Abstract
Protein synthesis rates are commonly measured by using isotopic tracers to quantify the incorporation of a labelled amino acid into muscle proteins. Here we provide evidence supporting our hypothesis that the non-isotopic SUnSET technique is a valid and accurate method for the measurement of in vivo changes in protein synthesis at the whole muscle and single muscle fiber levels.
Keywords: skeletal muscle, translation, hypertrophy, atrophy, puromycin, muscle fiber type, myosin heavy chain
Introduction
Skeletal muscle is crucial for movement and whole body metabolism, and therefore, the maintenance of skeletal muscle mass is essential for mobility, disease prevention and quality of life (20, 30). Skeletal muscle mass is ultimately determined by the net balance between the rate of protein degradation and the rate of protein synthesis (11). Thus, identifying the molecular mechanisms that regulate protein degradation and protein synthesis is critical for the development of effective exercise programs and potential pharmacological interventions that could inhibit muscle atrophy and / or promote hypertrophy.
Protein synthesis rates in skeletal muscle have traditionally been measured using various radioactive isotope (e.g. 3H-phenyalanine or 35S-methionine), or stable isotope (e.g.15N-lysine, 13C-leucine or [ring-13C6]-phenylalanine), tracers. These tracers are either constantly infused, or given as a flooding dose, and the incorporation of the labelled amino acid into muscle proteins over time is then measured (for further reviews see (4, 19, 25)). More recently, the non-amino acid stable isotope deuterium oxide (2H2O) has also been used to assess skeletal muscle protein synthesis rates (6). While these techniques have been very successful, they are expensive and time consuming, and typically do not allow for the measurement of protein synthesis at the single cell level. Recently, however, a non-isotopic technique known as SUnSET, which involves the use of the compound puromycin, was developed for measuring protein synthesis in cultured cells (29). Subsequently, we developed a methodology for using the SUnSET technique to measure in vivo rates of protein synthesis in skeletal muscle and other tissues (9, 10). Based on these studies, we hypothesize that the SUnSET technique is a valid and accurate method for measuring in vivo changes in protein synthesis in whole muscles and at the single muscle fiber level. This review will explain the properties of puromycin and the principles of the SUnSET technique, and then describe our in vivo SUnSET methodology and the evidence that supports the validity and accuracy of this technique for measuring skeletal muscle protein synthesis in vivo. Finally, we will discuss some of the potential limitations of the SUnSET technique.
Puromycin
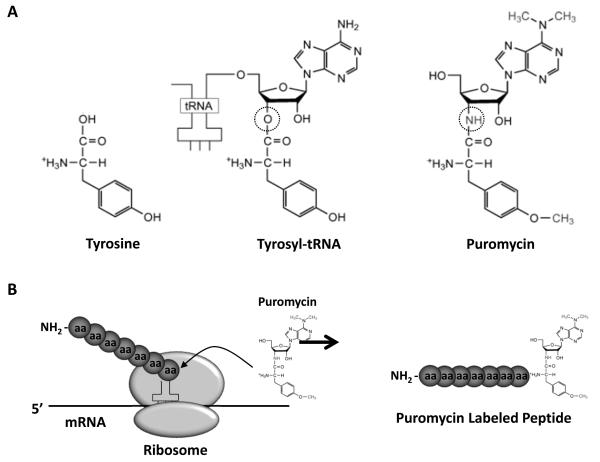
Puromycin is an aminonucleoside antibiotic produced by the bacterium Streptomyces alboniger. For many years, puromycin has been an important tool in molecular biology by acting as a selection agent for cultured cells that express the enzyme puromycin-N-acetyl-transferase (33). Importantly, puromycin is a structural analogue of aminoacyl-transfer RNA (aminoacyl-tRNA; specifically tyrosyl-tRNA; Figure 1A) and, as such, can be incorporated into elongating peptide chains via the formation of a peptide bond (23). However, whereas aminoacyl-tRNAs contain a hydrolyzable ester bond between their tRNA ribose moiety and the attached amino acid molecule, puromycin has a non-hydrolyzable amide bond in the equivalent position (Figure 1A). Thus, the binding of puromycin to a growing peptide chain prevents a new peptide bond from being formed with the next aminoacyl-tRNA. As a consequence, puromycin binding results in the termination of peptide elongation, and leads to the release of the truncated puromycin bound peptide from the ribosome (Figure 1B) (34). At very high concentrations, puromycin effectively shuts down the elongation phase of translation and thus inhibit protein synthesis (35); however, at very low concentrations, that do not inhibit the overall rate of translation, the rate at which puromycin-labelled peptides are formed reflects the overall rate of protein synthesis (29). This later property makes puromycin a potential tool for the measurement of changes in protein synthesis rates. Indeed, Nakano and Hara (1979) were the first to investigate the use of 3H-puromycin to measure changes in protein synthesis rates in vivo and demonstrated that puromycin could be used to effectively detect starvation- and low protein diet-induced decreases in protein synthesis rates in whole tissues including skeletal muscle (22). However, it took another 30 years, with the development of the SUnSET technique (29), to renew interest in the use of puromycin for detecting changes in protein synthesis.
Figure 1. Puromycin structure and mechanism of action.
