Abstract
In the past 30 years, the numbers and types of fluoroscopically-guided (FG) procedures have increased dramatically. The objective of the present study is to provide estimated radiation doses to physician specialists, other than cardiologists, who perform FG procedures. We searched Medline to identify English-language journal articles reporting radiation exposures to these physicians. We then identified several primarily therapeutic FG procedures that met specific criteria: well-defined procedures for which there were at least five published reports of estimated radiation doses to the operator, procedures performed frequently in current medical practice, and inclusion of physicians from multiple medical specialties. These procedures were percutaneous nephrolithotomy (PCNL), vertebroplasty, orthopedic extremity nailing for treatment of fractures, biliary tract procedures, transjugular intrahepatic portosystemic shunt creation (TIPS), head/neck endovascular therapeutic procedures, and endoscopic retrograde cholangiopancreatography (ERCP). We abstracted radiation doses and other associated data, and estimated effective dose to operators. Operators received estimated doses per patient procedure equivalent to doses received by interventional cardiologists. The estimated effective dose per case ranged from 1.7 – 56μSv for PCNL, 0.1 – 101 μSv for vertebroplasty, 2.5 – 88μSv for orthopedic extremity nailing, 2.0 – 46μSv for biliary tract procedures, 2.5 – 74μSv for TIPS, 1.8 – 53μSv for head/neck endovascular therapeutic procedures, and 0.2 – 49μSv for ERCP. Overall, mean operator radiation dose per case measured over personal protective devices at different anatomic sites on the head and body ranged from 19 – 800 (median = 113) μSv at eye level, 6 – 1180 (median = 75)μSv at the neck, and 2 – 1600 (median = 302) μSv at the trunk. Operators’ hands often received greater doses than the eyes, neck or trunk. Large variations in operator doses suggest that optimizing procedure protocols and proper use of protective devices and shields might reduce occupational radiation dose substantially.
Keywords: interventional procedure, fluoroscopically-guided procedure, occupational exposure, radiation protection
INTRODUCTION
The term “fluoroscopically-guided (FG) procedures” refers to procedures where real-time radiological images (fluoroscopy) of a patient’s internal structures are used for diagnostic or therapeutic purposes. FG procedures are utilized to treat a growing range of diseases and injuries by a variety of physician specialists, including interventional radiologists, neuroradiologists, cardiologists, electrophysiologists, orthopedic surgeons, urologists and gastroenterologists. Examples of structural or functional conditions treated using FG procedures include disorders of the heart, blood vessels, gastrointestinal system, biliary tract, bladder, ureters and kidneys. Fluoroscopic imaging has also been employed in minimally invasive hip fracture plating, nailing, external fixation and other orthopedic procedures.
The National Council on Radiation Protection and Measurements (NCRP) has estimated that an average of 17 million interventional fluoroscopic procedures were performed in 2006, including 4.6 million cardiac procedures, 3.4 million vascular non-cardiac procedures, and 8.6 million nonvascular procedures (NCRP 2009). Not included in these estimates were radiographic fluoroscopy procedures (e.g., barium enemas). The number of FG procedures has increased by about 8.5% annually (Bhargavan 2008), increasing approximately 4.7-fold between 1986 and 2005, with cardiac procedures (16% annual increase) and spinal procedures (15% annual increase) demonstrating the greatest growth. Of the FG procedures carried out in 2005, 33% were vascular procedures, 29% cardiac, 23% spinal 3.1% gastrointestinal, 1.8% urinary, 0.8% extremity procedures and 9.9% all others.
In contrast to other radiological modalities, such as conventional radiography, computed tomography and nuclear medicine, operators who perform FG procedures stand in close proximity to the patient and the x-ray tube, and are therefore exposed to substantial scattered radiation from the patient. Although radiation doses to operators from scattered radiation are much smaller than patient doses (Koenig et al. 2001, Vano et al. 2001, Miller et al. 2003a, Miller et al. 2003b, Neofotistou et al. 2003), the cumulative dose from many procedures performed over an operator’s career may be substantial. In addition, there appears to be an increasing workload per operator, as the number of practitioners performing FG procedures has not kept pace with the substantial increases in the numbers of FG procedures (Vano et al. 1998b)
Clinical reports and a case-control epidemiologic study have suggested an increased risk of brain tumors and skin cancers in physicians who use fluoroscopy (Finkelstein 1998, Hardell et al. 2001, Eagan and Jones 2010). Clinical and epidemiologic studies have also suggested a possible excess occurrence of radiation-related cataracts in physicians who perform FG procedures (Vano et al. 1998a, RSNA 2004, Ciraj-Bjelac et al. 2010, Shore et al. 2010, ICRP 2011b).
Recently, we reported estimated radiation doses to cardiologists who perform the most common FG cardiac procedures, based on a comprehensive assessment of the literature (Kim et al. 2008). Our review of exposure data demonstrated notable variations, ranging up to 1000-fold from minimum to maximum, in estimated radiation doses for each procedure - diagnostic cardiac catheterization (DC), percutaneous coronary intervention (PCI), radiofrequency ablation, and implantable cardioverter defibrillator (ICD) and pacemaker (PM) placement. Patient, operator, fluoroscopic equipment, equipment operation and shielding factors all influenced operator dose to different degrees (Kim and Miller 2009). An assessment of temporal trends revealed absent to modest dose reductions over time, likely reflecting dose increases due to the increasing complexity of medical procedures that offset dose reductions due to technological improvements. The International Atomic Energy Agency (IAEA) has begun an Information System on Occupational Exposure in Medicine, Industry and Research (ISEMIR). Its Working Group on Interventional Cardiology (WGIC) has proposed establishment of an international database of occupational exposures of staff working in interventional cardiology facilities (Padovani et al. 2011).
The objectives of the present study are to provide a comprehensive and systematic summary of estimated radiation doses received by operators performing non-cardiac FG procedures and to identify the primary factors influencing occupational radiation dose for these procedures.
MATERIALS AND METHODS
We carried out a preliminary review of the literature on radiation dose to operators performing non-cardiac FG procedures. We identified several procedures, primarily therapeutic in nature, which met the following criteria: well-defined procedures for which there were at least five published reports of estimated radiation doses to the operator, procedures performed frequently in current medical practice, and inclusion of physicians from multiple medical specialties. The procedures selected for this review were percutaneous nephrolithotomy (PCNL), vertebroplasty, orthopedic extremity nailing (for treatment of fractures), biliary tract procedures, transjugular intrahepatic portosystemic shunt creation (TIPS), head/neck endovascular therapeutic procedures, and endoscopic retrograde cholangiopancreatography (ERCP). We excluded studies for which it was difficult to interpret the reported data or to estimate dose on a per case basis. An example of a reason for exclusion was because the published report grouped together different procedures in one general category (i.e., peripheral arteriography and renal arteriography were grouped together as vascular procedures).
We conducted a comprehensive literature search using Medline to identify articles in English on occupational radiation dose from the selected procedures. We used broad search terms such as “(dos* or exposure or radiation) and (occupational or personnel or staff or operator or physician or doctor) and (fluoroscop* or intervention)”. The references cited in each useful publication were traced to locate other relevant publications.
From each publication we abstracted the total number of procedures reported within each major procedure category, dose assessment methods, reported doses to various anatomic sites on the operator, fluoroscopy time, kerma area product (KAP), and other data associated with radiation doses. Radiation doses to operators can be assessed by direct personnel monitoring during clinical procedures (Cohen et al. 1997, Derdeyn et al. 1999) or by indirect methods such as dose rate measurement or computer simulations (Schultz et al. 2003, Siiskonen et al. 2007). Our previous investigation demonstrated that dose estimates using indirect methods generally deviated more from the observed trend than did doses estimated from direct dose measurements (Kim et al. 2008). Therefore, in the current study we only abstracted dose data from direct monitoring.
Different dosimetric quantities and units have been used in the literature to describe occupational doses. To simplify our data analysis and to reduce the data to a single consistent metric of exposure, we transformed the different units and quantities to personal dose equivalent HP(10) and HP(0.07), as defined by the International Commission on Radiation Units and Measurements (ICRU) (ICRU 1993). Measurements obtained from personal monitors under a lead apron were converted to personal dose equivalent HP(10) and doses obtained from personal monitors near the operator’s eye and hand were converted to personal dose equivalent HP(0.07). HP(0.07) is more appropriate for the skin and the eye than HP(10) because doses to the skin and the lens of the eye are defined at a depth of 0.07 mm and 3 mm in tissue, respectively. The difference between HP(10) and HP(0.07) for a given procedure was minor because all procedures studied involved x-ray energies from fluoroscopy (Simon et al. 2006).
Effective doses were estimated using a systematic approach for conversion of the reported doses to comparable measures. Many strategies have been developed to estimate effective dose using personal monitors (Niklason et al. 1994, NCRP 1995, von Boetticher et al. 2003, Clerinx et al. 2008). A comprehensive review of different dosimetry algorithms used to determine effective doses for interventional radiology staff revealed that the Niklason algorithm estimated effective dose well and could provide good estimates of dose for operators regardless of whether or not they wore a thyroid shield (Niklason et al. 1994, Jarvinen et al. 2008). According to a review study, there were significant differences in the effective dose estimations by different algorithms (Jarvinen et al. 2008). The algorithms were generally developed for radiation protection purposes and thus resulting in conservatively high dose estimation (NCRP 1995). For this study we used the Niklason algorithm to well estimate effective dose based on two dosimeter readings, with one dose measured under the lead apron and the other measured over the lead apron or thyroid shield. When the dosimeter reading under the apron was not available, a modified Niklason approach was employed (Padovani and Rodella 2001).
The conversion algorithms are given below:
| (1) |
| (2) |
and
| (3) |
| (4) |
where E is effective dose, Hos is shallow dose measured over the thyroid shield at the neck, and Hu is the under apron dose. If the badge dose at the neck was not available, then the eye dose or trunk dose measured over the apron was substituted. There were only small differences among radiation doses over protective devices at the neck, at the eye and at the trunk for a given procedure. Radiation dose measured at the hand was not used to estimate effective dose because the radiation dose measured at the hand may be much greater than doses at the neck, eye, or trunk for these procedures. The use of hand doses tends to substantially overestimate effective doses to operators.
Since the operator’s head and neck are generally unshielded or poorly shielded during FG procedures, organs and tissues in the head and neck receive high radiation doses (Kuon et al. 2003, Ciraj-Bjelac et al. 2010). Effective dose is substantially affected by the use of a thyroid shield, because the thyroid shield protects the underlying skin, esophagus, vertebrae, and bone marrow as well as the thyroid gland. According to the Niklason algorithm, effective dose is reduced by about 50 percent when a thyroid shield is used. In our analysis, effective dose was calculated assuming no use of a thyroid shield. The assumption was made to facilitate comparisons of different studies. Most reports of occupational radiation exposure from fluoroscopic procedures lack detailed information about radiation protection measures, and especially about use of a thyroid shield. Forty-five of the publications reviewed reported that physicians wore lead aprons during procedures. Of those 45 publications, only 17 reported use of a thyroid shield.
Absorbed doses to the lens of the eye, thyroid, brain, and bone marrow were estimated assuming the operators wore a lead apron but no thyroid shield or leaded glasses. Organ absorbed doses were reconstructed with dose measurements at different anatomic sites based on an organ dose conversion algorithm (Simon 2011):
| (5) |
where DT is tissue or organ dose, Hp(d) is personal dose equivalent, and Ka is air kerma. Calculated dose conversion coefficients (DT per Ka) for the lens of the eye, thyroid, and brain for the general x-ray beam quality of fluoroscopy systems were 1.26, 1.17, and 0.262, respectively. Bone marrow dose was estimated based on the bone marrow fraction that might be assumed to be protected by a lead apron using the bone marrow distribution reported by Cristy (Cristy 1981, ICRP 1995). About 17% of bone marrow was found to be unprotected by standard lead aprons (Simon 2011).
Aprons of different lead equivalent thicknesses, ranging from 0.25 to 0.5 mm lead equivalence, were reported in the reviewed literature. We assumed that an apron with 0.5 mm lead equivalent thickness was worn most commonly. Our assumption was based on 26 publications (included in Tables 1–7) of which 17 reported an apron thickness of 0.5 mm lead equivalence.
Table 1.
