Abstract
Bacteria belonging to the Streptococcus anginosus group (Streptococcus intermedius, Streptococcus constellatus and Streptococcus anginosus) are capable of causing serious pyogenic infections, with a tendency for abscess formation. The present article reports a case of S anginosus group pyomyositis in a 47-year-old man. The pathogen was recovered from one of two blood cultures obtained from the patient, but speciation was initially not performed because the organism was considered to be a contaminant (viridans streptococci group). The diagnosis was ultimately confirmed using 16S ribosomal DNA sequencing of purulent fluid obtained from a muscle abscess aspirate. The present case serves to emphasize that finding even a single positive blood culture of an organism belonging to the S anginosus group should prompt careful evaluation of the patient for a pyogenic focus of infection. It also highlights the potential utility of 16S ribosomal DNA amplification and sequencing in direct pathogen detection from aspirated fluid in cases of pyomyositis in which antimicrobial therapy was initiated before specimen collection.
Keywords: 16S rDNA, Pyomyositis, Sequencing, Streptococcus anginosus group
Abstract
Les bactéries qui appartiennent au groupe Streptococcus anginosus (streptocoques intermedius, constellatus et anginosus) peuvent causer de graves infections à pyogènes qui ont tendance à former des abcès. Le présent article contient un cas de pyomyosite du groupe S anginosus chez un homme de 47 ans. Le pathogène a été décelé récupéré dans une des deux cultures sanguines prélevées sur le patient, mais au départ, la spéciation n’a pas été effectuée parce que l’organisme était considéré comme un contaminant (groupe de streptocoques viridians). Le diagnostic a finalement été confirmé au moyen du séquençage de l’ADN ribosomal 16S du fluide purulent obtenu dans l’aspirat d’un abcès musculaire. Le présent cas fait ressortir le fait que la détection d’une seule culture sanguine positive dans un organisme appartenant au groupe S anginosus devrait susciter une évaluation attentive du patient afin de trouver un foyer d’infection à pyogènes. Il fait également ressortir l’utilité potentielle de l’amplification et du séquençage de l’ADN ribosomal 16S pour la détection directe d’un pathogène dans le fluide aspiré en cas de pyomyosite lorsque le traitement aux antibiotiques a commencé à être administré avant le prélèvement.
CASE PRESENTATION
A 47-year-old man with a medical history significant only for hypercholesterolemia and excess alcohol consumption presented to a rural hospital in Manitoba with new onset of fever. The patient reported that he began feeling unwell three days before seeking medical attention. His symptoms started with anorexia, malaise and prominent myalgias in the thighs, with the right thigh affected to a greater extent than the left. He reported rigors, headache and fatigue in addition to fever. He also complained of cramping lower abdominal pain. There was no history of nausea, vomiting or diarrhea. The patient had been visiting a cottage four days before becoming ill. While at the cottage he had consumed a significant quantity of alcohol. The patient denied any recent travel outside of Manitoba. He also denied any infectious contacts. The only medication that he received regularly was rosuvastatin. He denied use of recreational drugs.
On physical examination, the patient was febrile, with a temperature of 38.3°C. His other vital signs were within normal limits. The patient’s abdomen was mildly tender on palpation. The remainder of the physical examination was unremarkable. Serum electrolyte levels were within normal limits, as was a complete blood count, with a white blood cell count of 8.1×109/L (normal range 4.5×109/L to 11×109/L). The patient’s aspartate aminotransferase level was elevated (60 U/L; normal range 10 U/L to 32 U/L) and his alanine aminotransferase level was elevated (85 U/L; normal <30 U/L). His total bilirubin was also elevated (59 μmol/L; normal range 3 μmol/L to 19 μmol/L). No abnormality was detected on chest and abdominal radiographs. Blood cultures were obtained from two sites and the patient received a single dose of ciprofloxacin 500 mg orally. He was subsequently referred to the Health Sciences Centre (HSC), a tertiary care hospital located in Winnipeg, Manitoba, for further evaluation. At the HSC, the patient remained clinically stable over a period of 24 h. It was believed that his symptoms were most likely secondary to mild alcoholic hepatitis or a viral infection. The patient was discharged home with instructions to return to the emergency department if his symptoms progressed.
