Abstract
BACKGROUND
Improved tests are needed for detection and management of prostate cancer. We hypothesized that differential gene expression in prostate tissue could help identify candidate blood biomarkers for prostate cancer and that blood from men with advanced prostate disease could be used to verify their presence in circulation.
METHODS
Candidate markers were identified using mRNA expression patterns from laser-capture microdissected prostate tissue. Tissue expression was confirmed using immunohistochemistry (IHC) for the subset of candidates having commercial antisera. Tissue extracts were analyzed with tandem mass spectrometry (MS/MS). Blood concentrations were measured using immunoassays and MS/MS of trypsin-digested, immuno-extracted peptides.
RESULTS
Thirty-five novel candidate prostate adenocarcinoma biomarkers were selected. Tissue expression was confirmed for all of the 13 markers having commercial antisera for IHC and six of these markers showed statistical discrimination between normal and malignant tissue. Only 5 of these markers were detected in tissue extracts using MS/MS. Sixteen of the 35 candidate markers were successfully assayed in blood. Four of eight biomarkers measured with ELISA and 3 of 10 biomarkers measured by targeted MS showed statistically significant increases in blood concentrations of advanced prostate cancer cases, compared to controls.
CONCLUSION
Seven novel biomarkers identified by gene expression profiles in prostate tissue were shown to have statistically significant increased levels in blood from men with advanced prostate adenocarcinoma compared to controls: APOC1, ASPN, COMP, CXCL11, CXCL9, F5, and PCSK6.
Keywords: Prostate Cancer, Mass Spectrometry, Biomarkers, Verification, Blood assays
Introduction
Prostate cancer (PCa) is the second leading cause of cancer-related death in American men [1], with >90% of this cancer consisting of adenocarcinoma subtype [2] .Typically, prostate adenocarcinoma undergoes slow growth; however, in a subset of men the cancer can rapidly develop, leading to multiple health problems and ultimately death. Autopsy studies have shown many older men who died of other causes, also had undetected prostate cancer [3]. Roughly one million men undergo prostate needle biopsy each year (often based on elevated blood PSA ), but only one fifth are malignant and one-tenth of those die of prostate disease [4, 5]. Better biomarkers to supplement PSA could help in diagnosis and management of prostate cancer.
The discovery of biomarkers in blood that are sensitive and specific for prostate cancer is difficult due to the large number of proteins and protein metabolic products with potentially very low concentrations. On the other hand, measurements of expressed mRNA from prostate tissue extracts are relatively robust due to commercial arrays and gene amplification techniques. Also, bioinformatics tools can provide guidance on which genes encode for proteins likely to be found in blood. The differential expression of genes in prostate tissue from men with benign prostatic hypertrophy compared with prostate tumors with low aggression characteristics versus tumors with aggressive characteristics provides a potential strategy for identifying candidate protein biomarkers. That is, the gene expression data could provide a treasury map to help look for specific candidate biomarkers in blood. This is the strategy used in this project. Three techniques- immunohistochemistry (IHC), immunoassay, and tandem mass spectrometry (MS/MS) were used to look for these proteins in tissue and blood. We identified 70 candidate biomarkers based on mRNA changes and pursued 35 candidates that had no prior proven utility in prostate cancer. Some of these proteins had been previously investigated, so antisera were available, whereas others had none. When available, commercial antisera and immunoassay reagents were utilized. For other markers, MS/MS reagents and techniques were developed.
Validation of potential biomarkers in blood is difficult. Blood contains proteins, protein precursor forms, and protein metabolites from many tissue sources. Also the dilution of proteins from a small organ into a large blood volume, combined with numerous blood clearance mechanisms make many of the blood concentrations very low. Validation of protein biomarkers in tissue is easier due to tissue specificity and relatively higher concentrations-so this is a potential intermediary step.
Two potential clinical roles for prostate biomarkers are early detection of cancer and identification of aggressive forms of cancer. For early detection very sensitive assay systems are needed to measure the low biomarker concentrations generated by the small tumor masses. The identification of aggressive forms of cancer requires longitudinal follow-up with stable preserved blood specimens. Since blood assays were not available which could measure very low concentrations of many of these proteins and long term stable blood specimens were not available, it was impractical to fully evaluate the utility of these candidate biomarkers. Therefore we elected to determine if these markers could be measured in blood specimens from men with advanced prostate cancer and whether those concentrations were different from the levels in blood from men with benign prostate disease and/or early cancer.
