Abstract
Objective
To compare adjunctive risperidone long-acting injectable + treatment as usual (RLAI+TAU) vs. TAU alone for relapse, rehospitalization, and urgent care events in bipolar disorder patients in routine care settings.
Methods
This was a 12-month randomized, open comparison of RLAI+TAU (n=20) and TAU alone (n=25) in adults with rapid cycling, MINI-confirmed bipolar I/II disorder and ≥ 4 illness relapses in the preceding 12 months. Clinical outcome was assessed every 2 weeks using the LIFE instrument. Psychopathology and quality of life were assessed monthly using the YMRS, MADRS, QIDS-SR-16 and Q-LES-Q. Relapse was defined using symptom severity, necessary clinical adjustment of medications, and urgent care referrals. Relapse rates and duration were calculated per person per year of follow-up. All treatments were provided by community-based clinicians.
Results
There were no significant between-groups differences in total number or duration of relapse events (any cause), or number of manic or depressive relapses. Thirteen of 14 urgent care events (hospitalization, ER visit, intensive outpatient or respite care referral) occurred with TAU alone (92.3%). Urgent care referral (p < 0.04) and necessary medication change rates (p = 0.01) were significantly lower in the RLAI+TAU group. There were no significant between-groups differences in duration of follow-up, hospitalization rates, or psychopathology over time.
Conclusions
Rates of any-cause relapse may not differ significantly between RLAI+TAU and TAU alone; however, RLAI may reduce the need for urgent care referrals or the frequency of medication adjustments to prevent relapse in community-treated patients with rapid cycling bipolar disorder. Additional investigation is warranted.
Keywords: atypical antipsychotics, bipolar disorder, mood relapse, risperidone long-acting injectable
Introduction
Bipolar disorder is a severe and recurring mood disorder that is associated with profound morbidity, mortality, and economic burden.1-3 Due to its recurring nature, long-term maintenance pharmacotherapy is often needed to prevent illness relapses in patients with bipolar disorder.4,5 Several pharmacotherapies, alone or in combination, may be effective for preventing mainly manic/mixed episode relapses during maintenance treatment.6 However, even with maintenance treatment, relapse rates in persons with bipolar disorder remain high.7
One important mechanism for acute illness relapse in bipolar disorder is poor adherence to treatment..8-10 Indeed, premature discontinuation of pharmacotherapy is the leading cause of bipolar relapse and recurrence,8,11 and is associated with higher psychiatric hospitalization risk, hospital bed-day consumption, and treatment costs in bipolar patients.12
A potential pragmatic solution to the problem of poor treatment adherence in bipolar disorder is the use of long-acting injectable medication during maintenance phase therapy. The use of long-acting typical neuroleptics for this indication is limited by several factors, including the risk of depressive symptom exacerbation13-15 and development of tardive dyskinesia.16,17 Long-acting injectable risperidone (RLAI), an atypical antipsychotic drug, is approved in the U.S. for the maintenance treatment of bipolar I disorder.18 Data from three randomized relapse prevention trials in patients with predominantly bipolar I disorder indicate that RLAI is effective for preventing acute mania relapses during maintenance phase treatment,19-21 without worsening depressive symptoms.19
There are some limitations to the generalizability of these study findings to patients who receive care in routine treatment settings. None of these studies were conducted exclusively in community-based settings, and all excluded patients with substance use disorders. Only one study enrolled patients with frequent illness relapses20 or those with bipolar II disorder.21 The objective of this study was to compare the effects of adjunctive RLAI (added to treatment-as-usual [TAU]) with TAU alone on rates of relapse, psychiatric hospitalization, and urgent care referral in adults with rapid cycling bipolar disorder in a community practice setting over 12 months.
Materials and Methods
Participants
Male and female patients (aged 18-64 years) who met Diagnostic and Statistical Manual, 4th Edition, Text Revision (DSM-IV-TR) criteria for bipolar I or II disorder were recruited from a single large community-based treatment center. Clinical diagnoses were confirmed using the Mini International Neuropsychiatric Interview (MINI).22 Current mood state was not a determinant of eligibility. Eligible subjects had a Young Mania Rating Scale (YMRS)23 or Hamilton Depression (17-item) Rating Scale (HAM-D)24 scores of ≥ 8 at screening and a history of ≥ 4 symptomatic relapses in the year prior to study participation, with at least one symptomatic relapse in the previous 6 months. Eligible patients were physically healthy, capable of providing informed consent, and able to complete self-administered questionnaires.
Subjects were excluded if they had active psychotic symptoms (hallucinations or delusions) at the time of study entry, history of non-response to RLAI, high suicide risk (rating of 4 on the suicide item [item 3] of the Hamilton Depression Rating Scale,24 or determination by the investigator of significant suicide risk), a psychiatric hospitalization between screening and baseline visits, or medical contraindication or hypersensitivity to oral risperidone or RLAI. Patients were not excluded on the basis of past risperidone treatment, or history of risperidone non-response or intolerance. Pregnant or nursing patients were ineligible.
Psychiatric comorbidity and recent substance abuse were not considered exclusionary, as long as bipolar disorder was the principal condition under treatment and the treating clinician and principal investigator deemed RLAI treatment was safe.
Design and Procedures
Eligible subjects were randomized 1:1 (via a computer-generated randomization list, maintained off-site by the study coordinator) to RLAI+TAU or TAU alone. Individuals who were assigned to RLAI+TAU were initiated on oral risperidone at a minimum dose of 0.5 mg daily. RLAI was initiated at a dose of either 12.5 mg or 25 mg IM every 2 weeks. For those started on 12.5 mg, RLAI was titrated to 25 mg every 2 weeks as tolerated. Further dose adjustments (to 37.5 or 50 mg every 2 weeks) were made at study clinicians’ discretion, or if: 1) the YMRS score was > 12; or 2) there was clinical evidence of an impending relapse. TAU consisted of oral antipsychotics (other than risperidone) and/or mood stabilizers, with or without concomitant antidepressants, at the discretion of the study physicians. Clinicians were instructed to optimize TAU medications as vigorously as required in order to effectively treat symptoms while maintaining acceptable tolerability. Compliance was monitored by patient self-report. Anticholinergic medications and benzodiazepines were allowed on a temporary basis to manage adverse effects, as clinically indicated.