The patient returned to the HSC emergency department three days later with persistent fever and worsening abdominal pain. The pain was now described as sharp in nature, and was most prominent in the lower abdomen. On repeat physical examination, the patient was again febrile, with a documented temperature of 39.1°C. Significant tenderness was now present on abdominal examination. The right inner thigh was also noted to be swollen and warm to palpation. An urgent computed tomographic scan of the abdomen revealed free intraperitoneal air. The patient was urgently taken to the operating room where he was diagnosed with diverticulitis with an associated perforation, as well as inflammation of the appendix with a perforation. Purulent free fluid was observed in the abdomen and specimens for aerobic and anaerobic culture were collected. A surgical resection of the rectosigmoid colon with closure of the rectal stump and formation of an end colostomy (Hartman’s procedure) was performed. Postoperatively, the patient was transferred to a general surgical ward in stable condition. Antimicrobial therapy with a combination of ciprofloxacin and metronidazole was initiated.
On postoperative day 4, one of two blood cultures collected at the peripheral hospital at which the patient initially presented was reported to be growing an alpha-hemolytic streptococcus belonging to the viridans streptococcus group. Further identification of the organism was not performed because the isolate was considered to represent a contaminant. By this time, the patient was improving clinically; however, he continued to experience focal swelling of the right thigh, as was originally documented on admission to hospital. To investigate this further, a magnetic resonance imaging scan of the right lower extremity was performed, which revealed multiple small lesions in the vastus medialis, adductor magnus and hamstring muscles consistent with abscesses (Figure 1). One of the lesions was aspirated under ultrasound guidance. A Gram stain of the purulent fluid obtained from the lesion demonstrated 4+ polymorphonuclear neutrophils and 3+ Gram-positive cocci. Aerobic and anaerobic bacterial cultures were sterile, likely due to previous antimicrobial exposure.
Figure 1).
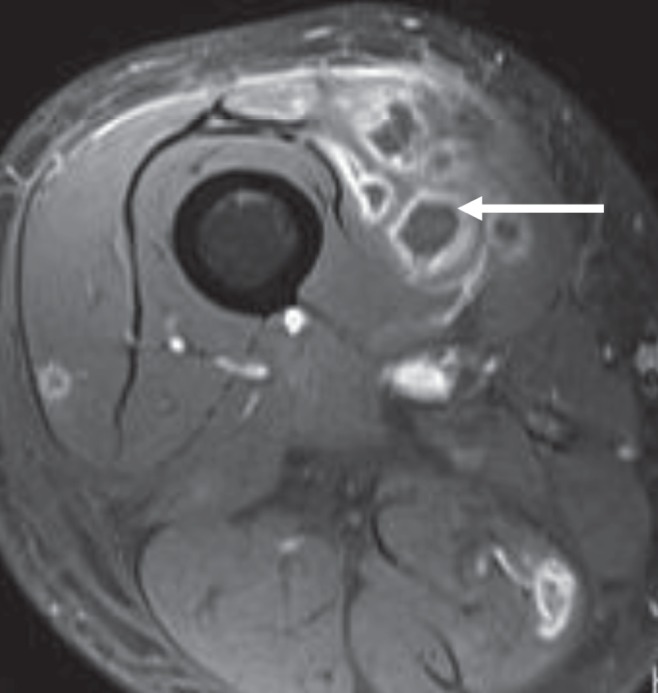
Magnetic resonance image (axial T1-weighted) of the patient’s right thigh, demonstrating low-signal-intensity lesions with ring enhancement post-gadolinium administration (arrow), consistent with small abscesses
To determine the etiology of the thigh abscess, amplification and sequencing of bacterial 16S ribosomal DNA (rDNA) was attempted. Isolation of bacterial DNA from the aspirated purulent fluid was performed using the QIAamp DNA Mini Kit (Qiagen, Canada). The isolated DNA was then used as a template for polymerase chain reaction amplification and sequencing of an 800 base-pair fragment of 16S rDNA using broad-range primers 8FPL (5′-AGTTTGATCCTGGCTCAG-3′) and 806R (5′-GGACTACCAGGGTATCTAAT-3′). The sequence obtained was compared with sequences deposited in the GenBank (National Center for Biotechnology Information, Bethesda, Maryland, USA) and Ribosomal Database Project (Michigan State University, Detroit, Michigan, USA) databases, and was found to be consistent with Streptococcus intermedius (Streptococcus anginosus group) (99.1% sequence identity with S intermedius American Type Culture Collection 27335; GenBank accession number AF104671). The alpha-hemolytic streptococcus recovered on blood culture was subsequently evaluated further and the isolate was identified as a member of the S anginosus group. Additionally, culture of the purulent peritoneal fluid obtained at the time of the surgery yielded a member of the S anginosus group, as well as several anaerobes. A white blood cell scan did not demonstrate any additional foci of infection beyond the right thigh abscesses. A transthoracic echocardiogram was negative for vegetations. The patient was ultimately diagnosed with right thigh pyomyositis caused by S intermedius. It is believed that his right thigh was seeded during bacteremia from an abdominal focus of infection (diverticulitis). The patient was treated with four weeks of intravenous ceftriaxone at a dose of 2 g once daily via a community home intravenous therapy program, as well as oral metronidazole for two weeks. When evaluated at the completion of antimicrobial therapy, he was clinically well. An ultrasound examination of the right thigh at the end of treatment demonstrated complete resolution of the abscesses.
