Abstract
In this study we investigated the effect of 2 weeks of robot-aided virtual reality therapy for the paretic upper limb in stroke patients on changes in brain activation. Brain activation was acquired during the resting state and during visually-guided hand movement. fMRI analysis focused on characterizing functional connectivity with ipsilesional primary motor cortex (iM1) at rest and during execution of paretic hand movement. Two subjects who sustained a stroke more than 6 months ago participated. Before and after the training period, motor function was evaluated (Wolf Motor Function Test [WMFT], Jebsen Test of Hand Function [JTHF]). After the training period, clinical outcomes (WMFT and JTHF) improved in both subjects. The resting state functional connectivity (rsFC) maps and task-related functional connectivity with iM1 showed different magnitudes of activation, however, the general directionality of the pattern (increases versus decreases) was similar. Specifically, both the rsFC and the task-related functional connectivity between iM1 and contralesional primary motor cortex (cM1) decreased after the therapy for the first subject and increased for the second subject. Our preliminary data suggest that resting state functional connectivity may be a useful measure of brain reorganization, particularly for subjects with limited volitional control of the paretic limb.
Keywords: stroke, fMRI connectivity, brain reorganization, hand rehabilitation
I. Introduction
Resting state fMRI is a measure of low frequency fluctuations in BOLD activity when the subject is at rest. Analysis of the resting state functional connectivity (rsFC) characterizes brain areas that are co-activated with a defined region of interest (ROI); thus identifying a functionally-connected network. Since the first rsFC study published by Biswal et al. in 1995 [1], rsFC has been used to evaluate a variety of hypotheses. For example, rsFC has been used as a measure of baseline BOLD activity to predict individual task-induced BOLD activity [2] and as a means of scaling the task-induced BOLD activity to obtain more accurate BOLD signal [3-5]. In the field of stroke and stroke rehabilitation research, rsFC has been used to characterize longitudinal brain recovery after stroke [6], and in a recent case study, as measure of brain reorganization after rehabilitation therapy [7]. In addition, analysis of rsFC can be combined with studies of structural connectivity [8, 9] and effective connectivity [10] to more accurately predict recovery after stroke [11]. Given the relationship between functional recovery and brain reorganization [12, 13], the use of rsFC to characterize brain reorganization and predict recovery in response to therapeutic interventions is particularly appealing. Identifying the neural mechanisms of recovery is crucial for developing more effective, neuroscience-grounded, rehabilitation interventions.
There are at least two important challenges posed to longitudinal fMRI studies investigating rehabilitation outcomes. The first relates to possible confounds induced by changes in motor performance across experimental sessions and testing days. One way to mitigate the effects of this confound is to monitor hand movement in real-time using MRI-compatible data gloves, and provide subjects with online visual feedback of their movement in the scanner. Doing so encourages consistent motion during scanning and allows for offline analysis of movement kinematics that were acquired during scanning. The second challenge is how to acquire imaging data on stroke patients who are unable to produce volitional motion. In this regard, rsFC imaging designs and analyses may be the answer since no overt movement is necessary. However, the degree to which functional networks and their activation patterns (increases versus decreases) identified with rsFC and task-related functional connectivity overlap, in the case of stroke patients undergoing intervention, is unknown.
In this pilot study, we compared changes in connectivity patterns with iM1 using both resting-state fMRI and task-related fMRI. To our knowledge, there are no published studies comparing functional connectivity of ipsilesional motor cortex during a movement task versus resting state functional connectivity. fMRI data was acquired before and after 2-weeks of intensive training of the affected upper extremity using the New Jersey Institute of Technology-Robot-Assisted Virtual Rehabilitation (NJIT-RAVR) adaptive training system [14]. This protocol focused on retraining hand and arm movements, including reaching, grasping, finger individuation, and coordination.
II. Methods
A. Subjects
Two male stroke (1 right hemiplegic) subjects with upper extremity hemiplegia participated after signing informed consents approved by the institutional IRB. Subjects were right handed before the stroke [15]. Table I shows the clinical characteristics of the two subjects based on the Chedoke-McMaster [16] and Ashworth motor scales. Figure 1 shows that the lesion was localized to the left pons for Subject 1 (S1) and the right thalamus for Subject 2 (S2). S2 also had an older lesion in left temporal lobe from a prior stroke.
