Abstract
Objective
To test whether the association between depression and inflammation differs by race and sex. Depressive symptoms have been associated with higher levels of CRP. However few studies have examined this association in samples including a significant number of African Americans, or whether the association differs by race and gender.
Methods
Depressive symptoms and CRP were assessed in 512 African American and White participants, age 30–65 years, as part of the community-based META-Health Study. Depression was determined by responses to the Beck’s Depression Inventory-II (BDI-II). Multivariable linear regression models were used to adjust for demographic and metabolic risk factors.
Results
African American men had higher total BDI-II scores than White men (p=0.03), while there was no difference in women. There was a significant race X gender X depression interaction in predicting CRP levels (p=0.02). White women with mild to severe depressive symptoms had higher levels of CRP compared to those with minimal to no depressive symptoms (p<0.05). There were no differences in levels of CRP by severity of depressive symptoms in White men or African Americans of either sex. Higher BDI-II scores were related to higher CRP levels in White women after adjusting for age and level of education (β=0.227, p=0.006). However the association was eliminated after further adjustment for metabolic risk factors (β=0.077, p=0.35).
Conclusions
Although depressive symptoms are associated with inflammation, the association varies by race and gender.
Keywords: depression, inflammation, race/ethnicity, gender
INTRODUCTION
Depressive symptoms are linked to incident cardiovascular disease (CVD), and patients with CVD are at risk for depression (1–3). Inflammation is a well-established mechanism in the pathogenesis of both clinical depression and atherosclerotic vascular disease, and may play a role in the association between these conditions (4–7). Consistent with this premise, several studies have found that individuals with depressive symptoms exhibit elevations of pro-inflammatory cytokines and acute phase proteins such as CRP, interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) (8–10). However, other studies have failed to find an association between depression and inflammation (11–13), while additional studies have found an inverse association between depression and circulating inflammatory biomarkers (11). Previous literature has also been inconsistent about whether the relationship between depression and inflammation differs by sex. While some studies have shown a significant association of depressive symptoms with inflammation in men only (9, 14, 15), others have found similar results for both men and women (8, 16).
Most of the existing data on the association between depression, inflammation, and CVD are based on predominantly White samples. African Americans exhibit differences in the expression of depressive symptoms compared to Whites. Specifically, African Americans are less likely than Whites to express depressed mood, and more likely to display somatic symptoms such as sleep disturbances and loss of appetite (17, 18) which have shown more robust associations with inflammation and with CVD than affective symptoms in a number of studies (19, 20). In addition, African Americans exhibit higher levels of CRP compared to Whites, even after controlling for body mass index (BMI), statin use, and smoking (21–23). Recent data from the Coronary Artery Risk Development in Young Adults (CARDIA) confirmed that higher scores on the depressed affect and somatic subscales of the Centers for Epidemiologic Studies Depression Scale in African Americans, but not Whites (24). These data suggest important race/ethnic differences in the phenotypic expression of both depression and inflammation that may underlie differences in pathophysiology and risk assessment of both depression and CVD in different demographic groups. If such differences exist, the inconsistencies in previous literature regarding the link between depression and inflammation could be due differences in the racial and gender mix of the populations studied. We hypothesized that there would be both race and gender differences in the association of depressive symptoms and inflammatory markers, with a stronger correlation in African Americans compared to Whites, and women compared to men, even after controlling for CVD risk factors.
METHODS
Study sample
The Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) Study was a two-stage cross-sectional study of both traditional and psychosocial risk factors for CVD that recruited participants during years 2005 to 2010. The first stage was a random digit dialing survey of African American and White residents of metropolitan Atlanta, ages 30 to 65 years (n=3391); the second stage included a subset of participants (n=753) who agreed to come to either Emory or Morehouse Schools of Medicine for a study visit. Detailed information on demographics and anthropometrics was collected. Blood pressure was measured with a sphygmomanometer after five minutes of rest, and was based on the average of the final two of three readings measured five minutes apart. Height, weight, and waist circumference were measured. Waist circumference was based on the average of three readings. Smoking history, obtained using standardized questionnaires, was defined as current, or never/former (no cigarettes within the past 30 days). Pregnant women were excluded. In addition, all participants were questioned regarding recent cold-like symptoms or pain to exclude those with any acute illnesses. The study was approved by the Emory University and Morehouse University Institutional Review Committees. Informed consent was obtained from all participants.