Clinical assessments occurred at baseline and every 2 weeks during the study period. Follow-up was continued for 52 weeks, even if a relapse or psychiatric hospitalization occurred. Gaps in follow-up were allowed during the 52-week study period; however, missed study visits did not count towards total person-time. Thus, if a patient missed one study visit but was otherwise continuously followed for the full 52 weeks, s/he contributed only 50 weeks (0.96 person-years) of follow up.
This study was approved by the institutional review boards of Vanderbilt University Medical Center, and was conducted in accordance with the ethical principles originating from the Declaration of Helsinki and good clinical practices. All participants provided written informed consent.
Efficacy Measures and Endpoints
Clinical outcome was assessed every two weeks using the Longitudinal Interval Follow-up Evaluation (LIFE).25 Psychopathology and quality of life were measured at baseline and monthly thereafter using the Young Mania Rating Scale (YMRS),23 Montgomery-Asberg Depression Rating Scale (MADRS),26 Quick Inventory of Depressive Symptoms-Self Report (QIDS-SR),27 and Quality of Life, Enjoyment, and Satisfaction Questionnaire (Q-LES-Q).28 The YMRS and MADRS were administered by trained and experienced research staff whose inter-rater reliability was assessed on a quarterly basis as approximately 95%.
The main endpoints of interest were the number and duration relapse events per person-year of follow up. Relapse events were defined as occurrence of any of the following at any study visit: (1) YMRS score > 14 or MADRS score ≥ 15; (2) > 20% increase in YMRS or MADRS scores from the previous study visit for patients with MADRS ≥ 10 or YMRS ≥ 8 at the current study visit; (3) urgent care visit/referral (psychiatric hospitalization, emergency room visit, or referral for respite care, partial hospitalization or intensive outpatient treatment) due to worsening mood symptoms; (4) CGI-S score ≥ 4; (5) syndromal relapse (DSM-IV-TR criteria for manic, hypomanic, major depressive or mixed episode met); (6) withdrawal from the study due to inefficacy; and (7) necessary clinical medication adjustments (NCAs). Relapse event duration was based on the number of weeks that any relapse criteria were met. However, discrete events (medication changes, ER visits) were treated as count data only. NCAs were defined as change in medication treatment intended to prevent worsening of clinical symptoms, and included addition of a new mood stabilizer, non-risperidone antipsychotic, or antidepressant; substitution of an existing bipolar medication with a new drug; and/or a ≥ 20% change in dose of any current medication (excluding routine increases in dose due to titration).
Thus, the main study endpoint was relapse for any of the above specified reasons (any-cause relapse event). Exploratory endpoints included the occurrence of each of the seven individual relapse event types, and scores on the aforementioned psychopathology and quality of life measures.
Safety and Tolerability Measures
Treatment-emergent adverse events (TEAEs) were ascertained by spontaneous report and changes in laboratory values (fasting glucose, lipids, serum chemistries, prolactin concentration). Anthropometric measures were not formally assessed. Neuromuscular adverse effects were assessed using the Abnormal Involuntary Movement Scale (AIMS),29 Barnes Akathisia Scale (BARS),30 and Simpson-Angus Rating Scale (SAS).31
Data Analytic Approach
The modified intent to treat (ITT) sample included all persons who were randomized, received study medication, and had at least one follow-up visit. All subjects were analyzed according to their allocated treatment group. Because of the variable follow-up time for individual subjects, both the number of relapse events and duration of relapses during follow-up were calculated as the number (or duration [weeks]) of events per person per year of follow-up.
Categorical baseline characteristics were compared between conditions with chi-square tests. Continuous and ordinal baseline data were analyzed via t-test and Wilcoxon rank-sum tests, respectively. Mean number and duration of relapse events normalized to person-time, as defined earlier, were compared between groups using t-tests. A mixed model repeated measures (MMRM) approach was used to model the effects of treatment (RLAI+TAU vs. TAU alone) on continuous psychopathology and safety measures at each time point. To account for baseline differences in these measures, the MMRM analyses were adjusted for baseline dependent measure values.
All tests were conducted with a significance level of 0.05 (2-sided), with no adjustments for multiplicity, using SAS statistical software (SAS Institute, Inc., 1994).
Results
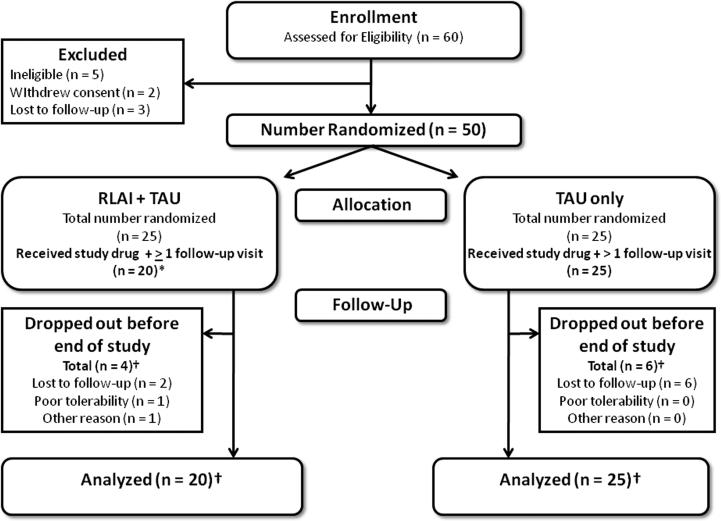
Fifty of 60 screened patients were randomized to receive RLAI+TAU (n = 25) or TAU alone (n = 25) (Figure 1). Of these, 20 patients in the RLAI+TAU group and 25 in the TAU alone group received at least one dose of study drug, and had at least one post-baseline follow-up assessment. Thus, 45 subjects were included in the analysis. The mean RLAI dose at study endpoint was 27.0 ± 10.4 mg/2 weeks. None of the RLAI-treated patients required titration to 50 mg/day. Very few patients required low-dose benzodiazepines (n = 1 in each group) or anticholinergic medications (RLAI+TAU, n = 1; TAU only, n = 3). For the majority of RLAI+TAU patients, RLAI was the only antipsychotic drug taken (Appendices 2 and 3); however, some patients continued their pre-study oral antipsychotics (n = 4 at 6 months, n = 2 at 12 months) or started oral antipsychotics after randomization (n = 2 at 6 months).
