Abstract
Racial disparities persist in access to renal transplantation in the United States, but the degree to which patient and neighborhood socioeconomic status (SES) impacts racial disparities in deceased donor renal transplantation access has not been examined in the pediatric and adolescent end-stage renal disease (ESRD) population. We examined the interplay of race and SES in a population-based cohort of all incident pediatric ESRD patients <21 years from the United States Renal Data System from 2000 to 2008, followed through September 2009. Of 8 452 patients included, 30.8%were black, 27.6% white-Hispanic, 44.3% female and 28.0% lived in poor neighborhoods. A total of 63.4% of the study population was placed on the waiting list and 32.5% received a deceased donor transplant. Racial disparities persisted in transplant even after adjustment for SES, where minorities were less likely to receive a transplant compared to whites, and this disparity was more pronounced among patients 18–20 years. Disparities in access to the waiting list were mitigated in Hispanic patients with private health insurance. Our study suggests that racial disparities in transplant access worsen as pediatric patients transition into young adulthood, and that SES does not explain all of the racial differences in access to kidney transplantation.
Keywords: Access to transplantation, health insurance, kidney transplant waiting list, neighborhood poverty, pediatric kidney transplantation, racial and ethnic disparities, United States Renal Data System
Introduction
Kidney transplantation is the optimal treatment for end-stage renal disease (ESRD) patients and is associated with increased quality of life and reduced morbidity and mortality compared to dialysis (1). However, the current demand for organs is higher than the supply (2). Despite a nationally regulated system for organ allocation, racial disparities exist in various stages of the renal transplant process in the United States, including referral, evaluation, waitlisting and organ receipt (3–5).
Most studies examining racial disparities in access to deceased donor kidney transplantation have focused on adults, but the few that have examined the pediatric ESRD population have identified similar disparities (6,7). Furth et al. examined racial differences in access to the waiting list among incident pediatric ESRD patients 1988–1996, finding that black patients were 12% less likely to waitlist than whites. Previous research suggests that these disparities are not entirely explained by clinical factors (8–10), and that socioeconomic variations may contribute to racial differences (11). Among adults, reduced access to renal transplant has been reported among minority patients living in high poverty neighborhoods (12–14), those with public insurance and lower educational attainment (15).
The degree to which individual and neighborhood-level socioeconomic factors might contribute to racial disparities observed in access to both waitlisting and deceased donor renal transplantation has not been well described among the national pediatric ESRD population. It has been well documented that adolescents and young adults have an increased risk of graft failure, with black adolescents identified as a particularly high risk group (2,16). The purpose of this study was to determine whether racial differences exist in access to transplant in a national pediatric ESRD cohort and to investigate how these disparities are influenced by socioeconomic status (SES). We considered incident ESRD patients until the age of 21 years because we hypothesized that racial differences in access to the waiting list and transplantation might differ in this particular age subset compared with younger pediatric ESRD patients, not only due to the influence of allocation policies but also perhaps in consideration of practice variation in the provider decision-making process.
Materials and Methods
Study population and data sources
Incident, pediatric (age < 21 years) ESRD patients who entered the Medicare ESRD program between January 2000 through September 2008, followed through September 2009, from United States Renal Data System (USRDS)were included in this analysis (2). Demographic data were obtained from the Centers for Medicare and Medicaid Services (CMS) Form-2728, completed on all incident ESRD patients. Follow-up data on waitlisting and deceased donor transplant were obtained from United Network for Organ Sharing (UNOS) files. Data on neighborhood poverty were obtained from Census 2000.
There were 11 458 incident ESRD patients < 21 years who entered the ESRD program from 2000 through September 2008. Patients were excluded if they were missing residential zip code (n = 595), received a previous renal transplant (n = 601), or their race/ethnicity was reported as other than black, white Hispanic, or white non-Hispanic (n = 999). Patients transplanted with living donor transplants without prior dialysis or waitlisting (i.e. preemptively transplanted) were excluded (n = 811). A total of 8452 pediatric ESRD patients were included in the final study population.
Study variables
The primary outcome was time (in days) from ESRD start to receipt of deceased donor renal transplant. We further examined two distinct steps: (1) time from ESRD start to waitlisting and (2) time from waitlisting to transplant. A total of 845 patients were placed on the waiting list before initiating dialysis and were defined as preemptively waitlisted; these patients were included in analyses and their time to waitlisting was counted as one day. For all time to event analyses, patients were censored at death, living donor transplant or end of study (September 30, 2009).
The primary exposure variable was CMS 2728-reported race/ethnicity, defined as non-Hispanic white (white), white-Hispanic (Hispanic) or non-Hispanic black (black). We defined SES using two variables: health insurance reported on the CMS 2728 form as a proxy for individual SES and residential zip code-level poverty at the time of ESRD start as a proxy for neighborhood SES. Health insurance was categorized as private (employer), public (Medicaid, Medicare, Veterans Affairs or combination), other or none. Neighborhood poverty was estimated as the proportion of individuals residing below the federal poverty level within a five-digit zip code.
Demographic and clinical covariates obtained from the CMS 2728 form included patient age (years), sex, organ procurement region (1–11), etiology of ESRD (glomerulonephritis, secondary glomerulonephritis, cystic/hereditary disease or other), BMI >85th percentile and clinical variables, including predialysis erythropoiesis-stimulating agent (ESA) use (yes/no), hemoglobin (<11 g/dL vs. ≥ 11 g/dL) and serum albumin (<3.5 g/dL vs. ≥ 3.5 g/dL).
For waitlisted patients, blood type (A, B, AB, or O) and peak panel reactive antibody (PPRA) (0, 1–19.9% and ≥ 20%) were obtained from UNOS. Because the UNOS ‘Share 35’ policy was enacted on September 28, 2005 to prioritize allocation of young (<35 years) deceased donor organs to patients less than 18 years, we categorized our cohort into two eras: pre-share 35 versus share 35 era. We examined whether a patient was inactive (yes/no) on the waiting list using UNOS status codes “4099” (temporarily inactive) and “4999” (old temporarily inactive).
Data analysis
Chi-square tests and t-tests (or nonparametric equivalents of the t-test) were used to compare demographic and clinical characteristics by race. To examine whether racial differences exist in transplant access, we examined each step using Kaplan–Meier methods and by fitting separate Cox proportional hazards regression models to obtain cause-specific hazard ratios (HR) and corresponding 95% confidence intervals (CI) for deceased donor transplant. Pediatric (age 0–17) and young adult (18–20) ESRD patients were analyzed separately in multivariable analyses due to the preferential allocation of patients <18 years under current kidney allocation policies.