Measurement of depression
Of the 753 participants enrolled in the META-Health study, 591 completed the Beck Depression Inventory (BDI)-II, a self-administered validated 21-item scale that assesses depressive symptoms experienced during the previous 2 weeks (25). Compared to those who completed the assessment, there was a higher percentage of women (71% vs. 51%, p<0.001) and Whites (59% vs. 43%) in the 162 participants who did not complete the BDI-II. Each item in the BDI-II contains four statements reflecting varying degrees of symptom severity, and participants are instructed to circle the number (0 to 3) which corresponds to the statement that best describes them. Ratings are summed to give a total BDI-II score, providing a continuous scale of depressive symptoms from 0 to 63. Higher scores indicate more severe depressive symptoms, with scores 0–13, minimal to no depression; 14–19, mild depression; 20–28, moderate depression; and 29–63 severe depression. If more than 2 BDI-II questions were missing, the score was set to missing (n=1); however, if only 1 to 2 questions were missing, the mean response from the non-missing questions was substituted for the missing values (n=35)(2). Questions regarding sadness, pessimism, past failure, guilty feelings, punishment, self-dislike, self-criticalness, suicidal thoughts or wishes, and worthlessness were summed to generate the cognitive subscale. Questions regarding loss of pleasure, crying, agitation, loss of interest, indecisiveness, loss of energy, changes in sleep patterns, irritability, changes in appetite, difficulty with concentration, tiredness or fatigue, and loss of interest in sex were summed to generate the somatic subscale
Measurement of sleep quality
Of the 555 participants eligible for this analysis based on their BDI-II scores, 476 also completed the Pittsburgh Sleep Quality Index (PSQI). The PSQI is a self-administered, validated 19-item scale that assesses overall sleep-quality and sleep-related symptoms experienced during the previous 1 month (26). The 19 items yield 7-component scores that reflect the frequency of sleep problems. The sum of the 7 components yields a global score that ranges from 0 to 21, with higher scores indicating poorer sleep quality.
Blood Specimens
Participants were instructed to fast for 12 hours before the study visit. Venous blood was collected in sodium heparin tubes, and the following serum measurements were made. Serum levels of high density lipoprotein cholesterol (HDL-C), triglycerides, and glucose were measured by spectrophotometry.
Measurement of inflammatory markers
Plasma was frozen at −70°C for subsequent measurement of inflammatory markers. High sensitivity C-reactive protein (CRP) was measured by immunonephelometry (Siemens/Dade Behring). Participants were excluded from this analysis for missing values (n=59) or values >2.5 standard deviations (SD) above the mean (n=20).
Statistical methods
Study variables are described as the mean±SD for normally distributed continuous variables, median and interquartile range for skewed continuous variables, or as proportions for categorical variables. In the main analyses, total BDI-II score was analyzed as a continuous variable; however, to provide a clinical perspective, descriptive analyses with BDI-II categories (minimal to no depression vs. mild to severe) were also conducted. Age, waist circumference, systolic and diastolic blood pressure, HDL-C, triglycerides, and glucose were used as continuous variables. Smoking history, education, and gender were categorical variables. Continuous variables were tested for normality using the Kolmogorov-Smirnov criterion. Because the distribution of BDI-II scores and CRP were skewed, natural log transformed levels were used for any parametric analysis. Chi-square tests were used to compare categorical variables, while unpaired t-tests or Wilcoxon rank sum tests were utilized for continuous variables.
In order to define the presence of an interaction of depressive symptoms with race and/or gender with CRP levels, linear regression models were conducted. The initial model tested the main effect of Total BDI-II score on CRP levels, after adjusting for race and gender. The second model added terms for the race*gender*total BDI-II score interaction, including all first and second order terms. The third fully adjusted model added terms for demographic factors (age, education) and metabolic risk factors (smoking, history of diabetes, waist circumference, blood pressure, triglycerides, and HDL-C to examine the associations of race, gender, total BDI-II score, and CRP. Covariates were selected based on their known association with CRP levels, as well as with depressive symptoms. History of diabetes was chosen rather than glucose because study visits were performed after fasting overnight, such that the use of serum glucose levels may overlook well treated diabetics. Stratifying by race and gender, analyses were conducted in the following steps: 1) total BDI-II score as the sole explanatory variable; 2) demographic factors were added; 3) metabolic risk factors were added. Further exploratory analyses were performed by adding the total PSQI score to Model 3 to test any effects of sleep quality on the associations. In order to display the magnitude of the difference in CRP levels with each adjustment, standardized beta coefficients were used. We computed covariate adjusted levels for CRP from multivariable models 2 and 3, and tested the significance of any differences in reverse-log transformed levels using Wilcoxon rank sum tests. All tests of statistical significance were 2-tailed, and P values <0.05 were considered significant. Statistical analyses were performed using SPSS, Inc. v17.0.