METHODS and MATERIALS
Study Design
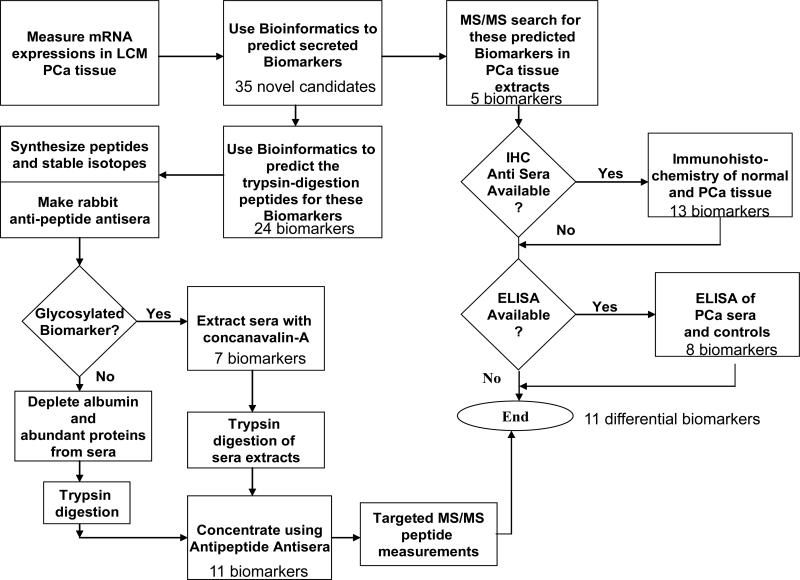
The study design is illustrated in Figure 1. Gene expression data generated in a discovery project funded by the Minnesota Partnership was used for hypothesis generation and selection of an initial candidate biomarker list [6, 7]. Briefly, frozen prostate tissue samples from consenting patients were procured from the Mayo Clinic Prostate SPORE tissue bank. Tumor cells or adjacent non-neoplastic prostate epithelial cells from 100 patients with prostate cancer were extracted by laser capture microdissection mRNA was extracted, amplified and measured on Affymetrix U133 Plus 2.0 microarray chips. Biomarker candidates were selected by comparing expression profiles. This candidate list was filtered to identify 70 genes with prostate-tissue specific expression, which encoded proteins localized to cellular membrane or extracellular space (Supplemental File 1-A). From this list, 35 novel candidate biomarkers were selected which had no prior literature or patent claims for utility in prostate disease. Intermediary studies were performed to evaluate protein expression in prostate tissue using IHC and MS/MS. Where available, ELISA assays were used to measure blood concentrations of candidate markers. MS/MS methods were developed to measure blood levels of candidate markers that possessed favorable characteristics. Prior to MS/MS measurement, the blood was depleted of high abundance proteins and/or glycated proteins. The depleted samples then were trypsin digested and the target peptides were extracted using rabbit anti-peptide polyclonal antibodies.
Figure 1.
Flow diagram illustrating processes used to identify novel candidate biomarkers and measure proteins in tissue and blood.
In-silico candidate marker selection
The initial candidate markers were selected for differential tumor-to-normal expression or high prostate tissue-specific expression profiles. Tissue specific expression was defined using a numeric score derived from existing cross-tissue expression data sets [8]. The candidates were filtered to identify markers encoding proteins localized to the extracellular space (secreted) or cellular membrane. Localization was defined by UniProt annotations [9]. For candidate proteins lacking annotation, the cellular localization was predicted using an integration of SignalP, TargetP and TMHMM predictions [10]. Candidate biomarkers were further filtered to select for protein targets with a high propensity for assay development, using a multi-step bioinformatic workflow [11]. Briefly, the protein sequences were computationally digested using PeptideCutter [12]. Tryptic peptide sequences were then compared to the human proteome using BLAST (blastp) [13], to ascertain parent protein specificity. Post-translational glycosylation and phosphorylation sites were extracted for each candidate marker from the UniProt database. This information was augmented using NetNGlyc 1.0, NetOGlyc 3.1 [14] and NetPhos 2.0 predictions [15]. Peptide immunogenic potential was determined on the basis of hydrophilicity, surface probability, and flexibility, using the GCG peptide structure prediction algorithm [16].
Evaluation of Protein Expression in Tissue
MS/MS of Tissue Extracts
Fresh frozen prostate adenocarcinoma and adjacent normal tissue were obtained from two men with high Gleason score prostate cancer: #81 ( PSA of 3.5 ng/mL, Gleason 9, and T3aN0+ tumor) and #143 (PSA of 7.1 ng/mL, Gleason 7, and T3aN0- tumor). The main focus of these tissue extract measurements was to evaluate if the gene expressions were associated with protein expressions. Only two patients were evaluated because of the limited sensitivity of MS/MS to detect low concentrations even with prior fractionation. Tissue was pulverized while frozen and homogenized with a POLYTRON® in 1M HEPES buffer pH 7.3 with protease inhibitors. The samples were electrophoresed on 10-14% SDS-PAGE gels, stained with Coomassie Blue, and sliced horizontally into strips. The expected biomarkers for each gel band are illustrated in Figure 2. The gel bands were destained with 50mM Tris, pH 8.1 / 50% acetonitrile, reduced with 20 mM DTT/50mM Tris, pH 8.1 at 55ºC for 40 minutes, and alkylated with 40mM iodoacetamide at room temperature for 40 minutes in the dark. The proteins were digested in-situ with 30μl (0.004μg/μl) trypsin (Promega Corporation, Madison WI) in 20 mM Tris pH 8.1 / 0.0002% Zwittergent 3-16, at 37°C overnight followed by peptide extraction with 60μL of 2% trifluoroacetic acid, then 60uL of acetonitrile. The pooled extracts were concentrated to less than 5μl on a SpeedVac spinning concentrator (Savant Instruments, Holbrook NY) and then brought up in 0.1% formic acid. MS/MS using nano-flow liquid chromatography electrospray on a ThermoFinnigan LTQ Orbitrap Hybrid Mass Spectrometer (ThermoElectron Bremen, Germany) was used to look for the targeted peptides for each gel band.
Figure 2.
Coomassie stained SDS-PAGE Gel of tissue extracts showing protein loading and gel slices used to evaluate target peptides on Thermo LTQ Orbitrap MS/MS. The predicted location of the 35 candidate biomarkers, based on molecular weights, are illustrated on this figure.