Mean exposure and effective dose to the operator per case from percutaneous nephrolithotomy
| Author (Publication Year) a |
Physician b | No of Cases c |
Fluoroscopy Time d (min) |
Protective Measures e |
Mean Badge Dose per Case (μSv) d, f |
Effective Dose k (μSv) |
Note | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Apron (mm) | Thyroid Shield (mm) |
Hand g | Eye Level h |
Neck i | Trunk j
|
|||||||
| Over Apron |
Under Apron |
|||||||||||
| Safak et al. (2009) | Urologist | 20 | 11.7 (1.5–31.2) | 0.5 | 0.5 | 33 | 26 | 48 | - | 12 | 14.2 | |
| Kumari et al. (2006) | Urologist | 50 | 6.0 (1.8–12.2) | 0.5 | 0.5 | 280 (±130) | - | - | 24.9 (7.4–50.2) | - | 1.7 | |
| Hellawell et al. (2005) | Urologist | 6 | 6.8–23 | 0.35 | 0.35 | 48 (±12) | 40 (±10) | - | - | - | 2.8 | |
| Yang et al. (2002) | Urologist | 6 | 12.8 | O | - | - | - | 88 | - | - | 6.2 | Without ceiling-suspended shield |
| Yang et al. (2002) | Urologist | 6 | 12.8 | O | - | - | - | 25 | - | - | 1.8 | With ceiling-suspended shield |
| Bowsher et al. (1992) | Urologist | 6 | 2.0 (0.3–2.8) | - | - | 50 (±40) | 30 (±15) | - | - | - | 2.1 | Under-couch system |
| Bowsher et al. (1992) | Urologist | 8 | 2.0 (0.3–2.8) | - | - | 230 (±120) | 190 (±120) | - | - | - | 13.3 | Over-couch system |
| Nowak and Jankowski (1991) | NS | 54 | - | 0.25 | - | 41 | 34 | - | - | - | 2.4 | |
| Ramsdale et al. (1990) | Radiologist | 42 | 22 (±13) | - | - | 520 (±750) | 320 (±360) | 270 (±220) | - | - | 18.9 | Over-couch system |
| Geterud et al. (1989) | Urologist + Radiologist | 11 | 14 (3.0–29) | 0.3 | X | 210 (14–710) | - | 99 (15–260) | - | 8.6 (2.1–18) | 14.0 | |
| Inglis et al. (1989) | Urologist | 55 | 4.4 (1.2–13) | - | - | 342 | - | 35 | - | - | 2.5 | |
| Rao et al. (1987) | Urologist + Radiologist | 18 | 22 (0.9–45) | O | - | 5800 | 800 | - | - | - | 56.0 | Over-couch system |
| Lowe et al. (1986) | Urologist + Radiologist | 15 | 28 | 0.5 | O | 83 (±84) | - | 45 (±48) | - | - | 3.2 | |
| Bush et al. (1985) | Urologist + Radiologist | 94 | 18 (4–65) | 0.5 | 0.5 | 300 (100–2000) | - | 100 (20–320) | - | - | 7.0 | |
| Bush et al. (1984) | Urologist | 51 | 8 (2–30) | 0.5 | X | - | - | 100 (10–380) | - | - | 7.0 | |
There is no column for kerma-area product (KAP) data because no KAP data were included in these reports.
References are arranged by publication year.
NS: Not specified.
Number of cases in the report.
Numbers in parenthesis are standard deviation (±) or minimum-maximum (−). Superscripts of ‘md’, and ‘iq’ indicate median value and inter-quartile.
Lead-equivalent thickness of protective measures. O indicates that protective measures were used but their thicknesses were not given. X indicates that protective measures were not used.
Exposure unit (R) in some studies was converted into Hp(10) for trunk dose under apron (conversion factor 11,600 μSv R−1) and into Hp(0.07) for doses outside shield (conversion factor 11,900 μSv R−1).
Measurements obtained at the wrist, hand, or finger outside shield.
Measurements obtained at the eye, forehead, glabella, maxilla, or temple outside shield.
Measurements obtained at the neck, collar, clavicle, or shoulder outside shield.
Measurements obtained at the chest, sternum, umbilicus, waist, or abdomen over or under apron.
Effective doses were calculated using the Niklason (2 dosimeters) and Padovani et al. (1 dosimeter) algorithms, assuming no use of a thyroid shield (see text for details). If no measurement over the thyroid shield was available, the measurement at eye level or outside the apron at trunk level was used, in that order of preference.
Table 7.
Mean exposure and effective dose to the operator per case from endoscopic retrograde cholangiopancreatography
| Author (Publication Year) a | Physician Type b | No of Cases c | KAP d, l (Gy cm2) | Fluoroscopy Time d (min) | Protective Measures e | Mean Badge Dose per Case (μSv) d, f | Effective Dose k (μSv) | Note | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Apron (mm) | Thyroid Shield (mm) | Hand g | Eye Level h | Neck i | Trunk j
|
||||||||
| Over Apron | Under Apron | ||||||||||||
| ERCP (Diagnostic) | |||||||||||||
| Chen et al. (1996) | Endoscopist | 4 | - | - | O | O | - | - | - | 2.5 (±5) | - | 0.2 | With ceiling-suspended shield |
| Chen et al. (1996) | Endoscopist | 4 | - | - | O | O | - | - | - | 15 (±19) | - | 1.1 | Without ceiling-suspended shield |
| Cohen et al. (1979) | Endoscopist | 15 | - | 10 (±4.4) | 0.5 | - | <30 | <30 | <30 | - | - | 2.1 | |
| ERCP (Therapeutic) | |||||||||||||
| Buls et al. (2002) | NS | 25 | 50(24–60) iq | 6 (3.6–8.3) iq | 0.5 | X | 640 (200–880)iq | 550 (160–660)iq | 450 (170–600)iq | - | - | 31.5 | Over-couch fluoroscopy |
| Chen et al. (1996) | Endoscopist | 6 | - | - | O | O | - | - | - | 2.8 (±4.4) | - | 0.2 | With ceiling-suspended shield |
| Chen et al. (1996) | Endoscopist | 6 | - | - | O | O | - | - | - | 32 (±45) | - | 2.2 | Without ceiling-suspended shield |
| Krueger and Hoffman (1992) | Endoscopist | 10 | - | 7.5 | 0.5 | - | - | - | 6.2 | 3.3 | 0 | 0.4 | |
| ERCP (Diagnostic + Therapeutic) | |||||||||||||
| Olgar et al. (2009) | NS | 31 | - | - | 0.5 | 0.5 | 835 | 94 | 75 | - | 0 | 4.5 | |
| Oonsiri et al. (2007) | Radiologist | 10 | 35 (9.6–105) | 1.7–23 | O | O | - | - | 170 (98–318) | - | - | 11.9 | |
| Naidu et al. (2005) | NS | 61 | - | 4.8 | 0.25 | X | - | - | 457 | - | 23 | 49 | Over-couch system |
| Heyd et al. (1996) | NS | 25 | - | - | - | - | - | - | - | 6 | - | 0.4 | |
See footnotes to Table 1.
Kerma-area product.
Radiation doses measured at eye level were converted to absorbed dose to the brain and the lens of the eye; doses measured at the neck were converted to absorbed dose to the thyroid. If measurements were not available for either site, measurement data from one site were used to estimate absorbed doses to all of these organs because there were small differences in radiation doses measured at eye level versus those measured at the neck. Average ratio of radiation doses measured at eye and neck was 1.1 ± 0.5 (see results).
The radiation dose data from the literature for non-cardiac procedures were tabulated by procedure type. For each procedure type, the reported radiation doses were designated according to the anatomic sites where dosimeters were placed. From the anatomic site-specific dose measurements, effective doses were estimated. Patient doses (as KAP and as fluoroscopy time) were also abstracted because occupational dose is strongly related to patient dose. Some dosimetry studies reported dose results under different conditions and compared the findings to determine if there were differences. The detailed data collected for various aspects of the FG procedures were evaluated to identify and quantify effects of dose-influencing factors.
The large variations in radiation intensity at different points around the periphery of the patient table and at different heights above the floor during a FG procedure may result in substantial variations in dose at different anatomic sites on the operator (Schueler et al. 2006). Ratios of doses measured at different pairs of anatomic sites were calculated for those studies that provided measurement data over personal protective shields at more than two different anatomic sites.
RESULTS
In general, there were substantially fewer reports of occupational doses associated with non-cardiac procedures than we had identified in our earlier study of occupational doses from cardiac procedures.
Table 1 summarizes radiation doses to operators during PCNL, a procedure for removing large renal calculi (kidney stones). Under fluoroscopic guidance, a needle is inserted percutaneously and guided to the renal stone. A tract is created and the stone is manipulated and removed or broken into very small fragments (Ko et al. 2008). The procedure is typically performed by a urologist or a radiologist. Although the initial needle placement and tract dilation results in high operator exposure, PCNL is generally associated with low or moderate radiation exposure unless the fluoroscope is placed in an oblique position. In general, the operator is usually positioned within 25 – 60 cm of the patient.
Mean fluoroscopy times were relatively short, usually ranging from 2 – 28 (median = 13) min per case (Table 1). Radiation doses to the hand (33 – 5800 μSv per case) were greater than radiation doses measured at the trunk or head (25 – 800μSv per case). Effective dose estimates ranged from 1.7 – 56 (median = 6.2) μSv per case. High radiation doses at the level of the eye, and thus high effective dose estimates, were reported in some studies in which over-couch fluoroscopy systems (tube-over-table geometry) were used (Rao et al. 1987, Ramsdale et al. 1990, Bowsher et al. 1992). A comparison of measured radiation doses using over-couch versus under-couch systems revealed that radiation doses to the forehead and finger were about 5–6-fold greater for over-couch than for under-couch systems (Bowsher et al. 1992). Yang et al. measured radiation dose with and without a leaded screen shield between the patient and the operator and found that the shield reduced the radiation dose to the forehead (about 50 cm from the radiation source) by an average of 70 percent (Yang et al. 2002).
Table 2 summarizes radiation doses to operators from vertebroplasty, a procedure involving injection of bone cement through a needle into an abnormal vertebral body, using fluoroscopy for guidance (Garfin et al. 2001). The procedure is generally performed by orthopedic surgeons or radiologists. Vertebroplasty, which has become widely used in the past decade, generally results in low or moderate exposure to operators. The operator is typically about 40 cm from the operative field (Ortiz et al. 2006), and the operator’s hands are approximately 25 – 30 cm from the surgical site (Kruger and Faciszewski 2003). With lateral fluoroscopy guidance, a cement injection system allows operators’ hands to remain 34 cm outside the fluoroscopy field (Komemushi et al. 2005).
Table 2.
Mean exposure and effective dose to the operator per case from vertebroplasty
| Author (Publication Year) a | Physician b | No of Cases c | KAP d, l (Gy cm2) | Fluoroscopy Time d (min) | Protective Measurese | Mean Badge Dose per Case (μSv) d, f | Effective Dose k (μSv) | Note | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Apron (mm) | Thyroid Shield (mm) | Hand g | Eye Level h | Neck i | Trunk j
|
||||||||
| Over Apron | Under Apron | ||||||||||||
| Tappero et al. (2009) | Neuroradiologist | 10 | - | - | 0.5 | - | - | - | - | - | 7.1 (±5.1) | 8.1 | |
| Fitousi et al. (2006) | Orthopedics/radiologist | 35 | - | 28 (±7.0) | O | - | 1661 | 328 | - | - | - | 23.0 | |
| Oritz et al. (2006) | Neuroradiologist | 82 | - | 8.0 (±2.2) | 0.5 | - | - | - | - | 15.4 (±13.3) | - | 1.1 | With cement delivery system |
| Oritz et al. (2006) | Neuroradiologist | 20 | - | 5.4 (±2.6) | 0.5 | - | - | - | - | 1.7 (±1.9) | - | 0.1 | With syringe |
| Synowitz and Kiwit (2006) | Neurosurgeon | 20 | 12.8 | 1.9 | - | - | 490 (±400) | - | - | - | - | - | Left hand protected |
| Synowitz and Kiwit (2006) | Neurosurgeon | 21 | 10.5 | 2 | - | - | 1810 (±1310) | - | - | - | - | - | Left hand unprotected |
| Harstall et al. (2005) | Spine surgeon | 136 | 28 (±9.1) | 8.0 (±2.0) | O | 0.5 | 453 | 84 | 222 | - | - | 15.5 | |
| Komemushi et al. (2005) | NS | 19 | - | 7.54 (±3.5) | 0.5 | - | - | - | - | 321 (±232) | 14.5 (±11.3) | 32.9 | 1 mL syringe group |
| Komemushi et al. (2005) | NS | 16 | - | 6.7 (±2.4) | 0.5 | - | - | - | - | 116 (±93) | 7.8 (±9.7) | 14.3 | Cement injector group |
| Mehdizade et al. (2004) | Neuroradiologist | 11 | - | 10–60 | O | - | 500–8500 | - | - | 22–3250 | 10–470 | - | |
| Kallmes et al. (2003) | NS | 19 | - | 8.7 | X | X | 1280 (±1610) | - | - | - | - | - | 1 ml syringe, without ceiling-suspended shield |
| Kallmes et al. (2003) | NS | 20 | - | 12 | X | X | 980 (±900) | - | - | - | - | - | Injection device, with ceiling-suspended shield |
| Kruger and Faciszewski (2003) | Surgeon | 18 | - | 6.5 | O | - | 2040 | - | - | 1440 | - | 100.8 | Before implementation of exposure reduction techniques and devices |
| Kruger and Faciszewski (2003) | Surgeon | 18 | - | 6.5 | O | - | 74 | - | - | 4 | - | 0.3 | After implementation |
See footnotes to Table 1.
Kerma-area product.