Table I.
SUBJECTS’ CLINICAL INFORMATION
| subject | Age | CMA | CMH | Ashworth |
|---|---|---|---|---|
| 1 | 51 | 5 | 4 | 6 |
| 2 | 66 | 4 | 5 | 5 |
CMA:Chedoke-McMaster Arm scale; CMH: Chedoke-McMaster Hand scale
Fig. 1.
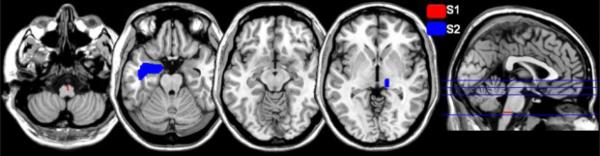
Mapping of subjects’ brain lesion after stroke.
B. Training Protocol
NJIT-RAVR training is an intensive upper extremity training protocol, where subjects play video games in a robot-assisted virtual reality setup. This setup works on retraining subjects’ hand/arm coordination, reaching, grasping, and finger individuation. The training was conducted three hours/day, four days/week, for two weeks. The protocol is described in detail elsewhere [14].
C. Outcome Measurements
The following outcome measurements were taken two weeks before training (Pre1, only for S1), one day before training (Pre2) and one day after training: clinical scales (WMFT & JTHF) and fMRI data.
Subjects were scanned in the MRI scanner while 1) performing whole paretic hand finger flexion movements (task fMRI) and 2) resting with eyes closed (resting fMRI). During the task fMRI, subjects were wearing an MRI compatible data glove (5DT) on each hand to 1) record the finger joint angle for offline analysis, and to 2) stream these kinematics to a display in real-time thus providing subjects with online feedback of their movement via a virtual reality environment (see [17, 18] for more description). One fMRI run and three task-related fMRI runs were acquired on each day (session). Resting state scans were always acquired after the structural scan and before the task fMRI, in order to avoid possible effects of movement on rsFC.
D. fMRI Data Acquisition
The MRI scanner used to acquire fMRI data was 3-T Siemens Allegra head-only scanner with a Siemens standard head coil. For each subject, both high resolution structural images (TR=2000 ms, TE= 4.38, voxel size 0.938×0.938×1 mm, 176 slices, 1 mm slice thickness) and functional images (TR=2000 ms, TE=30 ms, FOV 100 mm, voxel size= 3×3×3 mm, number of slides 32, interslice time 62 ms, 156 volumes) were acquired. All functional scans used a T2* weighted echo planar imaging sequence. Task fMRI data were preprocessed and analyzed using SPM8, resting fMRI data were preprocessed using SPM8 and analyzed using CONN toolbox [19]. Each subject's functional volume was realigned to the first volume and co-registered with the structural image. All images were normalized to the SPM8 Montreal Neurological Institute template, and functional images were smoothed with an 8 mm Gaussian kernel. The scans of S1 were flipped about the mid-sagittal plane before preprocessing so that the right hemisphere represents the lesioned side for both subjects.
E. Extent of Activation
A General Linear Model (GLM) was created for each subject in SPM8, with scans from different testing days modeled as separate sessions. The movement kinematics (angular excursion, and angular velocity) were included in the GLM as parametric modulators in order to measure any correlation between BOLD activity and change in movement kinematics across days.
F. Resting-state Functional Connectivity Analysis
The resting state fMRI scans were preprocessed using SPM8. The data was then low-pass filtered with a 1Hz cutoff frequency and used to create a GLM (CONN SPM Toolbox). A region of interest for functional connectivity was defined as the right (ipsilesional) primary motor cortex (iM1) at MNI coordinates [35 -20 50]. The correlation coefficient of each voxel in the brain with iM1 was calculated using the CONN toolbox correlation bivariate analysis. The correlation coefficient values were then Fischer transformed. Difference in connectivity with iM1 between testing days was computed using imcalc SPM tool.