IHC
Paraffin-embedded, formalin–fixed tissue sections from 20 men having prostate cancer [17] were used to evaluate protein expression for thirteen candidate biomarkers which had commercially available IHC antisera. For each case, matched normal and malignant tissue sections were analyzed. Slides were placed in a preheated 1mM EDTA, pH 8.0 retrieval buffer for 30 minutes and then cooled. After the heat activated epitope retrieval step, slides were placed on the DAKO Autostainer and incubated with the candidate biomarker specific antibodies (see Supplemental File 1-B). Staining intensity of the prostate tumor and matched normal prostate tissue were scored using an ordinal scale of 0-3, with 0 representing no staining and 3 representing heavy staining. For each antisera, the number of cases where the tumor stain intensity score exceeded the normal stain intensity score, or visa versa, were enumerated (Table 2).
Table 2.
Immunohistochemistry grading of staining.
| CCL19 | CDH7 | COL2A1 | COL9A2 | COMP | CXCL14 | CXCL9 | EFNA4 | F5 | GPR116 | NRN1 | PCSK6 | PRG3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glea | C | N | C | N | C | N | C | N | C | N | C | N | C | N | C | N | C | N | C | N | C | N | C | N | C | N |
| 6 | 1 | 2 | -- | 1 | 3 | 3 | 1 | 2 | 0 | 0 | 1 | 1 | -- | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | -- | 1 | -- | 1 |
| 6 | 2 | 1 | 1 | 3 | 3 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 3 | 1 | 1 | 1 | 2 | 1 | 2 | 2 |
| 6 | 3 | 1 | 2 | 1 | 3 | 1 | 3 | 1 | 1 | 0 | -- | -- | 1 | 1 | 2 | 1 | 2 | 0 | 3 | 1 | 2 | 1 | 2 | 0 | 2 | 1 |
| 6 | 2 | 2 | 1 | 1 | 3 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | -- | -- | 1 | 0 | 0 | 0 | 3 | 2 | 2 | 1 | 2 | 1 | 2 | 2 |
| 6 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 2 | 1 | 1 | 1 |
| 6 | 1 | 1 | 0 | 1 | 2 | 1 | 2 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 |
| 6 | 1 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 |
| 6 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 0 | 1 | 1 | -- | -- | 1 | 0 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 0 | 2 | 1 |
| 7 | 2 | 1 | 1 | 2 | 3 | 2 | 2 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 2 | 1 | 1 | 0 | 3 | 1 | 1 | 1 | 3 | 0 | 2 | 1 |
| 7 | 2 | 2 | 1 | 1 | 3 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| 7 | 3 | 2 | 1 | 1 | 3 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 0 | 3 | 2 | 3 | 1 | 3 | 1 | 3 | 2 |
| 7 | 2 | 2 | 2 | 2 | 3 | 2 | 3 | 2 | 2 | 0 | 3 | 1 | 2 | 1 | 3 | 0 | 3 | 1 | 3 | 1 | 2 | 1 | 3 | 1 | 2 | 2 |
| 7 | 3 | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 0 | 3 | 1 | 3 | 1 | 3 | 1 | 3 | 1 | 2 | 2 |
| 7 | 1 | 1 | 1 | 2 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 3 | 1 | 2 | 2 |
| 7 | 2 | 1 | 1 | 1 | 3 | 1 | 2 | 2 | 2 | 1 | -- | -- | 2 | 1 | 2 | 1 | 3 | 1 | 3 | 1 | 2 | 1 | 3 | 1 | 2 | 2 |
| 8 | 2 | 1 | 2 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 0 | 2 | 2 |
| 8 | 2 | 1 | 3 | 1 | 3 | 2 | 2 | 2 | 2 | 1 | 3 | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 |
| 9 | 2 | 2 | 1 | 2 | 3 | 1 | 2 | 2 | 1 | 0 | 3 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 3 | 2 | 1 | 1 | 3 | 1 | 2 | 2 |
| 10 | 1 | 1 | 2 | 1 | 3 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 0 | 1 | 2 | 2 | 2 | 1 | 3 | 1 | 2 | 1 |
| 10 | 2 | 1 | 0 | -- | 3 | --- | 1 | -- | 1 | 1 | 2 | 1 | 2 | -- | 1 | -- | 1 | -- | 2 | -- | 1 | 1 | 3 | 0 | 2 | -- |
| #C>N | 9 | 5 | 16* | 8 | 9* | 9* | 6 | 13* | 8 | 12* | 8 | 17* | 6 | |||||||||||||
| #N>C | 2 | 7 | 0 | 1 | 0 | 0 | 1 | 1 | 5 | 1 | 1 | 0 | 1 | |||||||||||||
| pval | 0.03 | 0.39 | <0.01 | 0.02 | <0.01 | <0.01 | 0.06 | <0.01 | 0.29 | <0.01 | 0.02 | <0.01 | 0.06 | |||||||||||||
C = prostate cancer tissue, N = adjacent normal tissue, 0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = heavy staining.
One-tail Sign Test, p<0.01.
ELISA assays
Commercial ELISAs were used to measure the blood levels of eight candidate biomarkers. Frozen aliquots (-70 C) of serum and EDTA samples from men with advanced prostate cancer (50) or men with recent prostate biopsies (26) were obtained from the Mayo Prostate SPORE. Many of the men in the advanced cancer series subsequently died of prostate cancer. Blood samples from men undergoing prostate biopsy, matched by PSA levels, were used as controls. These controls included 13 biopsies showing no cancer and 13 biopsies showing early prostate cancer. The analytic performances of the kits were verified and controls with known target ranges were assayed on every plate. The reagents are listed in Supplemental File 1-C.