DISCUSSION
The S anginosus group, formerly referred to as the Streptococcus milleri group, is comprised of three distinct species: S anginosus, S intermedius and Streptococcus constellatus (1). These species are catalase-negative Gram-positive cocci, as with other members in the genus Streptococcus. Colonies are typically <0.5 mm in diameter after 24 h incubation and may demonstrate alpha, beta or gamma hemolysis on sheep blood agar, with variation observed among different species (1). Members of the S anginosus group can be phenotypically differentiated from beta-hemolytic streptococci by the colony size and a negative glucuronidase test (1,2). Differentiation from other viridans group streptococci can be made by the ability of these bacteria to hydrolyze arginine and esculin, and by a positive Voges-Proskauer test (1). Furthermore, colonies of S anginosus group have a distinct caramel odour, related to the production of diacetyl (3).
Members of the S anginosus group are considered to be part of the normal oropharyngeal, urogenital and gastrointestinal flora (1). However, they are also capable of causing serious pyogenic infections with a tendency for abscess formation (1,4–7). Infection may occur in the head and neck, central nervous system, abdomen and thoracic cavity (pneumonia, empyema) (4–7). Endocarditis has also been described (4). Pyomyositis due to S anginosus group is an uncommon manifestation of infection, but it has been well documented in the literature (8–10). Of the three species, S intermedius and S constellatus appear to be more likely to cause deep abscesses than S anginosus (5).
The laboratory identification of bacteria belonging to the S anginosus group from blood cultures may be problematic for isolates that demonstrate alpha hemolysis on subculture to sheep blood agar. Alpha-hemolytic, catalase-negative Gram-positive cocci in chains recovered from one of multiple blood cultures are often classified as viridans group streptococci and considered to represent a contaminant (11). Indeed, this occurred in the current case. It should be emphasized that finding an S anginosus group isolate in even a single positive blood culture should not be regarded as contamination. Rather, the patient should be carefully evaluated for the presence of a deep-seated/pyogenic source of infection (4,7). Implementation of bacterial identification using matrix-assisted laser desorption ionization time-of-flight mass spectrometry may assist clinical microbiology laboratories in rapidly ruling out members of the S anginosus group in cases in which viridans streptococci are recovered from one of multiple blood cultures (12).
In the present case, the diagnosis of pyomyositis was confirmed by direct amplification and sequencing of bacterial 16S rDNA present in purulent fluid aspirated from the right thigh. 16S rDNA sequencing is a molecular diagnostic technique that is being used with increased frequency in the clinical microbiology laboratory (13,14). This method has the advantage over bacterial culture in that it does not require viable organisms in the clinical sample. Direct sequencing may prove to be particularly useful in pathogen detection from a normally sterile site when a specimen is collected after the initiation of antimicrobial therapy, as described in the current case (13,14). However, positive results must always be interpreted in the appropriate clinical context because false-positive results may occur due to contamination of reagents or the clinical specimen with small amounts of bacterial DNA.