Mean fluoroscopy times for vertebroplasty were relatively short, ranging from 2 – 35 (median = 8) min per case, but the operator’s hands may be within the x-ray field during needle placement. Radiation doses measured at the level of the body and the head ranged from 2 – 1600 μSv per case. Radiation doses measured at the hands ranged from 74 – 4500 μSv per case. The range of effective dose estimates was 0.1 – 101 (median = 14.3) μSv per case. Comparison of operator radiation exposure when using syringes versus other cement delivery systems has shown inconsistent findings as to which approach was associated with greater radiation doses (Kallmes et al. 2003, Ortiz et al. 2006). The inconsistency may be due to differences in hand location during the procedure. Use of leaded gloves reduced radiation dose by 75% (Synowitz and Kiwit 2006). Kruger et al. evaluated the effect of modified practice habits and use of radiation shielding (exposure-reducing fluoroscopy equipment configurations, fluoroscopy operational modes and dose rate considerations; minimization of fluoroscopy time; maximization of operator distance from the primary beam; improvements in placement of leaded shields and use of lead aprons) on occupational dose (Kruger and Faciszewski 2003). Implementation of multiple modifications to reduce radiation doses reduced operator whole-body dose per vertebroplasty procedure from 1440 μSv to 4 μSv.
Table 3 summarizes radiation doses to operators from orthopedic extremity nailing, which has been widely used for 30 years to treat long bone shaft fractures (Miller et al. 1983). Fluoroscopic guidance is required to reduce the fracture, place nails, and fix screws. The procedure is performed by orthopedic surgeons. Of concern is radiation exposure to the operator’s hands, which are in close proximity to the direct x-ray beam during the procedure (Hafez et al. 2005). Radiation doses measured at the surgeon’s hands within the direct beam were 100 times greater than doses to the operator’s hands at 15 cm from the beam (Arnstein et al. 1994, Blattert et al. 2004). Mean fluoroscopy times were shortest among the various types of procedures reviewed in the present study, and ranged from 1.2 – 15 (median = 4) minutes per case. Radiation doses to the hands (37 – 2100 [median = 553] μSv per case) were greater than the measured doses at the level of the body and the head (19 – 1180 [median = 70] μSv per case). Despite the short fluoroscopy times, effective dose estimates were relative high, ranging from 2.5–88 (median = 9.8) μSv per case, likely due to the proximity of the operator to the patient during the procedure. Comparison of radiation doses to trainees versus experienced operators revealed significantly greater radiation doses to trainees, perhaps resulting from closer proximity of the trainee’s hands to the x-ray beam rather than the differences in procedure length (Hafez et al. 2005). Mean fluoroscopy time for moderately experienced orthopedic surgeons was more than 2-fold longer than fluoroscopy time of senior surgeons (Madan and Blakeway 2002). Radiation dose to the surgeons’ hands was 4-fold greater for femoral nailing than for tibial nailing. An increase from 15 cm to 60 cm in the distance of the operator’s hands from the patient resulted in a more than 10-fold decrease in operator hand dose. Fluoroscopy time associated with use of the Marchetti-Vincenzi nail was significantly shorter than that associated with use of the Russell-Taylor nail (Madan and Blakeway 2002).
Table 3.
Mean exposure and effective dose to the orthopedic surgeon per case from orthopedic extremity nailing
| Author (Publication Year) a | Procedure | No of Cases c | KAP d, l (Gy cm2) | Fluoroscopy Time d (min) | Protective Measures e | Mean Badge Dose per Case (μSv) d, f | Effective Dose k (μSv) | Note | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Apron (mm) | Thyroid Shield (mm) | Hand g | Eye Level h | Neck i | Trunk j
|
||||||||
| Over Apron | Under Apron | ||||||||||||
| Kirousis et al. (2009) | Tibia intramedullary nailing | 25 | 0.75 (±0.5) | 1.2 (±0.7) | O | - | - | - | 1180 | - | - | 87.6 | |
| Hafez et al. (2005) | Intramedullary nailing | 6 | - | 2.6 (±0.34) | O | O | 1860 | - | - | - | - | - | Operating trainee |
| Hafez et al. (2005) | Intramedullary nailing | 19 | - | 1.5 | O | O | 37 | - | - | - | - | - | Consultant |
| Muzaffar et al. (2005) | Femoral interlocking nailing | 10 | - | 3.9 (±1.8) | - | - | 250 (±110) | 90 (±50) | - | - | - | 6.3 | |
| Blattert et al. (2004) | Intramedullary nailing | 12 | - | 4.4 (±2.0) | O | - | 776 (±879) | 42 (±43) | 57 (±80) | 80 (±87) | 15 (±27) | 17.5 | Senior group |
| Blattert et al. (2004) | Intramedullary nailing | 10 | - | 7.0 (±4.3) | O | - | 1397 (±1886) | 38 (±36) | 70 (±80) | 108 (±134) | 8 (±9) | 11.7 | Junior group |
| Madan and Blakeway (2002) | Intramedullary nailing | 99 | - | - | O | - | 330 | - | - | - | - | - | Tibia nailing |
| Madan and Blakeway (2002) | Intramedullary nailing | 85 | - | - | O | - | 1272 | - | - | - | - | - | Femoral nailing |
| Fuchs et al. (1998) | Intramedullary nailing | 8 | - | 7.5 (4.3–12) | O | - | 42 (±12) | 19 (±11) | 35 (±15) | - | - | 2.5 | |
| Muller et al. (1998) | Intramedullary nailing | 41 | - | 4.6 (0.9–15) | - | - | 1270 | - | - | - | - | - | |
| Goldstone et al. (1993) | Intramedullary nailing | 4 | - | 2.9 (2.9–3.0) | - | - | 69 (10–157) | - | - | - | - | - | |
| Sanders et al. (1993) | Intramedullary nailing | 21 | - | 3.6 | O | - | 280 | - | - | - | - | - | |
| Coetzee and Merwe (1992) | Intramedullary fixation | 15 | - | 15 (1.4–27) | O | - | 2100 (0–8780) | 140 (0–800) | 140 (0–520) | - | 50 (±0–170) | 9.8 | |
| Levin et al. (1987) | Intramedullary nailing | 30 | - | 8.0 | 0.5 | - | - | - | 70 | - | - | 4.9 | |
See footnotes to Table 1.
Kerma-area product.
Table 4 summarizes radiation dose to operators during biliary tract procedures, including drainage, stenting or both. Biliary tract procedures are commonly performed for treatment of bile duct occlusion or stenosis. These procedures are performed by radiologists. Fluoroscopy times were relatively short, ranging from 5 – 23 (median = 9.5) min per case. Radiation doses measured at the hands (105 – 1290 [median = 460] μSv per case) were much higher than those at the level of the body and the head (20 – 660 [median = 103] μSv per case). Effective dose estimates ranged from 2 – 46 (median = 5) μSv per case. Use of leaded under-couch shield decreased occupational radiation exposure at the level of the abdomen 8-fold (Stratakis et al. 2006). A comparison of radiation doses to the operator’s hands during biliary tract procedures, TIPS, angioplasty, stent placement, embolization, angiography, and cardiac procedures revealed that biliary tract procedures resulted in the highest hand doses. This was attributed to the proximity of the operators’ hands to the x-ray field during catheter manipulation (Martin and Whitby 2003). Radiation dose to the operator’s neck, normalized to KAP, was 7.4 times greater for biliary tract drainage procedures than for other procedures. Again, this was attributed to the very close proximity of the operator’s head and neck to the x-ray field (Williams et al, 1997).
Table 4.
Mean exposure and effective dose to the operator per case from biliary tract procedures
| Author (Publication Year) a | Physician b | No of Cases c | KAP d, l (Gy cm2) | Fluoroscopy Time d (min) | Protective Measures e | Mean Badge Dose per Case (μSv) d, f | Effective Dose k (μSv) | Note | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Apron (mm) | Thyroid Shield (mm) | Hand g | Eye Level h | Neck i | Trunk j
|
||||||||
| Over Apron | Under Apron | ||||||||||||
| Oonsiri et al. (2007) | Radiologist | 9 | 18 (2.8–32.7) | 1.9–14 | O | O | - | 110 (23–282) | 63 (1–200) | - | - | 4.4 | |
| Stratakis et al. (2006) | Radiologist | 35 | 20 | 7.8 | 0.5 | 0.5 | 430 | 83 | 60 | 23 | - | 4.2 | Drainage only, with under-couch shield |
| Stratakis et al. (2006) | Radiologist | - | - | - | 0.5 | 0.5 | - | 180 | 135 | 182 | - | 9.5 | Drainage only, without under-couch shield |
| Stratakis et al. (2006) | Radiologist | 17 | 25 | 11 | 0.5 | 0.5 | 507 | 96 | 70 | 27 | - | 4.9 | Drainage + stenting, with under-couch shield |
| Stratakis et al. (2006) | Radiologist | - | - | - | 0.5 | 0.5 | - | 212 | 160 | 215 | - | 11.2 | Drainage + stenting, without under-couch shield |
| Stratakis et al. (2006) | Radiologist | 19 | 17 | 5.7 | 0.5 | 0.5 | 278 | 72 | 52 | 20 | - | 3.6 | Stenting only, with under-couch shield |
| Stratakis et al. (2006) | Radiologist | - | - | - | 0.5 | 0.5 | - | 159 | 120 | 162 | - | 8.4 | Stenting only, without under-couch shield |
| Martin and Whitby (2003) | Radiologist | 17 | - | - | O | - | 800 (400–550) | - | - | - | - | - | Biliary procedure |
| Whitby and Martin (2003) | Radiologist | 11 | - | - | O | - | 950 | - | - | - | - | - | Biliary procedure |
| Williams (1997) | Radiologist | 86 | 43 (19–61) iq | - | 0.35/0.5 m | - | 105 | - | 38 | - | 2.1 | 4.3 | Biliary drainage |
| Williams (1997) | Radiologist | 74 | 51 (15–63) iq | - | 0.35/0.5 m | - | 124 | - | 45 | - | 2.5 | 5.1 | Biliary drainage + stent |
| Vehmas (1993) | Radiologist | 4 | 18 | 19 | - | - | 228 | - | - | - | - | - | |
| Vehmas and Tikkanen (1992) | Radiologist | 2 | - | 18 | O | - | 367 | - | 28 | - | - | 2.0 | |
| Nowak and Jankowski (1991) | NS | 29 | - | - | 0.25 | - | 488 | 213 | - | - | - | 14.9 | X-ray control of biliary route |
| Ramsdale et al. (1990) | Radiologist | 16 | - | 23 (±16) | - | - | 1290 (±1980) | 310 (±400) | 660 (±1000) | - | - | 46.2 | Biliary drainage and stent |
| Burgess and Burhenne (1984) | Radiologist | 33 | - | 5 | - | - | 600 | - | - | - | - | - | Biliary procedures |
See footnotes to Table 1.
Kerma-area product.
One operator wore an apron of 0.5 mm lead-equivalent thickness. The others wore aprons of 0.35 mm lead-equivalent thickness.
Table 5 summarizes radiation dose to operators during TIPS, a procedure in which a new vascular channel is created in the liver between the portal vein and a hepatic vein. The procedure is performed by interventional radiologists under fluoroscopic guidance. The procedure requires long fluoroscopy times, ranging from 32 – 78 (median = 59) min per case. As a result, effective dose estimates are among the highest for the procedures reviewed in this study, ranging from 2.5 – 74 (median = 17) μSv per case. Although the operator’s hands are relatively far from the x-ray field, the long fluoroscopy time for the procedure results in substantial hand doses, e.g., 447 –1350 (median = 935) μSv per case. The range of radiation doses measured at the level of the body and the head was 35 – 589 (median = 205) μSv. Comparison of radiation doses for two different fluoroscopy systems, where manual adjustment of fluoroscopy peak potential and tube current setting was possible for one system but not the other, demonstrated that increasing tube potential and lowering tube current resulted in a significant dose reduction for patient and staff (Zweers et al. 1998).
Table 5.
Mean exposure and effective dose to the operator per case from transjugular intrahepatic portosystemic shunt creation
| Author (Publication Year) a | Physician b | No of Cases c | KAP d, l (Gy cm2) | Fluoroscopy Time d (min) | Protective Measures e | Mean Badge Dose per Case (μSv) d, f | Effective Dose k (μSv) | Note | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Apron (mm) | Thyroid Shield (mm) | Hand g | Eye Level h | Neck i | Trunk j
|
||||||||
| Over Apron | Under Apron | ||||||||||||
| Pinto et al. (2007) | Radiologist | 12 | 340 | - | - | - | 1350 (900–1750) | - | - | - | - | - | |
| Hidajat et al. (2006) | Radiologist | 18 | 446 (±280) | 77.8 (±66.3) | 0.35 | X | - | 403 (±328) | 589 (±721) | - | 41 (±25) | 73.9 | |
| Martin and Whitby (2003) | Radiologist | 17 | - | - | O | - | 900 (50–2000) | - | - | - | - | - | |
| Whitby and Martin (2003) | Radiologist | 15 | - | - | O | - | 970 | - | - | - | - | - | |
| Zweers et al. (1998) | Radiologist | 14 | 226 (111–354) | 32 (9–79) | 0.5 | O | - | - | - | 205 (92–495) md | - | 14.4 | Automatic kV and mAs |
| Zweers et al. (1998) | Radiologist | 9 | 77 (7–240) | 59 (26–115) | 0.5 | O | - | - | - | 35 (18–177) md | - | 2.5 | Adjustment of kV and mAs |
| Williams (1997) | Radiologist | 56 | 182 (103 –237) iq | - | 0.35/0.5 m | - | 447 | - | 162 | - | 9.1 | 18.9 | |
See footnotes to Table 1.