RESULTS
Subject characteristics
Demographic and clinical characteristics of the 512 subjects are presented in Table 1. Compared to Whites, African Americans were younger, less educated, and more likely to be smokers. African American women had higher BMI and blood pressure than White women. African Americans had lower triglyceride levels than Whites, while African American men had higher HDL-C levels than White men. Overall, women had higher median CRP levels than men, although this difference only reached statistical significance in African Americans (p=0.002). African American women had higher median CRP levels than White women, while there was no difference in CRP levels between African American and White men. African American women had higher median PSQI scores than White women, and there was a trend towards higher PSQI scores in African American men compared to White men.
Table 1.
Subject characteristics by race and sex.
| White Women N=166 | African American Women N=147 | p | White Men N=104 | African American Men N=95 | p | |
|---|---|---|---|---|---|---|
| Age (yrs) | 52±9 | 50±9 | 0.17 | 52±9 | 48±9 | 0.003 |
| Education | <0.001 | <0.001 | ||||
| --College Graduate | 113 (72%) | 61 (42%) | 64 (70%) | 25 (28%) | ||
| --Some college | 35 (22%) | 43 (30%) | 17 (18%) | 29 (33%) | ||
| --High School or GED | 10 (6%) | 40 (28%) | 11 (12%) | 34 (39%) | ||
| Current smokers | 16 (10%) | 24 (17%) | 0.08 | 13 (14%) | 29 (32%) | 0.005 |
| History of diabetes | 8 (5%) | 19 (13%) | 0.01 | 7 (8%) | 11 (13%) | 0.28 |
| Waist circumference (cm) | 92±17 | 99±15 | <0.001 | 103±14 | 101±16 | 0.36 |
| SBP (mm Hg) | 118±17 | 126±20 | <0.001 | 121±16 | 123±18 | 0.46 |
| DBP (mm Hg) | 75±10 | 80±12 | <0.001 | 79±11 | 81±11 | 0.39 |
| Triglycerides (mg/dL) | 127±80 | 96±37 | 0.001 | 155±91 | 100±45 | <0.001 |
| HDL-C (mg/dL) | 65±19 | 61±14 | 0.18 | 49±15 | 52±13 | 0.02 |
| Glucose (mg/dL) | 89±16 | 96±34 | 0.62 | 99±33 | 92±13 | 0.10 |
| CRP (mg/L) | 1.5 (0.6–3.6) | 2.2 (1.3–5.1) | <0.001 | 1.3 (0.8–2.8) | 1.4 (0.5–4.0) | 0.93 |
| Total BDI-II score | 5 (2–10) | 6 (2–13) | 0.79 | 5 (1–8) | 6 (2–13) | 0.03 |
| Moderate to severe depression | 9 (5%) | 14 (10%) | 0.17 | 11 (11%) | 5 (5%) | 0.17 |
| Total PSQI score | 5 (4–7) | 7 (4–9) | 0.002 | 5 (3–8) | 6 (4–10) | 0.056 |
Values shown are mean±SD, N (%), or median (IQR). Comparisons between Whites and African Americans were made using t-tests, Wilcoxon rank sum tests, or χ2. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; CRP, high sensitivity C-reactive protein; PSQI, Pittsburgh Sleep Quality Index.
Univariate associations between risk factors and depression
Lower levels of education were associated with higher BDI-II scores in both African American and White women, while history of diabetes and higher triglycerides were associated with higher BDI-II scores in White women only. Higher waist circumference was associated with higher BDI-II scores in Whites only. Poorer sleep quality was associated with higher BDI-II scores in all groups.
Association between depression and inflammation by race and gender
African American men had higher total BDI-II scores than White men, while there was no difference in women (Table 1). The overall prevalence of moderate to severe depression (BDI score ≥20), was low in the study population, and there was no difference by race or sex. Cronbach’s alpha coefficient of reliability was 0.9 for the entire population, with no significant differences by race or sex.