Figure 1. Disposition of subjects through the study.
*Of the 25 subjects randomized to RLAI + TAU, four did not receive study drug, while one received study drug but did not have any follow-up visits after their baseline visit. Data from the 20 remaining patients were used in the data analysis.
† Four pa ents randomized to RLAI + TAU and six pa ents randomized to TAU alone dropped out prior to the final study visit (at the end of month 12). All of these patients had at least one study visit after their baseline visit. Thus, all of these patients (20 in the RLAI + TAU group and 25 in the TAU alone group) were eligible to be included in the data analysis.
Abbreviations: RLAI = risperidone long-acting injectable; TAU = treatment as usual.
The mean duration of follow-up in the total sample was 43.1 ± 15.2 weeks, with no significant differences in follow-up time between treatment groups. Four patients assigned to RLAI+TAU group and six assigned to TAU alone dropped out prior to the end of month 12 and did not return for subsequent study visits (p = NS). Nearly all withdrawals in both groups were due to loss of follow-up (RLAI+TAU, n = 2; TAU only, n = 6). One subject in the RLAI+TAU group moved out of the area before completing the study, and one subject did not tolerate RLAI.
There were no significant between-group differences in demographic/clinical characteristics or baseline psychopathology measures, as shown in Table 1. The sample consisted predominantly of females, Caucasians, and patients with bipolar I disorder, although approximately one-fourth of participants were diagnosed with bipolar II disorder. More than three-quarters of the sample had a comorbid anxiety disorder, substance use disorder, or both. Prior to study entry, most patients were receiving an approved mood stabilizer, typically in combination with an oral atypical antipsychotic drug and/or antidepressant (Table 1). Doses of individual oral psychotropic medications used in both treatment groups were considered to be generally appropriate (see Appendices).
Table 1.
Clinical and demographic characteristics of study sample (N = 46)
| RLAI + TAU | TAU | |
|---|---|---|
| (n = 20) | (n = 25) | |
| Age, mean (SD) yrs. | 42.8 (8.7) | 38.2 (10.2) |
| Sex, n (%) female | 14 (70.0) | 16 (64.0) |
| Race, n (%) | ||
| Caucasian | 13 (65.0) | 17 (68.0) |
| African-American | 7 (35.0) | 8 (32.0) |
| Diagnosis, primary* | ||
| Bipolar I Disorder | 14 (70.0) | 19 (76.0) |
| Bipolar II Disorder | 6 (30.0) | 6 (24.0) |
| Duration of illness, mean (SD) yrs. | 26.4 (7.3) | 22.3 (9.7) |
| Age of illness onset, mean (SD) yrs. | 14.8 (5.3) | 16.4 (3.8) |
| Comorbidity, n (%)† | ||
| None | 2 (10.0) | 6 (24.0) |
| Any anxiety disorder | 10 (50.0) | 15 (60.0) |
| Any substance use disorder | 14 (70.0) | 13 (52.0) |
| Anxiety and substance use disorder | 7 (35.0) | 9 (36.0) |
| Young Mania Rating Scale, mean (SD) | 4.6 (4.0) | 6.9 (5.6) |
| Montgomery-Asberg Depression Rating Scale, mean (SD) | 13.7 (5.7) | 16.0 (8.0) |
| Quick Interview of Depressive Symptoms-Self Report, mean (SD) | 10.2 (4.0) | 11.2 (5.2) |
| Clinical Global Impression-Severity scale, mean (SD) | 2.8 (0.4) | 2.8 (0.8) |
| Pre-study medication treatment, n (%) | ||
| No pre-study medication | 1 (5.0) | 0 (0.0) |
| Monotherapy | ||
| Mood stabilizer (MS) | 4 (20.0) | 3 (12.0) |
| Atypical antipsychotic drug (AAPD) | 2 (10.0) | 2 (8.0) |
| Typical neuroleptics (TYP) | 0 (0.0) | 1 (4.0) |
| Antidepressant (AD) | 0 (0.0) | 2 (8.0) |
| Combination therapy | ||
| MS + AAPD or typical neuroleptics | 6 (30.0) | 4 (16.0) |
| MS + MS | 1 (5.0) | 0 (0.0) |
| MS + AD | 3 (15.0) | 3 (12.0) |
| AAPD + AD | 1 (5.0) | 3 (12.0) |
| AD + AD | 1 (5.0) | 1 (4.0) |
| MS + AAPD + AD | 1 (5.0) | 5 (20.0) |
| Other | 0 (0.0) | 1 (4.0) |
Primary and comorbid Axis I diagnoses according to the MINI are presented.
Refers to comorbid Axis I diagnoses according to the MINI for non-substance related diagnoses, and both the MINI and clinical records for substance use disorder diagnoses. Note that counts will not add up to 100% as categories are not mutually exclusive.
RLAI+TAU was associated with fewer mean any-cause relapse events, and shorter mean time spent in an any-cause relapse event, compared with TAU alone (Table 2). However, these differences were not statistically significant. When individual relapse events were considered separately, significantly fewer acute depressive episodes, urgent care events, and NCAs occurred in the RLAI+TAU group than in the TAU group. Altogether, 14 urgent care referrals occurred among 7 persons (RLAI+TAU, n = 1 [5.0%] vs. TAU alone, n = 6 [24.0%]; Fisher's exact, p = NS). Thirteen (92.3%) of the 14 urgent care events occurred in the TAU alone group. Nearly all subjects randomized to TAU alone (n = 24 [96.0%]) and the majority of subjects randomized to RLAI+TAU (n = 12 [60.0%]) required a NCA (Fisher's exact, p = 0.006). No significant between-group differences in number or duration of other types of relapse events were observed, including no significant differences with regard to duration of depressive relapses.
Table 2.
