Abstract
Objective
Depression and obesity share overlapping psychosocial and pathophysiological etiologies. Animal models suggest that impaired leptin production, or leptin resistance, may contribute to depression. The link between leptin and depression could be mediated by obesity, which is more common in depression and increases leptin production.
Methods
We administered the Beck Depression Inventory II (BDI-II) to 537 participants (mean age 51, standard deviation 9 years, 49% African American, 61% female) enrolled in the Morehouse-Emory Partnership to Eliminate Cardiovascular Health Disparities (META-Health) study. BDI-II scores of 0–13 indicated minimal to no depression, 14–19 mild depression, and 20–63 moderate to severe depression. Levels of leptin were examined as continuous, log-transformed values.
Results
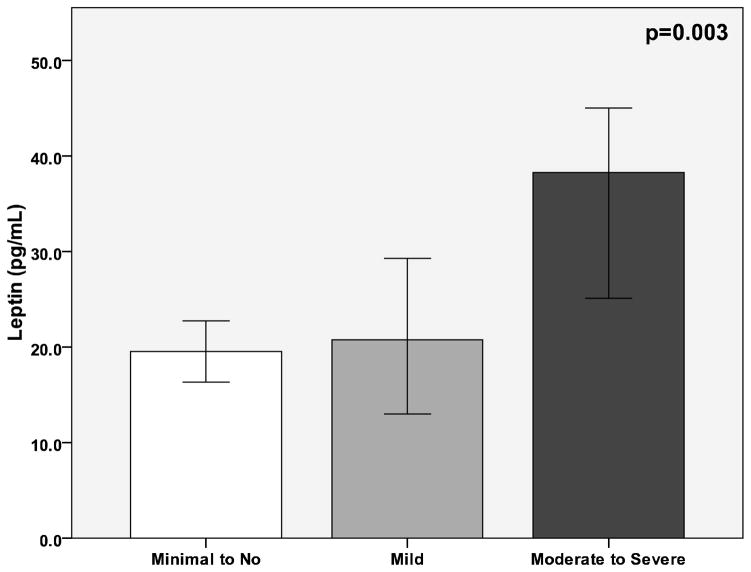
Participants with moderate to severe depression had higher levels of leptin (median 37.7, interquartile range [17.6–64.9] ng/mL) than those with mild depression (22.9 [7.0–57.9] ng/mL) or minimal to no depression (19.8 ng/mL, [7.8–39.1], p=0.003). Participants with moderate to severe depression had higher body mass index (BMI) than those with mild or minimal depression (33±8 vs. 31±9 vs. 29±7 kg/m2, p<0.001). After multivariate adjustment for age, gender, race, smoking, history of hypertension and diabetes, blood pressure, lipids, and CRP, the BDI-II score remained a significant predictor of leptin levels (β=0.093, p=0.01). Further adjustment for BMI eliminated the association between the BDI-II score and leptin (β=0.03, p=0.3). Adjusting for waist circumference in place of BMI revealed similar findings.
Conclusion
The association between depression and leptin appears to be mediated by increased adiposity in depressed individuals. Leptin may represent a pathway through which obese individuals may develop depression, or a common mechanism leading to both depression and obesity.
Keywords: leptin, depression, adiposity, adiponectin
BACKGROUND
Major depression affects approximately 14.8 million Americans age 18 and older each year, causing significant morbidity, and is a leading cause of disability in the US for adults ages 15–44 (1). Inflammation is being increasingly recognized as a mechanism that may contribute to the pathogenesis of clinical depression, as it has been documented that peripheral hormonal and inflammatory signals can access the brain and activate relevant cell types, such as microglia, that serve to amplify central inflammatory responses.
Leptin is a 16 kDa hormone secreted by adipose tissue that plays a key role in regulating energy intake and expenditure. Leptin is transported across the blood-brain barrier, and in the central nervous system interacts with specific receptors in the hypothalamus to maintain energy homeostasis (2). Energy deficient states result in reduced leptin levels, leading to activation of feeding stimulants such as neuropeptide Y and agouti-related protein, whereas states of energy excess results in increased leptin levels, and subsequent activation of appetite suppressants such as pro-opiomelanocortin (2, 3). Obesity is associated with higher levels of leptin, reflecting, in part, increased production by adipocytes. Moreover, levels of leptin may be higher in obese individuals due to leptin resistance, in a similar way that type 2 diabetics are resistant to insulin (2). In addition to its role in energy homeostasis, leptin has direct pro-inflammatory effects (4, 5). Previous research indicates that leptin activates circulating monocytes and upregulates in vivo production of interleukin (IL)-6 and tumor necrosis factor (TNF-) alpha (4, 6).