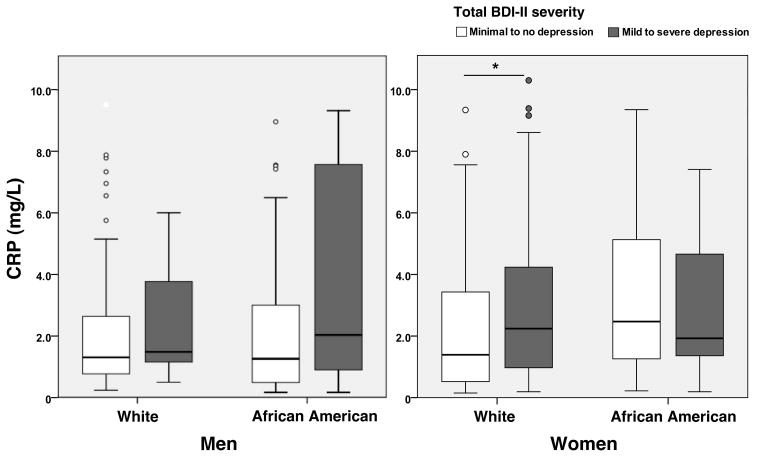
White women with depressive symptoms had higher levels of CRP compared to those with minimal to no depressive symptoms (p=0.03, Figure 1). However, there were no differences in levels of CRP by severity of depressive symptoms in White men or African Americans of either sex. In multivariable models, there was a significant race*gender*total BDI-II score interaction (β=−0.517, p=0.02). The interaction was attenuated (β=−0.423, p=0.07) after adjusting for age and education, and eliminated after adjusting for metabolic risk factors (β=−0.377, p=0.09).
Figure 1.
Median levels of CRP by severity of depression in men and women, stratified by race. Minimal to no depression was defined as total BDI-II score <14; mild to severe depression was defined as total BDI-II score ≥14. *p<.05. Samples include white men N=104, African American men N=95, white women N=166, and African American women N=147.
Race and gender stratified models confirmed a significant univariate association between the total BDI-II score and CRP levels in White women (β=0.263, p=0.001) but not African American women (β=−0.056, p=0.50), White men (β=0.092, p=0.35), or African American men (β=0.113, p=0.27). After adjusting for demographic factors in White women, total BDI-II score remained significantly associated with higher CRP levels (Table 3). However, this association was decreased after adjusting for metabolic risk factors. Variables associated with higher CRP levels in White women, which accounted for the association between BDI-II score and CRP, included waist circumference (β=0.361, p<0.001) and current smoking (β=0.183, p=0.02). In multivariate models, there was no association between the BDI-II score and CRP levels in African American women, or men of either race. Waist circumference was the strongest independent predictor of CRP levels in all groups, while age was an independent predictor of higher CRP in African American men (Tables 3 and 4).
Table 3.
Linear regression of BDI-II scores and lnCRP in women by race.
| White Women | African American Women | |||||
|---|---|---|---|---|---|---|
| β | t | p | β | t | p | |
| Model 1 | ||||||
| Total BDI | 0.263 | 3.47 | 0.001 | −0.056 | −0.68 | 0.50 |
|
| ||||||
| Model 2 | ||||||
| Total BDI | 0.227 | 2.76 | 0.006 | −0.055 | −0.64 | 0.53 |
| Age | 0.104 | 1.34 | 0.18 | −0.102 | −1.17 | 0.25 |
| Education | −0.022 | −0.27 | 0.79 | −0.008 | −0.09 | 0.93 |
|
| ||||||
| Model 3 | ||||||
| Total BDI | 0.077 | 0.93 | 0.35 | −0.099 | −1.22 | 0.23 |
| Age | −0.004 | −0.06 | 0.96 | −0.033 | −0.36 | 0.72 |
| Education | 0.116 | 1.43 | 0.15 | −0.003 | −0.03 | 0.98 |
| Smoking | 0.183 | 2.43 | 0.02 | 0.038 | 0.44 | 0.66 |
| Diabetes | 0.035 | 0.44 | 0.66 | −0.016 | −0.18 | 0.86 |
| Waist | 0.361 | 4.14 | <0.001 | 0.461 | 5.06 | <0.001 |
| SBP | 0.026 | 0.31 | 0.76 | −0.005 | −0.06 | 0.96 |
| TG | 0.109 | 1.19 | 0.24 | −0.129 | −1.31 | 0.19 |
| HDL-C | −0.086 | −0.99 | 0.33 | −0.162 | −1.74 | 0.08 |
Model 1 – unadjusted. Model 2 adjusts for Model 1 plus age and education. Model 3 adjusts for Model 2 plus metabolic risk factors (smoking, waist circumference, systolic blood pressure (SBP), triglycerides (TG), and HDL-C).
Table 4.