MS/MS Blood Measurements
A modified version of the SISCAPA ( Stable Isotope Standards with Capture by Anti-Peptide Antibodies) methodology published by Dr, Anderson was utilized. Serum specimens were trypsin digested and targeted peptides are immuno-extracted and quantitated by spectrometric multiple reaction monitoring (MRM) [18,19]. We utilized an API 5000 triple quadripole mass spectrometer (Applied Biosystems, Foster City, CA) with a higher injection volume (100 uL) compared to the published SISCAPA. Like Dr. Anderson, we used polyclonal rabbit anti-peptide antisera; however, our antibodies did not work well with whole serum, therefore we incorporated protein depletion prior to digestion. We multiplexed 5 to 7 antisera together to extract 1.0 mL of serum and did not reuse our anti-peptide extraction beads. The elution fraction from multiplexed immuno-affinity beads was evaporated on SpeedVac to approximately 100μl and mixture of internal standards (IS) was added to all samples just prior to MRM analysis. Four to five transitions were identified for each double charge parent ion but only the optimal two to three transitions were chosen for monitoring in order to have adequate resolution to simultaneously measure the 5 to 7 multiplexed peptides. Table 3 shows the parameters for each endogenous peptide and the corresponding isotopically labeled IS peptide.
Table 3.
Lists of anti-peptide rabbit antisera used on each of the multiplexed affinity extraction sets, along with the mass spectrometry transitions used for both the native and internal standard (IS) signals.
| Peptide Sequences | Native Transition | IS Transition | |
|---|---|---|---|
| Affinity Antibody Set A | |||
| APOF:232 | SGVQQLIQYYQDQK | 849.9/613.3 | 853.2/620.4 |
| CHD7:142 | IQDINDNEPK | 593.6/242.4 | 596.6/242.4 |
| COL2A1:871 | AGEPGLQGPAGPPGEK | 731.5/809.4 | 734.5/815.6 |
| PCDHB10_72 | QYLLLDSHTGNLLTNEK | 654/717.4 | 656.2/725.1 |
| PCDHGA4:265 | ATDPDEGANGDVTYSFR | 908.43/1058.5 | 913.15/1068.7 |
| Affinity Antibody Set B | |||
| ASPN:153 | LYLSHNQLSEIPLNLPK | 660.7+3/1010.6 | 662.6+3/1016.7 |
| B3GNT1:162 | YEAAVPDPR | 509.5/175.1 | 512.5/175.1 |
| F5:2151 | SYTIHYSEQGVEWK | 864.4/1125.5 | 867.2/1131.7 |
| OGDHL:673 | HHVLHDQEVDR | 693.1/275.4 | 696.1/275.4 |
| PCSK6:597 | AEGQWTLEIQDLPSQVR | 657.7/586.5 | 660/586.5 |
| Affinity Antibody Set C | |||
| ALSH3B2:121 | HLTPVTLELGGK | 633.2/251.1 | 636.5/251.1 |
| COL9A2:198 | GILGDPGHQGKPGPK | 729.84/1003.2 | 732.6/1008.9 |
| COMP:485 | LVPNPGQEDADR | 656.1/550.3 | 659.1/552.9 |
| OGDHL:673 | HHVLHDQEVDR | 693.1/275.4 | 696.1/275.4 |
| PCSK6:597 | AEGQWTLEIQDLPSQVR | 657.7/586.5 | 660/586.5 |
| PGLS:214 | ILEDQEENPLPAALVQPHTGK | 767.4+3/1118.7 | 769.4+3/971.5 |
| RPL22L1:15 | FNLDLTHPVEDGIFDSGNFEQFLR | 938+3/262.4 | 940+3/262.4 |
Serum Extraction and Trypsin digestion of Biomarkers prior to MS/MS
To improve detection of peptides, all serum samples were either lectin-extracted or depleted of high abundance proteins followed by trypsin digestion and immuno extraction with antipeptide beads (Figure 1). For the first protocol, 1.0 mL of sera to be tested was passed over a Concanavalin A lectin column (Sigma #C7555) to separate human N-glycosylated proteins from non-N-glycosylated proteins. Glycosylated proteins were eluted from the column with a gradient of methyl a-D-mannopyranoside in 20mM Tris, 0.5M NaCl, 1mM MgCl2, 1mM MnCl2, 1mM CaCl2, pH 7.5, trypsin digested, and serially extracted using two anti-peptide bead sets (A and B). The second protocol depleted albumin and 13 other high abundance proteins from a second set of 1.0 mL serum samples using MARS14 columns (Agilent Technologies, New Castle, DE). After trypsin digestion, peptides were extracted using the multiplexed antipeptide bead sets. For trypsin digestion, urea was added to obtain a final 6M concentration. The samples were incubated with 10mM of DTT at 37°C for 1h followed by alkylation with 30mM iodoacetamide at room temperature for 1h in the dark. Samples were diluted with 25mM NH4HCO3 with 0.001% Zwittergent 3-16 to 1M of urea. Proteins were digested with 0.5mg trypsin (Sigma) at 37°C overnight with 1:10 ratio, and the reaction was stopped by lima bean inhibitor of trypsin (Worthington).