Depression and obesity share overlapping psychosocial and pathophysiological etiologies, and an association between the two conditions has been seen in many population-based studies. (7, 8). Potential explanations for this association are known to be complex and not entirely clear (8). Conceptually, the association of leptin with psychiatric disorders may relate both to its metabolic properties and neurobiological functions (9). Animal models suggest that impaired leptin production may contribute to depression (10, 11). Rats or mice exposed to chronic stress that display behavioral deficits similar to human depression show decreased levels of leptin. In these mice, systemic administration of leptin reversed those behavioral deficits (10, 12). Prior studies in humans have been unable to reach a consensus, and have found both high and low levels of leptin in depressed patients, while others have shown no association (13–17). We sought to investigate the association of depression and leptin in a community-based population, with the hypothesis that the association between leptin and depression is modulated by adiposity.
METHODS
Study sample
The Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) Study was a two-stage cross-sectional study of both traditional and psychosocial risk factors for cardiovascular disease (CVD). Participants were recruited from March 2006 to October 2009. The first stage was a random digit dialing survey of African American and White residents of metropolitan Atlanta, ages 30 to 65 years (n=3391); the second stage included participants (n=753) who agreed to come to either Emory or Morehouse Schools of Medicine for a detailed study visit. Detailed information on demographics and anthropometrics was collected. Blood pressure was measured with a sphygmomanometer after five minutes of rest, and was based on the average of the final two of three readings measured five minutes apart. Height, weight, and waist circumference were measured. Waist circumference was based on the average of three readings taken at the level of the iliac crest. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Smoking history, obtained using standardized questionnaires, was defined as current, or never/former (no cigarettes within the past 30 days). Pregnant women and those with acute illnesses were excluded. The study was approved by the Emory University and Morehouse University Institutional Review Committees. Informed consent was obtained from all participants.
Measurement of depression
Of the 753 participants enrolled in the META-Health study, 597 completed the Beck Depression Inventory (BDI)-II, a self-administered validated 21-item scale that assesses depressive symptoms experienced during the previous 2 weeks (18). Compared to those who completed the assessment, there was a higher percentage of males (61% vs. 38%, p<0.001) and African Americans (66% vs. 49%, p<0.001) in the 156 participants who did not complete the BDI-II. The BDI-II score provides a continuous scale of depressive symptoms from 0 to 63. Higher scores indicate more severe depressive symptoms; scores 0–13 denote minimal to no depression; scores 14–19 mild depression; scores 20–28 moderate depression; and scores 29–63 severe depression. If more than 2 BDI-II questions were missing, the score was set to missing (n=2); however, if only 1 to 2 questions were missing, the mean response from the non-missing questions was substituted for the missing values (n=35)(19). The internal consistency (Cronbach’s alpha) of the scale was 0.9 in our study population.
Laboratory analyses
Participants were instructed to fast for 12 hours before the study visit. Venous blood was collected in sodium heparin tubes, and serum levels of high density lipoprotein cholesterol (HDL-C), triglycerides, and glucose were measured by spectrophotometry. Plasma was frozen at −70°C for subsequent measurement of adipokines and inflammatory markers. High sensitivity C-reactive protein (CRP) was measured by immunonephelometry (Siemens/Dade Behring). Leptin and adiponectin were measured using a Fluorokine MAP cytokine multiplex kit (R&D Systems, Minneapolis MN) designed for the Luminex 100 (BioRad Bioplex). Briefly, samples are incubated with color-coded beads that are pre-coated with analyte-specific capture antibodies. Expression levels are determined following incubation with a biotinylated detection antibody and streptavidin-conjugated phycoerythrin (PE). Using a Luminex® analyzer, independent lasers determine the color of each bead and the magnitude of the PE-derived signal, which is directly proportional to the levels of bound analyte.
Statistical methods
In the main analyses, total BDI-II score was analyzed as a continuous variable; however, to provide a clinical perspective, analyses with BDI-II categories (minimal depression vs. mild to severe) were also conducted. Age, BMI, waist circumference, systolic and diastolic blood pressure, HDL-C, triglycerides, and glucose were used as continuous variables. Continuous variables were tested for normality using the Kolmogorov-Smirnov criterion. Because the distribution of leptin was skewed, natural log transformed levels were used for any parametric analysis.