Linear regression of BDI-II scores and lnCRP in men by race.
| White Men | African American Men | |||||
|---|---|---|---|---|---|---|
| β | t | p | β | t | p | |
| Model 1 | ||||||
| Total BDI | 0.092 | 0.94 | 0.35 | 0.113 | 1.10 | 0.27 |
|
| ||||||
| Model 2 | ||||||
| Total BDI | 0.119 | 1.12 | 0.27 | 0.113 | 1.06 | 0.29 |
| Age | 0.131 | 1.24 | 0.22 | 0.239 | 2.27 | 0.03 |
| Education | −0.032 | −0.31 | 0.76 | 0.074 | 0.69 | 0.49 |
|
| ||||||
| Model 3 | ||||||
| Total BDI | 0.052 | 0.41 | 0.69 | 0.158 | 1.46 | 0.15 |
| Age | 0.184 | 1.49 | 0.14 | 0.239 | 2.14 | 0.04 |
| Education | 0.054 | 0.39 | 0.69 | −0.009 | −0.08 | 0.94 |
| Smoking | 0.217 | 1.63 | 0.11 | 0.096 | 0.88 | 0.38 |
| Diabetes | 0.077 | 0.61 | 0.55 | −0.168 | −1.44 | 0.15 |
| Waist | 0.243 | 1.73 | 0.09 | 0.458 | 3.88 | <0.001 |
| SBP | −0.033 | −0.26 | 0.79 | −0.185 | −1.69 | 0.09 |
| TG | 0.102 | 0.77 | 0.45 | −0.026 | −0.22 | 0.83 |
| HDL-C | 0.046 | 0.35 | 0.73 | −0.074 | −0.60 | 0.55 |
Model 1 – unadjusted. Model 2 adjusts for Model 1 plus age and education. Model 3 adjusts for Model 2 plus metabolic risk factors (smoking, waist circumference, systolic blood pressure (SBP), triglycerides (TG), and HDL-C).
Association of cognitive and somatic subscales, and sleep quality with inflammation
Race and gender stratified models confirmed a significant univariate association between the cognitive (β=0.218, p=0.005) and somatic (β=0.249, p=0.001) subscales and CRP levels in White women only. There were no associations of either subscale with CRP levels in African Americans, or White men. Consistent with the findings of the total BDI-II scores, both subscales remained significantly associated with CRP levels in White women after adjusting for demographic factors, but the association was eliminated after adjusting for metabolic risk factors. Similarly, adding the total PSQI scores to Model 3 to test for any additional effects of sleep quality on the associations between total BDI-II scores and CRP did not alter the findings.
DISCUSSION
In this bi-racial, community based population, we found an association between depression and CRP in White women only, and this association was mediated partly by waist circumference. Depression scores did not appear to contribute significantly to CRP levels in African Americans as compared to Whites, contrary to our hypothesis. These results demonstrate that the association between depression and inflammation depends on race and gender specific subgroups. They may also help explain why the association between depression and inflammation has been inconsistent in previous studies, since those study samples may have varied in their demographic composition.
Due to the cross-sectional design of the study, it is uncertain whether depressive symptoms lead to inflammation that then causes CVD, or whether inflammation secondary to CVD and/or its risk factors leads to depressive symptoms. It has been documented that peripheral inflammatory signals can access the brain and activate relevant cell types, such as microglia, that serve to amplify central inflammatory responses. Multiple mechanisms have been proposed by which inflammatory molecules access the brain and contribute to depression, including passage through the blood-brain barrier, transport via receptors, or activation of endothelial cells lining the cerebral vasculature (5, 27). However, it remains unclear whether inflammation originating in the periphery (e.g. as a function of medical illness) activates inflammatory pathways in the central nervous system (CNS) during depression, or whether psychological stress or other processes yet to be indentified induce inflammatory responses directly within the brain (5).
Multiple community based studies have shown that the association between depression and CRP varies by gender. Analyses from studies that only included women confirm an association between depressive symptoms and higher CRP (2, 28). Although some sex-specific analyses have confirmed an association between depression and inflammation in males (14, 15, 29), others have not (30). Indeed, a recent meta-analysis suggests that the relationship between depression and CRP may be stronger in males than females (31). We did not observe a significant association between depression and CRP in men, despite similar total BDI-II scores and prevalence of depressive symptoms among males and females in our sample.
Although we found an association between the total BDI-II score and subscales with CRP among White women, the association of depression with CRP was eliminated after controlling for metabolic risk factors in this group. These results suggest that behavioral and metabolic risk factors are, at least in part, mediators of this relationship. For example, studies that adjusted for waist and/or BMI have shown a much smaller association between depression and CRP than those studies that did not (14, 31, 32). This is similar to our findings, as waist circumference had the strongest association with CRP levels in all groups. Other analyses have shown significant effects of smoking, physical inactivity, and alcohol consumption on the association between depression and CRP (33). Furthermore, self-perception of overweight is more common in Whites than African Americans, and may be linked to depressive symptoms (24, 34). In particular, White women are more likely to be concerned and/or dissatisfied with their weight, and feel more pressure to be slim than are African American women (35, 36). Therefore, in White female participants, we found that the relationship between CRP and depression was driven by waist circumference and smoking, indicating that behavioral and metabolic factors that are often associated with depression are potential mechanisms underlying the association between depression and inflammation.