(A) Comparison of the molecular structure of tyrosine, tyrosyl-tRNA and puromycin. The hydrolyzable ester bond in tyrosyl-tRNA and the non-hydrolyzable amide bond in puromycin are highlighted with a circle. Modified from Trends in Biochemical Sciences, 28(3), TT Takahashi, RJ Austin and RW Roberts, “mRNA display: ligand discovery, interaction analysis and beyond”, pp. 159-165, Copyright 2003, with permission from Elsevier. (B) Puromycin’s mechanisms of action involves it’s incorporation into growing peptide chains via the formation of a peptide bound. Once bound, the puromycin-labelled peptide is unable to undergo further elongation and is released from the ribosome.
SUnSET
The SUnSET, or SUrface SEnsing of Translation, technique specifically involves the use of an anti-puromycin antibody for the immunological detection of puromycin-labelled peptides (29). Originally developed for use in cultured cells, SUnSET allows for the detection of changes in protein synthesis in whole cell lysates using western blotting (WB), in multiple live cells using fluorescence-activated cell sorting (FACS), and at the single cell level with immunohistochemistry (IHC) (29). SUnSET in cell culture has been shown to have a similar dynamic range as protein synthesis measurements performed using 35S-methionine. Furthermore, the dose of puromycin used in these cell culture studies (up to 18.4 μM) was shown to not interfere with the overall rate of protein synthesis (29). Importantly, SUnSET is able to detect increases and decreases in protein synthesis that are essentially indistinguishable from those obtained using 35S-methionine (29). Thus, SUnSET has been shown to be a valid alternative to the use of radioisotopes for measuring changes in protein synthesis in cell culture and provides a clear advantage in allowing for the visualization of protein synthesis at the single cell level (13, 29).
Using SUnSET to Measure Changes in Skeletal Muscle Protein Synthesis
Due to our ongoing interest in the regulation of skeletal muscle mass, and specifically the role of the mammalian target of rapamycin (mTOR) in regulating protein synthesis and muscle hypertrophy (8, 15), we were very interested in determining if SUnSET could be used to detect changes in protein synthesis in whole skeletal muscles under in vivo conditions. Thus, to investigate the validity and accuracy of the SUnSET technique, we performed a number of experiments using WB and IHC to measure changes in in vivo protein synthesis rates at the whole muscle and single muscle fiber levels (10).
Western Blot SUnSET
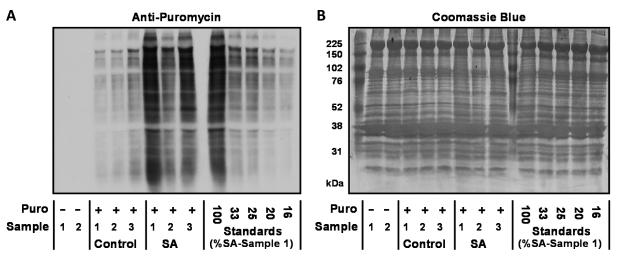
First, we set out to determine whether the WB version of SUnSET (WB-SUnSET) could be used to detect an increase in protein synthesis induced by bilateral synergist ablation (SA) surgery, and whether this increase would be similar to that detected using a traditional radioactive technique. To accomplish this, mice were subjected to SA or sham surgeries, and after 7 days, the plantaris muscles were extracted and then incubated in an ex vivo bath for 30 min with media that contained either a flooding dose of 3H-phenylalanine or puromycin (1 μM). Muscles incubated with puromycin were then analyzed for the amount of puromycin-labelled peptides by WB. During our preliminary studies, we used a mouse anti-puromycin primary antibody in conjunction with a general anti-mouse immunoglobulin G (IgG) secondary antibody. With these conditions, we observed relatively intense non-specific bands at both 50 and 25 kilodaltons. We were able to determine that these non-specific bands were coming from the secondary antibody, and we reasoned that they resulted from the detection of the endogenous mouse IgG heavy and light chains that are present in mouse tissue. Therefore, to overcome this problem, we took advantage of the fact that our anti-puromycin primary antibody was a monoclonal subtype 2a IgG (i.e. IgG2a) (29). Specifically, we switched our secondary antibody to an anti-mouse IgG2a specific antibody that only detects the crystallizable fragment (Fc) of the IgG2a heavy chain. Because 2a IgGs make up only a relatively small fraction of the total IgG pool, this secondary antibody enabled us to dramatically lower the intensity of the 50 kilodalton endogenous IgG heavy chain band and completely remove the presence of the 25 kilodalton light chain band. Thus, with this refined approach, the contribution of the endogenous IgG pool to the overall anti-puromycin signal was effectively eliminated (Figure 2). We also made an effort to improve the quantitative reliability of our WB analysis by running a series of standards on the same gel as our experimental samples (Figure 2). The standards were made from the homogenate of a puromycin treated SA plantaris muscle that was diluted 3-, 4-, 5- and 6-fold with homogenate from a non-puromycin treated muscle (Figure 2). The presence of these standards was important because it allowed us to confirm that our quantification was being performed on an image that had signal intensities within the linear range of the film. Specifically, an image in which the differences in puromycin signal intensity between the standards matched the actual fold dilutions was selected for final quantification. Once the appropriate image had been selected, the puromycin signal intensity in the lane for each SA sample was then expressed relative to the mean puromycin signal from the sham control lanes. The results demonstrated that SA induced a 3.6-fold increase in puromycin-labelled peptides, a result that was indistinguishable from the 3.4-fold increase in protein synthesis that we obtained with the traditional 3H-phenylalanine flooding dose methodology (10). Hence, these data confirmed that WB-SUnSET could be used to accurately detect increases in protein synthesis under ex vivo conditions.