Multivariable linear regression models were conducted in order to test the association of depressive symptoms with leptin levels after adjusting for covariates. Covariates were selected a priori, based on their known or potential association with leptin levels, as well as with depressive symptoms. Analyses were conducted in the following steps: Model 1) total BDI-II score as the sole explanatory variable; Model 2) demographic and metabolic risk factors (age, gender, race, smoking status, history of hypertension and diabetes, blood pressure, triglycerides, HDL-C, and glucose) were added; Model 3) CRP was added; Model 4) BMI was added. Additional analyses were performed to evaluate the importance of waist circumference, so the linear regression models were repeated using waist in place of BMI in Model 4. The Sobel test was performed to estimate the total, direct, and indirect effects of the total BDI-II score on leptin levels with adiposity as a mediator variable (20, 21). Because of the known association of leptin with adiponectin, the association of depression with adiponectin was examined as well. Further exploratory analyses were performed in order to define the presence of an interaction of depressive symptoms with BMI. Additional analyses were conducted with BMI treated as a categorical variable classified as low to normal, BMI ≤ 24.9 kg/m2; overweight, BMI 25–29.9 kg/m2; and obese, BMI ≥ 30 kg/m2. All tests of statistical significance were 2-tailed, and P values <0.05 were considered significant. Statistical analyses were performed using SPSS, Inc. v17.0.
RESULTS
Subject characteristics
Of the 597 participants with BDI-II data, 57 were excluded because of missing leptin values, and 3 were excluded because the values were potential outliers (leptin values >2.5 standard deviations (SD) above the mean). Demographic and clinical characteristics of the remaining 537 participants are presented in Table 1. The mean age was 51±9 years. Females comprised 61% of the sample, and 49% were African American.
Table 1.
Subject characteristics by categories of severity of depressive symptoms.
| Minimal to no Depression N=415 | Mild Depression N=63 | Moderate to Severe Depression N=59 | p | |
|---|---|---|---|---|
| Age, (yrs) | 51±9 | 53±9 | 51±9 | 0.18 |
| Female, N (%) | 246 (60) | 42 (67) | 37 (63) | 0.55 |
| African American, N (%) | 192 (47) | 37 (59) | 32 (54) | 0.14 |
| Current smokers, N (%) | 62 (16) | 17 (29) | 12 (21) | 0.05 |
| Hypertension, N (%) | 141 (37) | 28 (49) | 32 (58) | 0.004 |
| Diabetes, N (%) | 28 (7) | 8 (14) | 9 (16) | 0.04 |
| SBP (mm Hg) | 120±17 | 125±20 | 126±18 | 0.005 |
| DBP (mm Hg) | 78±11 | 77±12 | 83±14 | 0.01 |
| Triglycerides (mg/dL) | 114±61 | 120±63 | 141±114 | 0.20 |
| HDL-C (mg/dL) | 59±17 | 57±15 | 54±15 | 0.16 |
| Glucose (mg/dL) | 93±25 | 96±31 | 95±17 | 0.34 |
| CRP (mg/L) | 1.6 (0.7, 4) | 2 (1, 5) | 2 (1,6) | 0.018 |
| BMI (kg/m2) | 29±7 | 31±9 | 33±8 | <0.001 |
| Waist circumference (cm) | 96±16 | 101±20 | 105±18 | 0.001 |
| Total BDI-II score | 5±4 | 16±2 | 26±7 | <0.001 |
Values shown are mean ± standard deviation, median (interquartile range) or N (%). BDI-II scores 0–13, minimal to no depression; 14–19, mild depression; 20–63, moderate to severe depression. SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; CRP, C-reactive protein; BMI, body mass index.
Univariate associations between metabolic risk factors and depression
Total BDI-II scores correlated positively with history of hypertension and diabetes, BMI, waist circumference, blood pressure, triglycerides, and CRP, and there was a tendency towards a positive correlation with glucose (p=0.058). Leptin levels correlated positively with female gender, African American race, history of hypertension and diabetes, BMI, waist circumference, blood pressure, triglycerides, glucose, and CRP, and there was a tendency towards a positive correlation with age (p=0.055). Leptin levels were inversely correlated with current smoking and adiponectin levels.
Associations between depression, leptin levels, and adiposity
There was an increase in leptin levels with increasing BDI-II score. Participants with moderate to severe depression had higher levels of leptin than those with mild, or minimal to no depression (Figure 1). With BDI-II as a continuous variable, a 1-point increase in BDI-II score was associated with 17% higher leptin levels (Table 2). After multivariate adjustment for age, gender, race, history of hypertension and diabetes, blood pressure, lipids, glucose, and CRP, the BDI-II score remained a significant predictor of leptin levels (Table 3). However, further adjustment for BMI eliminated the association between the BDI-II score and leptin. BMI explained 59% of the variance in leptin levels, based on the partial R2 from the regression model. The Sobel test confirmed that BMI significantly mediated the relationship between the BDI-II score and leptin levels (test statistic= 4.9, p<0.0001). Other significant predictors of higher leptin levels after multivariate adjustment included age, female gender, triglycerides, and CRP, while current smoking was associated with lower leptin levels (Table 3).
Figure 1.