Kerma-area product.
One operator wore an apron of 0.5 mm lead-equivalent thickness. The others wore aprons of 0.35 mm lead-equivalent thickness.
Table 6 summarizes radiation dose to operators for head/neck endovascular therapeutic procedures. These procedures are performed by neuroradiologists and neurosurgeons and include vascular embolization to treat tumors and some vascular disorders (e.g., aneurysms, arteriovenous malformations), and thrombolytic and other procedures to treat other vascular disorders (e.g., arterial stenosis, stroke). Vascular procedures performed in the head and neck can be diagnostic or therapeutic. Both kinds of procedures demonstrate substantial variability in radiation dose to the operator (data not shown for diagnostic procedures). In one study, radiation doses for embolization were approximately 2-fold greater than for cerebral angiography (Marshall et al. 1995). There are limited dosimetry data on the radiation exposure of operators who perform therapeutic head and neck vascular procedures. The complexity of many of these procedures results in lengthy fluoroscopy time, with mean fluoroscopy times ranging from 35 –100 (median = 60) min per case. The operator’s hands are located relatively far from the x-ray field. Radiation doses measured at the level of the hand ranged from 71 to 208 (median = 197)μSv per case. Radiation doses measured at the level of the body and the head ranged from 25 to 337 (median = 98) μSv per case. Effective dose estimates ranged from 1.8 – 53 (median = 5.2)μSv per case.
Table 6.
Mean exposure and effective dose to the operator per case from head/neck endovascular therapeutic procedures
| Author (Publication Year) a | Physician b | No of Cases c | KAP d, l (Gy cm2) | Fluoroscopy Time d (min) | Protective Measures e | Mean Badge Dose per Case (μSv) d, f | Effective Dose k (μSv) | Note | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Apron (mm) | Thyroid Shield (mm) | Hand g | Eye Level h | Neck i | Trunk j
|
||||||||
| Over Apron | Under Apron | ||||||||||||
| Moritake et al. (2008) | NS | 25 | - | 56 (±37) | 0.2 | 0.2 | 208 (±341) | 254 (±338) | 72 (±71) | 152 (±260) | 9 (±21) | 17.6 | Neurointerventional procedures |
| Persliden (2005) | NS | 4 | 251 (106–433) | 100 (52–172) | 0.5 | O | - | - | - | - | - | 4.5 | Neuro-cranial procedures |
| Kemerink et al. (2002) | Radiologist | 31 | 228 (±131) | 35 (±13) | 0.35 | 0.5/X m | 71 (±46) | 79 (±52) | 74 (±59) | - | - | 5.2 | Neurointerventional procedures |
| Marshall et al. (1995) | Radiologist | 15 | 122 | - | 0.35 | 0.35 | - | - | - | 25 (14–53) iq | - | 1.8 | Arterial embolization |
| Kuwayama et al. (1994) | NS | 15 | - | 73 (±24) | O | O | - | 337 (±234) | 297 (±256) | - | 37 (±126) | 52.6 | Endovascular surgery of head and neck |
| Berthelsen et al. (1991) | Radiologist | 5 | - | 60 (±27) | 0.3 | - | 197 (±190) | 116 (±71) | 74 (±32) | - | - | 5.2 | Embolization of intracerebral arteriovenous malformation |
See footnotes to Table 1.
Kerma-area product.
One operator usually wore a thyroid shield of 0.5 mm lead-equivalent thickness. The other operators did not wear a thyroid shield.
Table 7 summarizes radiation dose to operators for ERCP, which combines the use of endoscopy and fluoroscopy to diagnose and treat certain obstructions and other disorders of the biliary and pancreatic ductal systems. These procedures are performed by endoscopists, primarily gastroenterologists. The operator can visualize the stomach and duodenum through the endoscope, and can inject contrast material into the biliary and pancreatic ducts so that they can be seen on x-rays. ERCP can be diagnostic or therapeutic. Radiation doses to operators and patients are higher for therapeutic than for diagnostic ERCP procedures, because the former are more complex, and require more fluoroscopy time (Chen et al. 1996, Olgar et al. 2009). In the dosimetry studies examined, fluoroscopy time was relatively short, ranging from 5 to 12 (median = 8) min per case. Radiation doses measured at the level of the hands ranged from <30 to 835 (median = 640) μSv per case and doses measured at the level of the body and the head ranged from 3 to 550 (median = 32) μSv per case. The limited number of studies revealed 10-fold differences in hand dose compared with doses to the body and head (Buls et al. 2002, Olgar et al. 2009). Substantially higher radiation doses were reported in studies in which over-couch fluoroscopy systems were used (Buls et al. 2002, Naidu et al. 2005). Effective dose estimates ranged from 0.2 – 49 (median = 1.1) μSv per case.
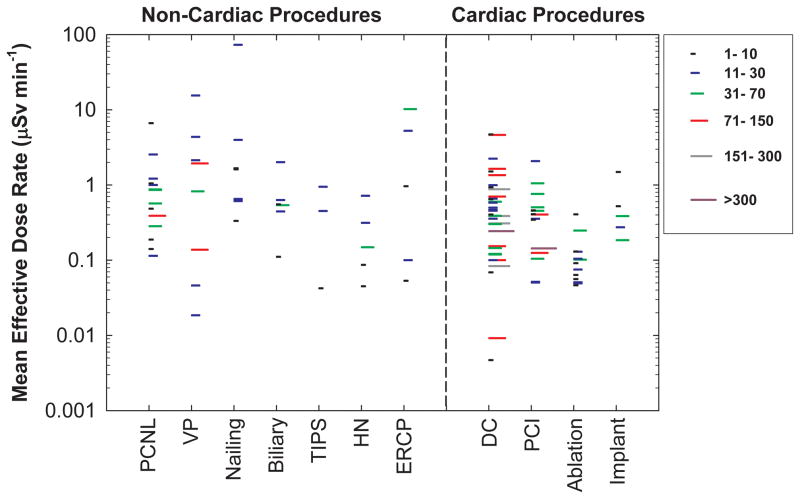
Figure 1 presents effective dose estimates, by procedure type, for the non-cardiac procedures included in the current study. For comparison, we also include radiation doses for cardiac procedures from our earlier study (Kim et al. 2008). The length of each line represents the number of cases in each report. We did not find any relationship between radiation dose and study size. Reported radiation doses varied by 1 – 3 orders of magnitude among studies. Since the data shown are the mean effective dose estimates from each study, not the range of individual measurements, the variation for individual measurements is even greater. Even within the same institution, for a given procedure there was a wide variation in individual measurements. Not uncommonly this variation was as much as 10-fold. Comparisons of mean values should be made with caution because the exposure conditions are specific to each procedure type and each published report. Direct comparisons are most appropriate when comparing doses for the same procedure and under similar exposure conditions.
Figure 1.
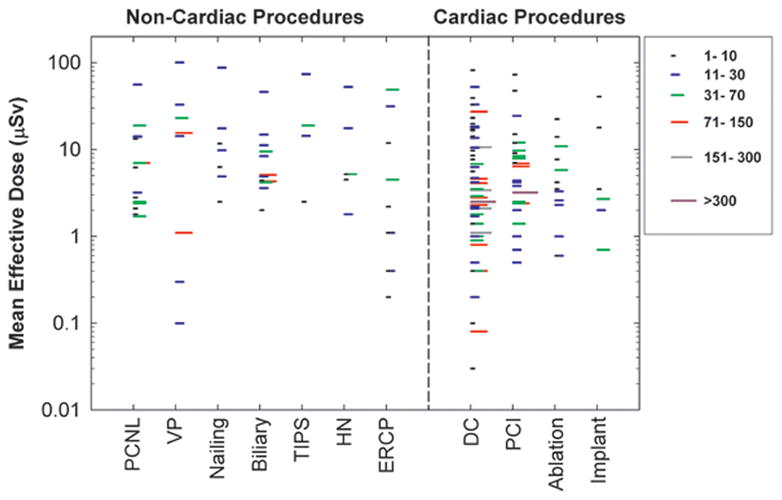
Mean effective dose estimates per case for operators performing various types of FG procedures. Each line represents the mean value from one published study under similar exposure conditions. The length of each line represents the number of cases in each study. Effective dose estimates for cardiac procedures are also depicted for comparison. PCNL (percutaneous nephrolithotomy), VP (Vertebroplasty), TIPS (transjugular intrahepatic portosystemic shunt creation), HN (Head/neck endovascular therapeutic procedures), ERCP (endoscopic retrograde cholangiopancreatography), DC (diagnostic catheterization), PCI (percutaneous coronary intervention).
Figure 2 presents operator effective dose normalized by patient dose (as fluoroscopy time). Even with normalization, wide variations in operator dose were observed. Operator effective dose normalized by fluoroscopy time varied by several orders of magnitude, ranging from 0.02μSv min−1 to 73μSv min−1depending on the study. The median values for mean effective dose rate were 1.6 μSv min−1 for orthopedic extremity nailing, 1.4μSv min−1 for vertebroplasty, 1.0 μSv min−1 for ERCP, 0.7 μSv min−1 for PCNL, 0.5 μSv min−1 for biliary tract procedures, 0.5 μSv min−1 for TIPS, and 0.1 μSv min−1 for head/neck endovascular therapeutic procedures. In comparison, the median values for mean effective dose rate for cardiac procedures are generally lower (0.4 μSv min−1 for DC, 0.4 μSv min−1 for PCI and implant, and 0.1 μSv min−1 for ablation) than those for non-cardiac procedures (Kim et al. 2008).
Figure 2.
Mean effective dose rate. Effective dose rate estimates are normalized by fluoroscopy time. Each line represents the mean value from one published study under similar exposure conditions. The length of each line represents the number of cases in each study. Effective dose rate estimates for cardiac procedures are also depicted for comparison. PCNL (percutaneous nephrolithotomy), VP (Vertebroplasty), TIPS (transjugular intrahepatic portosystemic shunt creation), HN (Head/neck endovascular therapeutic procedures), ERCP (endoscopic retrograde cholangiopancreatography), DC (diagnostic catheterization), PCI (percutaneous coronary intervention).
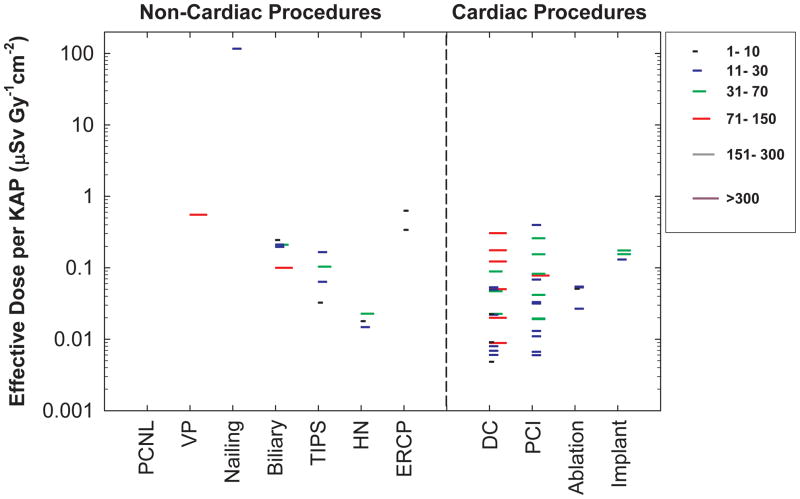
Some studies provided patient dose as KAP. Operator dose normalized by KAP also showed wide variation, ranging from 0.01 μSv Gy−1cm−2 to 0.63μSv Gy−1 cm−2, with the exception of a single outlier (Figure 3). Although the available data are limited, the normalized operator doses for non-cardiac procedures, except for TIPS and head/neck endovascular therapeutic procedures, appear higher than those for cardiac procedures (ranging from 0.006μSv Gy−1 cm−2 to 0.4μSv Gy−1 cm−2).
Figure 3.
Mean effective dose normalized by patient radiation dose (as kerma area product). Each line represents the mean value from one published study under similar exposure conditions. The length of each line represents the number of cases in each study. Data for cardiac procedures are also depicted for comparison. PCNL (percutaneous nephrolithotomy), VP (Vertebroplasty), TIPS (transjugular intrahepatic portosystemic shunt creation), HN (Head/neck endovascular therapeutic procedures), ERCP (endoscopic retrograde cholangiopancreatography), DC (diagnostic catheterization), PCI (percutaneous coronary intervention).