To assess whether racial disparities varied across SES for waitlisting and deceased donor transplant outcomes, we examined interactions between race, health insurance and neighborhood poverty in adjusted Cox models using the likelihood ratio test to assess statistical significance (17). To determine whether SES explained the impact of race on kidney transplant waitlisting and organ receipt, we examined the effect of sequentially adjusting for patient and demographic factors then SES factors in Cox models. If interaction was not detected, pooled hazard ratios were calculated. For all Cox models, both patient- and zip-code level variables were considered as potential confounders. We evaluated confounding by comparing meaningful changes in point estimates from a full model containing all a priori covariates to all other potential models (18,19), and by examining directed acyclic graphs (20). We used the robust sandwich variance estimator using zip code as the cluster variable to examine neighborhood poverty and individual level covariates simultaneously while accounting for potential correlation of patients within neighborhoods (21). In multivariable analyses, we used the Markov Chain Monte Carlo method for multiple imputation methods for missing covariate information.
Sensitivity analyses
We included living donor transplant recipients in our main analyses and censored patients at the time of organ receipt. However, because death and living donor transplantation may preclude a patient from waitlisting or transplantation, we explored the effect of living donor transplant in several ways. We used competing risk Cox proportional hazards models to calculate cause-specific hazard ratios for living donor transplant and death (22). We also examined the effect of excluding all living donor transplant recipients from analyses and considering both living and deceased donor transplants as events of interest.
To assess whether inactive waitlisting influenced access to deceased donor transplantation, we excluded patients who were listed inactive at any given time (n = 916). Finally, we examined the impact of excluding patients with “other” health insurance (n = 1 199) since this group is a potentially heterogenous group of insured patients. Two-tailed p-values <0.05 were considered statistically significant. All analyses were performed with SAS software version 9.2. The Emory University Institutional Review Board approved this study.
Results
Study population
Among the 8452 pediatric (<21 years) incident ESRD patients included in this study, the mean age was 13.4 ± 6.2 years, 41.7% were white, 27.6% were Hispanic, 30.8% were black, 44.7% had public insurance and 28.0% lived in impoverished communities (Table 1). Racial differences in baseline clinical and demographic factors were evident. Minorities were significantly older compared to whites and less likely to receive ESAs prior to dialysis. Compared to whites, Hispanics and blacks were more likely to have public or no health insurance. Similarly, minorities had a fourfold higher likelihood of living in poor neighborhoods (p < 0.0001 for all comparisons) (Table 1). The distribution of neighborhood poverty was similar among patients aged 0–17 and 18–20, but patients aged 18–20 were significantly more likely to be uninsured (20.9% of whites, 33.8% of Hispanics and 25.4% of blacks) than patients aged 0–17 (3.5% of whites, 13.0% of Hispanics and 4.2% of blacks) (data not shown). Waitlisted patients differed demographically and clinically by race. A greater proportion of blacks were older, had higher BMI at listing, PPRA ≥ 20% and B blood group compared to whites (Table 2). Among transplanted patients, blacks were older and more likely to have B blood type, public health insurance and to live in the most impoverished neighborhoods compared to whites. Similar SES differences were noted among Hispanics compared to whites (Table 3).
Table 1.
Baseline characteristics of study population at ESRD start by race
| Study population N = 8452 |
White N = 3521 (41.7%) |
Hispanic N = 2330 (27.6%) |
Black N = 2601 (30.8%) |
p-Value | |
|---|---|---|---|---|---|
| Patient-level characteristics | |||||
| Age, mean (SD), years | 13.4 ± 6.2 | 12.4 ± 6.7 | 13.5 ± 5.9 | 14.8 ± 5.5 | <.001 |
| Age category, N (%), years | <0.001 | ||||
| <1 year | 631 (7.5%) | 386 (11.0%) | 131 (5.6%) | 114 (4.4%) | |
| 1–5 years | 679 (8.0%) | 353 (10.0%) | 184 (7.9%) | 142 (5.5%) | |
| 6–10 years | 893 (10.6%) | 399 (11.3%) | 287 (12.3%) | 207 (8.0%) | |
| 11–17 years | 3373 (39.9%) | 1316 (37.4%) | 977 (41.9%) | 1080 (41.5%) | |
| 18–20 years | 2876 (34.0%) | 1067 (30.3%) | 751 (32.2%) | 1058 (40.7%) | |
| Female, N (%) | 746 (44.3%) | 1542 (43.8%) | 1007 (43.3%) | 1197 (46.0%) | 0.101 |
| Cause of ESRD, N (%) | <0.001 | ||||
| GN1 | 1172 (13.9%) | 486 (13.8%) | 398 (17.1%) | 288 (11.1%) | |
| Secondary GN | 481 (5.7%) | 309 (8.8%) | 94 (4.0%) | 78 (3.0%) | |
| Cystic/hereditary | 2213 (26.2%) | 1092 (33.9%) | 567 (24.3%) | 454 (17.5%) | |
| FSGS2 | 1162 (13.8%) | 360 (10.2%) | 255 (10.9%) | 547 (21.0%) | |
| Lupus nephritis | 655 (7.8%) | 122 (3.5%) | 169 (7.3%) | 364 (14.0%) | |
| Other | 2769 (32.8%) | 1052 (29.8%) | 847 (36.4%) | 870 (33.5%) | |
| Health insurance coverage | <0.001 | ||||
| Public | 3779 (44.7%) | 1232 (35.0%) | 1153 (49.5%) | 1394 (53.6%) | |
| Private | 2474 (29.0%) | 1491 (42.4%) | 379 (16.3%) | 604 (23.2%) | |
| Other | 1199 (14.2%) | 533 (15.1%) | 363 (15.6%) | 303 (11.7%) | |
| None | 1000 (11.8%) | 265 (7.5%) | 435 (18.7%) | 300 (11.5%) | |
| Share 35 policy era | 1852 (21.9%) | 762 (21.6%) | 585 (25.1%) | 505 (19.4%) | <0.001 |
| Clinical and laboratory measures | |||||
| BMI > 85%3 | 1073 (12.7%) | 350 (9.9%) | 240 (10.3%) | 483 (18.6%) | <0.001 |
| Albumin < 3.5 g/dL* | 5571 (65.9%) | 2253 (64.0%) | 1465 (62.8%) | 1853 (71.2%) | <0.001 |
| Hemoglobin < 11 g/dL* | 6474 (76.6%) | 2541 (72.2%) | 1816 (77.9%) | 2117 (81.4%) | <0.001 |
| Predialysis ESA4* | 2982 (35.3%) | 1405 (39.9%) | 773 (33.2%) | 804 (30.9%) | <0.001 |
| Zip code-level characteristics for patient residence at ESRD start | |||||
| Neighborhood poverty (% zip below poverty) | <0.001 | ||||
| 0–4.9% | 1027 (12.2%) | 755 (21.4%) | 114 (4.9%) | 158 (6.1%) | |
| 5–9.9% | 2116 (25.0%) | 1213 (34.5%) | 421 (18.1%) | 482 (18.5%) | |
| 10–14.9% | 1670 (19.8%) | 768 (21.8%) | 432 (18.5%) | 470 (18.1%) | |
| 15–19.9% | 1271 (15.0%) | 425 (12.1%) | 428 (18.4%) | 418 (16.1%) | |
| >20% | 2368 (28.0%) | 360 (10.2%) | 935 (40.1%) | 1073 (41.3%) | |
Glomerulonephritis.