Median levels of leptin by categories of depression. BDI-II scores 0–13, minimal depression; 14–19, mild depression; 20–28, moderate depression; and 29–63 severe depression.
Table 2.
Spearman correlations between total BDI-II scores, leptin levels, and demographic and metabolic variables.
| Total BDI-II | Leptin | |
|---|---|---|
| Age | 0.035 | 0.083 |
| Female gender | 0.041 | 0.471** |
| African American race | 0.057 | 0.159** |
| Current Smoking | 0.070 | −0.127* |
| History of hypertension | 0.163** | 0.228** |
| History of diabetes | 0.115* | 0.108* |
| BMI (kg/m2) | 0.162** | 0.651** |
| Waist circumference (cm) | 0.152** | 0.483** |
| SBP (mm Hg) | 0.153** | 0.195** |
| TG (mg/dL) | 0.119** | 0.131* |
| HDL-C (mg/dL) | 0.061 | 0.008 |
| Glucose (mg/dL) | 0.083 | 0.130* |
| CRP (mg/L) | 0.109* | 0.444* |
| Adiponectin (ng/mL) | −0.034 | −0.086* |
p<0.05,
p≤0.001.
BMI, body mass index, SBP, systolic blood pressure; TG, triglycerides; HDL-C, high density lipoprotein cholesterol; CRP, C-reactive protein.
Table 3.
Linear regression of risk factors with ln leptin levels.
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| BDI-II score | 0.158** | 0.094* | 0.093* | 0.033 |
| Age | -- | 0.023 | 0.025 | 0.066* |
| Sex | -- | 0.520** | 0.518** | 0.476** |
| African American Race | -- | 0.166** | 0.124* | 0.038 |
| Smoking | -- | −0.177** | −0.202** | −0.110** |
| Hypertension | -- | 0.205** | 0.164** | 0.052 |
| Diabetes | -- | −0.002 | 0.010 | −0.027 |
| SBP | -- | 0.044 | 0.055 | −0.008 |
| Triglycerides | -- | 0.145** | 0.133* | 0.072* |
| HDL-C | -- | −0.116* | −0.102* | −0.014 |
| Glucose | -- | 0.072 | 0.066 | 0.026 |
| CRP | -- | -- | 0.225** | 0.115** |
| BMI | -- | -- | -- | 0.529** |
Values shown are standardized beta coefficients. Model 2, further adjusted for age, sex, race, smoking status, history of hypertension and diabetes, blood pressure, lipids, and glucose. Model 3, further adjusted for CRP. Model 4, further adjusted for BMI.
p≤0.05,
p≤0.001.
To further examine the association of depression with adiposity, waist circumference was substituted for BMI in multivariable model 4. Adjusting for waist circumference revealed similar findings, in that the association between the BDI-II score and leptin was eliminated. Waist circumference remained independently associated with leptin levels (β=0.506, p<0.001) after adjusting for covariates, and explained 54% of the variance in leptin levels. The Sobel test confirmed that waist also significantly mediates the relationship between the BDI-II score and leptin levels (test statistic= 4.5, p<0.0001).
Association between depression and adiponectin levels
There was no difference in adiponectin levels among participants with minimal to no depression, mild depression, or moderate to severe depression (67.5 [38.6, 156.4], 67.8 [30.0, 136.1], 79.8 [43.0, 169.9] ng/mL, p=0.4).
Secondary analyses
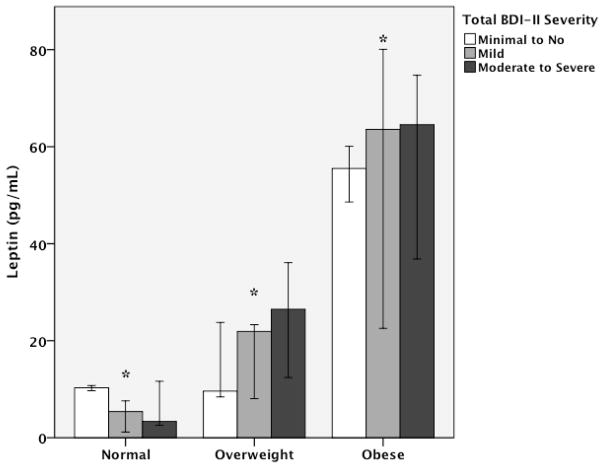
In the adjusted model including the same covariates as above, there was a trend towards a significant interaction effect of BMI and BDI-II scores (p=0.06). As seen in Figure 2, stratifying participants according to obesity status revealed that low to normal weight participants with moderate to severe depression had lower levels of leptin than those with mild or minimal to no depression (p<0.05). However, overweight and obese participants with moderate to severe depression had higher levels of leptin than those with mild or minimal to no depression (p=0.01 and 0.004, respectively). After adjusting for covariates, BDI-II scores showed a borderline significant association with higher leptin levels (β=0.107, p=0.06) in obese participants. There were no interactions based on race or gender.