SUMMARY
Members of the S anginosus group are capable of causing serious pyogenic infections with abscess formation, including pyomyositis. The present case serves to emphasize that finding even a single positive blood culture for an organism belonging to the S anginosus group should prompt careful evaluation of the patient for a pyogenic focus of infection. It also highlights the potential utility of direct 16S rDNA amplification and sequencing in pathogen detection from aspirated fluid in cases of pyomyositis in which antimicrobial therapy was initiated before specimen collection.
REFERENCES
- 1.Spellerberg B, Brandt C. Streptococcus. In: Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW, editors. Manual of Clinical Microbiology. 10th edn. Washington, DC: ASM Press; 2011. pp. 331–49. [Google Scholar]
- 2.Cimolai N, Mah D. β-D-Glucuronidase activity assay for rapid differentiation of species within β-haemolytic group C and G streptococci. J Clin Pathol. 1991;44:824–5. doi: 10.1136/jcp.44.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chew TA, Smith JM. Detection of diacetyl (caramel odor) in presumptive identification of the “Streptococcus milleri” group. J Clin Microbiol. 1992;30:3028–9. doi: 10.1128/jcm.30.11.3028-3029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infect Dis. 1998;27:385–7. doi: 10.1086/514658. [DOI] [PubMed] [Google Scholar]
- 5.Clarridge JE, III, Attorri S, Musher DM, Hebert J, Dunbar S. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (“Streptococcus milleri group”) are of different clinical importance and are not equally associated with abscess. Clin Infect Dis. 2001;32:1511–5. doi: 10.1086/320163. [DOI] [PubMed] [Google Scholar]
- 6.Whiley RA, Beighton D, Winstanley TG, Fraser HY, Hardie JM. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus (the Streptococcus milleri group): Association with different body sites and clinical infections. J Clin Microbiol. 1992;30:243–4. doi: 10.1128/jcm.30.1.243-244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salavert M, Gomez L, Rodriguez-Carballeira M, Xercavins M, Freixas N, Garau J. Seven-year review of bacteremia caused by Streptococcus milleri and other viridans streptococci. Eur J Clin Microbiol Infect Dis. 1996;15:365–71. doi: 10.1007/BF01690091. [DOI] [PubMed] [Google Scholar]
- 8.Calza L, Manfredi R, Briganti E, Attard L, Chiodo F. Iliac osteomyelitis and gluteal muscle abscess caused by Streptococcus intermedius. J Med Microbiol. 2001;50:480–2. doi: 10.1099/0022-1317-50-5-480. [DOI] [PubMed] [Google Scholar]
- 9.Yassin M, Yadavalli GK, Alvarado N, Bonomo RA. Streptococcus anginosus (Streptococcus milleri group) pyomyositis in a 50-year-old man with acquired immunodeficiency syndrome: Case report and review of the literature. Infection. 2010;38:65–8. doi: 10.1007/s15010-009-6002-9. [DOI] [PubMed] [Google Scholar]
- 10.Christin L, Sarosi GA. Pyomyositis in North America: Case reports and review. Clin Infect Dis. 1992;15:668–77. doi: 10.1093/clind/15.4.668. [DOI] [PubMed] [Google Scholar]
- 11.Garcia LS, Isenberg HD, editors. Clinical Microbiology Procedures Handbook. 2nd edn. Washington, DC: ASM Press; 2007. Blood cultures – general detection and interpretation; pp. 3.4.1.1–16. [Google Scholar]
- 12.Friedrichs C, Rodloff AC, Chhatwal GS, Schellenberger W, Eschrich K. Rapid identification of viridans streptococci by mass spectrometric discrimination. J Clin Microbiol. 2007;45:2392–7. doi: 10.1128/JCM.00556-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sotakke S, Cadenas MB, Maggi RG, Diniz PP, Breitschwerdt EB. Use of broad range 16S rDNA PCR in clinical microbiology. J Microbiol Methods. 2009;76:217–25. doi: 10.1016/j.mimet.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Petti CA. Detection and identification of microorganisms by gene amplification and sequencing. Clin Infect Dis. 2007;44:1108–14. doi: 10.1086/512818. [DOI] [PubMed] [Google Scholar]


