Abstract
BACKGROUND
Recurrent aphthous stomatitis (RAS) is characterized by painful recurrent oral ulcers and is typically diagnosed via history and clinical examination. Our aim was to validate a set of anamnestic diagnostic criteria (RASDX) to increase the accuracy of RAS diagnosis, particularly when a clinical examination is not feasible.
METHODS
Participants were enrolled during an unmatched case-control study. RASDX consisted of an initial phone screening using standardized questionnaires and recognition of RAS photos in the clinic. The proportion of agreement with an examination by an oral medicine expert was calculated.
RESULTS
A total of 115 participants were scheduled for a clinical diagnostic visit and 11 were withdrawn. The remaining 104 participants were aged 18–50 years, 54% female, 64% White and 20% Hispanic. Of these, all 49 controls with negative RASDX had no clinical ulcers. Of the 54 cases diagnosed with RAS by RASDX, 53 were clinically confirmed to have RAS lesions (99% agreement; exact 1-sided 95% CI=95–100%).
CONCLUSIONS
RASDX, based on a combination of history and photograph recognition, was highly accurate compared to a diagnosis that employed an oral examination.
Keywords: aphthous stomatitis, diagnosis, criteria, guidelines, mouth diseases
Introduction
Recurrent aphthous stomatitis (RAS) is an extremely common oral mucosal disease (1).The most comprehensive study of RAS prevalence was conducted on over 10,000 young adults in 21 different countries. Overall, 38.7% of men and 49.7% of women reported two or more previous occurrences of RAS. Approximately 25% of the participants reported at least one episode of RAS during the past year (2). Similar national figures have been reported for the United States (3, 4).
Smaller aphthae heal in about 2 weeks, although they may recur for up to 20 years or more (5), resulting in significant impact on quality of life (6). Larger ulcers may reach over 1 cm in diameter, can persist for several weeks or months and often heal with scarring. The pain associated with RAS lesions is often out of proportion to the size of the ulcers and can affect eating, speaking, and oral hygiene practices (7). For example, hot foods or beverages can increase RAS-associated pain scores by almost 60% (8).
The etiology of this prevalent condition is not clearly understood. Some of the most commonly investigated factors include family history, immune disturbances, vitamin deficiencies, trauma and stress (9, 10, 11, 12). The Surgeon General’s Report on Oral Health in America also identified RAS as a very common problem afflicting Americans. Further, this report emphasized the detrimental effects of oral disease on overall well-being and quality of life as measured along functional, psychosocial, and economic dimensions (13).
There is no laboratory test to diagnose RAS and histopathologic findings are also relatively non-specific. Therefore RAS is usually diagnosed based on history and clinical examination. For patients who have a current oral lesion, a clinical diagnosis of an active RAS lesion is often made based on the clinical appearance of the lesion and supporting history. For example, Rogers proposed that essential elements for a diagnosis for RAS include: recurrent ulcers on unattached oral mucosa, few lesions at a time, intense pain early then lessening, complete healing between episodes, and affliction of young people (14). However, for patients without clinically active lesions, diagnosis as a case or control is not straightforward. No validated diagnostic criteria exist for identifying patients with a history of RAS. We present validation of a new standardized diagnostic process (“RASDX”) developed for the purpose of identifying RAS patients for a research study, on the basis of history and RAS photo recognition alone.
Subjects and methods
Participants for this validation study were recruited as part of a larger unmatched case-control study examining specific risk factors for RAS. After approval by the University of Florida’s Institutional Review Board (UF IRB), participants were recruited throughout the UF campus using fliers.
The validation study consisted of a phone screening, RAS photo recognition and diagnostic clinical examination. Participants who completed all the validation steps were included in the analyses. Participants were eligible for a diagnostic visit if they (1) reported at least 2 episodes of idiopathic RAS in the prior 6 months and the presence of an aphthous/aphthous-like lesion less than 72 hours duration prior to the examination, or (2) reported a negative lifetime RAS history. When a diagnostic visit was conducted at the same time as a full visit for the main case-control study, participants were also required to abstain from drinking for 30 minutes (to limit variability in saliva collection), fast for at least 5 hours, be free of an acute infection in the prior 3 days, and avoid tobacco, alcohol or medications that affected the immune system for at least 24 hours prior to a visit.