The production of anti-peptide polyclonal immuno-affinity magnetic particles is outlined in Supplemental File 1-D. The anti-peptide bead sets were designed to provide good MS/MS separation of the peptides included in each elution. Bead sets A and B each contained multiplexed antisera for five peptides corresponding to glycosylated proteins (Table 1). Bead set C contained seven multiplexed antisera, including two antisera contained in bead set A (PCHHGA4, PCDHB10) and two antisera contained in bead set B ( OGDHL, PCSK6). Duplication of peptides between columns reflected uncertainty regarding the parent protein glycosylation status. Trypsin digested samples were added to the antibody conjugated beads and gently rocked for 4 hours at room temperature. The supernatant and the first 500 ul of a PBS wash were collected and either exposed to a second bead set or stored at -80C for future use. Prior to use, each multiplexed anti-peptide bead set was washed two times with the PBS buffer. The peptides were eluted with 5% acetic acid with 0.001% Zwittergen 3-16. Fresh bead sets were utilized for each patient sample processed.
Table 1.
Summary of test results.
| Biomarker | mRNA | Tissue | Blood Assays | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Num | Gene | Official Gene Name | MW | Select | MS | IHC | ELISA | Pep | #Ab | MS-A | MS-B | MS-C |
| 1 | ALDH3B2 | aldehyde dehydrogenase 3 family, member B2 | 42.7 | Diff Exp | 1 | 1 | ||||||
| 2 | APOC1 | apolipoprotein C-I | 9.3 | Diff Exp | 0/2 | 7/50* | ||||||
| 3 | APOF | apolipoprotein F | 33.5 | Diff Exp | 2 | 2 | 2/50 | |||||
| 4 | ASPN | asporin | 43.3 | Diff Exp | 1/2 | 2 | 1 | 26/50** | ||||
| 5 | B3GNT6 | UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 6 (core 3 synthase) | 42.7 | Diff Exp | 1 | 1 | 1/50 | |||||
| 6 | C1orf64 | chromosome 1 open reading frame 64 | 17.7 | Diff Exp | 1 | 1 | ||||||
| 7 | C4A/C4B | complement components 4A and 4B | 22.3 | Diff Exp | 0/2 | 4/50 | 3 | 3 | ||||
| 8 | CCL19 | chemokine (C-C motif) ligand 19 | 11.0 | Diff Exp | 9/20 | 2/50 | ||||||
| 9 | CDH10 | cadherin 10, type 2 (T2-cadherin) | 88.5 | Diff Exp | 2 | 1 | ||||||
| 10 | CDH7 | cadherin 7, type 2 | 87.1 | Diff Exp | 5/18 | 1 | 1 | 9/50 | ||||
| 11 | COL2A1 | collagen, type II, alpha 1 | 139.1 | Diff Exp | 16/19 | 3 | 3 | 5/50 | ||||
| 12 | COL9A2 | collagen, type IX, alpha 2 | 62.8 | Diff Exp | 8/19 | 3 | 3 | |||||
| 13 | COMP | cartilage oligomeric matrix protein | 82.8 | Diff Exp | 9/20 | 0/50 | 2 | 2 | 0/50 | |||
| 14 | CXCL11 | chemokine (C-X-C motif) ligand 11 | 82.8 | Diff Exp | 7/50* | |||||||
| 15 | CXCL14 | chemokine (C-X-C motif) ligand 14 | 10.4 | Diff Exp | 9/18 | 2/50 | ||||||
| 16 | CXCL9 | chemokine (C-X-C motif) ligand 9 | 11.8 | Diff Exp | 6/16 | 13/50** | ||||||
| 17 | EFNA4 | ephrin-A4 | 14.0 | Diff Exp | 13/19 | |||||||
| 18 | ESRP1 | epithelial splicing regulatory protein 1 (Prior RBM35A) | 50.0 | High E | 1 | 1 | ||||||
| 19 | F5 | coagulation factor V (proaccelerin, labile factor) | 22.4 | High E | 8/19 | 8/50 | 2 | 2 | 25/50*** | |||
| 20 | GPR116 | G protein-coupled receptor 116 | 18.6 | Diff Exp | 12/19 | |||||||
| 21 | KAZALD1 | Kazal-type serine peptidase inhibitor domain 1 | 149.2 | Diff Exp | 1 | 1 | ||||||
| 22 | LOC284591 | No name assigned | 32.9 | High E | ||||||||
| 23 | LOX | lysyl oxidase | 27.0 | High E | 2 | 2 | ||||||
| 24 | LRRN1 | leucine rich repeat neuronal 1 | 46.9 | Diff Exp | 1 | 1 | ||||||
| 25 | LSM14B | LSM14B, SCD6 homolog B (S. cerevisiae) (Prior FAM61B) | 251.7 | Diff Exp | ||||||||
| 26 | MS4A8B | membrane-spanning 4-domains, subfamily A, member 8B | 80.7 | Diff Eep | 1 | 1 | ||||||
| 27 | NKAIN1 | Na+/K+ transporting ATPase interacting 1 (Prior FAM77C) | 42.1 | High E | 1 | 0 | ||||||
| 28 | NRN1 | neuritin 1 | 26.3 | Diff Exp | 8/20 | 1 | 1 | |||||
| 29 | OGDHL | oxoglutarate dehydrogenase-like | 15.6 | Diff Exp | 1 | 1 | ||||||
| 30 | PCDHB10 | protocadherin beta 10 | 114.5 | Diff Exp | 2 | 2 | ||||||
| 31 | PCDHGA4 | protocadherin gamma subfamily A4 | 87.6 | Diff Exp | 2 | 2 | ||||||
| 32 | PCSK6 | proprotein convertase subtilisin/kexin type 6 | 100.6 | Diff Exp | 17/19 | 2 | 1 | 8/50 | 4/50* | |||
| 33 | PGLS | 6-phosphogluconolactonase | 106.4 | Diff Exp | 2/2 | 7/50 | ||||||
| 34 | PRG3 | proteoglycan 3 | 27.5 | High E | 6/18 | 1 | 1 | |||||
| 35 | RPL22L1 | ribosomal protein L22-like 1 | 80.3 | Diff Exp | 1/2 | 1 | 1 | 11/50 | ||||
Satterthwaite P-values for t-test separating advanced cancer from other groups
<0.05
<0.01
<0.001.