Duration and number of relapse events by treatment group
| A. Relapse duration (wks) | Mean (SD) duration (wks) | |||
|---|---|---|---|---|
| RLAI + TAU | TAU | Difference | p-value | |
| Any cause* | 20.0 (14.0) | 28.22 (2.69) | −8.22 | 0.052 |
| Acute manic episode | 1.81 (3.22) | 2.98 (4.58) | −1.17 | 0.34 |
| Acute depressive episode | 8.26 (8.94) | 13.28 (13.09) | −5.02 | 0.15 |
| Psychiatric hospitalization | 0 | 0.21 (0.85) | −0.21 | 0.28 |
| B. Number of events (no./yr. of follow up) | Mean (SD) no. events/yr. | |||
|---|---|---|---|---|
| RLAI + TAU | TAU | Difference | p-value | |
| Any cause* | 5.34 (2.35) | 6.26 (3.24) | −0.92 | 0.29 |
| Acute manic episode | 0.74 (1.03) | 1.64 (0.70) | −0.90 | 0.23 |
| Acute depressive episode | 2.71 (2.79) | 3.95 (3.19) | −1.25 | 0.018 |
| Psychiatric hospitalization | 0 | 0.21 (0.85) | −0.21 | 0.28 |
| Urgent care† | 0.05 (0.22) | 0.74 (1.57) | −0.69 | 0.038 |
| Necessary clinical adjustment (NCA)‡ | 1.64 (1.74) | 3.46 (2.57) | −1.82 | 0.01 |
Any-cause relapse was defined as one or more of the following at any study visit: (1) YMRS score > 14 or MADRS score > 15; (2) ≥ 20% increase in YMRS or MADRS scores from the previous study visit for patients with current MADRS ≥ 10 or YMRS ≥ 8; (3) urgent care visit/referral (psychiatric hospitalization, emergency room visit, or referral for respite care or intensive outpatient treatment) due to worsening mood symptoms; (4) CGI-S score ≥ 4; (5) syndromal relapse (DSM-IV-TR criteria for manic, hypomanic, major depressive or mixed episode met); (6) withdrawal from the study due to inefficacy; and/or (7) necessary clinical medication adjustments (NCAs).
Defined as psychiatric hospitalization, emergency room visit, or referral for respite care or intensive outpatient treatment due to worsening mood symptoms.
Defined as change in medication treatment intended to prevent worsening of clinical symptoms (any of the following): (1) addition of a new mood stabilizer, antipsychotic or antidepressant; (2) substitution of an existing bipolar medication with a new drug; and/or (3) a ≥ 20% change in dose of any current medication.
A significant time effect was observed for the QIDS-SR, indicating that patient-reported depressive symptoms improved in the whole sample (Table 3). However, there was no significant group x time interaction effect for any psychopathology measure, indicating that changes in these scores did not differ significantly by treatment group over time (Table 3). No significant changes in MADRS or QIDS-SR scores over time were observed in either group. There were also no significant differences in baseline to endpoint change in CGI-S or Q-LES-Q subscale scores (data not shown).
Table 3.
Change in psychopathology, quality of life and global clinical state scores over time
| Time pt. | (n = 20) | (n = 25) | p-value | Group | Time | Group*Time | |
|---|---|---|---|---|---|---|---|
| YMRS | Baseline | 5.3(1.0) | 6.5(0.8) | 0.31 | F(1,42)=0.27 | F(5,182)=1.2 | F(5,182)=2.1 |
| 1 month | 6.1(1.0) | 3.9 (0.8)* | 0.09 | P = NS | P = NS | P = NS | |
| 3 months | 5.2(1.0) | 3.9(0.9)* | 0.34 | ||||
| 6 months | 3.9(1.1) | 6.1(0.9) | 0.l1 | ||||
| 9 months | 4.0(1.1) | 4.1(0.9)* | 0.91 | ||||
| 12 months | 5.7(1.1) | 6.7(1.0) | 0.17 | ||||
| MADRS | Baseline | 14.4(1.6) | 15.6(1.4) | 0.60 | F(1,42)=3.6 | F(5,183)=2.0 | F(5,183)=0.5 |
| 1 month | 12.3(1.6) | 15.2(1.4) | 0.19 | P = 0.07 | P = NS | P = NS | |
| 3 months | 11.9(1.7) | 13.4(1.5) | 0.44 | ||||
| 6 months | 11.2(1.8) | 13.7(1.5) | 0.26 | ||||
| 9 months | 10.6(1.9) | 14.5(1.5) | 0.07 | ||||
| 12 months | 11.3(2.0) | 9.6(1.6)** | 0.86 | ||||
| QIDS SR | Baseline | 10.6(0.9) | 10.9(0.8) | 0.78 | F(1,42)=0.3 | F(5,135)=8.5 | F(5,135)=0.7 |
| 1 month | 9.9(0.9) | 9.5(0.8) | 0.71 | P = NS | P < 0.0001 | P = NS | |
| 3 months | 7.8(1.1)* | 9.4(0.9) | 0.27 | ||||
| 6 months | 7.9(1.1)* | 7.5(1.0)** | 0.79 | ||||
| 9 months | 7.0(1.2)** | 8.9(1.0)† | 0.23 | ||||
| 12 months | 6.0(1.1)*** | 5.5(1.0)**** | 0.76 | ||||
| CGI | Baseline | 2.8(0.2) | 2.8(0.2) | 0.94 | F(1,42)=3.1 | F(5,152)=0.4 | F(5,152)=1.2 |
| 1 month | 2.7(0.2) | 2.7(0.2) | 0.78 | P = NS | P = NS | P = NS | |
| 3 months | 2.6(0.2) | 2.8(0.2) | 0.45 | ||||
| 6 months | 2.6(0.3) | 3.1(0.2) | 0.10 | ||||
| 9 months | 2.6(0.3) | 3.0(0.2) | 0.21 | ||||
| 12 months | 2.4(0.3) | 3.0(0.2) | 0.04 |
All values are LS-mean (SE) unless otherwise specified.
We used a mixed model repeated measures (MMRM) approach with time (baseline, 1-, 3-, 6-, 9-, 12-month visits) as the within-subjects factor and treatment (RLAI+TAU vs. TAU alone) as the between-subjects factor. Analyses were adjusted for baseline dependent measure values. Reported p-values reflect between-group differences at the corresponding time point. Statistically significant within-group changes at each time point (relative to baseline values) are represented with asterisks:
p ≤ 0.0001
p ≤ 0.001
p≤0.01
p < 0.05
p < 0.08 (trend) for within group comparison for each time point against the baseline values.