Figure 2. Quantification of SA-induced increases in protein synthesis with WB-SUnSET.
WB-SUnSET measurements were performed by incubating sham (Control) and synergist ablated (SA) muscles for 30 min in an ex vivo bath that contained media supplemented with 1 μM puromycin. (A) Representative image of the SUnSET WB used to quantify the amount of puromycin-labelled peptides in sham and SA muscles. To demonstrate the specificity in the anti-puromycin signal, two non-puromycin treated muscle samples (Puro -) were also included (two far left lanes). To guide the selection of the final image for quantification, a series of standards were also loaded (last five lanes on the right). Specifically, a SA sample (SA, Sample 1) was diluted 3-, 4-, 5- and 6-fold with homogenate from a non-puromycin treated muscle (Puro -, Sample 1). A series of images were then acquired with different exposure times and an image that contained signal intensities within the linear range of the film was then used for the final quantification. In this quantification procedure, the puromycin signal of sham and SA samples was expressed relative to the mean puromycin signal obtained in the sham samples. (B) Following the image acquisition, the WB membrane was stained with Coomassie Blue to verify equal loading of total protein in all lanes.
We next asked whether WB-SUnSET could also be used to detect a similar change in protein synthesis under in vivo conditions. Based on the previous work of Nakano and Hara (22), and our own preliminary dose response and time course experiments, we administered a puromycin dose of 0.04 μmol/g body mass via an intraperitoneal (IP) injection and collected the muscles 30 minutes post-injection. Using the same approach that we used to control for image exposure time in the ex vivo experiments (see above), our results showed that SA induced a 2.9-fold increase in puromycin-labelled peptides (10). This result was not significantly different from the increase detected ex vivo and therefore suggested that WB-SUnSET could also be used to accurately detect an increase in protein synthesis in vivo (10). However, one further concern we had with performing SUnSET, under in vivo conditions, was that differences in the delivery/uptake of free puromycin may produce differences in the amount of puromycin-labelled proteins that are not the result of differences in the rate of protein synthesis. For example, it was possible that SA promoted an increase in blood flow to the plantaris muscles which, in-turn, could promote a greater uptake of puromycin, and therefore, a greater incorporation of puromycin into nascent peptides. To address this possibility, we developed an enzyme-linked immunosorbent assay (ELISA) to measure the free puromycin pool in sham and SA muscles, and using this technique we found no difference in the free puromycin pool between sham and SA muscles (10). Therefore, our data provide strong evidence that, with appropriate controls, WB-SUnSET can be used to accurately detect increases in protein synthesis under ex vivo and in vivo conditions.
During the course of developing the WB-SUnSET technique, we also performed experiments to determine whether we could detect an experimentally induced decrease in protein synthesis in vivo. Specifically, we food deprived (FD) mice for 48 hours and then collected various skeletal muscles at 30 minutes after an IP injection of puromycin (0.04 μmol/g body mass). The WB-SUnSET analysis revealed a 65% decrease in puromycin-labelled peptides, and thus protein synthesis, compared to muscles from ad lib fed control mice. Importantly, the magnitude of the FD-induced decrease in protein synthesis measured by WB-SUnSET is very similar to what has been reported with traditional radioactive techniques (21, 22, 24). In order to rule out any effect of FD on the delivery/uptake of puromycin into the muscle we used our ELISA assay to measure the free puromycin content and found no difference between muscles from FD and ad lib control animals. Therefore, the results from these experiments demonstrated that WB-SUnSET can indeed be used to detect a decrease in protein synthesis.
Taken together, this series of experiments provided several lines of evidence which indicate that the WB-SUnSET technique is a valid and accurate tool for measuring changes in protein synthesis at the whole muscle level.