G. Psychophysiological Interaction Analysis
Functional connectivity of iM1 during the movement task was studied using psychophysiological interaction analysis (PPI) using the SPM-based gPPI toolbox [20]. The region of interest was a cluster of 8 voxels in iM1, selected based on the peak activity in iM1 in the move>rest contrast. Regression analysis was done between PPI parameters (cross correlation of the model of the hemodynamic response to the task and time series of the seed) and the activity in each voxel in the brain. Changes in PPI connectivity with iM1 after training were defined using contrasts of PPI connectivity (e.g. post > pre contrast).
III. Results
Clinical scores
Both subjects showed improvement in motor function after the 2 weeks of rehabilitation training (see Table II), though gains were notably larger for S1 than for S2.
Table II.
PERCENT IMPROVEMENT IN CLINICAL SCORES
| subject | WMFT % | JHFT % |
|---|---|---|
| 1 | 40 | 10 |
| 2 | 6 | 3 |
WMFT: Wolf Motor Function Test; JHFT: Jebsen Hand Function Test.
Movement kinematics during task fMRI
Movement kinematics of the paretic hand during the fMRI experiment were compared between days (before and after therapy), and experimental sessions within each scanning day. In particular, we compared finger movement duration, angular excursion, and angular velocity. Repeated measures ANOVA revealed that subjects’ movements varied across sessions and testing days. Angular excursion for S1 was significantly higher in pre1 versus pre2 and post (F2,15=392, p<0.0001) and significantly differed between fMRI runs 1-3 (F2,15=42.5, p<0.0001). Similarly, peak angular velocity was lower in pre1 than pre2 and post (F2,15=114, p<0.0001) and differed between fMRI runs (F2,15=47, p<0.0001). Movement duration was longer in pre1 versus pre2 and post by 220 milliseconds (F2,15=28.7, p<0.0001), and differed between fMRI runs (F2,15=28.7, p<0.0001). Similarly, S2 had significant differences in angular excursion (days: F1,14=41.2, p<0.0001; runs: F1,14=73.8, p<0.0001), angular velocity (days: F1,14=80.4, p<0.0001, runs: F1,14=4.489, p<0.0197), and movement duration (days: F1,14=1423.2, p<0.0001; but runs: p>0.05).
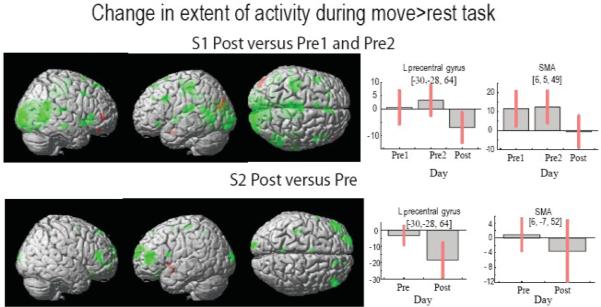
Extent of activation
The extent of activation after therapy decreased for both subjects. Fig.2. shows a bilateral decrease in the extent of activation at a threshold p<0.01 and k=10. The bar plots to the right show a decrease in activity in cM1 and supplementary motor area (SMA). To account for the possibility that these decreases may have been attributed to varied movement kinematics, we reanalyzed this data by using an exclusive mask of regions that showed significant parametric modulation by kinematics. These results remained consistent with those described above, suggesting that the variability in movement kinematics was not a major confound on the BOLD activity changes noted across days.
Fig 2.
The extent of activation in the move>rest contrast is shown in green color for the decrease in the activity after training and in red color for the increase in the activity after training. The bar plots to the right show change in the beta estimate of the two regions of interests, contralesional primary motor cortex (cM1) and supplementary motor area (SMA). Red bars denote the 95% confidence interval.