Individuals were excluded if they were less than 13 years old, unable or unwilling to consent, pregnant, had a blood relative already enrolled, reported a history of a rare systemic disease (inflammatory bowel disease, celiac disease, Behçet’s syndrome, cyclic neutropenia, low white blood cell counts, mouth and genital ulcers with inflamed cartilage [MAGIC] syndrome, periodic fever, aphthous stomatitis, pharyngitis and adenitis [PFAPA] syndrome, Sweet’s syndrome, systemic lupus erythematosus, Reiter’s disease, HIV/AIDS or severe immunodeficiency), had gastrectomy or gastric by-pass surgery, took systemic steroids in the prior month or were interested in the study because they had perioral lesions that they believed to be RAS (this exclusion prevented overrepresentation of herpetic lesions in controls).
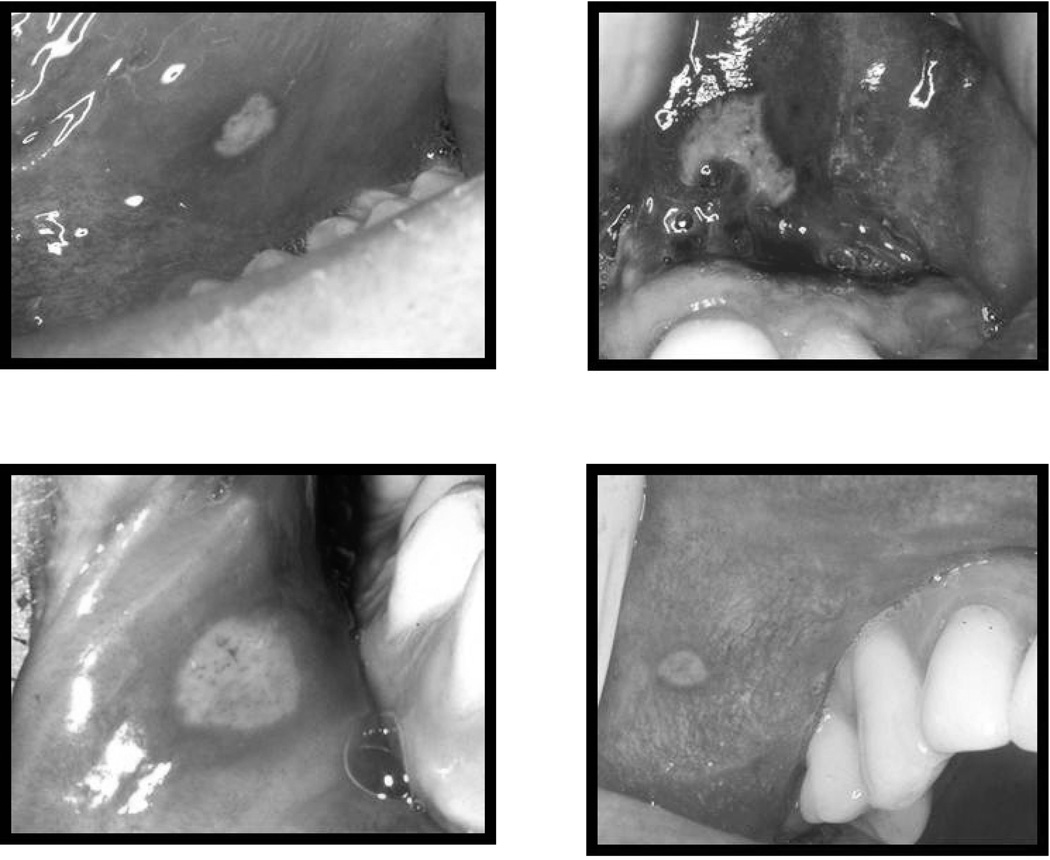
The validation process occurred in two phases: a RASDX screening phase (phone questionnaire and RAS photo recognition) and a clinical examination phase. The phone screening using standardized questionnaires (Table 2) was completed by research assistants specifically trained on the screening procedures, but without formal dental training. Because no validated diagnostic criteria existed at the time of screening, an oral medicine expert with significant experience in RAS diagnosis was available for assistance as needed. Participants were then shown 4 photos of aphthous ulcers of variable size and location (Figure 1) during the clinical visit and were asked if they ever had similar lesions. The 4 RAS photos were selected based on the prevalence of the three clinical forms. The majority of patients (3 out of 4) are affected by minor RAS, followed by major RAS (15). After the RASDX screening phase, participants were classified as RASDX positive, negative or undetermined. The last validation step consisted of an oral soft tissue examination by an oral medicine expert, during which participants were classified as exam positive (at least one visible aphtha) or negative.