Immunohistochemical SUnSET
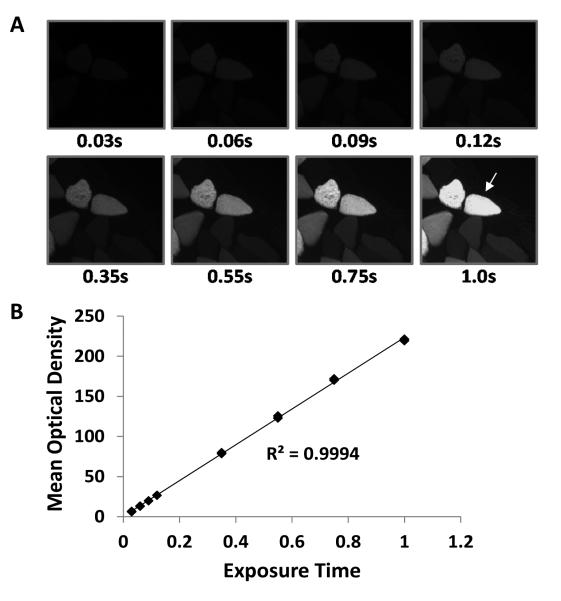
Immunohistochemistry (IHC) is an extremely valuable tool for investigating region specific differences across whole tissue sections and for examining changes in single cells. As such, we were very interested to determine whether IHC-SUnSET could be used to detect changes in protein synthesis in whole muscle cross-sections and at the single muscle fiber level. To address this, we first had to extensively optimize our IHC-SUnSET staining conditions. For example, our anti-puromycin antibody was derived in a mouse, and thus, we had to overcome the endogenous IgG background signal that would appear after incubating mouse skeletal muscle sections with an anti-mouse IgG secondary antibody. To accomplish this we first applied an unconjugated anti-mouse IgG Fab (heavy and light chain) antibody to sections for 1 hour prior to applying the anti-puromycin primary antibody. This approach effectively blocks all binding sites on the endogenous population of IgGs and removes most of the background signal that would normally be present after the application of an anti-mouse IgG secondary antibody (10). Similar to our WB-SUnSET protocol, we further reduced the endogenous IgG background signal by employing an anti-mouse IgG2a Fc specific secondary antibody, and with this combined approach, we were able to essentially eliminate all of the non-specific background (10). We were also able to demonstrate that the puromycin signal could be detected within the muscle fibers, but it was not detected within the interstitial space or within the lumen of blood vessels. This was important because it helped to establish that our IHC-SUnSET conditions did not detect free puromycin, but was instead specific for the detection of puromycin-labelled peptides (10). Finally, we also quantified the dynamic range of the image acquisition system that we used for measuring differences in the fluorescence signal intensity, and thus, the magnitude of changes that we could validly detect. As shown in Figure 3, our analysis demonstrated a greater than 33-fold working linear range (r = 0.9994), a range that far exceeded the greatest distribution of signal intensities that were obtained in any of the analyses performed in our experiments (the largest was a 10-fold distribution). Hence, with our optimized IHC-SUnSET protocol, and the wide dynamic range of our image acquisition system, we were confident that any detected changes in the puromycin signal would accurately reflect changes in protein synthesis.
Figure 3. Defining the linear range of the image acquisition system.
A Nikon 80i microscope coupled to a high sensitivity charged-couple device (CCD) camera (Nikon DS-QiMc) was used for the acquisition of fluorescent images (10). (A) To define the linear range of this system, images of a muscle transfected with GFP were captured with various exposure times (0.03 – 1.0 s). (B) The mean optical density of the fiber identified with the arrow was quantified in each image and plotted against the exposure time. Based on this analysis it was concluded that the image acquisition system had a greater than 33-fold working linear range.
To begin testing the quantitative reliability of our IHC-SUnSET measurements we examined the effect of FD on protein synthesis in randomly selected fibers from whole muscle sections. Specifically, in these experiments, both FD and ad lib muscle sections were mounted side by side on the same slide as this ensured that both sections received identical staining and image acquisition conditions. With this approach, we found that the mean puromycin signal in fibers from FD muscles was 53% lower than in fibers from ad lib muscles, a value that was not statistically different from the reduction in protein synthesis measured with the WB-SUnSET technique (10). This indicated that IHC-SUnSET can be used to detect changes in protein synthesis in muscle cross-sections, and that IHC- and WB-SUnSET produce quantitatively similar results when they were used to measure relative changes in the rate of protein synthesis in vivo.
We next determined whether IHC-SUnSET could be used to detect differences in protein synthesis between individual fibers within the same muscle section. In this experiment, in vivo transient transfection, via the electroporation of plasmid DNA, was used to overexpress two signaling molecules that are known to induce skeletal muscle hypertrophy, in part, by stimulating protein synthesis: constitutively active (ca)-Akt (3, 31) and Rheb (12). An important strength of this approach was that the non-transfected fibers served as controls, and therefore, allowed for the puromycin signal in transfected fibers to be expressed relative to the non-transfected fibers from the same muscle section (Figure 4). Furthermore, to control for the effects of electroporation per se, we also transfected muscles with green fluorescent protein (GFP), which presumably would not stimulate protein synthesis. As expected, we found that ca-Akt and Rheb induced relatively large increases in the puromycin signal (155% and 60%, respectively), while GFP only had a very minor effect (5-7%) (10). When compared to Rheb, the greater effect size of ca-Akt was also expected because ca-Akt has the potential to induce an increase in protein synthesis through the combined activation of mTOR signaling (3) and the inhibition of glycogen synthase kinase-3 β (GSK3β) (1, 16, 27), while Rheb only activates mTOR signaling (12). Taken together, these results demonstrated that the IHC-SUnSET technique can be used to detect changes in protein synthesis of different magnitudes at the single fiber level.
Figure 4. IHC-SUnSET combined with in vivo transient transfection of constitutively active-Akt.
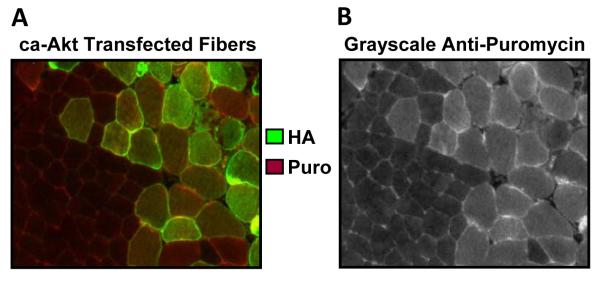
Tibialis Anterior muscles were transfected with plasmid DNA encoding hemagglutinin(HA)-tagged ca-Akt, and at 3 d after transfection, mice were injected with puromycin 30 min prior to the collection of the muscles. The muscles were then subjected to IHC-SUnSET by staining for puromycin (red) and the HA tag (green). (A) Representative merged image of the anti-HA and anti-puromycin signals. (B) Grayscale image of the signal for puromycin shown in A.