Epidemiologic studies have shown that African American race, in addition to female sex, is a significant predictor of higher levels of CRP (21, 22). However, there is a paucity of literature examining the association between depression and inflammation in racial/ethnic groups other than Whites. In the Multi-Ethnic Study of Atherosclerosis, depression scores tended to be associated with higher levels of CRP, IL-6, and fibrinogen, but only differences for IL-6 reached statistical significance (37). Although the authors stated that there were no consistent differences in the results by race/ethnicity or gender, they also suggested that the results were sensitive to the inclusion or exclusion of Chinese subjects in their analysis. Thus, analyses that adjust for race and sex may mask differences between depression and inflammation in these subgroups. More recent data from the CARDIA study prospectively confirmed that depressive symptoms at Year 15 predicted levels of CRP at Year 20 in African Americans, not Whites (24). The authors conclude that depressive symptoms may be more closely linked to inflammation in African Americans than Whites, which was our working hypothesis. However, there are distinct differences between this analysis and our data which could account for the conflicting findings. Compared to our study population, participants from CARDIA were younger, and had less comorbid conditions known to be associated with both depression and CRP. Because the association of depression and CRP is generally modest compared to that of other cardiovascular risk factors that are more prevalent in African Americans, such as obesity, diabetes and hypertension, it may be more difficult to detect an association between depression and CRP in this group. Furthermore, the authors note that the cross-sectional correlations between depressive symptoms and CRP at Years 15 and 20 were small, and failed to achieve statistical significance when examined separately in African Americans and Whites. Thus, the prospective association between depressive symptoms and CRP seems to have significance which may not be captured in our cross-sectional study.
We acknowledge a few limitations of our study. The overall prevalence of moderate to severe depression was low in our population, and we lacked information on a clinical diagnosis of major depression. However, even mild to modest levels of depressive symptoms have been associated with CRP and CVD in previous studies (31). We also lacked information on use of oral contraceptives and/or hormonal therapy, which are important determinants of CRP levels in women. According to a recent meta-analysis, the relationship between CRP and depression was much weaker in studies that utilized self-report instruments than in studies where depression was assessed using a clinical interview (31). Furthermore, although the BDI-II has been validated in small studies with African American populations (38), there are limited data in this group compared with Whites. These limitations may have increased the random error in our results, especially the results in African Americans. Moreover, we have only studied the association of depression and inflammation in two races, and these investigations need to be expanded to other ethnic groups. Finally, we excluded participants with a CRP of >15 mg/L, which was 2.5 SDs above the mean of our population. CRP >10 mg/L may be more specific for current infection(39), yet previous cross-sectional studies examining depressive symptoms and CRP have been inconsistent with regards to cutoff values for CRP. However, repeating the analysis excluding participants with CRP >10 mg/L (n=5) did not substantially change our findings.
In conclusion, ours is the first community-based study to report on the link between depression and inflammation separately in African American and Whites, as well as in men and women. We have described an association between depressive symptoms and CRP among White women; however we were unable to show that this association exists to a significant degree in African Americans or White men. Our findings emphasize the importance of performing research in diverse populations, as data in predominantly White cohorts may not be generalizable to other racial and ethnic groups.
Table 2.
Univariate Pearson correlations between total BDI-II scores and demographic and metabolic variables stratified by race and sex.
| White Women N=166 | African American Women N=147 | White Men N=104 | African American Men N=95 | |
|---|---|---|---|---|
| Age | −0.006 | −0.042 | 0.156 | −0.044 |
| Education | −0.243* | −0.216* | 0.004 | −0.153 |
| Smoking | 0.100 | 0.062 | −0.064 | 0.150 |
| History of diabetes | 0.204* | 0.012 | 0.141 | 0.106 |
| Waist circumference (cm) | 0.300** | 0.141 | 0.240* | 0.014 |
| SBP (mm Hg) | 0.120 | 0.140 | 0.147 | 0.093 |
| TG (g/dL) | 0.179* | 0.074 | 0.096 | 0.005 |
| HDL-C (g/dL) | −0.146 | −0.083 | 0.022 | 0.003 |
| Total PSQI score | 0.434** | 0.531** | 0.575** | 0.476** |
| CRP (mg/L) | 0.262** | −0.062 | 0.001 | 0.086 |
p<0.05,
p<0.001.
SBP, systolic blood pressure; TG, triglycerides; HDL-C, high density lipoprotein cholesterol, PSQI, Pittsburgh Sleep Quality Index.