Table 2.
RASDX questionnaire with percent of affirmative answers by final case (n=53) or control (n=50) status.a
| Diagnostic criteria | Case (% Yes) |
Control (% Yes) |
|---|---|---|
| SECTION 1 – Self-reported diagnosis and study exclusions.b | ||
| Q1. Have you ever had canker sores in your life, including when you were a child? | 100 | 2c,d |
| Q2. [If Q1=Yes] Did you have at least 2 distinct episodes of canker sores in the past 6 months? | 100e | Skip |
| Q3. Are you interested in this study because you have recurring sores around your lips (OUTSIDE your mouth)? | 0e | 0e |
| SECTION 2 – Major criteria: Have you ever had canker sores or other sores that… | ||
| M1. Were painful | 100e | 14 |
| M2. Were recurrent (they would come and go, at least 2 episodes in life) | 100e | 12 |
| M3. Occurred inside your mouth (never outside the lips) | 100e | 18 |
| M4. Were “sores” or ulcers (e.g., they did not contain a liquid and then ruptured) | 100e | 8f |
| M5. Occurred for no reason (idiopathic, mostly unable to establish a specific cause except for factors traditionally believed to be associated with canker sores- e.g., you may wake up with a canker sore and your sores are not always secondary to orthodontic treatment, pizza burn and aspirin burns, etc.) | 100e | 8 |
| SECTION 3 – [If M1-M5 =Yes] Minor criteria: Which of the following statements is true regarding your sores? | ||
| N1. You were told by a doctor/dentist that you had canker sores after looking inside your mouth | 51 | Skip |
| N2. They began before age 25 | 100 | Skip |
| N3. They do NOT always occur on firm tissues like the anterior part of the roof of your mouth or very close to your teeth* | 98 | Skip |
| N4. They mostly occur in areas like the lower lip and/or inside the cheek | 96g | Skip |
| N5. They do NOT always occur in the exact same spot (they can occur in the same AREA)* | 100 | Skip |
| N6. They sometimes occur in areas that you could NOT have bitten into (e.g., in the fold between your lips and teeth or cheek and teeth, back of your mouth, below tongue) | 98 | Skip |
| N7. Is at least one of the following 3 statements true about your sores? | 100 | Skip |
| N7.1. They are never or rarely triggered by accidental biting | 83g | Skip |
| N7.2. If triggered by biting the pain remains sustained after the biting episode | 68a | Skip |
| N7.3. You think that your canker sores are very different from simple cheek/lip or tongue biting | 96g | Skip |
| N8. If sores are less than half an inch (or < 1 cm) they usually last less than 2 weeks | 98 | Skip |
| N9. They are not usually associated with other general conditions (e.g., fever, sores or blisters of the skin or other areas) | 96 | Skip |
| N10. You have had 5 or more canker sore episodes in life | 100 | Skip |
| N11. You usually have less than 5 sores per episode | 100 | Skip |
| N12. You are certain that you ever had canker sores (e.g., got info from others, books, internet, photos) | 100 | Skip |
If a participant answers “No” but satisfies all other criteria, classify as “tentative case” and confirm case status by oral exam (must have clinical RAS) or exclude if clinical confirmation is not possible
One participant with undetermined final diagnosis (i.e., a RASDX positive participant with clinical erythema but no frank ulceration) is not listed. This individual answered “Yes” to all the criteria except N1
This section contained a preliminary assessment of RAS status and RAS-specific study exclusions related to the original case-control study
Only the control with a clinical mucocele answered “Yes”
4 answered “Unknown”
Required for entry into the study
2 answered “Unknown”
1 answered “Unknown”
Figure 1.