Focal segmental glomerulosclerosis.
Body mass index.
Erythropoiesis-stimulating agent.
Missing data: 16.7% missing albumin, 7.4% missing hemoglobin, 4.7% missing ESA use.
Table 2.
Characteristics of patients who were waitlisted for renal transplantation
| Waitlisted population N = 5356 |
White N = 2082 (38.9%) |
Hispanic N = 1605 (30.0%) |
Black N = 1669 (31.2%) |
p-Value | |
|---|---|---|---|---|---|
| Patient level characteristics | |||||
| Age at listing, mean (SD), years | 14.5 ± 5.9 | 13.8 ± 6.2 | 14.2 ± 5.9 | 15.7 ± 5.4 | <0.001 |
| Age at listing category, N (%), years | <0.001 | ||||
| <1 year | 51 (0.9%) | 30 (1.4%) | 17 (1.1%) | 4 (0.2%) | |
| 1–5 years | 577 (10.8%) | 272 (13.1%) | 181 (11.3%) | 124 (7.4%) | |
| 6–10 years | 557 (10.4%) | 250 (12.0%) | 176 (11.0%) | 131 (7.9%) | |
| 11–17 years | 2196 (41.0%) | 825 (39.6%) | 693 (43.2%) | 678 (40.6%) | |
| 18–20 years | 1368 (25.5%) | 509 (24.5%) | 373 (23.2%) | 486 (29.1%) | |
| 21–29 | 607 (11.3%) | 196 (9.4%) | 165 (10.3%) | 246 (14.7%) | |
| Female, N (%) | 2329 (43.5%) | 898 (43.1%) | 698 (43.5%) | 733 (43.9%) | 0.890 |
| Health insurance coverage at time of ESRD start, N (%) | <0.001 | ||||
| Public | 2368 (44.2%) | 709 (34.1%) | 821 (51.2%) | 838 (50.2%) | |
| Private | 1643 (30.7%) | 892 (42.8%) | 296 (18.4%) | 455 (27.3%) | |
| Other | 809 (15.1%) | 332 (16.0%) | 266 (16.6%) | 211 (12.6%) | |
| None | 536 (10.0%) | 149 (7.2%) | 222 (13.8%) | 165 (9.9%) | |
| Share 35 era | 975 (18.2%) | 385 (18.5%) | 351 (21.9%) | 239 (14.3%) | <0.001 |
| Pre-emptive waitlist | 845 (15.8%) | 456 (21.9%) | 175 (10.9%) | 214 (12.8%) | <0.001 |
| Inactive waitlist | 916 (17.1%) | 355 (17.1%) | 265 (16.5%) | 296 (17.7%) | 0.647 |
| Clinical and laboratory measures | |||||
| BMI> 85% at listing | 407 (7.6%) | 154 (7.4%) | 86 (5.4%) | 167 (10.0%) | <0.001 |
| Peak panel reactive antibody at time of listing, N (%) | <0.001 | ||||
| 0% | 3597 (69.6%) | 1442 (71.9%) | 1087 (70.9%) | 1068 (65.6%) | |
| 1–20% | 1061 (20.5%) | 395 (19.7%) | 315 (20.5%) | 351 (21.6%) | |
| >20% | 509 (9.9%) | 168 (8.1%) | 132 (8.2%) | 209 (12.5%) | |
| Missing | 77 (3.7%) | 71 (4.4%) | 41 (2.5%) | 41 (2.5%) | |
| ABO blood group, N (%) | <0.001 | ||||
| A | 1734 (32.4%) | 834 (40.1%) | 437 (27.2%) | 463 (27.7%) | |
| B | 682 (12.7%) | 215 (10.3%) | 152 (9.5%) | 315 (18.9%) | |
| AB | 165 (3.1%) | 67 (3.2%) | 44 (2.7%) | 54 (3.2%) | |
| O | 2775 (51.8%) | 966 (46.4%) | 972 (60.6%) | 837 (50.2%) | |
| Zip code-level characteristics for patient residence at ESRD start | |||||
| Neighborhood poverty (% zip below poverty), N (%) | <0.001 | ||||
| 0–4.9% | 639 (11.9%) | 452 (21.7%) | 70 (4.4%) | 117 (7.0%) | |
| 5–9.9% | 1322 (24.7%) | 712 (34.2%) | 279 (17.4%) | 331 (19.8%) | |
| 10–14.9% | 1037 (19.4%) | 439 (21.1%) | 292 (18.2%) | 306 (18.3%) | |
| 15–19.9% | 821 (15.3%) | 257 (12.3%) | 299 (18.6%) | 265 (15.9%) | |
| 20% | 1537 (28.7%) | 222 (10.7%) | 665 (41.4%) | 650 (39.0%) | |
Table 3.