Overall, our experiments have provided evidence that IHC-SUnSET can detect changes in protein synthesis that are quantitatively similar to those detected using WB-SUnSET. Furthermore, IHC-SUnSET can be combined with other powerful in vivo molecular techniques, such as transient transfection, to determine the effects of various signaling molecules on protein synthesis at the single fiber level.
Muscle Fiber Type-Dependent Differences in the Regulation of Protein Synthesis
When performing the IHC-SUnSET experiments on sections from FD and ad lib fed animals, we noticed a distinct mosaic pattern of puromycin staining in the ad lib sections, suggesting that there may be fiber type-dependent differences in basal rates of protein synthesis. Although a significant body of knowledge exists with regards to the genetic, structural, functional, metabolic and adaptive characteristics of different fiber types (28), technical limitations have meant that comparatively very little is known about possible fiber type-dependent differences in the regulation of protein synthesis (32). Thus, we set out to determine whether IHC-SUnSET could be used to reveal novel information about the basal protein synthesis rates in different fiber types found within the same whole muscle. To accomplish this, we combined IHC-SUnSET with myosin heavy chain (MHC) specific antibodies to identify the puromycin signal in type 1, 2A, 2X and 2B fibers found within a resting mouse plantaris muscle. To allow for comparison between the fiber types, we normalized the puromycin signal for a given fiber type to the mean puromycin signal found in the type 2A fibers. Thus, for each section we measured the puromycin signal in type 2A fibers and one of either type 1, 2X or 2B fibers. Using this approach, we demonstrated for the first time that basal protein synthesis rates vary in a fiber type-dependent manner with fast-twitch glycolytic type 2B fibers having lower rates of protein synthesis than slow-twitch oxidative type 1 and 2A fibers found within the same muscle (Figure 5) (9). These data are supported by whole muscle studies using radioactive techniques that show muscles composed predominantly of fast-twitch glycolytic fibers have lower basal rates of protein synthesis than muscles predominantly composed of slow-twitch oxidative fibers (7). Fast-twitch muscles also have lower amounts total RNA, of which >85% is ribosomal RNA (rRNA), and thus have a lower translational capacity compared with slow-twitch muscles (7). In this context, our IHC-SUnSET data is also supported by the study of Habets et al (14) which demonstrated that the content of 28S rRNA in single muscle fibers varies in a similar fiber type-dependent manner as our protein synthesis results. Furthermore, we have also measured fiber type-dependent levels of the ribosomal S6 protein, and in agreement with 28S rRNA data (14), ribosomal S6 protein also varied in a fiber-type dependent manner that was essentially the same as for protein synthesis rates (9). Together, these data demonstrated that basal rates of protein synthesis are regulated in a fiber type-dependent manner and that there is a close fiber type-dependent association between basal protein synthesis rates and translational capacity (i.e. levels of rRNA and ribosomal proteins).
Figure 5. IHC-SUnSET reveals muscle fiber type-dependent differences in basal rates of protein synthesis.
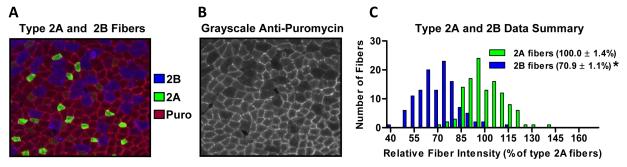
Resting mice were anaesthetized and injected with puromycin. Tibialis Anterior muscles were then collected 30 min later and subjected to IHC-SUnSET. (A) Representative image of a section triple stained for puromycin (Puro; red), type 2A fibers (green), and type 2B fibers (blue). (B) Grayscale image of the puromycin signal from the same image shown in A. (C) The puromycin staining intensity in the type 2A and type 2B fibers was expressed relative to the mean value obtained in the type 2A fibers of a given section, and these values were then plotted on a histogram. Inset values are means ± SE; n = 110–120 fibers/group from 4 independent muscles. *P < 0.05 vs. type 2A fibers.
Our finding of fiber-type dependent differences in basal protein synthesis rates next prompted us to ask whether IHC-SUnSET could also be used to detect fiber type-dependent changes in protein synthesis. To do this, we again employed the FD model to decrease protein synthesis and then mounted FD sections on the same slide as the ad lib sections (9). Our results revealed that FD induced a fiber type-dependent decrease in protein synthesis with type 2X and 2B fibers having a larger decrease than type 1 and 2A fibers (9). Moreover, only types 2X and 2B fibers displayed a significant decrease in fiber cross sectional area (CSA). This IHC-SUnSET data is supported by our previous data using WB-SUnSET (10), and by studies using traditional radiolabel techniques (2, 18), which have shown that protein synthesis in muscles composed predominantly of glycolytic fast-twitch fibers is more sensitive to FD than in muscles that primarily contain more oxidative slow-twitch fibers. Thus, our finding that IHC-SUnSET can detect changes in protein synthesis at the single fiber level, that are consistent with those found using more traditional techniques at the whole muscle level, provides further evidence of SUnSET’s validity and reliability.