The most common TEAEs in the RLAI+TAU group were sedation (n = 3) and subjective weight gain (n = 2). Two patients experienced potential prolactin-related adverse events (PPRAEs, glactorrhea, n = 1; menstrual changes, n = 1). The most common TEAEs in the TAU only group were sedation (n = 4), somnolence (n = 4), and subjective weight gain (n = 3). None of the patients in the TAU only group reported PPRAEs. Side-effects related to basal ganglia dysfunction (including extrapyramidal effects), were reported by 4 subjects in the RLAI+TAU group and 6 subjects in the TAU alone group.
There were no significant group or group × time interaction effects for AIMS or BARS scores (Table 4). Significant time and group × time interaction effects were observed for SAS scores. There were no significant changes in SAS scores from baseline in the RLAI+TAU group; however, in the TAU alone group, there was a significant increase (worsening) in SAS scores at 9 months. Increases in SAS scores of ≥ 5 points were observed in 5 TAU patients who were taking haloperidol (n = 1), quetiapine (n = 3), or ziprasidone (n = 1). There were no significant differences in AIMS, BARS or SAS scores baseline, 1-, 3-, 6- or 12 months between RLAI+TAU patients who received concomitant oral antipsychotics, RLAI+TAU patients who did not receive oral antipsychotics, and those who received TAU only (data not shown).
Table 4.
Change in AIMS, BARS and SAS scores over time
| groups | |||||||
|---|---|---|---|---|---|---|---|
| Time pt. | (n = 20) | (n = 25) | p-value | Group | Time | Group*Time | |
| AIMS | Baseline | 0.9(0.3) | 0.9(0.3) | 0.99 | F(5,42)=2.8 | F(5,134)=1.6 | F(5,134)=0.2 |
| 1 month | 0.5(0.3) | 0.8(0.3) | 0.48 | P = NS | P = NS | P = NS | |
| 3 months | 0.1(0.4)† | 0.4(0.3) | 0.48 | ||||
| 6 months | 0.04(0.4)† | 0.4(0.3) | 0.40 | ||||
| 9 months | 0.2(0.4) | 0.8(0.3) | 0.26 | ||||
| 12 months | 0.04(0.4)† | 0.5(0.3) | 0.37 | ||||
| BARS | Baseline | 0.2(0.1) | 0.4(0.1) | 0.38 | F(1,42)=0.2 | F(5,136)=0.7 | F(5,136)=0.7 |
| 1 month | 0.4(0.2) | 0.1(0.1) | 0.19 | P = NS | P = NS | P = NS | |
| 3 months | 0.02(0.2) | 0.3(0.2) | 0.33 | ||||
| 6 months | 0.03(0.2) | 0.07(0.2) | 0.89 | ||||
| 9 months | 0.1(0.2) | 0.3(0.2) | 0.85 | ||||
| 12 months | 0.1(0.2) | 0.04(0.2) | 0.81 | ||||
| SAS | Baseline | 1.1(0.4) | 1.0(0.3) | 0.57 | F(1,41)=3.3 | F(5,131)=2.4 | F(5,131)=2.5 |
| 1 month | 0.7(0.4) | 0.8(0.4) | 0.99 | P = NS | P = 0.04 | P = 0.04 | |
| 3 months | 0.8(0.5) | 1.2(0.4) | 0.58 | ||||
| 6 months | 0.7(0.5) | 0.7(0.4) | 0.92 | ||||
| 9 months | 0.6(0.6) | 3.2(0.4)**** | 0.0003 | ||||
| 12 months | 1.6(0.5) | 1.8(0.4) | 0.75 |
All values are LS-mean (SE) unless otherwise specified.
We used a mixed model repeated measures (MMRM) approach with time (baseline, 1-, 3-, 6-, 9-, 12-month visits) as the within-subjects factor and treatment (RLAI+TAU vs. TAU alone) as the between-subjects factor. Analyses were adjusted for baseline dependent measure values. Reported p-values reflect between-group differences at the corresponding time point. Statistically significant within-group changes at each time point (relative to baseline values) are represented with asterisks:
p ≤ 0.0001
*** p ≤ 0.001
** p≤0.01
* p < 0.05
p < 0.08 (trend) for within group comparison for each time point against the baseline values.
Baseline glucose values were available for only 56.5% of RLAI+TAU-treated patients and 50.0% of those who received TAU alone. Fasting lipid data were available for only 44.8% and 48.5% of RLAI+TAU- and TAU only-treated patients, respectively. For those with available data, no significant between-group differences in mean fasting glucose or lipid values at baseline or at study endpoint (data not shown). For those with complete follow-up data, there was no significant baseline to endpoint change in glucose or lipid measures in either group (data not shown).
No suicide attempts occurred for any of the study subjects. There were no significant between-groups differences in mean or median suicide item scores for either the QIDS-SR (item 12, 0-3 scale) or MADRS (item 10, 0-6 scale) at baseline. There were also no significant between-groups differences in the proportion of patients achieving a score of ≥ 2 on either the QIDS-SR (RLAI+TAU, n = 2 [10.0%] vs. TAU, n = 4 [16.0%], Fisher's exact, p = NS) or MADRS (RLAI+TAU, n = 5 [25.0%] vs. TAU, n = 9 [36.0%], Fisher's exact, p = NS) suicide items at any time during follow-up.
Discussion
This preliminary study is a first attempt at extending results of large phase III-type randomized, double-blind, placebo-controlled RLAI bipolar maintenance studies to a representative sample of rapid cycling, community-treated patients with bipolar I or II disorder over 52 weeks of follow-up. Neither the rate nor duration of any-cause relapse or episodic mood relapse, the primary study endpoints, differed significantly according to treatment group. However, a significantly lower number of urgent care events and NCAs per year of follow-up were observed with RLAI+TAU. There were fewer depressive relapses per person per year of follow-up in the RLAI+TAU group; however, no significant between-groups differences in the duration of depressive relapses or in psychopathology scores were observed. Between-group differences were significant only for secondary outcome measures; however, if supported by further research, these findings could indicate that bipolar patients who require repeated advanced care options such as hospitalizations, emergency room visits, or respite care might preferentially benefit from adjunctive RLAI.