Characteristics of patients who received a deceased donor transplant
| Transplanted population N = 2747 |
White n = 995 (36.2%) |
Hispanic n = 849 (30.9%) |
Black N = 903 (32.9%) |
p-Value | |
|---|---|---|---|---|---|
| Patient level characteristics | |||||
| Age, mean ± SD | 14.8 ± 6.0 | 14.4 ± 6.1 | 14.1 ± 5.9 | 16.0 ± 5.7 | <0.001 |
| Age at transplant category, N (%), years | <0.001 | ||||
| < 1 year | 2 (0.1%) | 1 (0.1%) | 1 (0.1%) | 0 (0%) | |
| 1–5 years | 278 (10.1%) | 116 (11.7%) | 101 (11.9%) | 61 (6.7%) | |
| 6–10 years | 320 (11.7%) | 131 (13.2%) | 114 (13.4%) | 75 (8.3%) | |
| 11–17 years | 1269 (46.2%) | 434 (43.6%) | 409 (48.2%) | 426 (47.2%) | |
| 18–20 years | 392 (14.3%) | 143 (14.4%) | 114 (13.4%) | 135 (15.0%) | |
| 21–29 years | 486 (17.7%) | 170 (17.1%) | 110 (13.0%) | 206 (22.8%) | |
| Female, N (%) | 1222 (44.5%) | 456 (45.8%) | 380 (44.8%) | 386 (42.8%) | 0.395 |
| Health insurance coverage at time of ESRD start, N (%) | <0.001 | ||||
| Public | 1350 (49.1%) | 388 (39.0%) | 464 (54.7%) | 498 (55.2%) | |
| Private | 784 (28.5%) | 401 (40.3%) | 150 (17.7%) | 233 (25.8%) | |
| Other | 380 (13.8%) | 144 (14.5%) | 135 (15.9%) | 101 (11.2%) | |
| None | 233 (8.5%) | 62 (6.2%) | 100 (11.8%) | 71 (7.9%) | |
| Share 35 era | 573 (20.9%) | 194 (19.5%) | 222 (26.2%) | 157 (17.4%) | <0.001 |
| Pre-emptive waitlist | 300 (10.9%) | 152 (15.3%) | 59 (7.0%) | 89 (9.9%) | <0.001 |
| Inactive waitlist | 145 (5.3%) | 65 (6.5%) | 31 (3.7%) | 49 (5.4%) | 0.022 |
| Clinical and laboratory measures | |||||
| BMI > 85% | 305 (11.1%) | 95 (9.6%) | 63 (7.4%) | 147 (16.3%) | <0.001 |
| Peak panel reactive antibody at time of listing, N (%) | 0.057 | ||||
| 0% | 1897 (69.1%) | 692 (69.6%) | 598 (70.4%) | 607 (67.2%) | |
| 1–20% | 555 (20.2%) | 204 (20.5%) | 159 (18.7%) | 192 (21.3%) | |
| >20% | 220 (8.0%) | 67 (6.7%) | 65 (7.7%) | 88 (9.8%) | |
| Missing | 75 (2.7%) | 32 (3.2%) | 27 (3.2%) | 16 (1.8%) | |
| ABO blood group, N (%) | <0.001 | ||||
| A | 885 (32.2%) | 380 (28.6%) | 243 (28.6%) | 262 (29.0%) | |
| B | 340 (12.4%) | 94 (9.5%) | 79 (9.3%) | 167 (18.5%) | |
| AB | 88 (3.2%) | 37 (3.7%) | 22 (2.6%) | 29 (3.2%) | |
| O | 1431 (52.1%) | 483 (48.5%) | 503 (59.3%) | 445 (49.3%) | |
| Zip code-level characteristics for patient residence at ESRD start | |||||
| Neighborhood poverty (% zip below poverty), N (%) | <0.001 | ||||
| 0–4.9% | 271 (9.9%) | 191 (19.2%) | 25 (2.9%) | 55 (6.1%) | |
| 5–9.9% | 654 (23.8%) | 345 (34.7%) | 140 (16.5%) | 169 (18.7%) | |
| 10–14.9% | 530 (19.3%) | 216 (21.7%) | 144 (17.0%) | 170 (18.8%) | |
| 15–19.9% | 432 (15.7%) | 136 (13.7%) | 167 (19.7%) | 129 (14.3%) | |
| >20% | 860 (31.3%) | 107 (10.8%) | 373 (43.9%) | 380 (42.1%) | |
Overall time from ESRD start to deceased donor transplantation
Overall, 2747 (32.5%) patients received a deceased donor transplant with a median time to transplant of 596 days (interquartile range [IQR] = 272, 984). Table 4 shows the effect of minority race on time to deceased donor transplant by age group. We found no significant interaction between race and individual-level SES or neighborhood-level SES in transplant access (likelihood ratio p > 0.05 for all comparisons). In multivariable-adjusted models, the rate of transplantation was lower among Hispanic and black patients compared to whites. Disparities were greatest among patients 18–20 years of age, where blacks had a 40% lower rate (HR = 0.60; 95% CI: 0.48–0.75) and Hispanics a 38% lower rate (HR = 0.62; 95% CI: 0.48–0.79) of transplant compared to whites. The effect of sequentially adjusting for SES on transplantation had little effect on the adjusted hazard ratios (Table 4, steps 1 and 2, models 1–3).
Table 4.
Multivariable-adjusted hazard ratios for rate of waitlisting (step 1) and rate of deceased donor transplant among waitlisted patients (step 2) by race and age group
| Hazard ratios (95% confidence intervals) | ||
|---|---|---|
| Transplant steps | Age 0–17 | Age 18–20 |
| Steps 1 and 2: Overall time from ESRD start to deceased donor transplantation1 | ||
| Black versus white | ||
| Model 1: Crude | 0.89 (0.80–0.98) | 0.51 (0.42–0.62) |
| Model 2: clinical + demographic factors | 0.88 (0.79–0.98) | 0.55 (0.45–0.68) |
| Model 3: clinical + demographic + SES factors | 0.90 (0.80–1.00) | 0.60 (0.48–0.75) |
| Hispanic versus white | ||
| Model 1: Crude | 0.94 (0.85–1.03) | 0.45 (0.37–0.56) |
| Model 2: clinical + demographic factors | 0.91 (0.82–1.01) | 0.59 (0.47–0.75) |
| Model 3: clinical + demographic + SES factors | 0.91 (0.81–1.02) | 0.62 (0.48–0.79) |
| Step 1: time from ESRD start to waitlisting1 | ||
| Black versus white | ||
| Model 1: Crude | 0.94 (0.87–1.02) | 0.72 (0.65–0.81) |
| Model 2: clinical + demographic factors | 0.92 (0.85–1.01) | 0.78 (0.68–0.84) |
| Model 3: clinical + demographic + SES factors | 0.92 (0.84–1.01) | 0.84 (0.74–0.96) |
| Hispanic versus white: (pooled estimates N/A)* | ||
| Step 2: time from waitlisting to deceased donor transplantation2 | ||
| Black versus white | ||
| Model 1: Crude | 0.84 (0.76–0.93) | 0.53 (0.44–0.64) |
| Model 2: clinical + demographic factors | 0.92 (0.83–1.02) | 0.60 (0.50–0.81) |
| Model 3: clinical + demographic + SES factors | 0.89 (0.80–0.99) | 0.61 (0.49–0.76) |
| Hispanic versus white | ||
| Model 1: Crude | 0.79 (0.71–0.87) | 0.52 (0.42–0.64) |
| Model 2: clinical + demographic factors | 0.98 (0.88–1.09) | 0.65 (0.50–0.85) |
| Model 3: clinical + demographic + SES factors | 0.97 (0.86–1.08) | 0.66 (0.51–0.86) |
Multivariable p-value for interaction between race and insurance p < 0.05 for both age groups.