Figure 2.
Median levels of leptin by categories of body mass index and severity of depressive symptoms. Values are adjusted for age, race, gender, blood pressure, lipids, and CRP. BDI-II scores 0–13, minimal to no depression; 14–19, mild depression; 20–63, moderate to severe depression. *p<0.05.
DISCUSSION
In this community-based sample, we found that the association between leptin and depression was mediated by adiposity. BMI accounted for 61% of the variance in leptin levels, and was a significant mediator in the association of BDI-II scores and leptin. Similarly, waist circumference was independently associated with leptin levels. Still, secondary analyses revealed a borderline significant interaction of obesity and depressive symptoms, such that low to normal weight participants with moderate to severe depression had lower levels of leptin than those with mild or minimal to no depression, while overweight and obese participants with moderate to severe depression had higher levels of leptin than those with mild or minimal to no depression. These results demonstrate that the association between depression and leptin varies based on obesity status.
Several neuropeptide systems are involved in the regulation of mood and body weight, and the hypothalamic-pituitary (HPA) axis seems to play a key role in the association of depression and obesity. The negative feedback response to cortisol mediated by the glucocorticoid receptor appears to be impaired in depressed patients, leading to a dysregulated HPA axis (22). Cortisol acts directly on adipose tissue, and increases leptin synthesis and secretion in humans. Leptin itself is considered a vital element in HPA regulation (23). Leptin is expressed in both the hypothalamus and pituitary gland, where it modulates corticotropin-releasing hormone (CRH) and adrenocorticotropin releasing hormone (ACTH), acting in an autocrine-paracrine manner to suppress the appetite stimulating effects of glucocorticoids (24).
It remains uncertain whether depressive symptoms lead to behaviors that promote weight gain, and the inflammation related to obesity further promotes depressive symptoms, or whether adipokines such as leptin upregulate the expression of inflammatory molecules that promote depressive symptoms. Miller et al. explored the inter-relationships between depression, adiposity, and inflammation in 100 young adults, 50 of whom were depressed while 50 served as healthy controls (25, 26). The authors found that depression was associated with increased expression of inflammatory markers, and that increased body mass was partially responsible for this relationship (25). Furthermore, the authors found a joint pathway model which suggested that depressive symptoms promote weight gain, which in turn activates an inflammatory response through at least two distinct pathways (26). The first pathway involves increased release of interleukin-6 (IL-6) from expanded adipose tissue, which in turn promotes increased expression of CRP. In addition, expanded adipose tissue contributes to elevated levels of leptin. Leptin in turn binds to its receptor on leukocytes and vascular endothelial cells, upregulating the release of IL-6, and CRP further downstream. Notably, the findings did not support a sickness behavior model whereby the inflammation arising from adiposity and leptin produced depressive symptoms. Previous work from our group and others has shown an association between CRP and depressive symptoms (27). Although CRP was significantly associated with leptin levels and depressive symptoms in our analysis, it did not account for the association between leptin and depressive symptoms.
Similar to our findings, higher levels of leptin have been noted in depressed patients compared to healthy controls in several previous studies. Zeman et al. noted higher levels of leptin in 38 depressed women compared with 38 healthy controls (28). Pasco et al. noted higher leptin levels in 83 depressed women compared with 427 non-depressed women (29). Moreover, women with incident depression over five years tended to have a higher BMI; however, elevated serum leptin predicted the development of depression even after adjusting for BMI. Similarly, Gecici et al reported higher levels of leptin in 57 patients with atypical depression compared to 27 healthy controls (30). In contrast, several other studies have noted lower levels of leptin in depressed patients. Kraus et al. noted lower levels of leptin in 62 patients with clinical depression compared to 64 healthy controls (15). In their analysis, there was no significant difference in BMI between the depressed patients (24.6±4.2) and the control patients (22.8±3.0); notably, this appeared to be a predominantly normal weight population and is consistent with our findings in normal weight individuals in our cohort. Jow et al. also noted lower levels of plasma leptin in depressed patients compared to healthy controls (31). Notably, the depressed patients in this analysis had lower BMI than both the schizophrenics and the healthy controls. Thus, although seemingly contradictory, our findings are similar to previous analyses in that depressed patients with low to normal BMI display lower levels of leptin; additionally, similar to the findings of Pasco et al.(29), we found that depressed patients with overweight and/or obesity display higher levels of leptin.