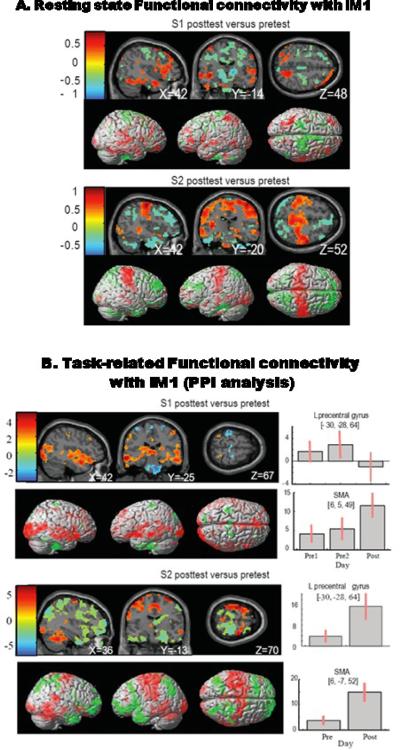
Functional Connectivity
S1 showed a pattern of decreased connectivity of iM1 with the sensorimotor cortex and increased connectivity with the temporal and occipital lobes (see Fig.3.). S2 showed an increase in iM1 connectivity with the sensorimotor cortex and a decrease in connectivity with the frontal occipital and temporal lobes. At the cluster level, both subjects showed increases in task related connectivity between iM1 and SMA (see Fig.3. bar plots). Additionally, S1 showed decreased connectivity with cM1 and S2 showed increased connectivity with cM1. This pattern of training-induced changes in functional connectivity matches the changes observed in rsFC where cM1 connectivity with iM1 decreased (pretest correlation coefficient R=0.85, posttest R=0.67, difference in R value after training is −0.18) for S1 and increased for S2 (pretest R= 0.54, posttest R=0.78, difference in R vale after training is 0.16).
Fig. 3.
A. Change in rsFC with the ipsilesional primary motor cortex; this is a change in correlation (Fischer transformed correlation coefficient values) with a threshold of 0.3. Sections are selected to highlight the pattern of change in deeper structures. B. Change in task-induced functional connectivity with iM1 (measured using PPI). Each subject's activation is plotted at a threshold of p<0.05 in order to compare the pattern of connectivity with the change in correlation (A). Bar plots show the beta estimate at each cluster with the whisker bars denoting the 95% confidence interval.
IV. Discussion
In just 2 weeks of NJIT-RAVR therapy, subjects showed notable improvements in movement, paralleled by changes in cerebral activation. It is interesting to find that each of the two subjects showed a different pattern of reorganization in functional connectivity with iM1. S1 had a predominantly decrease in connectivity between the contralesional sensorimotor cortex and iM1, while S2 had substantial increase in connectivity between bilateral sensorimotor / premotor areas and iM1. While the small sample size precludes us from drawing strong conclusions regarding the underlying mechanisms, we can speculate over several possibilities. First, S1 had a subcortical lesion while S2 had both cortical and subcortical lesions. Second, though the initial impairment level seems to be similar between subjects, S1 improved far more than S2. Thus, it is possible that the different patterns of change in functional connectivity may relate to lesion location or response to therapy. Recent work by Sergi et al [7] suggests that, at least for subcortical lesions, functional connectivity between contralesional sensorimotor cortex and iM1 does decreases after therapy (similar to S1 data in our study). Such hypotheses must be further tested in a larger cohort with variable lesions and motor ability.
To our surprise, despite providing subjects with real-time feedback of their movements, they nevertheless produced variable movements across fMRI runs and days. This underscores one of the main hurdles to the analysis of longitudinal task-related fMRI designs, where differences in motor performance are inevitable despite the best efforts and intentions to control for this. The observation that rsFC changes post-intervention are comparable to task-related changes in connectivity (at least in direction of effects and loci of connectivity, and to a lesser degree in the extent of the network) suggests that resting state fMRI may be a valuable addition to fMRI designs in stroke rehabilitation studies. This may be of clear use for subjects who are unable to produce movements, and may be an important baseline measure for higher-level patients as well [3-5].
Acknowledgments
This work was supported in part by NIH grants K01 HD059983 (ET), and R01 HD58301 (SA).
Contributor Information
Soha Saleh, New Jersey Institute of Technology Newark, NJ 07102 USA and the School of Biomedical Sciences at University of Medicine and Dentistry, Newark (shs25@njit.edu)..
Sergei V. Adamovich, New Jersey Institute of Technology Newark, NJ 07102 USA and the School of Biomedical Sciences at University of Medicine and Dentistry, Newark..