Data analysis
Significance of change in IHC staining intensity between tumor and normal tissue was assessed using a one-tail Sign test, excluding equivalent scoring samples, with a p-value threshold of 0.01. MS results for the tissue specimens were quantitated by counting unique peptides identified for each candidate marker. The ELISA and MS sera data was analyzed for group discrimination using a rank sum test and Satterthwaite t-test, with p-value thresholds of 0.05. To ascertain potential marker discrimination in a subset of advance prostate cancer sera samples, candidate markers were also characterized by the number of tumor samples with concentration values exceeding the maximum value from the control samples. To minimize false positives, only those biomarker concentrations which exceeded the highest concentration measured in the 26 control samples were considered positive on the cross plots.
RESULTS
The initial selection process identified 70 candidate serum biomarkers based on our bioinformatics selection criteria (Supplemental File 1). This list included known prostate cancer biomarkers such as PSA, AMACR, and hK2. Candidate markers were screened against the literature to identify 35 novel candidates, for which utility as a prostate cancer marker was previously unreported (Table 1). Twenty-six of these candidate markers were classified as extracellular and the remaining 9 as cellular membrane proteins. The predicted molecular weights ranged from 9.3 to 251.7 kD.
Mass spectrometry analysis of tissue extracts identified only five of the candidate biomarkers in the samples analyzed: APOC1 (gel 8), ASPN (gel 5), C4A (gel 8), PGLS (gel 7), and RPL22L1 (gel 8). ASPN, RPL22L1, and PGLS demonstrated some tumor-specific discrimination. IHC analysis of prostate tumor and normal tissue sections revealed that all 13 assayed proteins were expressed in at least one of the prostate cancer samples. Table 3 shows the assigned staining code for each sample. The aggregate counts at the bottom of each IHC column reports the number of samples in which the staining code was higher or lower for the cancer tissue compared to adjacent normal tissue. No marker demonstrated statistically significant differences between Gleason scores. Using a one-tail Sign test with a p-value < 0.01, six markers showed statistically significant increases in staining codes for the prostate cancer tissue compared to the matched control tissue (COL2A1, COMP, CXCL14, EFNA4, GPR116, and PCSK6).
The eight ELISA assays identified measurable levels of the biomarkers in a majority of samples analyzed. Statistically significant changes in four candidate marker concentrations between advance prostate cancer sera and control sera were found using a rank sum test with p-value < 0.05 (APOC1, CXCL11, CXCL9, and COMP). Statistical significance was confirmed with a Satterthwaite t-test for APOC1, CXCL11, and CXCL9. When selecting candidate markers on the basis of >10% of advanced prostate cancer sample values exceeding the maximum value of the control samples, the same three markers identified by the Satterthwaite t-test were found.
For the blood-based MS/MS assays, 39 peptides were synthesized, representing 24 of the protein candidate biomarkers. The remaining 11 biomarkers were not targeted by MS/MS analysis due to difficulties synthesizing peptides or the availability of ELISA assays (Table 1). Antisera was successfully developed for 38/39 peptides and 25 antibodies worked for immuno-extraction.
Eleven of the captured peptides resulted in weak or undetectable signal on MS/MS. Ultimately, MS/MS assays that could detect peptides in blood were obtained for 10 biomarkers.
Three multiplexed sets of MS/MS assays were run using the same sample set from men with advanced prostate cancer and associated controls which was used for the ELISA assays. Based on rank sum test (pvalue < 0.05), three markers discriminated the advanced prostate cancer samples from associated controls. ASPN and F5 were assayed on multiplexed bead set B and PCSK6 on multiplexed bead set C. Owing to the known heterogeneity in cancer, the candidate markers were also characterized for potential signal in a subset of advanced prostate cancer patients by identifying the number of samples in which the advanced prostate cancer sera concentration values exceeded the highest control value. Elevated values were identified for 3 markers in bead set A (APOF in 2/50, CDH7 in 9/50, and COL2A1 in 5/50), four markers in bead set B (ASPN in 26/50, B3GNT in 1/50, Factor 5 in 25/50 and PCSK6 in 8/50), and three markers in bead set C (PCSK6 in 4/50, PGLS in 7/50, and RPL22L1 in 11/50) (Figure 3). Results are summarized in Table 1.
Figure 3.
Plots of sequential measurements of selected analytes. Panel A shows ELISA assays. Panel B shows MS/MS assays from bead set A. Panel C shows MS/MS assays from bead set B. Panel D shows MS/MS assays from bead set C. Open symbols: squares are benign prostate, diamonds are QC pools, and triangles are early prostate cancer. Solid dots represent patients with advanced prostate cancer.