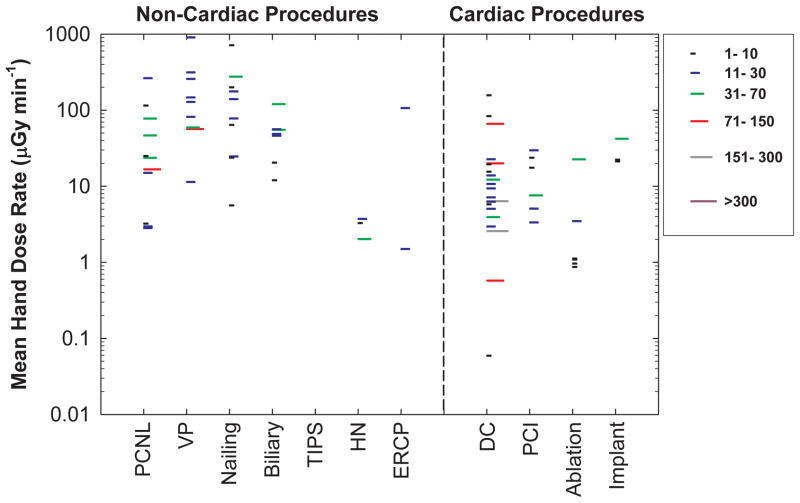
Figure 4 presents operator hand dose normalized by patient dose (as fluoroscopy time). Radiation dose rates to the operator’s hands for non-cardiac procedures demonstrated wide variation, ranging from 1.5 μGy min−1 to 905μGy min−1. The mean hand dose rates for certain non-cardiac procedures (i.e., vertebroplasty, nailing, ERCP, and biliary procedures) exceeded the dose rates for cardiac procedures. Median values of hand dose rates for non-cardiac procedures were 130 μGy min−1 for vertebroplasty, 110μGy min−1 for nailing, 54 μGy min−1 for ERCP, 49μGy min−1 for biliary procedures, 24 μGy min−1 for PCNL, and 3 μGy min−1 for head/neck procedures while the median values for cardiac procedures were 22μGy min−1 for pacemaker implant, 9μGy min−1 for DC, 8μGy min−1 for PCI, and 1μGy min−1 for ablation.
Figure 4.
Mean hand dose rate. Radiation dose rates measured at the operator’s hand are normalized by fluoroscopy time. Each line represents the mean value from one published study under similar exposure conditions. The length of each line represents the number of cases in each study. Data for cardiac procedures are also depicted for comparison. PCNL (percutaneous nephrolithotomy), VP (Vertebroplasty), TIPS (transjugular intrahepatic portosystemic shunt creation), HN (Head/neck endovascular therapeutic procedures), ERCP (endoscopic retrograde cholangiopancreatography), DC (diagnostic catheterization), PCI (percutaneous coronary intervention).
Fluoroscopy time varied with procedure type (Tables 1–7). In general, head/neck endovascular therapeutic procedures (35 – 100 [median = 60] minutes) and TIPS (32 – 78 [median = 59] min) were characterized by relatively long fluoroscopy time whereas PCNL (2 –28 [median = 13] min), biliary tract procedures (5 – 23 [median = 9] min), vertebroplasty (2 – 35 [median = 8] min), ERCP (5 – 12 [median = 8] min), and orthopedic extremity nailing (1 – 15 [median = 4] min) required less fluoroscopy time.
Patient dose, measured as KAP, generally showed a similar relationship with procedure type as did patient dose measured as fluoroscopy time (Tables 1–7). Reported mean KAP values were high for head/neck endovascular therapeutic procedures (120 – 250 [median = 230] Gy·cm2) and TIPS (77 – 450 [median = 230] Gy·cm2) and substantially less for the other procedures: 35 –50 (median=43) Gy·cm2 for ERCP, 17 – 51 (median=20) Gy·cm2 for biliary tract procedures, and 11 – 28 (median=13) Gy·cm2 for vertebroplasty.
Overall, mean operator radiation dose per case measured over personal protective devices at different anatomic sites on the head and body ranged from 19 – 800 (median = 113) μSv at eye level, 6 – 1180 (median = 75) μSv at the neck, and 2 – 1600 (median = 302) μSv at the trunk (Tables 1–7). Radiation doses measured at the hand were notably higher, ranging from 30 – 5800 (median = 450) μSv per case. Under-apron measurements at the trunk yielded the lowest doses, ranging from 0 to 240 (median = 9) μSv per case. The ratios of radiation doses between various anatomic sites were 1.1 ± 0.5 (±1σ) for eye to neck and 1.0 ± 0.5 (±1σ) for trunk to neck. However, the dose ratio between the hand and the eye, neck or trunk was substantially greater, e.g., 5.2 ± 5.7 (±1σ). Especially large differences between hand dose and eye, neck or trunk dose were observed frequently for PCNL, vertebroplasty, orthopedic nailing, and biliary tract procedures. For cardiac procedures, we previously reported that the corresponding average ratios between anatomic sites of the reported doses measured on eye to neck, trunk to neck, and hand to neck were 0.9, 1.0, and 1.3, respectively (Kim et al. 2008).
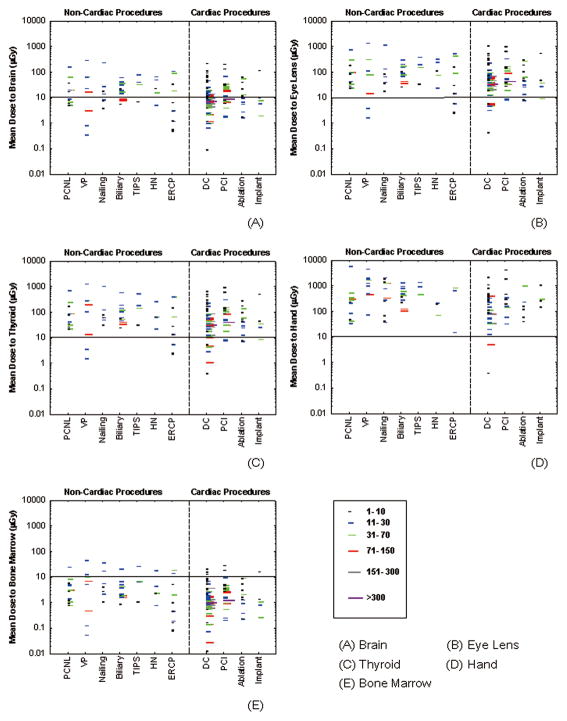
Figure 5 presents data on radiation dose to the brain, the lens of the eye, the thyroid, the hand, and bone marrow. Radiation dose was highest for the hand. Radiation doses to the lens of the eye and the thyroid were comparable to each other and much greater than effective dose, ranging from 1.5 to 1300μSv per case. The radiation dose to the brain was about 5 times smaller than the radiation dose to the lens of the eye, but still an order of magnitude greater than effective dose. The radiation dose to bone marrow was comparable to effective dose because most bone marrow (about 83%) is well protected by lead aprons (Boothroyd and Russell 1987, Simon 2011). The small fraction of bone marrow (about 17%) unprotected by the lead apron receives relatively high radiation doses.
Figure 5.
Mean organ dose estimates per case for operators performing various types of FG procedures. Each line represents the mean value from one published study under similar exposure conditions. The length of each line represents the number of cases in each study. Organ dose estimates for cardiac procedures are also depicted for comparison. PCNL (percutaneous nephrolithotomy), VP (Vertebroplasty), TIPS (transjugular intrahepatic portosystemic shunt creation), HN (Head/neck endovascular therapeutic procedures), ERCP (endoscopic retrograde cholangiopancreatography), DC (diagnostic catheterization), PCI (percutaneous coronary intervention).
DISCUSSION
Our comprehensive literature search for reports on radiation dose to operators who perform FG procedures revealed relatively few reports for non-cardiac FG procedures. Substantially more studies assessing occupational radiation doses have been reported for cardiac than for non-cardiac FG procedures (Padovani and Rodella 2001, Tsapaki et al. 2004, Lange and von Boetticher 2006). Cardiac FG procedures are more commonly performed than most non-cardiac FG procedures.
The non-cardiac procedures that met our criteria included PCNL, vertebroplasty, orthopedic extremity nailing, biliary tract procedures, TIPS, head/neck endovascular therapeutic procedures and ERCP. Radiation doses to operators performing these non-cardiac FG procedures varied by 1 to 3 orders of magnitude (10s – 1000s of times), depending on the type of procedure. While the average operator dose was quantitatively related to the average patient dose, we observed much greater variation in operator doses than in patient doses, as we previously reported for cardiac procedures (Kim et al. 2008). Longer fluoroscopy times and greater KAP were observed for head/neck endovascular therapeutic procedures and TIPS as compared with the other non-cardiac procedures. Radiation doses measured at the eye, neck, and trunk outside protective equipment were comparable. Radiation doses to operators’ hands were often much higher than those to the operator’s head or trunk.
We observed wide variations in operator dose within published reports as well as among reports. For a given procedure, the radiation dose to the operator varies, depending on factors such as patient characteristics, lesion characteristics, the experience and skill of the operator, and characteristics of the fluoroscopic equipment and its operation (Pantos et al. 2009). These dose-influencing factors may result in differences in fluoroscopy time, variation in the need for imaging during a procedure, and other determinants of differing radiation exposure to patients and associated differences in levels of radiation exposure to operators. For individual procedure types, occupational dose from FG procedures is strongly related to patient dose as fluoroscopy time (Delichas et al. 2003, Vano et al. 2009). However, variations in patient dose, as KAP or as fluoroscopy time, do not fully explain the greater variation in operator dose. For the same fluoroscopy time or KAP, data from our review revealed that occupational dose still varied widely (Figures 2 and 3).
Some dose-influencing factors affect both patient and operator dose (patient characteristics, lesion characteristics, the experience and skill of the operator, characteristics of the fluoroscopic equipment and its operation) and some factors affect only operator dose (operator position, use of protective measures such as protective garments and shielding). A shorter fluoroscopy time for certain non-cardiac FG procedures does not necessarily result in a lower radiation dose to the operator if the procedure requires the operator to stay in very close proximity to the x-ray field (Whitby and Martin 2005). In general, the distance between the operator and the patient during cardiac procedures is greater than that for many non-cardiac procedures (Vano et al. 1998b).
Factors that affect only operator dose are the principal causes for the wide variation in operator dose normalized by patient dose. Kim and Miller determined that operator dose could change several-fold depending on the operator’s position with respect to the patient, and up to an order of magnitude depending on the use of radiation shielding (Kim and Miller 2009). In addition, an operator’s awareness of radiation exposure could result in a marked decrease in his or her occupational dose (Kim et al. 2010).
We observed variation in KAP and in fluoroscopy time for the same procedure, although KAP data were limited in the reports we evaluated. This is consistent with the findings in other, larger studies of patient radiation dose. In an observational study of patient doses in interventional radiology procedures carried out at seven academic medical centers in the U.S. (Miller et al. 2003a, Miller et al. 2003b, Balter et al. 2004), Miller et al. found wide variations in KAP and fluoroscopy time. For example, the fluoroscopy time for TIPS ranged from 3.5 to 153 (mean = 39) min for 135 cases and KAP ranged from 14 to 1364 (mean = 335) Gy·cm2 for 135 cases. Based on KAP and fluoroscopy time in the current review, patient doses from FG procedures can be grouped into two patient dose groups. Head/neck therapeutic procedures and TIPS were associated with greater patient dose, while PCNL, vertebroplasty, nailing, biliary procedures, and ERCP were associated with low or moderate patient dose. However, it should be noted that the same KAP or fluoroscopy time may result in orders of magnitude differences in radiation doses to operators, depending on the effect of factors that influence operator dose (Hirshfeld et al. 2004, Kim and Miller 2009).
Radiation doses to the eye, neck, and trunk measured outside aprons or shields during FG procedures were comparable. The higher doses to operators’ hands observed for PCNL, vertebroplasty, orthopedic nailing, and biliary tract procedures can be attributed to the location of the operator’s hands with respect to the primary x-ray beam during these FG procedures. Operators perform these procedures with their hands relatively close to the x-ray field, in contrast to the location of the operator’s hands during head and neck procedures, TIPS, and cardiac procedures, where they are relatively far from the x-ray field. During PCNL, vertebroplasty, orthopedic nailing and biliary tract procedures, the operator may place his or her hands within the primary beam. The radiation dose to hands placed within the primary beam is substantially greater than the radiation dose to hands exposed for the same period of time to scatter radiation.
In this study, effective doses to operators were estimated using dose measurements and algorithms derived from the literature. Although estimated effective dose is useful for comparing doses from different FG procedures and for comparing radiation doses reported in different publications for the same types of FG procedures, effective dose does not describe the actual dose received by any particular organ or tissue. Because the operator’s organs and tissues receive heterogeneous radiation exposure during an FG procedure, radiation doses to specific organs are generally not well represented by effective dose. As a result, cancer risk to any specific tissue cannot be estimated. During FG procedures, the radiation dose to the hands, brain, lens of the eye, thyroid, and skin of the head and neck can be high. The hands are located close to or within the x-ray field and the other organs and tissues are generally unshielded or only partially shielded. Because of the relatively large doses to these organs and structures, they are at greater risk of stochastic effects than is suggested by the operator’s effective dose. The hands and lens of the eye are also at risk for deterministic effects (Dauer et al. 2010).