Using the same approach described above, we also found that SA induced fiber type-dependent changes in protein synthesis. Specifically, while SA induced an increase in protein synthesis in all fiber types, the most substantial increase occurred in the smaller type 1 and type 2A fibers, while the larger type 2B fibers had the lowest increase (9). Type 2B fibers also had the smallest SA-induced increase in CSA. Based on these observations, we proposed that these fiber type-dependent changes in protein synthesis may be due to differences in motor unit recruitment patterns or to mechanisms that may limit how large an individual fiber can grow before compromising oxygen delivery and/or an optimal myonuclei to cytoplasm ratio (9). In other words, it appears that the SA-induced changes in protein synthesis are likely due to a complex array of factors and our IHC-SUnSET data has now opened up a new field of inquiry aimed at identifying the molecular mechanisms that are responsible for these fiber type-dependent effects. Moreover, our results again highlight the potential that the IHC-SUnSET technique has for gaining insights into the regulation of protein synthesis that have not previously been possible to obtain with traditional isotopic techniques. Finally, these results also demonstrate that changes in protein synthesis detected at the whole muscle level may not accurately reflect the changes that occur within the individual muscle fiber types.
Protein Synthesis in Newly Formed Muscle Fibers
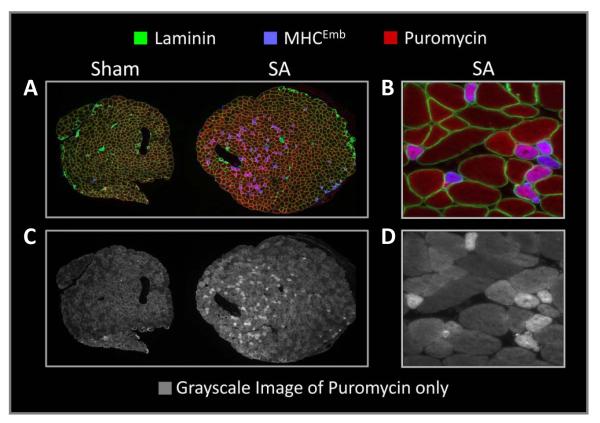
Recently, we reported that SA not only induces an increase in fiber size but also induces a significant increase (59%) in the total number of muscle fibers per cross-section (8). Furthermore, we found that SA induces an increase in the number of muscle fibers expressing the embryonic MHC isoform (MHCEmb), an isoform that is typically found in newly formed muscle fibers (8). We also noted that the increase in the number of MHCEmb positive fibers was similar to the increase in the total number of fibers. Based on these observations we concluded that SA induces the formation of new muscle fibers (i.e. hyperplasia) (8). This is important because when we performed the IHC-SUnSET technique on sections from SA muscles we noticed a population of smaller fibers that displayed very high puromycin signals (9). Thus, we hypothesized that these fibers were newly formed MHCEmb positive fibers and, as such, we were very interested to determine the relative rate of protein synthesis in these fibers. As expected, our results revealed that this population of smaller fibers did indeed express the MHCEmb isoform and that the puromycin signal in these fibers was 3.6-fold higher than the average signal in a mixed population of fibers from control sections (Figure 6) (9). In support of the high rates of protein synthesis, MHCEmb positive fibers also had very high levels of total ribosomal S6 protein and S6 Serine240/244 phosphorylation, which are crude markers of translational capacity and translational efficiency, respectively (9). Combined, these data suggested that, due to the constant mechanical stimulus placed on the muscle, SA induces the formation of new fibers and that these fibers undergo a rapid growth that is supported by very high rates of protein synthesis. Furthermore, as these MHCEmb positive fibers account for up to 30–40% of the total fibers in 14 day SA muscles (8), these fibers are likely to account for a significant proportion of the increases in total S6 protein, S6 Serine240/244 phosphorylation, and protein synthesis that is observed at the whole muscle level. Combined, these results provided a clear and striking example of fiber type-dependent differences in protein synthesis that would not be evident if analyses are performed at the whole muscle level.
Figure 6. MHCEmb positive fibers in SA muscles have very high rates of protein synthesis.
Plantaris muscles, obtained from puromycin injected mice that had been subjected to sham or synergist ablation (SA) surgeries, were frozen adjacent to one another, cross-sectioned, and then subjected to IHC-SUnSET by triple staining for puromycin (red), embryonic myosin heavy chain (MHCEmb; blue) and laminin (green). (A) Representative triple stained image of the sham and SA muscle sections. (B) Higher magnification image of a region in the SA muscle that was enriched with MHCEmb positive fibers. (C and D) Grayscale images of the puromycin signal shown in A and B, respectively. Modified from International Journal of Biochemistry and Molecular Biology, 43(9), TA Hornberger, “Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle”, pp.1267-1276, Copyright 2011, with permission from Elsevier.
In summary, our studies to date have shown that the IHC-SUnSET technique can be successfully used to make novel observations about the regulation of protein synthesis at the single fiber level. Furthermore, IHC-SUnSET can also be combined with other IHC analyses (e.g. total and phospho ribosomal S6 protein) to gain further insight into the mechanisms responsible for the changes in protein synthesis.