Figure 3. Panel A- ELISAs:
Figure 3. Panel B- MS/MS from bead Set A:
Figure 3. Panel C- MS/MS from bead set B:
Figure 3. Panel D- MS/MS from bead set C:
CONCLUSIONS AND DISCUSSION
Seven novel biomarkers were identified as statistically significant in discriminating protein concentrations in blood from men with advanced prostate cancer compared to controls: APOC1, ASPN, COMP, CXCL11, CXCL9, F5, and PCSK6. Four additional novel biomarkers were identified with elevated serum concentration values in at least 10% of advanced prostate cancer samples compared to controls: CDH7, COL2A1, PGLS, and RPL22L1. These blood proteins have not been previously advocated as markers of prostate cancer. The increased expression of mRNA in the prostate tissues and the biochemical characteristic predicting their presence in blood were the guiding principles for the targeted search. Although no single candidate biomarker was found in all men with advanced prostate cancer, measurements of combinations of these markers may have good clinical utility. However, verification of multivariate panels of biomarkers must await further studies because that requires larger numbers of patients.
Several studies have identified potential prostate cancer biomarkers based on differential gene expression in tissue or cell culture [20-22]. There is surprisingly little agreement across these studies. Part of this lack of concordance may be related to co-regulation of genes causing high correlations in gene expression, where one study may select one of the correlated genes and another study may select alternate genes. The NCI study identified and annotated 91 molecular markers with potential utility for prostate cancer including PSA and KLK2 [20]. None of our 35 novel potential biomarkers were included on this list.
Bai et al identified 37 overexpressed and 7 down-regulated genes for prostate cancer based on differential expression of mRNA transcripts. None of their gene products matched our list, although, only 13 of their sequences had reported identities in GenBank, the others were “expressed sequence tags” [21]. Sardana et al identified 65 extracellular candidate tumor markers in PC3 cell culture media. Only one marker, COL2A1, was also on our list; however, two other markers CXCL3 and C3 belonged to the same protein family as entries on our list [22]. Yang et al utilized integrative genomic mining for discovery of potential blood-borne cancer biomarkers [23]. None of their 33 markers for prostate, breast, or lung matched our list and only one (CXCL9) of their larger list of 178 general markers matched our list. None of the 27 novel prostate cancer markers reported by neither the Genome Sciences Centre nor any of the 16 markers reported by the Goulart's group were on our list [24, 25].
Coagulation factor V (F5) was elevated in many of our advanced prostate cancer samples. Elevations of other coagulation factors (prothrombin fragments and fibrous degradation products) have been similarly reported in advanced colon cancer [26]. Additionally, prostate tissue has been reported to be a rich reservoir of thrombin [27]. These observations are consistent with the findings reported here and suggest that coagulation markers may be important biomarkers for prostate cancer.
It is interesting that the chemokines CXCL9 and CXCL11 were found overexpressed in our laser microdissected tissues and showed discrimination for advanced prostate cancer. Localization of these markers to the prostate is expected, as chemokines are known to be constitutively expressed in epithelial cells of male urogenital track and present in seminal fluid [28]. Also, with Ewing sarcoma, CXCL9 was up regulated and was associated with tumor progression [29]. Hu et al showed that both CXCL12 and CXCL16 induced invasion with PC3 cell lines using the matrigel invasion assay [30], but they did not report investigating the effects of CXCL9, CXCL1, or CXCL14.
The preferred samples for evaluating the utility of blood markers for discriminating aggressive prostate cancer from slow growing prostate cancer would be blood collected at the time of diagnosis. The samples used in this study were collected many years after the diagnosis because earlier samples were not archived. Even if samples were originally saved, stability may be an issue after long storage. The candidate markers identified in this study will need further investigation to determine if they have early prognostic value. Also further study will be needed to confirm if multivariant combinations of these markers have additional utility.
Supplementary Material
Acknowledgments
Research Funding
This work was supported by two grants from Minnesota Partnership for Biotechnology and Medical Genomics and the NCI Mayo Clinic Prostate SPORE grant P50CA91956.
Nonstandard abbreviations
- IHC
immunohistochemistry
- IS
internal standard
- MRM
multiple reaction monitoring
- MS/MS
tandem mass spectrometry
- PCa
prostate cancer
- PSA
prostate specific antigen
- MRM
multiple reaction monitoring
- IS
internal standard
References
- 1.American Cancer Society . Cancer Facts & Figures 2010. American Cancer Society; Atlanta: 2010. [Google Scholar]
- 2.Dickinson SI. Premalignant and malignant prostate lesions: pathologic review. Cancer Control. 2010;17:214–22. doi: 10.1177/107327481001700402. [DOI] [PubMed] [Google Scholar]
- 3.Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol. 2004;17:292–306. doi: 10.1038/modpathol.3800054. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. Ca Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Wasson JH, Bubolz TA, Yao GL, Barry MJ. Prostate Biopsies in Men with Limited Life Expectancy. American College of Physicians. 2002 [PubMed] [Google Scholar]