Eugene Tunik, School of Health Related Professions and the school of Biomedical Sciences at the University of Medicine and Dentistry, Newark, 07107 (tunikeu@umdnj.edu)..
REFERENCES
- 1.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995 Oct;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Zhu XH, Chen W. Baseline BOLD correlation predicts individuals’ stimulus-evoked BOLD responses. NeuroImage. 2011 Feb 1;54:2278–86. doi: 10.1016/j.neuroimage.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. NeuroImage. 2008 May 1;40:1567–74. doi: 10.1016/j.neuroimage.2007.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kannurpatti SS, Motes MA, Rypma B, Biswal BB. Neural and vascular variability and the fMRI-BOLD response in normal aging. Magnetic resonance imaging. 2010 May;28:466–76. doi: 10.1016/j.mri.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kannurpatti SS, Motes MA, Rypma B, Biswal BB. Increasing measurement accuracy of age-related BOLD signal change: minimizing vascular contributions by resting-state-fluctuation-of-amplitude scaling. Human brain mapping. 2011 Jul;32:1125–40. doi: 10.1002/hbm.21097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, Kim YH. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke; a journal of cerebral circulation. 2011 May;42:1357–62. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sergi F, Krebs HI, Groissier B, Rykman A, Guglielmelli E, Volpe BT, Schaechter JD. Predicting efficacy of robot-aided rehabilitation in chronic stroke patients using an MRI-compatible robotic device. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2011 Aug;2011:7470–3. doi: 10.1109/IEMBS.2011.6091843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansari AH, Oghabian MA, Hossein-Zadeh GA. Assessment of functional and structural connectivity between motor cortex and thalamus using fMRI and DWI. Conf Proc IEEE Eng Med Biol Soc. 2011 Aug;2011:5056–9. doi: 10.1109/IEMBS.2011.6091252. [DOI] [PubMed] [Google Scholar]
- 9.Carter AR, Patel KR, Astafiev SV, Snyder AZ, Rengachary J, Strube MJ, Pope A, Shimony JS, Lang CE, Shulman GL, Corbetta M. Upstream dysfunction of somatomotor functional connectivity after corticospinal damage in stroke. Neurorehabil Neural Repair. 2012 Jan;26:7–19. doi: 10.1177/1545968311411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James GA, Lu ZL, VanMeter JW, Sathian K, Hu XP, Butler AJ. Changes in resting state effective connectivity in the motor network following rehabilitation of upper extremity poststroke paresis. Top Stroke Rehabil. 2009 Jul-Aug;16:270–81. doi: 10.1310/tsr1604-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010 Mar;67:365–75. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000 Nov;20:1513–28. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003 Nov;126:2476–96. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu Q, Ramirez DA, Saleh S, Fluet GG, Parikh HD, Kelly D, Adamovich SV. The New Jersey Institute of Technology Robot-Assisted Virtual Rehabilitation (NJIT-RAVR) system for children with cerebral palsy: a feasibility study. J Neuroeng Rehabil. 2009;6:40. doi: 10.1186/1743-0003-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 16.Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, Sanford J, Barreca S, Vanspall B, Plews N. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993 Jan;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 17.Adamovich SV, August K, Merians A, Tunik E. A virtual reality-based system integrated with fmri to study neural mechanisms of action observation-execution: a proof of concept study. Restor Neurol Neurosci. 2009;27:209–23. doi: 10.3233/RNN-2009-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh S, Bagce H, Qiu Q, Fluet G, Merians A, Adamovich S, Tunik E. Mechanisms of neural reorganization in chronic stroke subjects after virtual reality training. Conf Proc IEEE Eng Med Biol Soc. 2011 Aug;2011:8118–21. doi: 10.1109/IEMBS.2011.6092002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conn toolbox. available at: http://www.nitrc.org/projects/conn.
- 20.Mclaren D. A Generalized Form of Context-Dependent Psychophysiological Interactions (gPPI) 2011 doi: 10.1016/j.neuroimage.2012.03.068. Available at: http://www.brainmap.wisc.edu/PPI. [DOI] [PMC free article] [PubMed]