Very little maintenance-phase data is available regarding the effectiveness of antipsychotic drugs in patients with rapid cycling.6 Small, open-label studies and subgroup analyses of short-term randomized controlled trials suggest that olanzapine, quetiapine and aripiprazole may be more effective for treating manic/mixed than depressive symptoms in rapid cycling bipolar patients. 32 However, long-term follow-up studies focused on the maintenance effects of these drugs in rapid cycling bipolar patients are sparse. Prior randomized bipolar maintenance studies have documented significantly longer time to recurrence/relapse and lower episodic mood recurrence/relapse rates in patients treated with RLAI. The first of these, conducted by Macfadden and colleagues,20 was a 52-week randomized, double-blind comparison of RLAI+TAU continuation treatment (vs. TAU+placebo) in 124 frequently relapsing patients who had previously remitted during a 16-week open-label lead-in phase. The second study, conducted by Quiroz and colleagues,19 was a large, 24-month randomized, placebo-controlled, recurrence prevention study in 303 subjects who had previously achieved a stable positive treatment response during a 6-month stabilization phase. In both studies, relapse events were broadly defined, and included many of the same elements that were incorporated into our relapse definition. Generalizability of these results to community practice are limited by enrichment of the randomized, double-blind phases of each study with patients who had already responded favorably to RLAI, and exclusion of subjects with bipolar II disorder and concomitant substance use disorders. Only the Madfadden et al.20 study targeted frequently relapsing patients.
The only randomized study that did not include a pre-randomization lead-in phase and enrolled patients with bipolar II disorder was a 24-week open trial in which 23 subjects were switched to RLAI monotherapy and 26 continued their pre-study oral medication.21 No significant between-groups differences in manic or depressive symptoms were observed between treatment arms; however, this study focused only on symptom-based outcomes (not relapse events), and excluded patients with substance use disorders.
Although we were unable to replicate previous findings of a statistically significant advantage for RLAI related to any-cause relapse or episodic mood relapse, we report novel results for RLAI in a high-risk, community-treated population of patients with bipolar I and II disorder. Our selection criteria targeted frequently relapsing patients using criteria similar to that of Macfadden et al.20 However, unlike previous randomized studies, the majority of our sample had comorbid anxiety disorders, substance use disorders, or both at rates consistent with other community-based bipolar samples.33-36 Conducting intervention studies in such populations is important, given the well-known challenges imposed by high rates of anxiety and substance-related comorbidity.37-42 Thus, we were able to extend prior findings from more selective patient populations to one that is more representative of community-based practice. Moreover, unlike previous studies that employed time to event analytic designs that censored follow-up after the first post-randomization relapse event, patients in our study were continuously followed after relapse events occurred. This approach allowed us to collect data on multiple relapse events, an important design consideration given the high relapse rates encountered in routine practice. In addition, we were able to estimate the total person-time spent in a relapse event during a fixed (up to 52 weeks) period of follow-up, which served as a proxy measure of illness burden than could be compared between treatment groups.
Our finding of a statistically significant advantage of adjunctive RLAI for urgent care events and NCAs is also novel. Although urgent care events and NCAs were secondary endpoints, they are important therapeutic measures that are pertinent to “real world” clinical management. Our definition of urgent care event included a wide spectrum of services available in many community mental health systems. Because our urgent care definition also required documentation of worsening mood symptoms, this specific endpoint served as a pragmatic indicator of significant clinical worsening. Medication adjustment to prevent symptom worsening may be expected to occur frequently in community practice, particularly in highly relapse-prone patients. In our study, only medication changes or adjustments intended to prevent worsening of symptoms were defined as NCAs; thus, NCAs also served as a reasonable effectiveness indicator. The degree to which potential differences in medication adherence may have contributed to these differences is uncertain, since oral medication adherence was monitored by patient self-report.
In our study, oral antipsychotics were not restricted to the TAU alone group. A small number of RLAI+TAU patients received oral antipsychotics. The majority of oral antipsychotic use in the RLAI+TAU group represented continuation of pre-study medication. While there was no evidence of increased EPS burden associated with such treatment in our study, others have documented greater adverse effect burden with antipsychotic combination therapy in bipolar patients.43 Moreover, there is no evidence supporting the use of antipsychotic combinations in bipolar disorder treatment.
Our results are preliminary, and should be interpreted with additional limitations in mind. First, given the small sample size of our study, we cannot exclude the possibility of type II error for any-cause and episodic mood relapse. Second, because clinicians were allowed to adjust the doses of oral medications to optimize their effectiveness and tolerability, the use of TAU in both arms may have obscured between-group differences in relapse occurrence and symptom ratings. Our study was intended to provide preliminary effectiveness evidence for adjunctive RLAI under real-world treatment conditions, and any residual bias not addressed by randomization would be towards null findings. Adjustments intended to prevent relapse or clinical worsening were accounted for in the analysis by including a NCA endpoint. Third, data regarding index episode (the mood episode type present at the beginning of follow-up) was not ascertained. Index episode polarity appears to predict the polarity of mood episode relapses, and may influence therapeutic response to specific pharmacotherapies during maintenance treatment.44-46 Fourth, the finding of a statistically significant between-group difference in the number of depressive relapses favoring RLAI+TAU must be interpreted cautiously. Because neither oral risperidone nor RLAI has demonstrated value either acutely or prophylactically, respectively, for bipolar depression,19,47 the possibility of type I error cannot be excluded. Moreover, no significant differences between treatment groups were noted regarding the duration of depressive relapses, or scores on the MADRS or QIDS-SR. Fifth, weight data were not ascertained. Consistent with prior reports, compliance with fasting laboratory assessments was poor. Thus, potential benefits of long-term adjunctive RLAI treatment are difficult to assess in relation to the risk of metabolic adverse effects. Sixth, because costs of treatment were not estimated in this study, additional investigation is needed to determine the cost-outcome profile of RLAI for bipolar maintenance treatment. Next, because we desired to investigate the effects of adjunctive RLAI under conditions relevant to community practice, we chose not to conduct a blinded study. Finally, patient acceptance was not a specific study endpoint. In “real-world” practice, concerns about convenience, persistence of adverse effects, injection site pain and other factors may contribute to high discontinuation rates for RLAI and other long-acting injectable medications in patients with bipolar disorder.48-50
In conclusion, rates of any-cause relapse with RLAI+TAU were numerically, but not significantly, lower than that of TAU alone in our sample of community-treated adults with rapid cycling bipolar I or II disorder. However, RLAI as an adjunct to TAU may be beneficial for reducing urgent care episodes and NCAs during maintenance treatment. Replication and extension of these results in future large, randomized, rater-blinded studies is warranted.