Recently, Lu et al. proposed an explanation for the seemingly contradictory findings of both low and high leptin levels being associated with depressive symptoms (32). Although low leptin levels have been associated with depression, leptin insufficiency may occur in only a subset of depressed patients. In other patients, the high leptin levels observed with obesity may be indicative of leptin resistance. Leptin resistance could occur at several levels, including impaired transport of leptin across the blood-brain barrier, reduced function of the leptin receptor, and defects in signal transduction (33). Thus, despite higher levels of leptin in obese patients, functional leptin resistance could contribute to a similar phenotype seen in those who are leptin insufficient. These data, along with our own findings, suggest that it may not be the absolute concentration of leptin that is correlated with mood, but rather the ability of the hormone to induce an effect at the receptor or postreceptor level (9).
Recent experiments in mouse models confirm the hypothesis that the effect of leptin at the receptor level may be more important than its absolute concentration (34). In normal weight mice who exhibit depressive behavior, administration of leptin demonstrates anti-depressive effect and increases expression of neuronal activation markers, in the hippocampus. However in obese mice whose leptin levels were higher than normal weight mice, leptin neither induced anti-depressive effect, nor did it increase expression of activation markers in the hippocampus. Furthermore, diet substitution and weight loss in the obese mice normalized leptin levels, and restored the anti-depressive effect of leptin.
Previous studies examining the relationship between serum adiponectin levels and depression have produced conflicting results. Lehto et al. observed lower levels of adiponectin in 70 patients with major depression compared to healthy controls (35). Similarly, Leo et al. noted that patients with major depression displayed lower adiponectin levels compared to healthy controls, and that adiponectin significantly correlated with depression severity (36). Other studies, however, have found no association between depressive symptoms and adiponectin (37, 38). Similarly, we did not find an association between adiponectin and depression in our sample, and further investigation is needed to clarify the role of adiponectin in mood disorders.
We acknowledge a few limitations of our study. First, the study design was cross-sectional, thus temporal and/or causal associations between depression, adiposity, and leptin could not be examined. Second, depressive symptoms were obtained by self-report. However, the use of clinically trained interviewers to ascertain diagnoses of depression is prohibitively cumbersome in large community surveys. Furthermore, the diagnostic agreement of the BDI-II and psychiatric clinical interview is high (18). Third, the overall prevalence of moderate to severe depression was low in our population, and we lacked information on a clinical diagnosis of major depression or other axis I psychiatric conditions. Furthermore, we lacked information on the use of psychoactive medications, which have been shown to affect leptin levels in previous analyses (39). Finally, we lacked information on alcohol consumption, as elevated levels of leptin have been associated with alcohol dependence (9, 40, 41).
In conclusion, we have found that the association of depression and leptin is mediated by adiposity in this community-based sample. The correlation between depressive symptoms and obesity may occur through dysregulation of the HPA axis, and this relationship may further depend on obesity. Our results suggest that future investigations should focus on the ability of leptin to induce effects at the receptor level in order to truly understand its association with mood. The implications of our findings are that leptin as a potential treatment for depression may have different effects in patients whose depression is related to leptin insufficiency, compared to those whose depressive symptoms are related to leptin resistance. Further research is needed to investigate these relationships.
Acknowledgments
The authors thank the META-Health study population, and Emory and Morehouse GCRC staff for their assistance and participation. Sources of funding: This work was supported by funding from NIH/NHLBI 1 U01 HL079156-01 (Quyyumi) and 1 U01 HL79214-01 (Gibbons); NIH, National Center for Research Resources (NCRR) Grant M01-RR00039 for the Emory General Clinical Research Center (GCRC) and NIH/NCRR 5P20RR11104 for the Morehouse CRC; NIH K24HL077506-06 (Vaccarino); and NIH/NCRR 5U54RR022814 (Din).
ABBREVIATIONS
- BDI-II
Beck Depression Inventory II
- BMI
body mass index
- HDL-C
high density lipoprotein cholesterol
Footnotes
Financial Disclosures: The authors have no financial conflicts of interest to disclose.