Model 2 adjusted for age, sex, ESRD etiology, BMI, Share 35 era, OPO region, blood type, ESA, albumin and hemoglobin. Model 3 adjusted for all factors in Model 2 plus zip code poverty and insurance.
Model 2 adjusted for age, sex, ESRD etiology, BMI, Share 35 era, OPO region, blood type, ESA, albumin, hemoglobin and PPRA. Model 3 adjusted for all factors in Model 2 plus zip code poverty and insurance.
Step 1: Time from ESRD to waitlisting
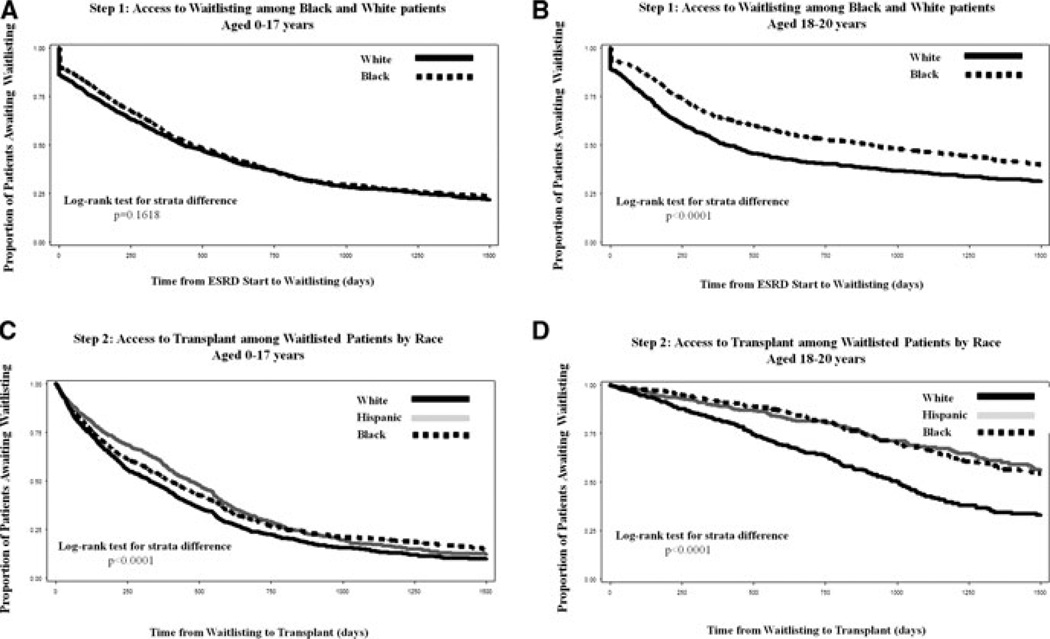
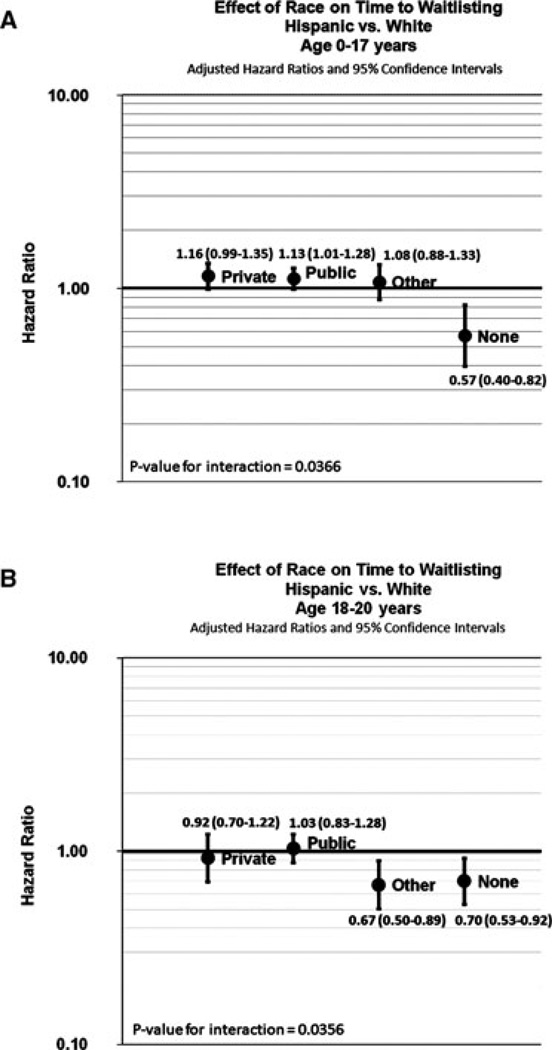
A total of 5356 (63.4%) patients were placed on the deceased donor waiting list throughout the study period, with the median time from ESRD start to listing of 233.5 days (IQR = 72, 516). In crude analyses, black patients 0–17 years had equivalent access (HR = 0.94, 95% CI: 0.85–1.02) but blacks 18–20 years (HR = 0.72; 95% CI; 0.65–0.81) had reduced access to the waiting list compared to whites (Table 4, step 1, model 1; Figure 1, Panel A–B). Adjusting for SES did not meaningfully change these effects (Table 4; model 3). Among Hispanics, however, the relationship between race and waitlisting was modified by health insurance for Hispanic versus white patients (p < 0.0001; Figure 2). Compared to whites with the same type of insurance, Hispanics with private or public health insurance had nearly equal access to waitlisting, but Hispanic patients with no insurance had a lower rate of waitlisting, both for patients aged 0–17 (HR = 0.57; 95% CI: 0.40–0.82) and patients aged 18–20 (HR = 0.70; 95% CI: 0.53–0.92) (Figure 2).
Figure 1. Kaplan–Meier estimates for time to waitlisting (step 1) and deceased donor renal transplantation (step 2) by race/ethnicity and age group.