Supplementary Material
Acknowledgements
Funding for the study was provided by an investigator-initiated grant from Janssen Pharmaceutica. In the past, WVB has received grant/research support from Cephalon, Inc., and has served on speaker bureaus for Janssen Pharmaceutica and Pfizer, Inc. RCS has received grant/research support from Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceutica, Pfizer, Sanofi-Aventia, Wyeth-Ayerst Laboratories, AstraZeneca Pharmaceuticals, and Abbott Laboratories; has served as a paid consultant for Pfizer, Janssen Pharmaceutica, and Sierra Neuropharmaceuticals; and has served on the speaker's bureau for Bristol-Myers Squibb, Eli Lilly and Company, Janssen Pharmaceutica, Pfizer, GlaxoSmithKline, Wyeth-Ayerst Laboratories, and Abbott Laboratories. RAE, AL, NB and TDP have no potential sources of conflict of interest to disclose. We are indebted to the patients and staff of the Mental Health Cooperative, Inc. (Nashville, TN), without whom this study would not have been possible. Study data were collected and managed using REDCap electronic data capture tools (1 UL1 RR024975) hosted at Vanderbilt University.
Footnotes
Trial Registration: clinicaltrials.gov Identifier: NCT00571688
Reference List
- 1.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 2.Dean BB, Gerner D, Gerner RH. A systematic review evaluating health-related quality of life, work impairment, and healthcare costs and utilization in bipolar disorder. Curr Med Res Opin. 2004;20:139–154. doi: 10.1185/030079903125002801. [DOI] [PubMed] [Google Scholar]
- 3.Dutta R, Boydell J, Kennedy N, Van OJ, Fearon P, Murray RM. Suicide and other causes of mortality in bipolar disorder: a longitudinal study. Psychol Med. 2007;37:839–847. doi: 10.1017/S0033291707000347. [DOI] [PubMed] [Google Scholar]
- 4.Hirschfeld RMA, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(Suppl.):1–50. [PubMed] [Google Scholar]
- 5.Suppes T, Dennehy EB, Hirschfeld RM, et al. The Texas implementation of medication algorithms: update to the algorithms for treatment of bipolar I disorder. J Clin Psychiatry. 2005;66:870–886. doi: 10.4088/jcp.v66n0710. [DOI] [PubMed] [Google Scholar]
- 6.Vieta E, Gunther O, Locklear J, et al. Effectiveness of psychotropic medications in the maintenance phase of bipolar disorder: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol. 2011;14:1029–1049. doi: 10.1017/S1461145711000885. [DOI] [PubMed] [Google Scholar]
- 7.Maj M, Pirozzi R, Magliano L, Bartoli L. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30–35. doi: 10.1176/ajp.155.1.30. [DOI] [PubMed] [Google Scholar]
- 8.Scott J, Pope M. Nonadherence with mood stabilizers: prevalence and predictors. J Clin Psychiatry. 2002;63:384–390. doi: 10.4088/jcp.v63n0502. [DOI] [PubMed] [Google Scholar]
- 9.Scott J, Pope M. Self-reported adherence to treatment with mood stabilizers, plasma levels, and psychiatric hospitalization. Am J Psychiatry. 2002;159:1927–1929. doi: 10.1176/appi.ajp.159.11.1927. [DOI] [PubMed] [Google Scholar]
- 10.Lingam R, Scott J. Treatment non-adherence in affective disorders. Acta Psychiatr Scand. 2002;105:164–172. doi: 10.1034/j.1600-0447.2002.1r084.x. [DOI] [PubMed] [Google Scholar]
- 11.Johnson RE, McFarland BH. Lithium use and discontinuation in a health maintenance organization. Am J Psychiatry. 1996;153:993–1000. doi: 10.1176/ajp.153.8.993. [DOI] [PubMed] [Google Scholar]
- 12.Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv. 2001;52:805–811. doi: 10.1176/appi.ps.52.6.805. [DOI] [PubMed] [Google Scholar]
- 13.Ahlfors UG, Baastrup PC, Dencker SJ, et al. Flupenthixol decanoate in recurrent manic-depressive illness. A comparison with lithium. Acta Psychiatr Scand. 1981;64:226–237. doi: 10.1111/j.1600-0447.1981.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 14.Esparon J, Kolloori J, Naylor GJ, McHarg AM, Smith AH, Hopwood SE. Comparison of the prophylactic action of flupenthixol with placebo in lithium treated manic-depressive patients. Br J Psychiatry. 1986;148:723–725. doi: 10.1192/bjp.148.6.723. [DOI] [PubMed] [Google Scholar]
- 15.White E, Cheung P, Silverstone T. Depot antipsychotics in bipolar affective disorder. Int Clin Psychopharmacol. 1993;8:119–122. doi: 10.1097/00004850-199300820-00007. [DOI] [PubMed] [Google Scholar]
- 16.Cavazzoni PA, Berg PH, Kryzhanovskaya LA, et al. Comparison of treatment-emergent extrapyramidal symptoms in patients with bipolar mania or schizophrenia during olanzapine clinical trials. J Clin Psychiatry. 2006;67:107–113. doi: 10.4088/jcp.v67n0116. [DOI] [PubMed] [Google Scholar]
- 17.Keck PE, Jr., McElroy SL, Strakowski SM, Soutullo CA. Antipsychotics in the treatment of mood disorders and risk of tardive dyskinesia. J Clin Psychiatry. 2000;61(Suppl 4):33–38. [PubMed] [Google Scholar]
- 18.Package insert. Risperdal Consta (risperidone) long-acting injection: US prescribing information. Janssen, a division of Ortho-McNeil-Janssen Pharmaceuticals, Inc.; Titusville, NJ: 2010. [Google Scholar]
- 19.Quiroz JA, Yatham LN, Palumbo JM, Karcher K, Kushner S, Kusumakar V. Risperidone Long-Acting Injectable Monotherapy in the Maintenance Treatment of Bipolar I Disorder. Biol Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 20.Macfadden W, Alphs L, Haskins JT, et al. A randomized, double-blind, placebo-controlled study of maintenance treatment with adjunctive risperidone long-acting therapy in patients with bipolar I disorder who relapse frequently. Bipolar Disord. 2009;11:827–839. doi: 10.1111/j.1399-5618.2009.00761.x. [DOI] [PubMed] [Google Scholar]