Acknowledgments
The authors thank the META-Health study population, and Emory and Morehouse GCRC staff for their assistance and participation. Sources of funding: This work was supported by funding from NIH/NHLBI 1 U01 HL079156-01 (Quyyumi) and 1 U01 HL79214-01 (Gibbons); NIH, National Center for Research Resources (NCRR) Grant M01-RR00039 for the Emory General Clinical Research Center (GCRC) and NIH/NCRR 5P20RR11104 for the Morehouse CRC; NIH K24HL077506-06 (Vaccarino); and NIH/NCRR 5U54RR022814 (Din).
Abbreviations
- CVD
cardiovascular disease
- BDI-II
Beck Depression Inventory-II
- CRP
C-reactive protein
- BMI
body mass index
- HDL-C
high density lipoprotein cholesterol
Footnotes
Conflicts of Interest: None
Financial Disclosures: The authors have no financial conflicts of interest to disclose.
References
- 1.Shimbo D, Chaplin W, Crossman D, Haas D, Davidson KW. Role of Depression and Inflammation in Incident Coronary Heart Disease Events. The American Journal of Cardiology. 2005;96:1016–21. doi: 10.1016/j.amjcard.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, Inflammation, and Incident Cardiovascular Disease in Women With Suspected Coronary Ischemia: The National Heart, Lung, and Blood Institute-Sponsored WISE Study. Journal of the American College of Cardiology. 2007;50:2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 3.Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar SH, Lichtman J, Wenger NK, Vaccarino V for the PRI Depressive Symptoms After Acute Myocardial Infarction. Evidence for Highest Rates in Younger Women. Arch Intern Med. 2006;166:876–83. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- 4.Dinan TG. Inflammatory markers in depression. Current Opinion in Psychiatry. 2009;22:32–6. doi: 10.1097/YCO.0b013e328315a561. [DOI] [PubMed] [Google Scholar]
- 5.Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biological Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM. Novel Inflammatory Markers of Coronary Risk: Theory Versus Practice. Circulation. 1999;100:1148–50. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- 7.Libby P, Theroux P. Pathophysiology of Coronary Artery Disease. Circulation. 2005;111:3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 8.Elovainio M, Keltikangas JÄrvinen L, Pulkki RÅback L, Kivim, Ki M, Puttonen S, Viikari L, Nen L, Mansikkaniemi K, Viikari J, Raitakari OT. Depressive symptoms and C-reactive protein: The Cardiovascular Risk in Young Finns Study. Psychological Medicine. 2006;36:797–805. doi: 10.1017/S0033291706007574. [DOI] [PubMed] [Google Scholar]
- 9.Danner M, Kasl SV, Abramson JL, Vaccarino V. Association Between Depression and Elevated C-Reactive Protein. Psychosom Med. 2003;65:347–56. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- 10.Suarez EC. C-Reactive Protein Is Associated With Psychological Risk Factors of Cardiovascular Disease in Apparently Healthy Adults. Psychosom Med. 2004;66:684–91. doi: 10.1097/01.psy.0000138281.73634.67. [DOI] [PubMed] [Google Scholar]
- 11.Whooley MA, Caska CM, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and Inflammation in Patients With Coronary Heart Disease: Findings from the Heart and Soul Study. Biological Psychiatry. 2007;62:314–20. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews KA, Schott LL, Bromberger J, Cyranowski J, Everson-Rose SA, Sowers MF. Associations Between Depressive Symptoms and Inflammatory/Hemostatic Markers in Women During the Menopausal Transition. Psychosom Med. 2007;69:124–30. doi: 10.1097/01.psy.0000256574.30389.1b. [DOI] [PubMed] [Google Scholar]
- 13.Steptoe A, Kunz-Ebrecht SR, Owen N. Lack of association between depressive symptoms and markers of immune and vascular inflammation in middle-aged men and women. Psychological Medicine. 2003;33:667–74. doi: 10.1017/s0033291702007250. [DOI] [PubMed] [Google Scholar]
- 14.Elovainio M, Aalto A-M, Kivimaki M, Pirkola S, Sundvall J, Lonnqvist J, Reunanen A. Depression and C-Reactive Protein: Population-Based Health 2000 Study. Psychosom Med. 2009;71:423–30. doi: 10.1097/PSY.0b013e31819e333a. [DOI] [PubMed] [Google Scholar]
- 15.Ford DE, Erlinger TP. Depression and C-Reactive Protein in US Adults: Data From the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–4. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 16.Davidson KW, Schwartz JE, Kirkland SA, Mostofsky E, Fink D, Guernsey D, Shimbo D. Relation of Inflammation to Depression and Incident Coronary Heart Disease (from the Canadian Nova Scotia Health Survey [NSHS95] Prospective Population Study) The American Journal of Cardiology. 2009;103:755–61. doi: 10.1016/j.amjcard.2008.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohl M, Lesser IRA, Smith M. Clinical Presentations of Depression in African American and White Outpatients; [DOI] [PubMed] [Google Scholar]