Limitations
While our evidence to date suggests that SUnSET is a valid and reliable technique for measuring relative rates of protein synthesis in rodent skeletal muscle, the technique does have some limitations. For example, while SUnSET has been shown to be valid for the measurement of relative rates, or relative changes in protein synthesis, it remains to be determined whether SUnSET could be used to calculate absolute or fractional synthesis rates similar to those measured with isotope techniques. Indeed, the development of a reliable methodology for determining fractional synthetic rates using SUnSET could allow for direct comparison between animal SUnSET studies and human isotope studies. Additional studies are therefore required to explore the feasibility of developing such a methodology. Another limitation hinges on the potentially adverse effects that puromycin might exert on kidney function (26). Hence, the use of SUnSET for the measurement of skeletal muscle protein synthesis in humans is very unlikely. As a result, sensitive measurements of protein synthesis in single human muscle fibers will likely require further refinements of current isotope-based methodologies (5, 17). A final limitation of SUnSET relates to the ability to measure the free puromycin pool. Indeed, one key assumption when measuring changes in protein synthesis, with either puromycin or isotopes, is that the experimental condition does not alter the equilibration kinetics of the free pool of puromycin or isotope when compared to the control condition. With sufficient amount of tissue, this assumption can be validated by measuring the amount of free puromycin in the muscle, however, quantification of free puromycin at the single cell level may not be feasible, especially with IHC. Thus, there remains the possibility that an apparent change in protein synthesis may, in part, be due to experimentally-induced changes in puromycin uptake, and not due to an increase in translation per se. Therefore, when free puromycin cannot be measured, additional complimentary measurements, such as changes in the phosphorylation of relevant signaling molecules, changes in markers of translational capacity or efficiency, and/or the use of specific pharmacological inhibitors, are recommended to assist in the interpretation of data derived using SUnSET.
Conclusion
Our experiments to date have provided strong evidence in support of our hypothesis that, with appropriate controls, SUnSET is a valid and accurate alternative to traditional isotope methods for measuring relative rates of protein synthesis in skeletal muscle. Furthermore, SUnSET offers the advantage of being able to investigate protein synthesis at the single fiber level. While SUnSET may not be appropriate for every experimental model, we believe that this technology will enable investigators to make novel discoveries about the regulation of protein synthesis and may ultimately assist in the discovery of interventions aimed at the preservation of skeletal muscle mass.
Acknowledgments
Funding: This work was supported by U.S. National Institutes of Health grant AR057347 to T.A.H.
Footnotes
Conflict of Interest: There is no conflict of interest for either Dr Craig A. Goodman or Dr Troy A. Hornberger
References
- 1.Armstrong DD, Esser KA. Wnt/{beta}-catenin signaling activates growth-control genes during overload-induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol. 2005;289:C853–9. doi: 10.1152/ajpcell.00093.2005. [DOI] [PubMed] [Google Scholar]
- 2.Baillie AG, Garlick PJ. Responses of protein synthesis in different skeletal muscles to fasting and insulin in rats. Am J Physiol - Endocrinol And Metab. 1991;260:E891–E6. doi: 10.1152/ajpendo.1991.260.6.E891. [DOI] [PubMed] [Google Scholar]
- 3.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 4.Davis TA, Reeds PJ. Of flux and flooding: the advantages and problems of different isotopic methods for quantifying protein turnover in vivo: II. Methods based on the incorporation of a tracer. Current opinion in clinical nutrition and metabolic care. 2001;4:51–6. doi: 10.1097/00075197-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson JM, Lee JD, Sullivan BE, Harber MP, Trappe SW, Trappe TA. A new method to study in vivo protein synthesis in slow- and fast-twitch muscle fibers and initial measurements in humans. J Appl Physiol. 2010;108:1410–6. doi: 10.1152/japplphysiol.00905.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasier H, Fluckey J, Previs S. The application of 2H2O to measure skeletal muscle protein synthesis. Nutrition & metabolism. 2010;7:31. doi: 10.1186/1743-7075-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg AL. Protein Synthesis in Tonic and Phasic Skeletal Muscles. Nature. 1967;216:1219–20. doi: 10.1038/2161219a0. [DOI] [PubMed] [Google Scholar]
- 8.Goodman CA, Frey JW, Mabrey DM, Jacobs BL, Lincoln HC, You J-S, Hornberger TA. The role of skeletal muscle mTOR in the regulation of mechanical load-induced growth. J Physiol. 2011;589:5485–501. doi: 10.1113/jphysiol.2011.218255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman CA, Kotecki JA, Jacobs BL, Hornberger TA. Muscle Fiber Type-Dependent Differences in the Regulation of Protein Synthesis. PLoS ONE. 2012;7:e37890. doi: 10.1371/journal.pone.0037890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, Hornberger TA. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011;25:1028–39. doi: 10.1096/fj.10-168799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodman CA, Mayhew DL, Hornberger TA. Recent Progress towards Understanding the Molecular Mechanisms that Regulate Skeletal Muscle Mass. Cellular signalling. 2011;23:1896–906. doi: 10.1016/j.cellsig.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Ge Y, Chen J, Hornberger TA. A Phosphatidylinositol 3-Kinase/Protein Kinase B-independent Activation of Mammalian Target of Rapamycin Signaling Is Sufficient to Induce Skeletal Muscle Hypertrophy. Mol. Biol. Cell. 2010;21:3258–68. doi: 10.1091/mbc.E10-05-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman CA, Pierre P, Hornberger TA. Imaging of protein synthesis with puromycin. Proceedings of the National Academy of Sciences. 2012;109:e989. doi: 10.1073/pnas.1202000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habets PE, Franco D, Ruijter JM, Sargeant AJ, Pereira JA, Moorman AF. RNA content differs in slow and fast muscle fibers: implications for interpretation of changes in muscle gene expression. J Histochem Cytochem. 1999;47:995–1004. doi: 10.1177/002215549904700803. [DOI] [PubMed] [Google Scholar]