Recurrent aphthous stomatitis (RAS) photos shown to study participants
To be classified as positive RASDX, a participant had to answer “yes” to Q2"yes” to all 5 questions in section 2 (major criteria), and “yes” to at least 7 of the 12 questions in section 3 (minor criteria). The participant also had to positively recognize at least one of 4 RAS photos. To be classified as negative RASDX, a participant had to answer “no” to at least one of the 5 questions in section 2 (major diagnostic criteria). In addition, the participant should have not recognized any of the 4 RAS photos. A participant was classified as undetermined RASDX if s/he answered “don’t know” to at least one of the major criteria and “yes” to the remaining major criteria. Positive or negative clinical exam classification was based on the presence or absence of aphthous ulcerations using the same clinical criteria as those utilized by the Centers for Disease Control and Prevention (CDC) during the US National Health and Nutrition Examination Survey III (NHANES III; http://www.cdc.gov/nchs/nhanes.htm).
Data were collected on hard copy forms and then transferred to the web-based Research Electronic Data Capture (REDCap) system hosted at the University of Florida (16). Double data entry checks and random third checks were performed for each data field. RASDX findings were compared with those from the oral examination using proportion of agreement and exact binomial 1-sided 95% confidence interval (95% CI) in SAS v9.3 (SAS Institute Inc., Cary, NC).
Results
A total of 338 potential participants who responded to the advertisement were screened by phone by one of 12 research assistants between June 2009 and February 2011. A total of 130 individuals were excluded during the phone screening for the following reasons: no RAS in the past 6 months (n=89), administrative (n=8), medical (n=14), other reasons (n=3), interested in the study because of perioral lesions (n=2), met 5/5 major but only 6/12 minor criteria (n=1), met 4/5 major criteria and described a mucocele that was diagnosed as RAS in the past by a physician (response to criterion M4=unknown, RASDX=undetermined, n=1). The remaining 12 individuals did not fulfill all 5 major criteria but were not enrolled as controls by the oral medicine expert for the following reasons: reported sustained pain after accidental biting (n=3), reported bothersome, but not painful, ill-defined lesions (n=1), felt rawness twice in life after citrus intake (n=1), or had other rare lesions in life (n=7).
A total of 93 participants were withdrawn because they could not be scheduled for a diagnostic visit. Some of the reasons included scheduling issues, waiting to discontinue medications or complete other research studies, moving out of town, or ulcers that did not recur before study closure.
An additional 11 participants were withdrawn after one visit, including 4 who did not follow instructions and 1 who was incorrectly scheduled for an “active” visit in the absence of any intraoral pain. Six of the 11 participants were initially classified as controls based on the questionnaire, but recalled a history of ulcers at the time of appointment and were withdrawn from the study with no exam data and an unknown final diagnosis. Of these, 5 recalled a history of one or more ulcers in their mouth similar to the ones shown in the photo and one participant reported one ulcer in life before seeing the photos.
The remaining 104 participants (Table 1) completed the full validation process (phone questionnaire, RAS photo recognition and oral examination) by March 2011. The final diagnosis was control (n=50), case (n=53), or undetermined (n=1).
Table 1.
Socio-demographic characteristics of participants (n=104).