- 6.Kube DM, Savci-Heijink CD, Lamblin AF, Kosari F, Vasmatzis G, Cheville JC, et al. Optimization of laser capture microdissection and RNA amplification for gene expression profiling of prostate cancer. BMC Mol Biol. 2007;8:25. doi: 10.1186/1471-2199-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vasmatzis G, Klee EW, Kube DM, Therneau TM, Kosari F. Quantitating tissue specificity of human genes to facilitate biomarker discovery. Bioinformatics. 2007;23:1348–55. doi: 10.1093/bioinformatics/btm102. [DOI] [PubMed] [Google Scholar]
- 8.Kosari F, Munz JAM, Savci-Hejink CD, Spiro C, Klee EW, Kube DM, et al. Identification of prognostic biomarkers for prostate cancer. Clin Cancer Res. 2008;14:1734–43. doi: 10.1158/1078-0432.CCR-07-1494. [DOI] [PubMed] [Google Scholar]
- 9.The Universal Protein Resource (UniProt) in 2010. Nucleic Acids Res. 2010;38:D142–8. doi: 10.1093/nar/gkp846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klee EW, Ellis LB. Evaluating eukaryotic secreted protein prediction. BMC Bioinformatics. 2005;6:256. doi: 10.1186/1471-2105-6-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerszten RE, Accurso F, Bernard GR, Caprioling RM, Klee EW, Klee GG, et al. Challenges in translating plasma proteomics from bench to bedside: update from the NHLBI Clinical Proteomics Programs. Am J Physiol Lung Cell Mol Physiol. 2008;295:L16–22. doi: 10.1152/ajplung.00044.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasteiger E, Hoogland C, Gattiker A, Duvand S, Wilkins MR, Appel RD, Bairoch A. The Proteomics Protocols Handbook. Humana Press; 2005. Protein Identification and Analysis Tools on the ExPASy Server. p. 38. [Google Scholar]
- 13.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 14.Julenius K, Molgaard A, Gupta R, Brunak S. Prediction, conservation analysis, and structural characterization of mammalian mucin-type O-glycosylation sites. Glycobiology. 2005;15:153–64. doi: 10.1093/glycob/cwh151. [DOI] [PubMed] [Google Scholar]
- 15.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–62. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 16.Jameson BA, Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988;4:181–6. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- 17.Sebo TJ, Cheville JC, Riehle DL, Lohse CM, Pankratz VS, Myers RP, et al. Predicting prostate carcinoma volume and stage at radical prostatectomy by assessing needle biopsy specimens for percent surface area and cores positive for carcinoma, perineural invasion, Gleason score, DNA ploidy and proliferation, and preoperative serum prostate specific antigen: a report of 454 cases. Cancer. 2001;91:2196–204. doi: 10.1002/1097-0142(20010601)91:11<2196::aid-cncr1249>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Anderson NL, Anderson GA, Haines LR, Hardie DB, Olafson RW, et al. Mass spectrometric quantitation of peptides and proteins using stable isotope standards and capture by anti-peptide antibodies (SISCAPA). J Proteome Res. 2004;3:235–44. doi: 10.1021/pr034086h. [DOI] [PubMed] [Google Scholar]
- 19.Anderson NL, Jackson A, Smith D, Hardie D, Borchers C, et al. SISCAPA peptide enrichment on magnetic beads using an in-line bead trap device. Mol Cell Proteomics. 2009;8:995–1005. doi: 10.1074/mcp.M800446-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tricoli JV, Schoenfeldt M, Conley BA. Detection of prostate cancer and predicting progression: current and future diagnostic markers. Clin Cancer Res. 2004;10:3943–53. doi: 10.1158/1078-0432.CCR-03-0200. [DOI] [PubMed] [Google Scholar]
- 21.Bai VU, Kaseb A, Tejwani S, Divine GW, Barrack ER, Menon M, et al. Identification of prostate cancer mRNA markers by averaged differential expression and their detection in biopsies, blood, and urine. Proc Natl Acad Sci U S A. 2007;104:2343–8. doi: 10.1073/pnas.0610504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sardana G, Marshall J, Diamandis EP. Discovery of candidate tumor markers for prostate cancer via proteomic analysis of cell culture-conditioned medium. Clin Chem. 53:429–37. doi: 10.1373/clinchem.2006.077370. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Iyer LK, Adelstein SJ, Kassis AI. Integrative genomic data mining for discovery of potential blood-borne biomarkers for early diagnosis of cancer. PLos ONE. 2008;3:1–8. doi: 10.1371/journal.pone.0003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romanuik TL, Ueda T, Le N, et al. Novel biomarkers for prostate cancer including noncoding transcripts. Am J Pathol. 2009;175:2264–76. doi: 10.2353/ajpath.2009.080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso V, Neves AF, Marangoni K, et al. Gene expression profile in the peripheral blood of patients with prostate cancer and benign prostatic hyperplasia. Cancer Detect Prev. 2009;32:336–7. doi: 10.1016/j.cdp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Iverson LH, Okholm M, Thorlacius-Ussing O. Pre- and postoperative state of coagulation and fibrinolysis in plasma of patients with benign and malignant colorectal disease–a preliminary study. Thromb Haemost. 1996;76:523–8. [PubMed] [Google Scholar]
- 27.Kohli M, Williams K, Yao JL, Dennis RA, Huang J, Reeder J, Ricke WA. Thrombin expression in prostate: a novel finding. Cancer Invest. 2011;29:62–7. doi: 10.3109/07357907.2010.535057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linge HM, Collin M, Giwercman A, Malm J, Bjartell A, Egesten A. The antibacterial chemokine MIG/CXCL9 is constitutively expressed in epithelial cells of the male urogenital tract and is present in seminal plasma. J Interferon Cytokine Res. 2008;28:191–6. doi: 10.1089/jir.2007.0100. [DOI] [PubMed] [Google Scholar]
- 29.Berghuis D, Santos SJ, Baelde HJ, Taminiau AH, Maarten Egeler R, Schilham MW, et al. Pro-inflammatory chemokine-chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8 (+) T-lymphocyte infiltration and affect tumour progression. J Pathol. 2011;223:347–357. doi: 10.1002/path.2819. [DOI] [PubMed] [Google Scholar]
- 30.Hu W, Zhen X, Xiong B, Wang B, Zhang W, Zhou W. CXCR6 is expressed in human prostate cancer in vivo and is involved in the in vitro invasion of PC3 and LNCap cells. Cancer Sci. 2008;99:1362–9. doi: 10.1111/j.1349-7006.2008.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.