The rate of waitlisting (step 1) was lower among black versus white patients age 18–20 years (p < 0.0001) and the rate of transplant (step 2) was lower among blacks and Hispanics of all ages (p < 0.0001).
Figure 2. Step 1: Effect of race on time to waitlisting within strata of health insurance.
Panel A shows the multivariable-adjusted effect of time to waitlisting among Hispanics versus whites (age 0–17 years) by health insurance coverage. Panel B shows the multivariable-adjusted effect of time to waitlisting among Hispanics versus whites (age 18–20) by health insurance coverage at the time of ESRD start.
Step 2: Time from waitlisting to deceased donor transplant
Among waitlisted patients, 51.3% received a deceased donor transplant with a median time from listing to transplant of 293 days (IQR = 98, 592). In crude analyses, a lower rate of deceased donor transplantation was observed among black and Hispanic patients (Figure 1, panels C and D). Once a patient was placed on the waiting list, the effect of race on receipt of a deceased donor transplant was not modified by individual or neighborhood SES (p > 0.05) among either race/ethnicities or age groups. After adjusting for demographic, clinical and socioeconomic characteristics, the racial disparity in access to deceased donor transplant among pediatric patients aged 0–17 was attenuated for blacks (HR = 0.89; 95% CI: 0.80–0.99) and eliminated among Hispanics (HR = 0.97; 95% CI: 0.86–1.08). Among patients aged 18–20, racial disparities were more pronounced, with the rate of transplant 39% lower among black (HR = 0.61; 95% CI: 0.49–0.76) and 34% lower among Hispanic (HR = 0.66; 95% CI: 0.51–0.86) versus white patients (Table 4, step 2, model 3). The effect of sequentially adjusting for SES was not different than the effect observed when adjusting for demographic and clinical characteristics only.
Sensitivity analyses
Throughout the study period, 2336 patients (27.6%) were censored due to living donor transplant and 1066 (12.6%) due to death. Minorities received a relatively higher fraction of deceased donor organs than white patients, primarily due to the high proportion of white patients censored for living donor transplantation (59.9% white, 23.4% Hispanic and 16.7% black living donor recipients). In multivariable analyses exploring the cause-specific HR for the effect of race on competing risks of living donor transplant and death, racial disparities were observed in access to living donor transplant, such that blacks were 69% less likely (HR = 0.31; 95% CI: 0.27–0.34) and Hispanics 53% less likely (HR = 0.47; 95% CI: 0.43–0.52) to receive a living donor transplant at any given time compared to whites. Similarly, excluding patients who eventually received living donor transplants (n = 2336) or including living donor transplant as an event of interest resulted in larger racial differences than the main study results (results not shown). No racial differences were observed in mortality among blacks (vs. whites), and Hispanics were 46% less likely to die compared to whites at any given time (HR = 0.54; 95% CI: 0.44–0.65).
Finally, excluding patients (1) who were inactive on the waiting list at any given time during follow up slightly overestimated racial disparities and (2) with “other” health insurance resulted in more pronounced racial disparities for blacks and similar effects among Hispanics, where blacks aged 0–17 had a 15% lower rate, blacks aged 18–20 a 48% lower rate, Hispanics aged 0–17 an 11% lower rate and Hispanics aged 18–20 a 49% lower rate of transplant compared to whites.
Discussion
In this national registry of pediatric ESRD patients, significant racial disparities in access to renal transplantation exist. Compared to white patients, the rate of deceased donor transplantation at any given time was lower among blacks and Hispanics, and disparities were magnified among older pediatric patients. The relationship between race and waitlisting varied by health insurance coverage for Hispanics, such that among uninsured patients, Hispanics were less likely to waitlist at any given time compared to whites. In contrast, racial disparities in waitlisting were diminished among Hispanic patients with private or public insurance. However, once patients were waitlisted, racial disparities persisted across all levels of health insurance for both black and Hispanic patients. Results of this study suggest that racial disparities that are unexplained by clinical factors or SES still exist in pediatric renal transplant access once patients are placed on the deceased donor waiting list, and that racial disparities increase as adolescents transition into young adulthood.
Several studies among adult ESRD patients have documented that the degree of disparity in transplant access is associated with lower SES (12–14,23,24). Similar disparities in access to waitlisting have been previously documented among the pediatric ESRD population. The disparity we observed among black patients with low SES is remarkably similar to what Furth et al. reported in the national pediatric ESRD population more than a decade ago, that black pediatric ESRD patients were 12% less likely to waitlist compared to whites (6).
We found that racial disparities in transplant access increase from pediatric ESRD into early adulthood. The reasons why these disparities increase from pediatrics to young adults are not entirely clear but are likely multifactorial. Clearly the change in allocation benefit at the age of 18 impacts the rate of transplantation, but it is unclear why this impacts racial groups differentially. Patient nonadherence with therapy may be one potential explanation. The risk of nonadherence is particularly high among adolescents, and is higher among patients who are responsible for their own medications compared with those who maintain parental oversight (25) and is more prevalent among black versus white patients (26). In a survey of nephrologists, nonadherence was associated with a lower odds of referral for renal transplant in children and adolescents and this effect was more pronounced among blacks (27). The time of transition from the pediatric to adult healthcare provider is also a period of increased risk for graft failure. While we do not have data on whether patients were cared for by pediatric versus adult providers or transitioned in this age group, certainly transfer of care at the time of ESRD could be problematic and potentially delay transplant evaluation. Physician racial bias may also play a role in that physicians have been reported as less likely to recognize the survival benefit of transplant for black (vs. white) patients (28) and this may be a more prevalent concept in the adult care setting where morbidity and mortality risks for the overall ESRD population are higher compared with pediatric ESRD. Patients’ personal and socioecultural preferences for transplant also may play a role (29). Finally, social networks may influence health behaviors and outcomes (30,31); it is possible that, in our study, minority patients with lower SES and less likelihood of a living donor transplant had limited social support networks.
Our observations that racial disparities in access to the waiting list exist among Hispanics with no health insurance, but are not apparent among those with public or private insurance raise the possibility that racial disparities prior to accessing the deceased donor waiting list may be reduced if Hispanics with chronic kidney disease had improved access to care prior to ESRD. Among adults, a growing body of literature has linked delayed referral for pre-ESRD care with worse patient outcomes, including increased risk of mortality (32,33), hospitalization (33,34) and decreased access to renal transplantation (35,36). In addition, both low SES and minority race have been linked with inadequate pre-ESRD care (37–39). In pediatric ESRD, minority race and lack of health insurance are both associated with late start of dialysis and decreased access to specialty care (40). In our study, minorities were less likely to have health insurance at the start of dialysis and receive predialysis ESA, suggesting delayed referral for nephrology care. While USRDS started recording receipt of pre-ESRD nephrology care in 2005, the majority (62%) of patients in our study initiated dialysis prior to this. Among patients who initiated dialysis in the 2005–2008 era, significant racial differences in pre-ESRD care were observed, with 68.7% of whites, 63.3% of blacks and 53.2% of Hispanics reporting pre-ESRD care. Additional follow-up of this pediatric cohort is needed to confirm these racial differences.