References
- 1.The World Health Organization. The global burden of disease: 2004 update, Table A2: Burden of disease in DALYs by cause, sex and income group in WHO regions, estimates for 2004. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 2.Brennan AM, Mantzoros CS. Drug Insight: the role of leptin in human physiology and pathophysiology[mdash]emerging clinical applications. Nat Clin Pract End Met. 2006;2:318–27. doi: 10.1038/ncpendmet0196. [DOI] [PubMed] [Google Scholar]
- 3.Chan JL, Mantzoros CS. Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. The Lancet. 2005;366:74–85. doi: 10.1016/S0140-6736(05)66830-4. [DOI] [PubMed] [Google Scholar]
- 4.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, Klein AS, Bulkley GB, Bao C, Noble PW, Lane MD, Diehl AM. Leptin regulates proinflammatory immune responses. The FASEB Journal. 1998;12:57–65. [PubMed] [Google Scholar]
- 5.Matarese G, Moschos S, Mantzoros CS. Leptin in Immunology. The Journal of Immunology. 2005;174:3137–42. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 6.Santos-Alvarez J, Goberna R, Sánchez-Margalet V. Human Leptin Stimulates Proliferation and Activation of Human Circulating Monocytes. Cellular Immunology. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 7.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is Obesity Associated with Major Depression? Results from the Third National Health and Nutrition Examination Survey. American Journal of Epidemiology. 2003;158:1139–47. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 8.Heo M, Pietrobelli A, Fontaine KR, Sirey JA, Faith MS. Depressive mood and obesity in US adults: comparison and moderation by sex, age, and race. Int J Obes. 2005;30:513–9. doi: 10.1038/sj.ijo.0803122. [DOI] [PubMed] [Google Scholar]
- 9.Zupancic ML, Mahajan A. Leptin as a Neuroactive Agent. Psychosomatic Medicine. 2011;73:407–14. doi: 10.1097/PSY.0b013e31821a196f. [DOI] [PubMed] [Google Scholar]
- 10.Lu X-Y, Kim CS, Frazer A, Zhang W. Leptin: A potential novel antidepressant. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1593–8. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu X-Y. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Current Opinion in Pharmacology. 2007;7:648–52. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim CS, Huang T-Y, Garza J, Ramos F, Frazer A, Liu F, Lu XY. Leptin induces antidepressant-like effects and activates specific signal transduction pathways in the hippocampus and amygdala of mice. Neuropsychopharmacology. 2006;31:S237–8. [Google Scholar]
- 13.Antonijevic IA, Murck H, Frieboes RM, Horn R, Brabant G, Steiger A. Elevated nocturnal profiles of serum leptin in patients with depression. Journal of Psychiatric Research. 1998;32:403–10. doi: 10.1016/s0022-3956(98)00032-6. [DOI] [PubMed] [Google Scholar]
- 14.Gecici O, Kuloglu M, Atmaca M, Tezcan AE, Tunckol H, EmÜL HM, Ustundag B. High serum leptin levels in depressive disorders with atypical features. Psychiatry and Clinical Neurosciences. 2005;59:736–8. doi: 10.1111/j.1440-1819.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- 15.Kraus T, Haack M, Schuld A, Hinze-Selch D, Pollmacher T. Low Leptin Levels but Norma Body Mass Indices in Patients with Depression or Schizophrenia. Neuroendocrinology. 2001;73:243–7. doi: 10.1159/000054641. [DOI] [PubMed] [Google Scholar]
- 16.Guey-Mei J, Tsung-Tsair Y, Chun-Lan C. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. Journal of affective disorders. 2006;90:21–7. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Deuschle M, Blum WF, Englaro P, Schweiger U, Weber B, Pflaum CD, Heuser I. Plasma Leptin in Depressed Patients and Healthy Controls. Horm Metab Res. 1996;28:714, 7. doi: 10.1055/s-2007-979885. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in Psychiatric Outpatients. Journal of Personality Assessment. 1996;67:588. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 19.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, Inflammation, and Incident Cardiovascular Disease in Women With Suspected Coronary Ischemia: The National Heart, Lung, and Blood Institute-Sponsored WISE Study. Journal of the American College of Cardiology. 2007;50:2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 20.Preacher K, Hayes A. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, and Computers. 2004;36:717–31. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 21.Hayes A. Sobel Macros and Codes for SPSS and SAS. 2011 Apr 4; http://www.afhayes.com/spss-sas-and-mplus-macros-and-code.html.