| Final diagnosis |
Overall n (%)a |
|||
|---|---|---|---|---|
| Case n (%)a |
Control n (%)a |
Undetermined n (%)a |
||
| Ageb | ||||
| Mean (±SD) | 23 (4) | 23 (6) | --- | 23 (5) |
| Range | 18–38 | 18–50 | --- | 18–50 |
| Gender | ||||
| Female | 27 (51) | 29 (58) | 0 (0) | 56 (54) |
| Male | 26 (49) | 21 (42) | 1 (100) | 48 (46) |
| Race | ||||
| White | 40 (75) | 26 (52) | 1 (100) | 67 (64) |
| Black | 2 (4) | 11 (22) | 0 (0) | 13 (13) |
| Asian | 9 (17) | 12 (24) | 0 (0) | 21 (20) |
| Other | 2 (4) | 1 (2) | 0 (0) | 3 (3) |
| Ethnicity | ||||
| Hispanic | 9 (17) | 12 (24) | 0 (0) | 21 (20) |
| Non-Hispanic | 44 (83) | 38 (76) | 1 (100) | 83 (80) |
| Occupationb | ||||
| Student | 40 (83) | 43 (86) | --- | 83 (85) |
| Other | 8 (17) | 7 (14) | --- | 15 (15) |
| Marital statusb | ||||
| Never married | 36 (75) | 47 (94) | --- | 83 (85) |
| Other | 12 (25) | 3 (6) | --- | 15 (15) |
| Total n | 53 | 50 | 1 | 104 |
Number and percent of cases or controls, unless otherwise specified
Data from 6 participants who had a diagnostic visit are missing because they did not return for additional main study visits that included more extensive questionnaires
Of 49 participants with a negative RASDX, all (100%) were also RAS negative upon examination and were classified as controls. One additional participant, who had initially reported a history of “canker sores” (question Q1=yes; Table 2), only had 4/5 major criteria positive, with criterion M4 (“Were sores or ulcers”) marked as “unknown”. Thus, she was classified as undetermined based on RASDX. Clinically, the participant was RAS negative and had a mucocele that she thought was RAS (final diagnosis: control).
Overall, 40/50 (80%) controls answered “No” to all 5 major criteria. The remaining 10 controls fulfilled only 1–4 major criteria. These controls reported lesions that occurred for a specific reason (n=6; M5=no), that did not occur inside the mouth (n=1; M3=no), or that were described as raised, round, tiny, white or clear “blisters” or “bumps” filled with liquid, that were not painful or that the participant often attempted to pop (n=3; M4=no/unknown or M1=no). None of the 50 controls recognized the RAS photos.
A total of 54 participants were RASDX positive. All of them answered positively when asked if they ever had RAS in life (question Q1) before beginning the RASDX process, although a “yes” answer for this question was not a required RASDX element. RASDX positive participants answered “yes” to all 5 major criteria and the majority of minor criteria (Table 2). Although only 7 positive criteria out of 12 were minimally required for a participant to be considered RASDX positive, the number of criteria answered positively was higher (range 10–12). All 54 participants with a positive RASDX questionnaire also recognized RAS photos.
Of the 54 participants with a positive RASDX, 53 (98%) were clinically confirmed to have one or more aphthous lesion and were classified as cases. The remaining participant had fulfilled all major and minor criteria, except N1 (i.e., prior diagnosis by a MD/DDS), and was examined once. At the time of the examination the participant had mild pain in two locations of the right and left lateral borders of the tongue, but only localized mild erythema and edema were present, with no visible ulceration (final diagnosis: undetermined).
Overall, there was almost complete agreement between the RASDX and clinical exam findings (percent agreement=99%; 95% CI=95–100%). Of the 103 participants initially classified as positive or negative by RASDX, 102 fell in the same disease category when a clinical exam was used (Table 3). The clinical exam did not improve the differential diagnostic power already provided by RASDX, except for one control (less than 1% of participants) who would have been excluded from the study if RASDX had been the only available instrument for diagnosis.
Table 3.
Agreement between a positive or negative RAS diagnosis based on history and photo recognition (RASDX) and intraoral visual examination.
| EXAM | ||||
|---|---|---|---|---|
| POSITIVE (n) |
NEGATIVE (n) |
Total (n) |
||
| RASDX | POSITIVE (n) | 53a | 1b | 54 |
| NEGATIVE (n) | 0 | 49c | 49 | |
| UNKNOWN (n) | 0 | 1c | 1 | |
| Total (n) | 53 | 51 | 104 | |
Final diagnosis=case
Final diagnosis=undetermined
Final diagnosis=control
All values represent number of subjects
Discussion
Traditional RAS diagnosis is based on a combination of patient’s history and clinical exam. Although both components have been considered necessary for an accurate diagnosis (see for example diagnostic criteria published by CDC), only specific clinical criteria, but not anamnestic criteria, have been developed. Furthermore, an accurate history is essential when repeated clinical examinations are not feasible or when assessing past or future RAS episodes. Direct clinical observation of aphthous lesions may not be feasible in large research studies involving multiple RAS episodes over long time periods and also in public health surveys such as the NHANES survey which involve many thousands of patients. A validated anamnesis is also needed in genetic studies where positive or negative lifetime history, rather than the presence of an ulcer on any one day, is the determining factor for diagnosis.