- 21.Yatham LN, Fallu A, Binder CE. A 6-month randomized open-label comparison of continuation of oral atypical antipsychotic therapy or switch to long acting injectable risperidone in patients with bipolar disorder. Acta Psychiatr Scand Suppl. 2007:50–56. doi: 10.1111/j.1600-0447.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 23.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 24.HAMILTON M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation. A comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 27.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 28.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 29.Lane RD, Glazer WM, Hansen TE, Berman WH, Kramer SI. Assessment of tardive dyskinesia using the Abnormal Involuntary Movement Scale. J Nerv Ment Dis. 1985;173:353–357. doi: 10.1097/00005053-198506000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- 31.Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 32.Muzina DJ. Pharmacologic treatment of rapid cycling and mixed states in bipolar disorder: an argument for the use of lithium. Bipolar Disord. 2009;11(Suppl 2):84–91. doi: 10.1111/j.1399-5618.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 33.Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2004;161:2222–2229. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- 34.Henry C, Van den BD, Bellivier F, Etain B, Rouillon F, Leboyer M. Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J Clin Psychiatry. 2003;64:331–335. [PubMed] [Google Scholar]
- 35.Young LT, Cooke RG, Robb JC, Levitt AJ, Joffe RT. Anxious and non-anxious bipolar disorder. J Affect Disord. 1993;29:49–52. doi: 10.1016/0165-0327(93)90118-4. [DOI] [PubMed] [Google Scholar]
- 36.Grant BF, Stinson FS, Dawson DA, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- 37.Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am J Psychiatry. 2004;161:2222–2229. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- 38.Coryell W, Solomon DA, Fiedorowicz JG, Endicott J, Schettler PJ, Judd LL. Anxiety and outcome in bipolar disorder. Am J Psychiatry. 2009;166:1238–1243. doi: 10.1176/appi.ajp.2009.09020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kauer-Sant'Anna M, Frey BN, Andreazza AC, et al. Anxiety comorbidity and quality of life in bipolar disorder patients. Can J Psychiatry. 2007;52:175–181. doi: 10.1177/070674370705200309. [DOI] [PubMed] [Google Scholar]
- 40.Salloum IM, Thase ME. Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disord. 2000;2:269–280. doi: 10.1034/j.1399-5618.2000.20308.x. [DOI] [PubMed] [Google Scholar]
- 41.Marangell LB, Bauer MS, Dennehy EB, et al. Prospective predictors of suicide and suicide attempts in 1,556 patients with bipolar disorders followed for up to 2 years. Bipolar Disord. 2006;8:566–575. doi: 10.1111/j.1399-5618.2006.00369.x. [DOI] [PubMed] [Google Scholar]
- 42.Sajatovic M, Ignacio RV, West JA, et al. Predictors of nonadherence among individuals with bipolar disorder receiving treatment in a community mental health clinic. Compr Psychiatry. 2009;50:100–107. doi: 10.1016/j.comppsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brooks JO, III, Goldberg JF, Ketter TA, et al. Safety and tolerability associated with second-generation antipsychotic polytherapy in bipolar disorder: findings from the Systematic Treatment Enhancement Program for Bipolar Disorder. J Clin Psychiatry. 2011;72:240–247. doi: 10.4088/JCP.09m05214yel. [DOI] [PubMed] [Google Scholar]
- 44.Calabrese JR, Vieta E, El-Mallakh R, et al. Mood state at study entry as predictor of the polarity of relapse in bipolar disorder. Biol Psychiatry. 2004;56:957–963. doi: 10.1016/j.biopsych.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro DR, Quitkin FM, Fleiss JL. Response to maintenance therapy in bipolar illness. Effect of index episode. Arch Gen Psychiatry. 1989;46:401–405. doi: 10.1001/archpsyc.1989.01810050015004. [DOI] [PubMed] [Google Scholar]
- 46.Tohen M, Sutton VK, Calabrese JR, Sachs GS, Bowden CL. Maintenance of response following stabilization of mixed index episodes with olanzapine monotherapy in a randomized, double-blind, placebo-controlled study of bipolar 1 disorder. J Affect Disord. 2008 doi: 10.1016/j.jad.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Cruz N, Sanchez-Moreno J, Torres F, Goikolea JM, Valenti M, Vieta E. Efficacy of modern antipsychotics in placebo-controlled trials in bipolar depression: a meta-analysis. Int J Neuropsychopharmacol. 2010;13:5–14. doi: 10.1017/S1461145709990344. [DOI] [PubMed] [Google Scholar]
- 48.Heres S, Hamann J, Kissling W, Leucht S. Attitudes of psychiatrists toward antipsychotic depot medication. J Clin Psychiatry. 2006;67:1948–1953. doi: 10.4088/jcp.v67n1216. [DOI] [PubMed] [Google Scholar]
- 49.Jaeger M, Rossler W. Attitudes towards long-acting depot antipsychotics: a survey of patients, relatives and psychiatrists. Psychiatry Res. 2010;175:58–62. doi: 10.1016/j.psychres.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Patel MX, Nikolaou V, David AS. Psychiatrists' attitudes to maintenance medication for patients with schizophrenia. Psychol Med. 2003;33:83–89. doi: 10.1017/s0033291702006797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.