- 22.Himmerich H, Zimmermann P, Ising M, Kloiber S, Lucae S, Kunzel HE, Binder EB, Holsboer F, Uhr M. Changes in the Hypothalamic-Pituitary-Adrenal Axis and Leptin Levels during Antidepressant Treatment. Neuropsychobiology. 2007;55:28–35. doi: 10.1159/000103573. [DOI] [PubMed] [Google Scholar]
- 23.Bornstein SR, Schuppenies A, Wong ML, Licinio J. Approaching the shared biology of obesity and depression: the stress axis as the locus of gene-environment interactions. Mol Psychiatry. 2006;11:892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- 24.Malendowicz LK, Rucinski M, Belloni AS, Ziolkowska A, Nussdorfer GG, Kwang WJ. International Review of Cytology. Vol. 263. Academic Press; 2007. Leptin and the Regulation of the Hypothalamic-Pituitary-Adrenal Axis; pp. 63–102. [DOI] [PubMed] [Google Scholar]
- 25.Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. The American Journal of Cardiology. 2002;90:1279–83. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- 26.Miller GE, Freedland KE, Carney RM, Stetler CA, Banks WA. Pathways linking depression, adiposity, and inflammatory markers in healthy young adults. Brain, Behavior, and Immunity. 2003;17:276–85. doi: 10.1016/s0889-1591(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 27.Morris AA, Zhao L, Ahmed Y, Stoyanova N, De Staercke C, Hooper W, Gibbons G, Din-Dzietham R, Quyyumi A, Vaccarino V. Association between depression and inflammation--differences by race and sex: the META-Health study. Psychosom Med. 2011;73:462–68. doi: 10.1097/PSY.0b013e318222379c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeman M, Jirak R, Jachymova M, Vecka M, Tvrizicka E, Zak A. Leptin, adiponectin, leptin to adiponectin ratio and insulin resistance in depressive women. Neuro Endocrinol Lett. 2009;30:387–95. [PubMed] [Google Scholar]
- 29.Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, Berk M. Leptin in depressed women: Cross-sectional and longitudinal data from an epidemiologic study. Journal of affective disorders. 2008;107:221–5. doi: 10.1016/j.jad.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Gecici O, Kuloglu M, Atmaca M, Tezcan AE, Tunckol H, Emul HM, Ustundag B. High serum leptin levels in depressive disorders with atypical features. Psychiatry and Clinical Neurosciences. 2005;59:736–8. doi: 10.1111/j.1440-1819.2005.01445.x. [DOI] [PubMed] [Google Scholar]
- 31.Jow GM, Yang TT, Chen CL. Leptin and cholesterol levels are low in major depressive disorder, but high in schizophrenia. Journal of affective disorders. 2006;90:21–7. doi: 10.1016/j.jad.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Lu XY. The leptin hypothesis of depression: a potential link between mood disorders and obesity? Current Opinion in Pharmacology. 2007;7:648–52. doi: 10.1016/j.coph.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munzberg H, Myers MG. Molecular and anatomical determinants of central leptin resistance. Nat Neurosci. 2005;8:566–70. doi: 10.1038/nn1454. [DOI] [PubMed] [Google Scholar]
- 34.Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, Nakao K. Impaired CNS Leptin Action Is Implicated in Depression Associated with Obesity. Endocrinology. 2011;152:2634–43. doi: 10.1210/en.2011-0004. [DOI] [PubMed] [Google Scholar]
- 35.Lehto SM, Huotari A, Niskanen L, Tolmunen T, Koivumaa-Honkanen H, Honkalampi K, Ruotsalainen H, Herzig KH, Viinamäki H, Hintikka J. Serum adiponectin and resistin levels in major depressive disorder. Acta Psychiatrica Scandinavica. 2010;121:209–15. doi: 10.1111/j.1600-0447.2009.01463.x. [DOI] [PubMed] [Google Scholar]
- 36.Leo R, Di Lorenzo G, Tesauro M, Cola C, Fortuna E, Zanasi M, Troisi A, Siracusano A, Lauro R, Romeo F. Decreased plasma adiponectin concentration in major depression. Neuroscience Letters. 2006;407:211–13. doi: 10.1016/j.neulet.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 37.Pan A, Ye X, Franco OH, Li H, Yu Z, Wang J, Qi Q, Gu W, Pang X, Liu H, Lin X. The Association of Depressive Symptoms with Inflammatory Factors and Adipokines in Middle-Aged and Older Chinese. PLoS One. 2008;3:e1392. doi: 10.1371/journal.pone.0001392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mamalakis G, Kiriakakis M, Tsibinos G, Hatzis C, Flouri S, Mantzoros C, Kafatos A. Depression and serum adiponectin and adipose omega-3 and omega-6 fatty acids in adolescents. Pharmacology Biochemistry and Behavior. 2006;85:474–79. doi: 10.1016/j.pbb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Kraus T, Haack M, Schuld A, Hinze-Selch D, Kühn M, Uhr M, Pollmächer T. Body Weight and Leptin Plasma Levels During Treatment With Antipsychotic Drugs. Am J Psychiatry. 1999;156:312–4. doi: 10.1176/ajp.156.2.312. [DOI] [PubMed] [Google Scholar]
- 40.Nicolás JM, Fernández-Solà J, Fatjó F, Casamitjana R, Bataller R, Sacanella E, Tobías E, Badía E, Estruch R. Increased Circulating Leptin Levels in Chronic Alcoholism. Alcoholism: Clinical and Experimental Research. 2001;25:83–8. [PubMed] [Google Scholar]
- 41.Kiefer F, Jahn H, Jaschinski M, Holzbach R, Wolf K, Naber D, Wiedemann K. Leptin: a modulator of alcohol craving? Biological Psychiatry. 2001;49:782–7. doi: 10.1016/s0006-3223(01)01081-2. [DOI] [PubMed] [Google Scholar]