Thus, in this study we compared the results of a standardized questionnaire, which we had developed while performing hundreds of interviews and exams, with those of a clinical soft tissue exam.
A consideration that emerged from the use of RASDX is that the diagnostic method affects the types of lesions that should be included in the primary differential diagnosis. Clinical differential diagnosis is primarily between traumatic lesions (e.g., cheek/lip bite) and an active aphthous ulcer. These are the two most common intraoral ulcerative lesions and can be easily differentiated based on the pathognomonic appearance of RAS. Other lesions, such as oral cancer, intraoral herpes or other ulcerative lesions are very rare and have a different appearance, natural history, symptomatology, anatomic location or demographic distribution (17). However, during patient’s history taking, recurrent mucocele and perioral herpes should also be considered, because some patients may report these lesions as if they were RAS. Our 5 major criteria excluded perioral herpes (question M3), mucocele (questions M4 and M1), and traumatic lesions (question M5). Additional questions also contributed to the differentiation between RAS and rare intraoral herpetic lesions: question M4 asked about the presence of vesicles which precede herpetic ulcers but not aphthous ulcers. Further, questions N3 and N4 differentiated between the two lesions based on the predilection of RAS for non-keratinized oral mucosa and of herpetic lesions for keratinized mucosa, such as the hard palate and attached gingiva.
Overall, RASDX performed very well. Our results indicate an almost complete agreement between the history and photo recognition diagnostic process (RASDX) and the clinical exam. The potential for disease misclassification was the same for both RASDX and the exam procedures, because the only participant with an undetermined final diagnosis could have been a true case with a healing ulcer (false negative exam) or a true control (false positive RASDX). A study limitation was the lack of blinding of the examiner to the RASDX diagnosis. However, most importantly, the RASDX diagnosis was made blinded to the participant’s clinical status. In addition, due to the inherent recurrent nature of the disease, a negative exam did not necessarily imply that a participant was a true control, because s/he could have been an inactive case. However, confirming a true control (negative lifetime history of RAS) is not feasible for any RAS study, because it would require examining a person daily for life.
Our main case-control study (thus also this validation study) excluded participants who reported certain systemic conditions. Extensive laboratory and medical work-up to confirm these exclusions was not feasible. Furthermore, the causal link between the highly common RAS and these fairly rare medical diseases is being debated, though certain diseases seem to exacerbate RAS (9, 18). Since severe (major) lesions were captured by RASDX and the validation focused on the agreement between history and exam within each patient, the workup would have not likely changed the outcome of the validation process in our sample.
In conclusion, there was almost complete agreement (99%) between the diagnosis made by clinical exam and the diagnosis based on RASDX. The results of this study suggest that RASDX is a useful diagnostic process for measurement of lifetime disease prevalence or in studies where direct clinical observation of an aphthous lesion is not always feasible. Future research studies should include RASDX validation in larger and more diverse demographic groups, as well as medically compromised populations.
Acknowledgements
The authors wish to thank study participants, volunteer research assistants and research staff at the University of Florida, Clinical and Translational Science Institute, Clinical Research Center. This research was supported by the National Institutes of Health (NIH), NIDCR grants R21DE018714 and R03DE016356, NCRR CTSA grants M011RR000082 and UL1RR029890 and NCTAS grant UL1TR000064.
Footnotes
Conflict of interest
The authors report no financial or other conflict of interest.