Radiation dose to the lens of the eye has been a topic of interest and concern. Recent publications have highlighted epidemiologic evidence supporting a lower threshold dose (and potentially no dose threshold) for radiation-induced cataracts than previously suspected (Kleiman 2007, Shore et al. 2010). The IAEA has coordinated surveys in Latin America and Asia of cardiologists and support staff working in catheterization laboratories. These surveys found that a high percentage of cardiologists and support staff had lens opacities characteristic of radiation exposure and attributable to occupational radiation exposure (Vano et al. 2008, Ciraj-Bjelac et al. 2010, Vano et al. 2010). As a result, the International Commission on Radiological Protection (ICRP) recently lowered the recommended annual dose limit for the lens of the eye (ICRP 2011b).
A limitation of our study was the difficulty of comparing dosimetry results from different studies. We found differences in the dosimetry methods used and often an absence of information associated with operator dose. Future studies on occupational exposure from FG procedure could benefit from standardization of dose estimation methods and detailed reporting of related information. It would be helpful for characterization of operator doses and for radiation protection purposes if there was standardization in the placement and numbers of personal dosimeters used. Another limitation was the paucity of KAP data in the studies we reviewed. As a result, the graph on operator doses normalized by KAP contains relatively little data.
Another potential limitation of our study is our assumption regarding the use of thyroid shields. We estimated effective dose and organ doses in order to compare doses reported in different studies. To do this, we assumed that thyroid shields were not used. This assumption could be a potential source of error in dose estimation. If a thyroid shield is used during a procedure, the radiation dose to the thyroid is substantially reduced and effective dose is reduced by about 50% (Niklason et al. 1994). For typical fluoroscopy beam energies, a 0.5 mm lead equivalent thyroid shield provides a reduction in thyroid exposure of more than 95% (Yaffe et al. 1991, Murphy et al. 1993, von Boetticher et al. 2009).
The number of cases in the studies included in our review of operator doses from non-cardiac procedures ranged from 2 to 136. We found that reported radiation doses varied widely. The data do not permit characterization of operator dose on a national or international basis. Larger dosimetry studies are needed to provide sufficient information to understand exposure conditions under different working conditions. A well-quantified relationship between dose-influencing factors and occupational dose could provide valuable insights to help optimize radiation protection. This quantification can be achieved through dosimetry standardization and systematic collection of data on dose-influencing factors.
Our finding of large variations in operator doses associated with the same patient dose suggests that radiation doses to operators during FG procedures could be substantially reduced with improved radiation protection practices. Operators who perform FG procedures with their hands close to the x-ray field should be careful to avoid positioning their hands within the primary beam during the procedure. Extremity dosimeters can provide useful information about doses to operators’ hands.
The studies we identified did not provide data that would enable estimation of cumulative dose, thereby impeding our ability to estimate typical annual or lifetime doses. We found that most studies provided radiation dose per case rather than annual or cumulative dose. A key difficulty in estimating physicians’ annual or lifetime cumulative doses from personal badges is the absence of a nationwide radiation dose registry or repository for badge readings. In the absence of such a registry, it is often not possible to obtain complete film badge data for individual operators. Another critical problem limiting determination of cumulative doses is the likely underestimation of doses for the unknown but non-trivial proportion of physicians who do not wear film badges consistently (Marx et al. 1992, Padovani et al. 2011).
Despite the increasing number of FG procedures, the high radiation dose from FG procedures, and the wide variation in radiation dose for the same type of procedure, national and international radiation protection organizations recommend that physicians who perform FG procedures be trained in radiation protection and radiation management, with regular refresher training (ICRP 2009, NCRP 2010). Currently, however, this training may not be easily available or provided to physician specialists other than radiologists. A growing number of non-radiologist physicians are performing FG procedures (ICRP 2011a). These physicians often lack knowledge in key areas of radiation science, including radiation dose management and radiation protection. These physicians need to be informed about their radiation dose, the key factors influencing their dose and those radiation protection measures that can reduce their dose. It has been shown that increasing operator awareness can lead to marked decreases in occupational dose (Pitney et al. 1994, Huyskens and Hummel 1995). Increasing physicians’ awareness of radiation dose levels, determinants of dose, and protective measures to reduce dose can be improved by providing regular training in radiation protection.
CONCLUSION
Occupational radiation dose to operators who perform selected non-cardiac FG procedures varied over a range of one to three orders of magnitude for a given procedure. The estimated occupational effective doses per case for these physicians were equivalent to those received by interventional cardiologists. Radiation doses to the operator’s hands, brain, lens of the eye and thyroid from non-cardiac procedures are much greater than the operator’s effective dose because the operator’s hands are often close to or within the direct beam, and the brain, lens of the eye and (if no thyroid shield is worn) the thyroid are typically less well shielded during FG procedures. Because of the relatively larger doses to these organs and structures, they are at greater risk of stochastic effects than is suggested by the operator’s effective dose. Large variations in operator dose for the same type of procedure suggest that optimizing procedure protocols and the use of protective measures might reduce occupational radiation doses substantially. Optimization and improved radiation protection measures can be achieved through continuing education and training of physicians in radiation physics and radiation protection.
Acknowledgments
Funding
This study was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health
References
- Arnstein PM, Richards AM, Putney R. The Risk from Radiation Exposure during Operative X-Ray Screening in Hand Surgery. Journal of Hand Surgery-British and European. 1994;19B:393–396. doi: 10.1016/0266-7681(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Balter S, Schueler BA, Miller DL, Cole PE, Lu HT, Berenstein A, Albert R, Georgia JD, Noonan PT, Russell EJ, Malisch TW, Vogelzang RL, Geisinger M, Cardella JF, St George J, Miller GL, Anderson J. Radiation doses in interventional radiology procedures: The RAD-IR study Part III: Dosimetric performance of the interventional fluoroscopy units. Journal of Vascular and Interventional Radiology. 2004;15:919–926. doi: 10.1097/01.RVI.0000130864.68139.08. [DOI] [PubMed] [Google Scholar]
- Berthelsen B, Cederblad A. Radiation-Doses to Patients and Personnel Involved in Embolization of Intracerebral Arteriovenous-Malformations. Acta Radiologica. 1991;32:492–497. [PubMed] [Google Scholar]
- Bhargavan M. Trends in the utilization of medical procedures that use ionizing radiation. Health Physics. 2008;95:612–27. doi: 10.1097/01.HP.0000327659.42618.c1. [DOI] [PubMed] [Google Scholar]
- Blattert TR, Fill UA, Kunz E, Panzer W, Weckbach A, Regulla DF. Skill dependence of radiation exposure for the orthopaedic surgeon during interlocking nailing of long-bone shaft fractures: a clinical study. Archives of Orthopaedic and Trauma Surgery. 2004;124:659–664. doi: 10.1007/s00402-004-0743-9. [DOI] [PubMed] [Google Scholar]
- Boothroyd AE, Russell JG. The lead apron: room for improvement? Br J Radiol. 1987;60:203–4. doi: 10.1259/0007-1285-60-710-203. [DOI] [PubMed] [Google Scholar]
- Bowsher WG, Blott P, Whitfield HN. Radiation Protection in Percutaneous Renal Surgery. British Journal of Urology. 1992;69:231–233. doi: 10.1111/j.1464-410x.1992.tb15518.x. [DOI] [PubMed] [Google Scholar]
- Buls N, Pages J, Mana F, Osteaux M. Patient and staff exposure during endoscopic retrograde cholangiopancreatography. British Journal of Radiology. 2002;75:435–443. doi: 10.1259/bjr.75.893.750435. [DOI] [PubMed] [Google Scholar]
- Burgess AE, Burhenne HJ. Finger Doses in Special Procedures. British Journal of Radiology. 1984;57:650–651. doi: 10.1259/0007-1285-57-679-650. [DOI] [PubMed] [Google Scholar]
- Bush WH, Brannen GE, Gibbons RP, Correa RJ, Elder JS. Radiation Exposure to Patient and Urologist during Percutaneous Nephrostolithotomy. Journal of Urology. 1984;132:1148–1152. doi: 10.1016/s0022-5347(17)50071-3. [DOI] [PubMed] [Google Scholar]
- Bush WH, Jones D, Brannen GE. Radiation-Dose to Personnel during Percutaneous Renal Calculus Removal. American Journal of Roentgenology. 1985;145:1261–1264. doi: 10.2214/ajr.145.6.1261. [DOI] [PubMed] [Google Scholar]
- Chen MYM, VanSwearingen FL, Mitchell R, Ott DJ. Radiation exposure during ERCP: Effect of a protective shield. Gastrointestinal Endoscopy. 1996;43:1–5. doi: 10.1016/s0016-5107(96)70250-x. [DOI] [PubMed] [Google Scholar]
- Ciraj-Bjelac O, Rehani MM, Sim KH, Liew HB, Vano E, Kleiman NJ. Risk for radiation-induced cataract for staff in interventional cardiology: is there reason for concern? Catheter Cardiovasc Interv. 2010;76:826–34. doi: 10.1002/ccd.22670. [DOI] [PubMed] [Google Scholar]
- Clerinx P, Buls N, Bosmans H, de Mey J. Double-dosimetry algorithm for workers in interventional radiology. Radiat Prot Dosimetry. 2008;129:321–7. doi: 10.1093/rpd/ncn148. [DOI] [PubMed] [Google Scholar]
- Coetzee JC, van der Merwe EJ. Exposure of surgeons-in-training to radiation during intramedullary fixation of femoral shaft fractures. S Afr Med J. 1992;81:312–4. [PubMed] [Google Scholar]
- Cohen G, Brodmerkel GJ, Lynn S. Absorbed doses to patients and personnel from endoscopic retrograde cholangiopancreatographic (ERCP) examinations. Radiology. 1979;130:773–775. doi: 10.1148/130.3.773. [DOI] [PubMed] [Google Scholar]
- Cohen RV, Aldred MA, Paes WS, Fausto AM, Nucci JR, Yoshimura EM, Okuno E, Garcia MEM, Tolosa LMEM. How safe is ERCP to the endoscopist? Surgical Endoscopy. 1997;11:615–617. doi: 10.1007/s004649900405. [DOI] [PubMed] [Google Scholar]
- Cristy M. Active bone marrow distribution as a function of age in humans. Physics in Medicine and Biology. 1981;26:389–400. doi: 10.1088/0031-9155/26/3/003. [DOI] [PubMed] [Google Scholar]
- Dauer LT, Thornton RH, Solomon SB, St Germain J. Unprotected operator eye lens doses in oncologic interventional radiology are clinically significant: estimation from patient kerma-area-product data. Journal of Vascular and Interventional Radiology. 2010;21:1859–61. doi: 10.1016/j.jvir.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Delichas M, Psarrakos K, Molyvda-Athanassopoulou E, Giannoglou G, Sioundas A, Hatziioannou K, Papanastassiou E. Radiation exposure to cardiologists performing interventional cardiology procedures. European Journal of Radiology. 2003;48:268–273. doi: 10.1016/s0720-048x(03)00007-x. [DOI] [PubMed] [Google Scholar]
- Derdeyn CP, Moran CJ, Eichling JO, Cross DT. Radiation dose to patients and personnel during intraoperative digital subtraction angiography. American Journal of Neuroradiology. 1999;20:300–305. [PMC free article] [PubMed] [Google Scholar]
- Eagan JT, Jr, Jones CT. Cutaneous cancers in an interventional cardiologist: a cautionary tale. J Interv Cardiol. 2010;24:49–55. doi: 10.1111/j.1540-8183.2010.00610.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM. Is brain cancer an occupational disease of cardiologists? Canadian Journal of Cardiology. 1998;14:1385–8. [PubMed] [Google Scholar]
- Fitousi NT, Efstathopoulos EP, Delis HB, Kottou S, Kelekis AD, Panayiotakis GS. Patient and staff dosimetry in vertebroplasty. Spine (Phila Pa 1976) 31:E884–9. doi: 10.1097/01.brs.0000244586.02151.18. discussioin E890; 2006. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Schmid A, Eiteljorge T, Modler M, Sturmer KM. Exposure of the surgeon to radiation during surgery. International Orthopaedics. 1998;22:153–156. doi: 10.1007/s002640050230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine (Phila Pa 1976) 2001;26:1511–5. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- Geterud K, Larsson A, Mattsson S. Radiation-Dose to Patients and Personnel during Fluoroscopy at Percutaneous Renal Stone Extraction. Acta Radiologica. 1989;30:201–206. [PubMed] [Google Scholar]
- Goldstone KE, Wright IH, Cohen B. Radiation exposure to the hands of orthopaedic surgeons during procedures under fluoroscopic X-ray control. Br J Radiol. 1993;66:899–901. doi: 10.1259/0007-1285-66-790-899. [DOI] [PubMed] [Google Scholar]
- Hafez MA, Smith RM, Matthews SJ, Kalap G, Sherman KP. Radiation exposure to the hands of orthopaedic surgeons: are we underestimating the risk? Arch Orthop Trauma Surg. 2005;125:330–5. doi: 10.1007/s00402-005-0807-5. [DOI] [PubMed] [Google Scholar]
- Hardell L, Mild KH, Pahlson A, Hallquist A. Ionizing radiation, cellular telephones and the risk for brain tumours. European Journal of Cancer Prevention. 2001;10:523–529. doi: 10.1097/00008469-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Harstall R, Heini PF, Mini RL, Orler R. Radiation exposure to the surgeon during fluoroscopically assisted percutaneous vertebroplasty - A prospective study. Spine. 2005;30:1893–1898. doi: 10.1097/01.brs.0000174121.48306.16. [DOI] [PubMed] [Google Scholar]
- Hellawell GO, Mutch SJ, Thevendran G, Wells E, Morgan RJ. Radiation exposure and the urologist: What are the risks? Journal of Urology. 2005;174:948–952. doi: 10.1097/01.ju.0000170232.58930.8f. [DOI] [PubMed] [Google Scholar]
- Heyd RL, Kopecky KK, Sherman S, Lehman GA, Stockberger SM. Radiation exposure to patients and personnel during interventional ERCP at a teaching institution. Gastrointestinal Endoscopy. 1996;44:287–292. doi: 10.1016/s0016-5107(96)70166-9. [DOI] [PubMed] [Google Scholar]
- Hidajat N, Wust P, Felix R, Schroder RJ. Radiation exposure to patient and staff in hepatic chemoembolization: risk estimation of cancer and deterministic effects. Cardiovasc Intervent Radiol. 2006;29:791–6. doi: 10.1007/s00270-005-0247-1. [DOI] [PubMed] [Google Scholar]
- Hirshfeld JW, Jr, Balter S, Brinker JA, Kern MJ, Klein LW, Lindsay BD, Tommaso CL, Tracy CM, Wagner LK, Creager MA, Elnicki M, Lorell BH, Rodgers GP, Weitz HH. ACCF/AHA/HRS/SCAI clinical competence statement on physician knowledge to optimize patient safety and image quality in fluoroscopically guided invasive cardiovascular procedures. A report of the American College of Cardiology Foundation/American Heart Association/American College of Physicians Task Force on Clinical Competence and Training. Journal of the American College of Cardiology. 2004;44:2259–82. doi: 10.1016/j.jacc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Huyskens CJ, Hummel WA. Data-Analysis on Patient Exposures in Cardiac Angiography. Radiation Protection Dosimetry. 1995;57:475–480. [Google Scholar]
- ICRP. Anatomical and physiological data for use in radiological protection. Part 1. Skeleton. International Commission on Radiological Protection. ICRP Publication 70. Ann ICRP. 1995;25(2) [PubMed] [Google Scholar]
- ICRP. Annals of the ICRP. 5. Vol. 39. New York, NY: International Commission on Radiological Protection; 2009. Education and training in radiological protection for diagnostic and interventional procedures. ICRP 113. [DOI] [PubMed] [Google Scholar]
- ICRP. [Accessed December 10, 2011.];Draft report for consultation: radiological protection in fluoroscopically guided procedures performed outside the imaging department. [online]. Available at: http://www.icrp.org/page.asp?id=126.
