this study is the ability of EC and VSMC to respond to IL-19. This is potentially paradigm altering as it suggests that resident vascular cells can respond to IL-19 to assume a Th2-like, anti-inflammatory phenotype to promote resolution of the local vascular response to injury. A limitation of the present study is that it can not identify if the protection imparted by IL-19 is mediated primarily by adaptive immune system polarization, a decrease in macrophage infiltrate into the lesion, or by direct anti-inflammatory effects on resident vascular cells, or combinations thereof. Future studies will elucidate the precise cellular mediator(s) of IL-19 effects.
Supplementary Material
SIGNIFICANCE.
Despite dietary modification and lipid reducing medications, atherosclerotic vascular syndromes account for 50% of all mortality in the United States and is increasing in the developing world. This manuscript describes expression of Interleukin-19, a recently described anti-inflammatory cytokine, in human atheromatous plaque. Administration of IL-19 can significantly decrease severity of atherosclerosis in several murine models by multiple pleiotropic mechanisms, including adaptive immune system polarization, decrease in leukocyte-endothelial interaction, and reduction in inflammatory gene expression in macrophage and resident vascular cells. Together these data support the hypothesis that expression of IL-19 by immune and resident vascular cells is a novel compensatory counter-regulatory mechanism which attenuates atherosclerosis, and suggests that IL-19 itself has therapeutic potential for inhibition of atherosclerosis.
ACKNOWLEDGEMENTS
We thank Ana Persson, Marie M.N. Nilsson, Mihaela Nitulescu and Anna-Maria Dutius Andersson for valuable assistance with the carotid plaque experiments.
Sources of funding: This work was supported by grants HL090885, HL115575, and HL117724 from the National Heart Lung, and Blood Institute of the National Institutes of Health to M.V.A. Also by grants HLF20090419 from the Swedish Heart and Lung Foundation to I.G; 2011-3900 to M.F.G. and 2010-2932 to I.G. from the Swedish Research Council; and by the Lund University Diabetes Centre, the Albert Påhlsson, Marianne & Marcus Wallenberg, and Knut & Alice Wallenberg foundations. S.E. supported by American Heart Association pre-doctoral fellowship12PRE12040331. K.G .is supported by American Heart Association post-doctoral fellowship 11POST7530001. R.S. is supported by grant # DK064344. . L.M.B. is supported by fellowships from the Swedish Society for Medical Research and EFSD (European Foundation for the Study or Diabetes).
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ross R. Atherosclerosis--an inflammatory disease. N Eng J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Mallat Z, Ait-Oufella H, Tedgui A. Regulatory T cell responses: potential role in the control of atherosclerosis. Curr Opin Lipidol. 2005;16:518–524. doi: 10.1097/01.mol.0000182532.11512.90. [DOI] [PubMed] [Google Scholar]
- 4.Schulte S, Sukhova GK, Libby P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol. 2008;172:1500–1508. doi: 10.2353/ajpath.2008.070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 6.von der Thüsen JH, Kuiper J, van Berkel TJ, Biessen EA. Interleukins in atherosclerosis: molecular pathways and therapeutic potential. Pharmacol Rev. 2003;55:133–166. doi: 10.1124/pr.55.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. doi: 10.1093/cvr/cvn120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinderski LJ, Fischbein MP, Subbanagounder G, Fishbein MC, Kubo N, Cheroutre H, Curtiss LK, Berliner JA, Boisvert WA. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient Mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–1071. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 9.Caligiuri G, Rudling M, Ollivier V, Jacob M, Michel J, Hansson G, Nicoletti A. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol Med. 2003;9:10–17. [PMC free article] [PubMed] [Google Scholar]
- 10.von der Thüsen JH, Kuiper J, Fekkes ML, De Vos P, Van Berkel TJ, Biessen EA. Attenuation of atherogenesis by systemic and local adenovirus-mediated gene transfer of interleukin-10 in LDLR−/− mice. FASEB J. 2001;15:2730–2742. doi: 10.1096/fj.01-0483fje. [DOI] [PubMed] [Google Scholar]
- 11.Potteaux S, Esposito B, van Oostrom O, Brun V, Ardouin P, Groux H, Tedgui A, Mallat Z. Leukocyte-derived interleukin 10 is required for protection against atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:1474–1488. doi: 10.1161/01.ATV.0000134378.86443.cd. [DOI] [PubMed] [Google Scholar]