References
- 1.Jurge S, Kuffer R, Scully C, Porter SR. Mucosal disease series. Number VI. Recurrent aphthous stomatitis. Oral Dis. 2006;12:1–21. doi: 10.1111/j.1601-0825.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 2.Embil JA, Stephens RG, Manuel FR. Prevalence of recurrent herpes labialis and aphthous ulcers among young adults on six continents. Can Med Assoc J. 1975;113:627–630. [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinman DV, Swango PA, Pindborg JJ. Epidemiology of oral mucosal lesions in United States schoolchildren: 1986–87. Community Dent Oral Epidemiol. 1994;22:243–253. doi: 10.1111/j.1600-0528.1994.tb01815.x. [DOI] [PubMed] [Google Scholar]
- 4.Shulman JD. An exploration of point, annual, and lifetime prevalence in characterizing recurrent aphthous stomatitis in USA children and youths. J Oral Pathol Med. 2004;33:558–566. doi: 10.1111/j.1600-0714.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S, Murphy R. Refractory aphthous ulceration treated with thalidomide: a report of 10 years' clinical experience. Clin Exp Dermatol. 2012;37:132–135. doi: 10.1111/j.1365-2230.2011.04169.x. [DOI] [PubMed] [Google Scholar]
- 6.Axell T, Henricsson V. The occurrence of recurrent aphthous ulcers in an adult Swedish population. Acta Odontol Scand. 1985;43:121–125. doi: 10.3109/00016358509046497. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson JM, Greenspan JS, Spritzler J, et al. Thalidomide for the treatment of oral aphthous ulcers in patients with human immunodeficiency virus infection. National Institute of Allergy and Infectious Diseases AIDS Clinical Trials Group. N Engl J Med. 1997;336:1487–1493. doi: 10.1056/NEJM199705223362103. [DOI] [PubMed] [Google Scholar]
- 8.Saxen MA, Ambrosius WT, Rehemtulaal KF, Russell AL, Eckert GJ. Sustained relief of oral aphthous ulcer pain from topical diclofenac in hyaluronan: a randomized, double-blind clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:356–361. doi: 10.1016/s1079-2104(97)90031-7. [DOI] [PubMed] [Google Scholar]
- 9.Baccaglini L, Lalla RV, Bruce AJ, et al. Urban legends: recurrent aphthous stomatitis. Oral Dis. 2011;17:755–770. doi: 10.1111/j.1601-0825.2011.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kozlak ST, Walsh SJ, Lalla RV. Reduced dietary intake of vitamin B12 and folate in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2010;39:420–423. doi: 10.1111/j.1600-0714.2009.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scully C, Gorsky M, Lozada-Nur F. The diagnosis and management of recurrent aphthous stomatitis: a consensus approach. J Am Dent Assoc. 2003;134:200–207. doi: 10.14219/jada.archive.2003.0134. [DOI] [PubMed] [Google Scholar]
- 12.Huling LB, Baccaglini L, Choquette L, Feinn RS, Lalla RV. Effect of stressful life events on the onset and duration of recurrent aphthous stomatitis. J Oral Pathol Med. 2012;41:149–152. doi: 10.1111/j.1600-0714.2011.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anonymous. Oral health in America: a report of the Surgeon General. J Calif Dent Assoc. 2000;28:685–695. [PubMed] [Google Scholar]
- 14.Rogers RS., 3rd Recurrent aphthous stomatitis: clinical characteristics and associated systemic disorders. Semin Cutan Med Surg. 1997;16:278–283. doi: 10.1016/s1085-5629(97)80017-x. [DOI] [PubMed] [Google Scholar]
- 15.Bagan JV, Sanchis JM, Milian MA, Penarrocha M, Silvestre FJ. Recurrent aphthous stomatitis. A study of the clinical characteristics of lesions in 93 cases. J Oral Pathol Med. 1991;20:395–397. doi: 10.1111/j.1600-0714.1991.tb00952.x. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shulman JD. Prevalence of oral mucosal lesions in children and youths in the USA. Int J Paediatr Dent. 2005;15:89–97. doi: 10.1111/j.1365-263X.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 18.Baccaglini L. Myths and evidence on the link between recurrent aphthous stomatitis and systemic diseases. Oral Dis. 2012 doi: 10.1111/j.1601-0825.2012.01900.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]