- ICRP. [Accessed December 10, 2011.];ICRP statement on tissue reactions. [online]. Available at: http://icrp.org/news.asp.
- ICRU. Quantities and units in radiation protection dosimetry. Vol. 51. Bethesda, MD: International Commission on Radiation Units and Measurements ICRU; 1993. [Google Scholar]
- Inglis JA, Tolley DA, Law J. Radiation safety during percutaneous nephrolithotomy. Br J Urol. 1989;63:591–3. doi: 10.1111/j.1464-410x.1989.tb05251.x. [DOI] [PubMed] [Google Scholar]
- Jarvinen H, Buls N, Clerinx P, Jansen J, Miljanic S, Nikodemova D, Ranogajec-Komor M, d’Errico F. Overview of double dosimetry procedures for the determination of the effective dose to the interventional radiology staff. Radiat Prot Dosimetry. 2008;129:333–9. doi: 10.1093/rpd/ncn082. [DOI] [PubMed] [Google Scholar]
- Kallmes DF, OE, Roy SS, Piccolo RG, Marx WF, Lee JK, Jensen ME. Radiation dose to the operator during vertebroplasty: Prospective comparison of the use of 1-cc syringes versus an injection device. American Journal of Neuroradiology. 2003;24:1257–1260. [PMC free article] [PubMed] [Google Scholar]
- Kemerink GJ, Frantzen MJ, Oei K, Sluzewski M, van Rooij WJ, Wilmink J, van Engelshoven JMA. Patient and occupational dose in neurointerventional procedures. Neuroradiology. 2002;44:522–528. doi: 10.1007/s00234-002-0780-4. [DOI] [PubMed] [Google Scholar]
- Kim C, Vasaiwala S, Haque F, Pratap K, Vidovich MI. Radiation safety among cardiology fellows. American Journal of Cardiology. 2010;106:125–128. doi: 10.1016/j.amjcard.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Kim KP, Miller DL. Minimising radiation exposure to physicians performing fluoroscopically guided cardiac catheterisation procedures: a review. Radiat Prot Dosimetry. 2009;133:227–33. doi: 10.1093/rpd/ncp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KP, Miller DL, Balter S, Kleinerman RA, Linet MS, Kwon D, Simon SL. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Physics. 2008;94:211–27. doi: 10.1097/01.HP.0000290614.76386.35. [DOI] [PubMed] [Google Scholar]
- Kirousis G, Delis H, Megas P, Lambiris E, Panayiotakis G. Dosimetry during intramedullary nailing of the tibia. Acta Orthop. 2009;80:568–72. doi: 10.3109/17453670903350057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman NJ. Radiation cataract. Radiation Protection 145 - EU Scientific Seminar 2006 New insights in radiation risk and basic safety standards. 2007:81–95. [Google Scholar]
- Ko R, Soucy F, Denstedt JD, Razvi H. Percutaneous nephrolithotomy made easier: a practical guide, tips and tricks. BJU Int. 2008;101:535–9. doi: 10.1111/j.1464-410X.2007.07259.x. [DOI] [PubMed] [Google Scholar]
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: Part 2, Review of 73 cases and recommendations for minimizing dose delivered to patient. American Journal of Roentgenology. 2001;177:13–20. doi: 10.2214/ajr.177.1.1770013. [DOI] [PubMed] [Google Scholar]
- Komemushi A, Tanigawa N, Kariya S, Kojima H, Shomura Y, Sawada S. Radiation exposure to operators during vertebroplasty. Journal of Vascular and Interventional Radiology. 2005;16:1327–1332. doi: 10.1097/01.RVI.0000179794.65662.01. [DOI] [PubMed] [Google Scholar]
- Krueger KJ, Hoffman BJ. Radiation Exposure during Gastroenterologic Fluoroscopy - Risk Assessment for Pregnant Workers. American Journal of Gastroenterology. 1992;87:429–431. [PubMed] [Google Scholar]
- Kruger R, Faciszewski T. Radiation dose reduction to medical staff during vertebroplasty - A review of techniques and methods to mitigate occupational dose. Spine. 2003;28:1608–1613. [PubMed] [Google Scholar]
- Kumari G, Kumar P, Wadhwa P, Aron M, Gupta NP, Dogra PN. Radiation exposure to the patient and operating room personnel during percutaneous nephrolithotomy. Int Urol Nephrol. 2006;38:207–10. doi: 10.1007/s11255-005-4972-9. [DOI] [PubMed] [Google Scholar]
- Kuon E, Birkel J, Schmitt M, Dahm JB. Radiation exposure benefit of a lead cap in invasive cardiology. Heart. 2003;89:1205–10. doi: 10.1136/heart.89.10.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama N, Takaku A, Endo S, Nishijima M, Kamei T. Radiation Exposure in Endovascular Surgery of the Head and Neck. American Journal of Neuroradiology. 1994;15:1801–1808. [PMC free article] [PubMed] [Google Scholar]
- Lange HW, von Boetticher H. Randomized comparison of operator radiation exposure during coronary angiography and intervention by radial or femoral approach. Catheterization and Cardiovascular Interventions. 2006;67:12–16. doi: 10.1002/ccd.20451. [DOI] [PubMed] [Google Scholar]
- Levin PE, Schoen RW, Browner BD. Radiation Exposure to the Surgeon during Closed Interlocking Intramedullary Nailing. Journal of Bone and Joint Surgery-American Volume. 1987;69A:761–766. [PubMed] [Google Scholar]
- Lowe FC, Auster M, Beck TJ, Chang R, Marshall FF. Monitoring radiation exposure to medical personnel during percutaneous nephrolithotomy. Urology. 1986;28:221–6. doi: 10.1016/0090-4295(86)90047-6. [DOI] [PubMed] [Google Scholar]
- Madan S, Blakeway C. Radiation exposure to surgeon and patient in intramedullary nailing of the lower limb. Injury-International Journal of the Care of the Injured. 2002;33:723–727. doi: 10.1016/s0020-1383(02)00042-6. [DOI] [PubMed] [Google Scholar]
- Marshall NW, Noble J, Faulkner K. Patient and staff dosimetry in neuroradiological procedures. British Journal of Radiology. 1995;68:495–501. doi: 10.1259/0007-1285-68-809-495. [DOI] [PubMed] [Google Scholar]
- Martin CJ, Whitby M. Application of ALARP to extremity doses for hospital workers. Journal of Radiological Protection. 2003;23:405–421. doi: 10.1088/0952-4746/23/4/004. [DOI] [PubMed] [Google Scholar]
- Marx MV, Niklason L, Mauger EA. Occupational radiation exposure to interventional radiologists: a prospective study. Journal of Vascular and Interventional Radiology. 1992;3:597–606. doi: 10.1016/s1051-0443(92)72903-0. [DOI] [PubMed] [Google Scholar]
- Mehdizade A, Lovblad KO, Wilhelm KE, Somon T, Wetzel SG, Kelekis AD, Yilmaz H, Abdo G, Martin JB, Viera JM, Rufenacht DA. Radiation dose in vertebroplasty. Neuroradiology. 2004;46:243–245. doi: 10.1007/s00234-003-1156-0. [DOI] [PubMed] [Google Scholar]
- Miller DL, Balter S, Cole PE, Lu HT, Berenstein A, Albert R, Schueler BA, Georgia JD, Noonan PT, Russell EJ, Malisch TW, Vogelzang RL, Geisinger M, Cardella JF, St George J, Miller GL, Anderson J. Radiation doses in interventional radiology procedures: The RAD-IR study - Part II: Skin dose. Journal of Vascular and Interventional Radiology. 2003a;14:977–990. doi: 10.1097/01.rvi.0000084601.43811.cb. [DOI] [PubMed] [Google Scholar]
- Miller DL, Balter S, Cole PE, Lu HT, Schueler BA, Geisinger M, Berenstein A, Albert R, Georgia JD, Noonan PT, Cardella JF, George JS, Russell EJ, Malisch TW, Vogelzang RL, Miller GL, Anderson J. Radiation doses in interventional radiology procedures: The RAD-IR Study - Part I: Overall measures of dose. Journal of Vascular and Interventional Radiology. 2003b;14:711–727. doi: 10.1097/01.rvi.0000079980.80153.4b. [DOI] [PubMed] [Google Scholar]
- Miller ME, Davis ML, Macclean CR, Davis JG, Smith BL, Humphries JR. Radiation Exposure and Associated Risks to Operating-Room Personnel during Use of Fluoroscopic Guidance for Selected Orthopedic Surgical-Procedures. Journal of Bone and Joint Surgery-American Volume. 1983;65:1–4. [PubMed] [Google Scholar]
- Moritake T, Matsumaru Y, Takigawa T, Nishizawa K, Matsumura A, Tsuboi K. Dose measurement on both patients and operators during neurointerventional procedures using photoluminescence glass dosimeters. AJNR Am J Neuroradiol. 2008;29:1910–7. doi: 10.3174/ajnr.A1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller LP, Suffner J, Wenda K, Mohr W, Rommens PM. Radiation exposure to the hands and the thyroid of the surgeon during intramedullary nailing. Injury-International Journal of the Care of the Injured. 1998;29:461–468. doi: 10.1016/s0020-1383(98)00088-6. [DOI] [PubMed] [Google Scholar]
- Murphy PH, Wu Y, Glaze SA. Attenuation properties of lead composite aprons. Radiology. 1993;186:269–72. doi: 10.1148/radiology.186.1.8416577. [DOI] [PubMed] [Google Scholar]
- Muzaffar TS, Imran Y, Iskandar MA, Zakaria A. Radiation exposure to the surgeon during femoral interlocking nailing under fluoroscopic imaging. Med J Malaysia. 2005;60(Suppl 26–9) [PubMed] [Google Scholar]
- Naidu LS, Singhal S, Preece DE, Vohrah A, Loft DE. Radiation exposure to personnel performing endoscopic retrograde cholangiopancreatography. Postgraduate Medical Journal. 2005;81:660–662. doi: 10.1136/pgmj.2004.031526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCRP. Use of personal monitors to estimate effective dose equivalent and effective dose to workers for external exposure to low-LET radiation. Bethesda, MD: National Council on Radiation Protection and Measurements; 1995. NCRP report No. 122. [Google Scholar]
- NCRP. Ionizing radiation exposure of the population of the United States. Bethesda, MD: National Council on Radiation Protection and Measurements; 2009. NCRP Report No. 160. [Google Scholar]
- NCRP. Radiation dose management for fluoroscopically-guided interventional medical procedures. Bethesda, MD: National Council on Radiation Protection and Measurements; 2010. NCRP Report No. 168. [Google Scholar]
- Neofotistou V, Vano E, Padovani R, Kotre J, Dowling A, Toivonen M, Kottou S, Tsapaki V, Willis S, Bernardi G, Faulkner K. Preliminary reference levels in interventional cardiology. European Radiology. 2003;13:2259–2263. doi: 10.1007/s00330-003-1831-x. [DOI] [PubMed] [Google Scholar]