- 12.King VL, Cassis LA, Daugherty A. Interleukin-4 does not influence development of hypercholesterolemia or angiotensin II-induced atherosclerotic lesions in mice. Am J Pathol. 2007;171:2040–2047. doi: 10.2353/ajpath.2007.060857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, Vazquez N, Pestka S, Donnelly RP, Kotenko SV. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10). Genes Immun. 2000;1:442–1450. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 15.Oral HB, Kotenko SV, Yilmaz M, Mani O, Zumkehr J, Blaser K, Akdis CA, Akdis M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–388. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 16.Gallagher G, Eskdale J, Jordan W, Peat J, Campbell J, Boniotto M, Lennon GP, Dickensheets H, Donnelly RP. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol. 2004;4:615–626. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher G. Interleukin-19: multiple roles in immune regulation and disease. Cytokine Growth Factor Rev. 2010;21:345–352. doi: 10.1016/j.cytogfr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Sabat R, Wallace E, Endesfelder S, Wolk K. IL-19 and IL-20: two novel cytokines with importance in inflammatory diseases. Expert Opin Ther Targets. 2007;11:601–612. doi: 10.1517/14728222.11.5.601. [DOI] [PubMed] [Google Scholar]
- 19.Tian Y, Sommerville LJ, Cuneo A, Kelemen SE, Autieri MV. Expression and suppressive effects of interleukin-19 on vascular smooth muscle cell pathophysiology and development of intimal hyperplasia. Am J Pathol. 2008;173:901–909. doi: 10.2353/ajpath.2008.080163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuneo AA, Herrick D, Autieri MV. Il-19 reduces VSMC activation by regulation of mRNA regulatory factor HuR and reduction of mRNA stability. J Mol Cell Cardiol. 2010;49:647–654. doi: 10.1016/j.yjmcc.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 22.Li HH, Lin YC, Chen PJ, Hsiao CH, Lee JY, Chen WC, Tzung TY, Wu JC, Chang MS. Interleukin-19 upregulates keratinocyte growth factor and is associated with psoriasis. Br J Dermatol. 2005;153:591–595. doi: 10.1111/j.1365-2133.2005.06665.x. [DOI] [PubMed] [Google Scholar]
- 23.Ghazizadeh R, Shimizu H, Tosa M, Ghazizadeh M. Pathogenic mechanisms shared between psoriasis and cardiovascular disease. Int J Med Sci. 2010;7:284–289. doi: 10.7150/ijms.7.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain S, Gabunia K, Kelemen SE, Panetti TS, Autieri MV. The anti-inflammatory cytokine interleukin 19 is expressed by and angiogenic for human endothelial cells. Arterioscler Thromb Vasc Biol. 2011;31:167–175. doi: 10.1161/ATVBAHA.110.214916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–1601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stary HC. Natural History and Histological Classification of Atherosclerotic Lesions: An Update. Arterioscler Thromb Vasc Biol. 2000;20:1177–1178. doi: 10.1161/01.atv.20.5.1177. [DOI] [PubMed] [Google Scholar]
- 27.Phipps RP. Atherosclerosis: The emerging role of inflammation and the cd40-cd40 ligand system. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6930–6932. doi: 10.1073/pnas.97.13.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulte S, Sukhova GK, Libby P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am J Pathol. 2008;172:1500–1508. doi: 10.2353/ajpath.2008.070776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, Hansson GK. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 30.England RN, Preston KJ, Scalia R, Autieri MV. Interleukin-19 Decreases Leukocyte-Endothelial Cell Interactions by Reduction in Endothelial Cell Adhesion Molecule mRNA Stability. Am J Physiol Cell Physiol. 2013 Apr 17; doi: 10.1152/ajpcell.00069.2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WY, Cheng BC, Jiang MJ, Hsieh MY, Chang MS. IL-20 is expressed in atherosclerosis plaques and promotes atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:2090–2095. doi: 10.1161/01.ATV.0000232502.88144.6f. [DOI] [PubMed] [Google Scholar]
- 32.Yeh CH, Cheng BC, Hsu CC, Chen HW, Wang JJ, Chang MS, Hsing CH. Induced interleukin-19 contributes to cell-mediated immunosuppression in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. Ann Thorac Surg. 2011;92:1252–1259. doi: 10.1016/j.athoracsur.2011.04.061. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. doi: 10.1084/jem.20071868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–1125. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taleb S, Romain M, Ramkhelawon B, Uyttenhove C, Pasterkamp G, Herbin O, Esposito B, Perez N, Yasukawa H, Van Snick J, Yoshimura A, Tedgui A, Mallat Z. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J Exp Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 39.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS One. 2007:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:2292–2301. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 41.Galéa P, Thibault G, Lacord M, Bardos P, Lebranchu Y. IL-4, but not tumor necrosis factor-alpha, increases endothelial cell adhesiveness for lymphocytes by activating a cAMP-dependent pathway. J Immunol. 1993;151:588–96. [PubMed] [Google Scholar]
- 42.Lisinski TJ, Furie MB. Interleukin-10 inhibits proinflammatory activation of endothelium in response to Borrelia burgdorferi or lipopolysaccharide but not interleukin-1beta or tumor necrosis factor alpha. J Leukoc Biol. 2002;72:503–311. [PubMed] [Google Scholar]
- 43.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.