SES appears to explain a modest proportion of the racial disparities observed in access to deceased donor transplantation among adults. Hall et al. reported that adjustment for health insurance coverage and zip code poverty accounted for 18% of the reduced rate of transplant among blacks and 14% of the reduced transplant rate among Hispanics. Once on the waiting list, however, health insurance and zip code poverty accounted for little if any of the racial disparities, indicating that SES influences the earlier step of access to the waiting list, but may not play a significant role after waitlisting (23). Similarly, in our study, despite lower SES among minorities, neither SES, clinical, nor laboratory measures explained the racial differences in transplant access once patients were placed on the waiting list. Further, adjusting for SES did not reduce the observed racial differences, and no interaction was detected across groups of SES, suggesting that racial disparities are consistent across all levels of SES once patients are listed.
The limitations to our observations should be noted. We were unable to account for patient health status at the time of waitlisting and/or transplant, and comorbid factors could have changed over time. We used zip-code level data to proxy neighborhood SES, but census-tract level analyses would have yielded more robust and sensitive results (41). Our proxy measure for individual SES, health insurance, likely does not completely capture a patient’s SES.
The data used in this study are from a national, population-based registry that is virtually 100% complete. The potential for misclassification of waitlisting and transplantation is small. Previous studies had limited power to report associations between transplant access for the Hispanic pediatric ESRD population, despite their high ESRD rate versus whites (2,42). To our knowledge, this is the first national study to examine racial disparities in access to both waitlisting and transplant receipt among the pediatric population, and the first to study how both individual- and neighborhood-level SES effect racial disparities in access to renal transplant.
Consistent with reports from more than a decade ago, racial disparities in access to renal transplantation are evident among children with ESRD, but remain poorly understood. The racial disparities in transplant access worsen as children progress through adolescence and young adulthood, where black ESRD patients 18–20 years of age have a 40% lower rate and Hispanics a 38% lower rate of deceased donor transplantation compared to whites. The disparity observed in access to waitlisting among Hispanics was eliminated with private health insurance, but once waitlisted, SES did not explain racial disparities in access to transplant. These findings deserve further exploration to identify the causes for continued racial disparities in access to renal transplantation for young ESRD patients.
Acknowledgments
We would like to acknowledge Paul Eggers, PhD, and Rebecca Zhang, MS, for assistance in USRDS data acquisition. The interpretation and reporting of the data presented here are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- CMS
Centers for Medicare and Medicaid Services
- ESA
erythropoiesis-stimulating agent
- ESRD
end-stage renal disease
- FSGS
focal segmental glomerulosclerosis
- GN
glomerulonephritis
- HR
hazard ratio
- IQR
interquartile range
- PPRA
peak panel reactive antibody
- SES
socioeconomic status
- UNOS
United Network for Organ Sharing
- USRDS
United States Renal Data System
Footnotes
A portion of this work was presented as free communication at the American Transplant Congress 2010 Annual Meeting (San Diego, CA, USA): Am J Transplantation 10, Suppl 4: 73. Abstract #113.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Young CJ, Gaston RS. Renal transplantation in black Americans. N Engl J Med. 2000;343:1545–1552. doi: 10.1056/NEJM200011233432107. [DOI] [PubMed] [Google Scholar]
- 2.USRDS. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2010. US Renal Data System. Annual data report: Atlas of chronic kidney disease and end-stage renal disease in the United States. [Google Scholar]
- 3.Nzerue CM, Demissochew H, Tucker JK. Race and kidney disease: Role of social and environmental factors. J Natl Med Assoc. 2002;98(8 Suppl):28S–38S. [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne C, Nedelman J, Luke RG. Race, socioeconomic status, and the development of end stage renal disease. Am J Kidney Dis. 1994;23:16–22. doi: 10.1016/s0272-6386(12)80806-7. [DOI] [PubMed] [Google Scholar]
- 5.Brancati FL, Whittle JC, Whelton PK, et al. The excess incidence of diabetic end-stage renal disease among blacks: A population-based study of potential explanatory factors. JAMA. 1992;268:379–384. [PubMed] [Google Scholar]
- 6.Furth SL, Garg PP, Neu AM, Hwang W, Fivush BA, Powe NR. Racial differences in access to the kidney transplant waiting list for children and adolescents with end-stage renal disease. Pediatrics. 2000;106:756–761. doi: 10.1542/peds.106.4.756. [DOI] [PubMed] [Google Scholar]
- 7.Omoloja A, Stolfi A, Mitsnefes M. Racial differences in pediatric renal transplantation-24-year single center experience. J Natl Med Assoc. 2006;98:154–157. [PMC free article] [PubMed] [Google Scholar]
- 8.Soucie JM, Neylan JF, McClellan W. Race and sex differences in the identification of candidates for renal transplantation. Am J Kidney Dis. 1992;19:414–419. doi: 10.1016/s0272-6386(12)80947-4. [DOI] [PubMed] [Google Scholar]
- 9.Alexander GC, Sehgal AR. Barriers to cadaveric renal transplantation among blacks, women, and the poor. JAMA. 1998;280:1148–1152. doi: 10.1001/jama.280.13.1148. [DOI] [PubMed] [Google Scholar]
- 10.Gaylin DS, Held PJ, Port FK, et al. The impact of comorbid and sociodemographic factors on access to renal transplantation. JAMA. 1993;269:603–608. [PubMed] [Google Scholar]
- 11.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55:992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall YN, Choi AI, Xu P, O’Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol. 2011;22:743–751. doi: 10.1681/ASN.2010080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall YN, O’Hare AM, Young BA, Boyko EJ, Chertow GM. Neighborhood poverty and kidney transplantation among US Asians and Pacific Islanders with end-stage renal disease. Am J Transplant. 2008;8:2402–2409. doi: 10.1111/j.1600-6143.2008.02413.x. [DOI] [PubMed] [Google Scholar]