- 15.Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol. 2011;43:1267–76. doi: 10.1016/j.biocel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferson LS, Fabian JR, Kimball SR. Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int J Biochem Cell Biol. 1999;31:191–200. doi: 10.1016/s1357-2725(98)00141-1. [DOI] [PubMed] [Google Scholar]
- 17.Koopman R, Gleeson B, Gijsen A, Groen B, Senden J, Rennie M, van Loon L. Post-exercise protein synthesis rates are only marginally higher in type I compared with type II muscle fibres following resistance-type exercise. European journal of applied physiology. 2011;111:1871–8. doi: 10.1007/s00421-010-1808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li JB, Goldberg AL. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976;231:441–8. doi: 10.1152/ajplegacy.1976.231.2.441. [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Barrett EJ. Human protein metabolism: its measurement and regulation. Am J Physiol Endocrinol Metab. 2002;283:E1105–12. doi: 10.1152/ajpendo.00337.2002. [DOI] [PubMed] [Google Scholar]
- 20.Lynch GS. Tackling Australia's future health problems: developing strategies to combat sarcopenia--age-related muscle wasting and weakness. Intern Med J. 2004;34:294–6. doi: 10.1111/j.1444-0903.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- 21.Millward DJ, Garlick PJ, Nnanyelugo DO, Waterlow JC. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. The Biochemical journal. 1976;156:185–8. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakano K, Hara H. Measurement of the protein-synthetic activity in vivo of various tissues in rats by using [3H]Puromycin. The Biochemical journal. 1979;184:663–8. doi: 10.1042/bj1840663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathans D. Puromycin Inhibition of Protein Synthesis: Incorporation of Puromycin into Peptide Chains. Proceedings of the National Academy of Sciences of the United States of America. 1964;51:585–92. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rannels DE, Pegg AE, Rannels SR, Jefferson LS. Effect of starvation on initiation of protein synthesis in skeletal muscle and heart. Am J Physiol Endocrinol Metab. 1978;235:E126–33. doi: 10.1152/ajpendo.1978.235.2.E126. [DOI] [PubMed] [Google Scholar]
- 25.Reeds PJ, Davis TA. Of flux and flooding: the advantages and problems of different isotopic methods for quantifying protein turnover in vivo: I. Methods based on the dilution of a tracer. Current opinion in clinical nutrition and metabolic care. 1999;2:23–8. doi: 10.1097/00075197-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Ryan GB, Karnovsky MJ. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975;8:219–32. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- 27.Schakman O, Kalista S, Bertrand L, Lause P, Verniers J, Ketelslegers JM, Thissen JP. Role of Akt/GSK-3{beta}/{beta}-Catenin Transduction Pathway in the Muscle Anti-Atrophy Action of Insulin-Like Growth Factor-I in Glucocorticoid-Treated Rats. Endocrinology. 2008;149:3900–8. doi: 10.1210/en.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schiaffino S, Reggiani C. Fiber Types in Mammalian Skeletal Muscles. Physiological reviews. 2011;91:1447–531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt EK, Clavarino G, Ceppi M, Pierre P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat Meth. 2009;6:275–7. doi: 10.1038/nmeth.1314. [DOI] [PubMed] [Google Scholar]
- 30.Srikanthan P, Karlamangla AS. Relative Muscle Mass Is Inversely Associated with Insulin Resistance and Prediabetes. Findings from The Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011;96:2898–903. doi: 10.1210/jc.2011-0435. [DOI] [PubMed] [Google Scholar]
- 31.Ueki K, R Yamamoto-Honda, Kaburagi Y, Yamauchi T, Tobe K, Burgering BMT, Coffer PJ, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential Role of Protein Kinase B in Insulin-induced Glucose Transport, Glycogen Synthesis, and Protein Synthesis. Journal of Biological Chemistry. 1998;273:5315–22. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 32.van Wessel T, de Haan A, van der Laarse W, Jaspers R. The muscle fiber type–fiber size paradox: hypertrophy or oxidative metabolism? European journal of applied physiology. 2010;110:665–94. doi: 10.1007/s00421-010-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vara JA, Portela A, Ortìn J, Jimènez A. Expression in mammalian cells of a gene from Streptomyces alboniger conferring puromycin resistance. Nucleic Acids Res. 1986;14:4617–24. doi: 10.1093/nar/14.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wool IG, Kurihara K. Determination of the number of active muscle ribosomes: effect of diabetes and insulin. Proceedings of the National Academy of Sciences of the United States of America. 1967;58:2401–7. doi: 10.1073/pnas.58.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yarmolinsky MB, Haba GL. Inhibition by Puromycin of Amino Acid Incorporation into Protein. Proceedings of the National Academy of Sciences of the United States of America. 1959;45:1721–9. doi: 10.1073/pnas.45.12.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]