- Niklason LT, Marx MV, Chan HP. The Estimation of Occupational Effective Dose in Diagnostic-Radiology with 2 Dosimeters. Health Physics. 1994;67:611–615. doi: 10.1097/00004032-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Nowak B, Jankowski J. Occupational exposure in operational radiology. Pol J Occup Med Environ Health. 1991;4:169–74. [PubMed] [Google Scholar]
- Olgar T, Bor D, Berkmen G, Yazar T. Patient and staff doses for some complex x-ray examinations. J Radiol Prot. 2009;29:393–407. doi: 10.1088/0952-4746/29/3/004. [DOI] [PubMed] [Google Scholar]
- Oonsiri S, Jumpangern C, Sanghangthum T, Krisanachinda A, Suriyapee S. Radiation dose to medical staff in interventional radiology. J Med Assoc Thai. 2007;90:823–8. [PubMed] [Google Scholar]
- Ortiz AO, Natarajan V, Gregorius DR, Pollack S. Significantly reduced radiation exposure to operators during kyphoplasty and vertebroplasty procedures: methods and techniques. AJNR Am J Neuroradiol. 2006;27:989–94. [PMC free article] [PubMed] [Google Scholar]
- Padovani R, Le Heron J, Cruz-Suarez R, Duran A, Lefaure C, Miller DL, Sim HK, Vano E, Rehani M, Czarwinski R. International project on individual monitoring and radiation exposure levels in interventional cardiology. Radiat Prot Dosimetry. 2011;144:437–441. doi: 10.1093/rpd/ncq326. [DOI] [PubMed] [Google Scholar]
- Padovani R, Rodella CA. Staff dosimetry in interventional cardiology. Radiation Protection Dosimetry. 2001;94:99–103. doi: 10.1093/oxfordjournals.rpd.a006490. [DOI] [PubMed] [Google Scholar]
- Pantos I, Patatoukas G, Katritsis DG, Efstathopoulos E. Patient radiation doses in interventional cardiology procedures. Curr Cardiol Rev. 2009;5:1–11. doi: 10.2174/157340309787048059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persliden J. Patient and staff doses in interventional X-ray procedures in Sweden. Radiation Protection Dosimetry. 2005;114:150–157. doi: 10.1093/rpd/nch539. [DOI] [PubMed] [Google Scholar]
- Pinto NGV, Braz D, Vallim MA, Filho LGP, Azevedo FS, Barroso RC, Lopes RT. Radiation exposure in interventional radiology. Nuclear Instruments and Methods in Physics Research Section A. 2007;580:586–590. [Google Scholar]
- Pitney MR, Allan RM, Giles RW, Mclean D, Mccredie M, Randell T, Walsh WF. Modifying Fluoroscopic Views Reduces Operator Radiation Exposure during Coronary Angioplasty. Journal of the American College of Cardiology. 1994;24:1660–1663. doi: 10.1016/0735-1097(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Ramsdale ML, Walker WJ, Horton PW. Extremity Doses during Interventional Radiology. Clinical Radiology. 1990;41:34–36. doi: 10.1016/s0009-9260(05)80929-8. [DOI] [PubMed] [Google Scholar]
- Rao PN, Faulkner K, Sweeney JK, Asbury DL, Sambrook P, Blacklock NJ. Radiation-Dose to Patient and Staff during Percutaneous Nephrostolithotomy. British Journal of Urology. 1987;59:508–512. doi: 10.1111/j.1464-410x.1987.tb04864.x. [DOI] [PubMed] [Google Scholar]
- RSNA. RSNA news - Interventional radiologies carries occupational risk for cataracts. RANA News. 2004;14:5–6. [Google Scholar]
- Safak M, Olgar T, Bor D, Berkmen G, Gogus C. Radiation doses of patients and urologists during percutaneous nephrolithotomy. J Radiol Prot. 2009;29:409–15. doi: 10.1088/0952-4746/29/3/005. [DOI] [PubMed] [Google Scholar]
- Sanders R, Koval KJ, DiPasquale T, Schmelling G, Stenzler S, Ross E. Exposure of the orthopaedic surgeon to radiation. J Bone Joint Surg Am. 1993;75:326–330. doi: 10.2106/00004623-199303000-00003. [DOI] [PubMed] [Google Scholar]
- Schueler BA, Vrieze TJ, Bjarnason H, Stanson AW. An investigation of operator exposure in interventional radiology. Radiographics. 2006;26:1533–41. doi: 10.1148/rg.265055127. [DOI] [PubMed] [Google Scholar]
- Schultz FW, Geleijns J, Spoelstra FM, Zoetelief J. Monte Carlo calculations for assessment of radiation dose to patients with congenital heart defects and to staff during cardiac catheterizations. British Journal of Radiology. 2003;76:638–647. doi: 10.1259/bjr/21647806. [DOI] [PubMed] [Google Scholar]
- Shore RE, Neriishi K, Nakashima E. Epidemiological Studies of Cataract Risk at Low to Moderate Radiation Doses: (Not) Seeing is Believing. Radiation Research. 2010:889–894. doi: 10.1667/RR1884.1. [DOI] [PubMed] [Google Scholar]
- Siiskonen T, Tapiovaara M, Kosunen A, Lehtinen M, Vartiainen E. Monte Carlo simulations of occupational radiation doses in interventional radiology. Br J Radiol. 2007;80:460–8. doi: 10.1259/bjr/26692771. [DOI] [PubMed] [Google Scholar]
- Simon SL. Organ-specific external dose coefficients and protective apron transmission factors for historical dose reconstruction for medical personnel. Health Physics. 2011;101:13–27. doi: 10.1097/HP.0b013e318204a60a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Weinstock RM, Doody MM, Neton J, Wenzl T, Stewart P, Mohan AK, Yoder RC, Hauptmann M, Freedman DM, Cardarelli J, Feng HA, Bouville A, Linet M. Estimating historical radiation doses to a cohort of U.S. radiologic technologists. Radiation Research. 2006;166:174–92. doi: 10.1667/RR3433.1. [DOI] [PubMed] [Google Scholar]
- Stratakis J, Damilakis J, Hatzidakis A, Theocharopoulos N, Gourtsoyiannis N. Occupational radiation exposure from fluoroscopically guided percutaneous transhepatic biliary procedures. Journal of Vascular and Interventional Radiology. 2006;17:863–871. doi: 10.1097/01.RVI.0000217959.86251.11. [DOI] [PubMed] [Google Scholar]
- Synowitz M, Kiwit J. Surgeon’s radiation exposure during percutaneous vertebroplasty. Journal of Neurosurgery-Spine. 2006;4:106–109. doi: 10.3171/spi.2006.4.2.106. [DOI] [PubMed] [Google Scholar]
- Tappero C, Barbero S, Costantino S, Bergui M, Ropolo R, Bradac G, Gandini G. Patient and operator exposure during percutaneous vertebroplasty. Radiol Med. 2009;114:595–607. doi: 10.1007/s11547-009-0385-7. [DOI] [PubMed] [Google Scholar]
- Tsapaki V, Kottou S, Vano E, Komppa T, Padovani R, Dowling A, Molfetas M, Neofotistou V. Occupational dose constraints in interventional cardiology procedures: the DIMOND approach. Physics in Medicine and Biology. 2004;49:997–1005. doi: 10.1088/0031-9155/49/6/010. [DOI] [PubMed] [Google Scholar]
- Vano E, Gonzalez L, Beneytez F, Moreno F. Lens injuries induced by occupational exposure in non-optimized interventional radiology laboratories. British Journal of Radiology. 1998a;71:728–733. doi: 10.1259/bjr.71.847.9771383. [DOI] [PubMed] [Google Scholar]
- Vano E, Gonzalez L, Fernández JM, Haskal ZJ. Eye lens exposure to radiation in interventional suites: caution is warranted. Radiology. 2008;248:945–53. doi: 10.1148/radiol.2482071800. [DOI] [PubMed] [Google Scholar]
- Vano E, Gonzalez L, Guibelalde E, Fernandez JM, Ten JI. Radiation exposure to medical staff in interventional and cardiac radiology. British Journal of Radiology. 1998b;71:954–960. doi: 10.1259/bjr.71.849.10195011. [DOI] [PubMed] [Google Scholar]
- Vano E, Gonzalez L, Ten JI, Fernandez JM, Guibelalde E, Macaya C. Skin dose and dose-area product values for interventional cardiology procedures. British Journal of Radiology. 2001;74:48–55. doi: 10.1259/bjr.74.877.740048. [DOI] [PubMed] [Google Scholar]
- Vano E, Kleiman NJ, Duran A, Rehani MM, Echeverri D, Cabrera M. Radiation cataract risk in interventional cardiology personnel. Radiation Research. 2010;174:490–495. doi: 10.1667/RR2207.1. [DOI] [PubMed] [Google Scholar]
- Vano E, Ubeda C, Leyton F, Miranda P, Gonzalez L. Staff radiation doses in interventional cardiology: correlation with patient exposure. Pediatr Cardiol. 2009;30:409–13. doi: 10.1007/s00246-008-9375-0. [DOI] [PubMed] [Google Scholar]
- Vehmas T. What Factors Influence Radiologists Finger Doses during Percutaneous Drainages under Fluoroscopic Guidance. Health Physics. 1993;65:161–163. doi: 10.1097/00004032-199308000-00005. [DOI] [PubMed] [Google Scholar]
- Vehmas T, Tikkanen H. Measuring radiation exposure during percutaneous drainages: can shoulder dosemeters be used to estimate finger doses? Br J Radiol. 1992;65:1007–1010. doi: 10.1259/0007-1285-65-779-1007. [DOI] [PubMed] [Google Scholar]
- von Boetticher H, Lachmund J, Hoffmann W. Cardiac catheterization: impact of face and neck shielding on new estimates of effective dose. Health Physics. 2009;97:622–7. doi: 10.1097/01.HP.0000363843.01041.99. [DOI] [PubMed] [Google Scholar]
- von Boetticher H, Meenen C, Lachmund J, Hoffmann W, Engel HJ. [Radiation exposure to personnel in cardiac catheterization laboratories] Z Med Phys. 2003;13:251–6. [PubMed] [Google Scholar]
- Whitby M, Martin CJ. Radiation doses to the legs of radiologists performing interventional procedures: are they a cause for concern? British Journal of Radiology. 2003;76:321–327. doi: 10.1259/bjr/65778215. [DOI] [PubMed] [Google Scholar]
- Whitby M, Martin CJ. A study of the distribution of dose across the hands of interventional radiologists and cardiologists. British Journal of Radiology. 2005;78:219–229. doi: 10.1259/bjr/12209589. [DOI] [PubMed] [Google Scholar]
- Williams JR. The interdependence of staff and patient doses in interventional radiology. British Journal of Radiology. 1997;70:498–503. doi: 10.1259/bjr.70.833.9227232. [DOI] [PubMed] [Google Scholar]
- Yaffe MJ, Mawdsley GE, Lilley M, Servant R, Reh G. Composite materials for x-ray protection. Health Physics. 1991;60:661–4. doi: 10.1097/00004032-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Yang RM, Morgan T, Bellman GC. Radiation protection during percutaneous nephrolithotomy: a new urologic surgery radiation shield. J Endourol. 2002;16:727–31. doi: 10.1089/08927790260472872. [DOI] [PubMed] [Google Scholar]
- Zweers D, Geleijns J, Aarts NJM, Hardam LJ, Lameris JS, Scultz FW, Kool LJS. Patient and staff radiation dose in fluoroscopy-guided TIPS procedures and dose reduction, using dedicated fluoroscopy exposure settings. British Journal of Radiology. 1998;71:672–676. doi: 10.1259/bjr.71.846.9849393. [DOI] [PubMed] [Google Scholar]