- 14.Patzer RE, Amaral S, Wasse H, Volkova N, Kleinbaum D, McClellan WM. Neighborhood poverty and racial disparities in kidney transplant waitlisting. J Am Soc Nephrol. 2009;20:1333–1340. doi: 10.1681/ASN.2008030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keith D, Ashby VB, Port FK, Leichtman AB. Insurance type and minority status associated with large disparities in prelisting dialysis among candidates for kidney transplantation. Clin J Am Soc Nephrol. 2008;3:463–470. doi: 10.2215/CJN.02220507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omoloja A, Mitsnefes M, Talley L, Benfield M, Neu A. Racial differences in graft survival: A report from the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) Clin J Am Soc Nephrol. 2007;2:524–528. doi: 10.2215/CJN.03100906. [DOI] [PubMed] [Google Scholar]
- 17.Freedman LS, Graubard BI, Schatzkin A. Statistical validation of intermediate endpoints for chronic diseases. Stat Med. 1992;11:167–178. doi: 10.1002/sim.4780110204. [DOI] [PubMed] [Google Scholar]
- 18.Kleinbaum DG, Mitchel K. Survival Analysis: A Self-Learning Text. 2nd edn. New York, NY: Springer; 2005. [Google Scholar]
- 19.Kleinbaum DG, Mitchel K. Logistic Regression: A Self-Learning Text. 2nd edn. New York, NY: Springer; 2002. [Google Scholar]
- 20.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Medl Res Methodol. 2008;8:1–15. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin DY, Wei LJ. The Robust inference for the cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 22.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall YN, Choi AI, Xu P, O’Hare AM, Chertow GM. Racial ethnic differences in rates and determinants of deceased donor kidney transplantation. J Am Soc Nephrol. 2011;22:743–751. doi: 10.1681/ASN.2010080819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders MR, Cagney KA, Ross LF, Alexander GC. Neighborhood poverty, racial composition and renal transplant waitlist. Am J Transplant. 2010;10:1912–1917. doi: 10.1111/j.1600-6143.2010.03206.x. [DOI] [PubMed] [Google Scholar]
- 25.Zelikovsky N, Schast AP, Palmer J, Meyers KE. Perceived barriers to adherence among adolescent renal transplant candidates. Pediatr Transplant. 2008;12:300–308. doi: 10.1111/j.1399-3046.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 26.Jarzembowski T, John E, Panaro F, et al. Impact of noncompliance on outcome after pediatric kidney transplantation: An analysis in racial subgroups. Pediatr Transplant. 2004;8:367–371. doi: 10.1111/j.1399-3046.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 27.Furth SL, Hwang W, Neu AM, Fivush BA, Powe NR. Effects of patient compliance, parental education and race on nephrologists’ recommendations for kidney transplantation in children. Am J Transplant. 2003;3:28–34. doi: 10.1034/j.1600-6143.2003.30106.x. [DOI] [PubMed] [Google Scholar]
- 28.Gordon EJ, Sehgal AR. Patient-nephrologist discussions about kidney transplantation as a treatment option. Adv Ren Replace Ther. 2000;7:177–183. doi: 10.1053/rr.2000.5268. [DOI] [PubMed] [Google Scholar]
- 29.Ayanian JZ, Cleary PD, Weissman JS, Epstein AM. The effect of patients’ preferences on racial differences in access to renal transplantation. N Engl J Med. 1999;341:1661–1669. doi: 10.1056/NEJM199911253412206. [DOI] [PubMed] [Google Scholar]
- 30.Ladin K, Hanto DW. Understanding disparities in transplantation: Do social networks provide the missing clue? Am J Transplant. 2010;10:472–476. doi: 10.1111/j.1600-6143.2009.02963.x. [DOI] [PubMed] [Google Scholar]
- 31.Clark CR, Hicks LS, Keogh JH, Epstein AM, Ayanian JZ. Promoting access to renal transplantation: The role of social support networks in completing pre-transplant evaluations. J Gen Intern Med. 2008;23:1187–1193. doi: 10.1007/s11606-008-0628-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinchen KS, Sadler J, Fink N, et al. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137:479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 33.Hedelin M, Chang ET, Wiklund F, et al. Association of frequent consumption of fatty fish with prostate cancer risk is modified by COX-2 polymorphism. Int J Cancer. 2007;120:398–405. doi: 10.1002/ijc.22319. [DOI] [PubMed] [Google Scholar]
- 34.Arora P, Kausz AT, Obrador GT, et al. Hospital utilization among chronic dialysis patients. J Am Soc Nephrol. 2000;11:740–746. doi: 10.1681/ASN.V114740. [DOI] [PubMed] [Google Scholar]
- 35.Cass A, Cunningham J, Snelling P, Ayanian JZ. Author reply: Late referral to a nephrologist reduces access to renal transplantation. American J Kidney Dis. 2003;42:1043–1049. doi: 10.1016/j.ajkd.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Winkelmayer WC, Mehta J, Chandraker A, Owen WF, Jr, Avorn J. Predialysis nephrologist care and access to kidney transplantation in the United States. Am J Transplant. 2007;7:872–879. doi: 10.1111/j.1600-6143.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 37.Prakash S, Rodriguez RA, Austin PC, et al. Racial composition of residential areas associates with access to pre-ESRD nephrology care. J Am Soc Nephrol. 2010;21:1192–1199. doi: 10.1681/ASN.2009101008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obialo CI, Ofili EO, Quarshie A, Martin PC. Ultralate referral and presentation for renal replacement therapy: Socioeconomic implications. Am J Kidney Dis. 2005;46:881–886. doi: 10.1053/j.ajkd.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Khosla N, Gordon E, Nishi L, Ghossein C. Impact of a chronic kidney disease clinic on preemptive kidney transplantation and transplant wait times. Prog Transplant. 2010;20:216–220. doi: 10.1177/152692481002000304. [DOI] [PubMed] [Google Scholar]
- 40.Seikaly MG, Salhab N, Browne R. Patterns and time of initiation of dialysis in US children. Pediatr Nephrol. 2005;20:982–988. doi: 10.1007/s00467-004-1803-7. [DOI] [PubMed] [Google Scholar]
- 41.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: A comparison of area-based socioeconomic measures–the public health disparities geocoding project. Am J Public Health. 2003;93:1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmer Alves T, Lewis J. Racial differences in chronic kidney disease (CKD) and end-stage renal disease (ESRD) in the United States: A social and economic dilemma. Clin Nephrol. 2010;74(Suppl 1):S72–S77. doi: 10.5414/cnp74s072. [DOI] [PubMed] [Google Scholar]