- 18.Ayalon L, Young MA. A Comparison Of Depressive Symptons In African Americans And Caucasian Americans. Journal of Cross-Cultural Psychology. 2003;34:111–24. [Google Scholar]
- 19.Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, Maisano C, Jones L, Murrah NV, Vaccarino V. Depressive Symptoms and Metabolic Syndrome: Is Inflammation the Underlying Link? Biological Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolderen KG, Spertus JA, Reid KJ, Buchanan DM, Krumholz HM, Denollet J, Vaccarino V, Chan PS. The Association of Cognitive and Somatic Depressive Symptoms With Depression Recognition and Outcomes After Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2009;2:328–37. doi: 10.1161/CIRCOUTCOMES.109.868588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albert MA, Glynn RJ, Buring J, Ridker PM. C-Reactive protein levels among women of various ethnic groups living in the United States (from the Women’s Health Study) The American Journal of Cardiology. 2004;93:1238–42. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 22.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians JFH, Grundy SM, de Lemos JA. Race and Gender Differences in C-Reactive Protein Levels. Journal of the American College of Cardiology. 2005;46:464–9. doi: 10.1016/j.jacc.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 23.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janicki Deverts D, Cohen S, DiLillo VG, Lewis CE, Kiefe C, Whooley MA, Matthews KA. Depressive Symptoms, Race, and Circulating C-Reactive Protein: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2010;72:734–41. doi: 10.1097/PSY.0b013e3181ec4b98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in Psychiatric Outpatients. Journal of Personality Assessment. 1996;67:588. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews KA, Schott LL, Bromberger JT, Cyranowski JM, Everson-Rose SA, Sowers M. Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain, Behavior, and Immunity. 24:96–101. doi: 10.1016/j.bbi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luukinen H, Jokelainen J, Hedberg P. The relationships between high-sensitivity C-reactive protein and incident depressed mood among older adults. Scandinavian Journal of Clinical & Laboratory Investigation. 70:75–9. doi: 10.3109/00365510903410548. [DOI] [PubMed] [Google Scholar]
- 30.Hung YJ, Hsieh CH, Chen YJ, Pei D, Kuo SW, Shen DC, Sheu WHH, Chen YC. Insulin sensitivity, proinflammatory markers and adiponectin in young males with different subtypes of depressive disorder. Clinical Endocrinology. 2007;67:784–9. doi: 10.1111/j.1365-2265.2007.02963.x. [DOI] [PubMed] [Google Scholar]
- 31.Howren MB, Lamkin DM, Suls J. Associations of Depression With C-Reactive Protein, IL-1, and IL-6: A Meta-Analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 32.Douglas KM, Taylor AJ, O’Malley PG. Relationship Between Depression and C-Reactive Protein in a Screening Population. Psychosom Med. 2004;66:679–83. doi: 10.1097/01.psy.0000138132.66332.85. [DOI] [PubMed] [Google Scholar]
- 33.Hamer M, Molloy GJ, de Oliveira C, Demakakos P. Persistent depressive symptomatology and inflammation: To what extent do health behaviours and weight control mediate this relationship? Brain, Behavior, and Immunity. 2009;23:413–8. doi: 10.1016/j.bbi.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Paeratakul S, White MA, Williamson DA, Ryan DH, Bray GA. Sex, Race/Ethnicity, Socioeconomic Status, and BMI in Relation to Self-Perception of Overweight. Obesity. 2002;10:345–50. doi: 10.1038/oby.2002.48. [DOI] [PubMed] [Google Scholar]
- 35.Powell AD, Kahn AS. Racial differences in women’s desires to be thin. International Journal of Eating Disorders. 1995;17:191–5. doi: 10.1002/1098-108x(199503)17:2<191::aid-eat2260170213>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 36.Kemper K, Sargent R, Drane J, Valois R, Hussey J. Black and white females’ perceptions of ideal body size and social norms. Obes Res. 1994;2:117–26. doi: 10.1002/j.1550-8528.1994.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 37.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, Ni H. Psychosocial Factors and Inflammation in the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2007;167:174–81. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 38.Grothe KB, Dutton GR, Jones GN, Bodenlos J, Ancona M, Brantley PJ. Validation of the Beck Depression Inventory-II in a Low-Income African American Sample of Medical Outpatients. Psychological Assessment. 2005;17:110–4. doi: 10.1037/1040-3590.17.1.110. [DOI] [PubMed] [Google Scholar]
- 39.Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: A Statement for Healthcare Professionals From the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]